Abstract
Serine/arginine-rich splicing factors (SR proteins) are substrates for serine phosphorylation that can regulate SR protein function. We have observed gross changes in SR protein phosphorylation during early development coincident with major zygotic gene activation in the nematode Ascaris lumbricoides. These differences correlate with large-scale changes in SR protein activity in promoting both trans- and cis-splicing. Importantly, inactive early stage extracts can be made splicing competent on addition of later stage SR proteins. These data suggest that changes in SR protein phosphorylation have a role in the activation of pre-mRNA splicing during early development.
Keywords: SR protein, splicing factor, phosphorylation, development
SR proteins are a family of conserved, essential pre-mRNA splicing factors (Zahler et al. 1992). Each of these related proteins contains an amino-terminal RNA recognition motif (RRM), followed, in some cases, by a second degenerate RRM, and end in a carboxy-terminal domain consisting of multiple RS dipeptides (for review, see Cáceres and Krainer 1997). SR proteins are modular in nature, with the RRM alone able to bind RNA (Tacke and Manley 1995) and the RS domain able to activate splicing even when fused to a heterologous RNA-binding domain (Graveley and Maniatis 1998). Functionally, SR proteins are required for basic, constitutive pre-mRNA splicing (Zahler et al. 1992), as well as for numerous alternative splicing events (for review, see Cáceres and Krainer 1997). Several properties of SR proteins contribute to these two roles. First, SR proteins are able to interact with other RS domain-containing splicing factors (Wu and Maniatis 1993; Kohtz et al. 1994), which can lead to functional association of 5′ and 3′ splice sites, even when those sites are present on separate molecules (Bruzik and Maniatis 1995; Chiara and Reed 1995; Stark et al. 1998). Second, SR proteins bind to cis-acting elements in alternatively spliced pre-mRNAs. These splicing enhancers are generally located in downstream exons, are purine rich, and have been demonstrated to directly interact with SR proteins in a number of instances (for review, see Manley and Tacke 1996). Interestingly, if the cis-acting element is positioned within the intron, interaction with SR proteins can actually lead to the opposite effect, that is, splicing repression (Kanopka et al. 1996). Finally, the RS domain of SR proteins is highly phosphorylated (Roth et al. 1990), with the level of serine phosphorylation able to alter SR protein activity (Cao et al. 1997; Xiao and Manley 1997; Kanopka et al. 1998). This last feature allows for the modulation of SR protein activity in either constitutive or alternative splicing events.
Changes in the phosphorylation of splicing components occurs at the level of the individual reaction as well as in a broader, cell-wide manner (for review, see Mermoud et al. 1994a). Phosphatase inhibitors have been used to demonstrate that dephosphorylation is important for single splicing reactions (Mermoud et al. 1992; Tazi et al. 1992). Specific phosphatases including protein phosphatase 1 (PP1) have been shown to affect in vitro splicing (Mermoud et al. 1992, 1994a,b; Cardinali et al. 1994; Cao et al. 1997). In addition, thiophosphorylation of splicing factors interferes with pre-mRNA splicing (Tazi et al. 1992, 1993; Xiao and Manley 1998). More directed experiments have demonstrated that the state of phosphorylation of SR proteins in particular can be linked to splicing activity in vitro. Several SR protein kinases have been described, including SR protein kinase 1 (SRPK1), Clk/Sty, SRPK2, and a kinase activity of DNA topoisomerase I (for review, see Misteli and Spector 1998). Hyperphosphorylation with SRPK1 can inhibit splicing (Gui et al. 1994a), as can dephosphorylation of SR proteins (Cao et al. 1997); thus, an intermediate level of phosphorylation is required for splicing in vitro (Cao et al. 1997; Xiao and Manley 1997). In addition, dephosphorylation of SR proteins appears to be required during constitutive splicing reactions but not for their ability to act as splicing activators in regulated splicing (Xiao and Manley 1998).
Broad changes in SR protein phosphorylation are also seen in response to events that affect the entire cell. Hyperphosphorylation of SR proteins occurs during M phase of the cell cycle in which splicing is quiescent (Roth et al. 1990; Gui et al. 1994a). Recently, large-scale dephosphorylation of SR proteins was observed in late adenovirus-infected HeLa cells (Kanopka et al. 1998). In Drosophila, defects in SR protein phosphorylation have been correlated with discrete phenotypes in the sex determination pathway (Du et al. 1998). Changes in SR protein phosphorylation have also altered their subnuclear localization, with increases in either SRPK1 or Clk/Sty leading to diffusion of speckles and PP1 also altering the speckled morphology (for review, see Misteli and Spector 1998). In addition, hyperphosphorylation of SF2/ASF leads to its accumulation in the cytoplasm (Cáceres et al. 1998).
The nematode Ascaris lumbricoides has been used as a model system to study both trans- and cis-splicing (Hannon et al. 1990, 1991). Active splicing extracts can be prepared from embryos that have been allowed to develop beyond the 16-cell stage (T. Nilsen, pers. comm.). We have demonstrated that SR proteins are required in vitro for both trans- and cis-splicing in A. lumbricoides by developing an SR protein-depleted whole-cell extract (Sanford and Bruzik 1999). Because SR proteins can act as overall regulators of pre-mRNA splicing activity (for review, see Cáceres and Krainer 1997), we examined changes in SR proteins over this developmental time span.
Here, we demonstrate that SR proteins in synchronously developing embryos of the nematode A. lumbricoides undergo dephosphorylation, reaching an intermediate state of phosphorylation concomitant with major zygotic gene activation (ZGA). Functionally, this decrease in phosphorylation leads to SR protein activity in both trans- and cis-splicing assays. Taken together, these observations suggest that regulation of SR protein phosphorylation has a role in the activation of pre-mRNA splicing during early development.
Results
A. lumbricoides SR protein activity is developmentally regulated
We took advantage of the ability to grow synchronous embryos from the nematode A. lumbricoides and prepared SR proteins at different early stages of development. The stages represented, from 1 to ∼32 cells, bracket the major activation of gene expression in A. lumbricoides embryos, which occurs at the 4- to 8-cell stage (Cleavinger et al. 1989; Spicher et al. 1994). Equal amounts of SR proteins from embryos at 1-, 2- to 4-, and ∼32-cell stages were examined by probing a Western blot with the anti-SR protein monoclonal antibody 104 (Roth et al. 1990) (Fig. 1A). The SR proteins isolated from A. lumbricoides display an array of sizes from ∼17 to ∼70 kD. Some SR proteins, that is, aSRp70, appear to generate a more intense signal over time; however, the bulk of the detectable proteins are present in similar amounts. As development proceeds, the mobility of these factors increases [Fig. 1A, cf. lanes 1–3, especially see the 30- to 38-kD protein(s)]. This experiment demonstrates that SR proteins are present in embryos prior to the major onset of gene expression.
Figure 1.
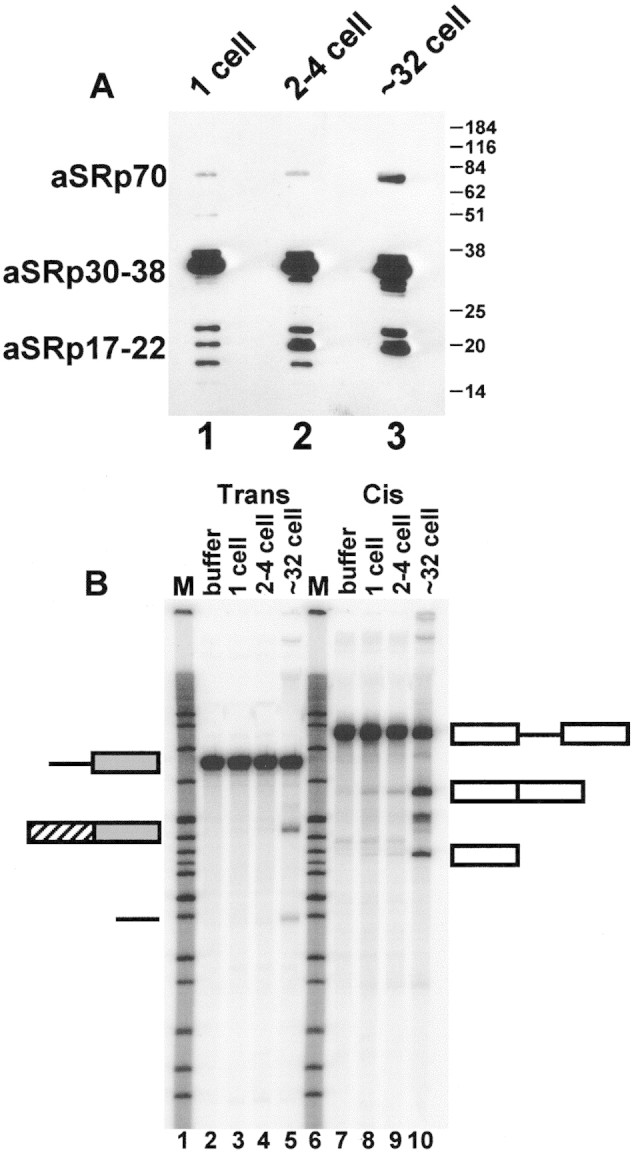
SR proteins are present throughout early development, but their activity changes. (A) Equal amounts of SR proteins prepared from 1-, 2- to 4-, and ∼32-cell embryos of the nematode A. lumbricoides were examined by Western blotting with mAb104 (Roth et al. 1990). (B) Only SR proteins from ∼32-cell embryos can rescue trans- and cis-splicing in an SR protein-depleted extract. Splicing reactions were performed as described. Appreciable levels of trans- and cis-splicing were rescued on addition of ∼32-cell-stage SR proteins but not with earlier-stage proteins. Appreciable kinase or phosphatase activity is not seen in the whole cell SR protein-depleted extract (J.R. Sanford and J.P. Bruzik, unpubl.). Open rectangles represent the cis-splicing construct, shaded rectangles represent trans-acceptor exon, and the hatched rectangle represents the trans-spliced leader exon.
SR proteins play a critical role in splice site interaction (for reviews, see Bruzik 1996; Reed 1996), as well as in spliceosome assembly (for review, see Will and Lührmann 1997). Therefore, we assayed the activity of SR proteins from each developmental stage in trans- and cis-splicing reactions. Because splicing extracts from A. lumbricoides can only be prepared from whole cells (Hannon et al. 1990), methods for making S100 (cytoplasmic) fractions, which are deficient in SR proteins, from mammalian cells are not applicable. We have developed an SR protein-depleted whole cell extract that has no splicing activity unless supplemented with exogenous SR proteins (Sanford and Bruzik 1999) (see, e.g., Fig. 1B, lanes 2 and 7 for trans- and cis-splicing, respectively). SR proteins from each of the three stages examined were added in equal amounts to a depleted whole cell extract (Fig. 1B). Only when SR proteins prepared from ∼32-cell-stage embryos were added to the reaction was appreciable trans- (lane 5) and cis-splicing (lane 10) activity observed. Depleted extract supplemented with one- or two- to four-cell-stage SR proteins shows less trans- (lanes 3,4) and cis-splicing activity (lanes 8,9). Also, addition of early (1-cell stage) SR proteins to splicing-competent ∼32-cell extracts does not inhibit pre-mRNA splicing (data not shown). These experiments demonstrate that, although SR proteins are present at each of these early stages, their activity in promoting in vitro trans- and cis-splicing is developmentally regulated.
Developmental regulation of SR protein phosphorylation
As indicated earlier, the phosphorylation state of SR proteins can have dramatic effects on pre-mRNA splicing (Cao et al. 1997; Xiao and Manley 1997, 1998; Du et al. 1998; Kanopka et al. 1998). Therefore, we hypothesized that the developmental control of SR protein activity observed in Figure 1B might be exerted at the level of SR protein phosphorylation. To test this hypothesis, we resolved SR proteins from 1-, 2- to 4-, and ∼32-cell stages on two-dimensional gels (Fig. 2). It is apparent that the diffuse signal in the acidic region of the gel present when 1-cell-stage SR proteins are examined (Fig. 2, top) shifts to an intermediate form at the 2- to 4-cell stage (center), and collapses into the more neutral region when the proteins are prepared from ∼32-cell embryos (bottom). Difficulties in visualizing some of the SR proteins at later stages is probably due to heterogeneity in their state of phosphorylation; however, the effects on the predominant SR proteins are clear. These mobility shifts are consistent with the occurrence of dephosphorylation during development.
Figure 2.
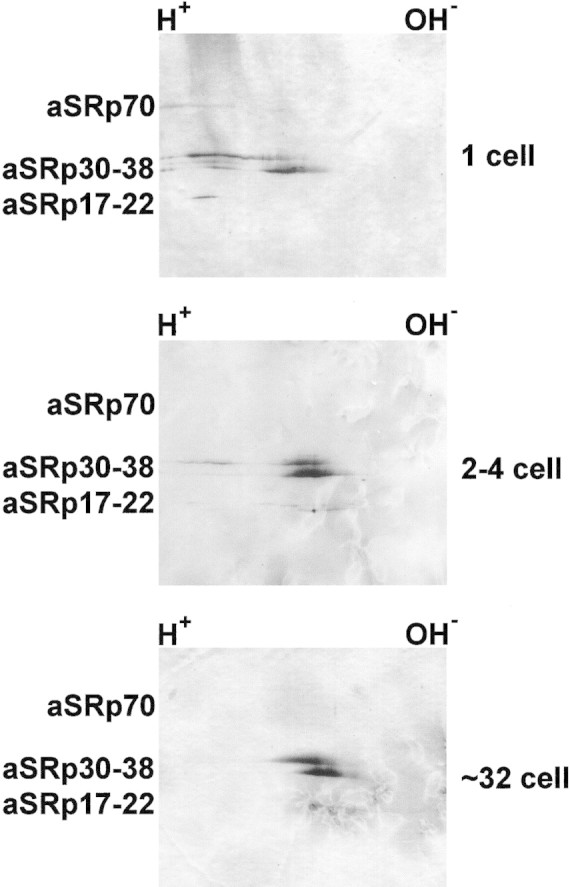
Direct analysis of changes in SR protein phosphorylation. Western blots of two-dimensional gels were probed with mAb104 (Roth et al. 1990). As in Fig. 1, equal amounts of SR proteins from the 1-, 2- to 4-, and ∼32-cell stages were examined. Proteins containing more phosphate run to the acidic (H+) side of the gel. Not all protein species are detectable at all developmental stages after separation in two dimensions.
To further assess their phosphorylation state, SR proteins at these different stages were phosphorylated with the SR protein-specific kinase SRPK1 (Gui et al. 1994a) and [γ32P]ATP (Fig. 3A). Over this developmental span, the SR proteins became progressively better substrates for SRPK1 (lanes 1–3). SR proteins that are hyperphosphorylated do not incorporate label, whereas the same proteins that have undergone partial dephosphorylation are labeled more efficiently. To demonstrate that this difference was the result of limited dephosphorylation and not a mass effect, we performed phosphorylation reactions after first dephosphorylating the SR preparations with PP1. Under these conditions, there is an equivalent amount of phosphate incorporated into SR proteins prepared from each of the three developmental stages (Fig. 3B, lanes 1–3). A mock reaction containing only PP1 and SRPK1 shows no detectable labeled proteins (lane 4). In summary, each of these assays indicates that SR proteins undergo dephosphorylation concomitant with the major activation of gene expression.
Figure 3.
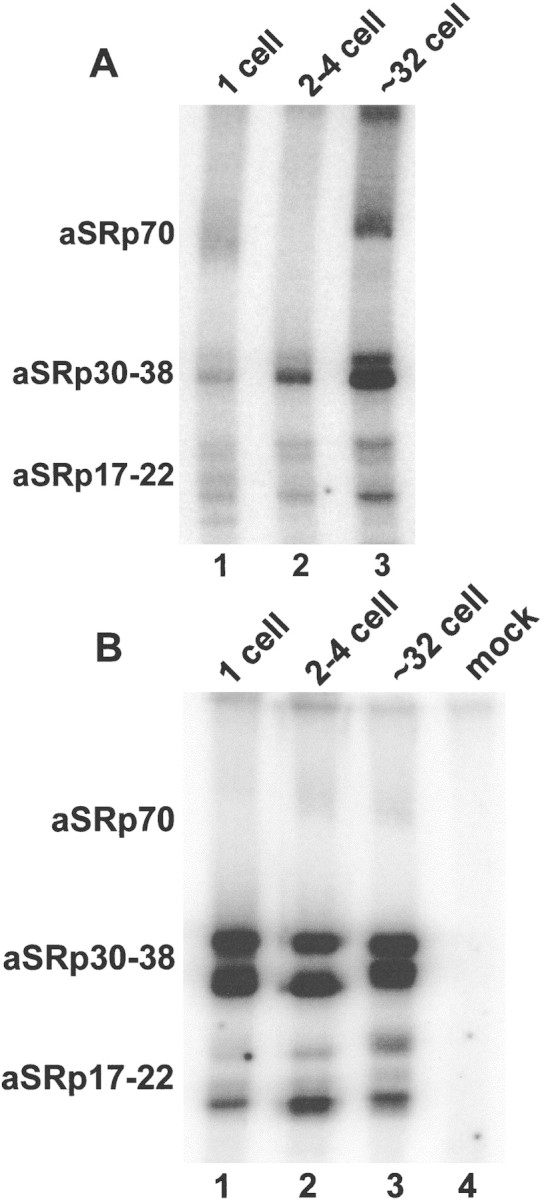
SR proteins become better substrates for SRPK1 during early development. (A) SR proteins from the indicated points in development were labeled with SRPK1 and [γ32P]ATP (Gui et al. 1994a). The amount of label incorporated is inversely proportional to the native level of phosphorylation. (B) The same amount of SR proteins labeled in A were first dephosphorylated with PP1 and then labeled with SRPK1 and [γ32P]ATP, demonstrating that equal amounts of protein are present in each lane of both A and B. Note that in both kinase assays (A,B) aSRp70 does not label efficiently.
Inactive early-stage whole cell extracts can be made splicing competent on addition of later-stage SR proteins
In addition to examining the activity of SR proteins prepared at different stages of development when added to depleted whole cell extracts, we have investigated whether SR proteins purified from later stages are able to rescue the splicing defects of early stage whole cell extracts. The splicing activites of 2- to 4- and ∼32-cell stage extracts are compared in Figure 4A. Extracts prepared from 2- to 4-cell embryos are deficient in both trans- (lanes 2,3) and cis-splicing (lanes 7,8), whereas extracts prepared from ∼32-cell embryos are active in both reactions (lanes 4,5, trans-splicing; lanes 9,10, cis-splicing) (Hannon et al. 1990). Extracts from 2- to 4-cell embryos can be rescued for trans- and cis-splicing activity on addition of SR proteins from ∼32-cell embryos (Fig. 4B, cf. lane 3 to lanes 4–6, trans-splicing; cf. lane 9 to lanes 10–12, cis-splicing). Earlier-stage SR proteins are inefficient at rescuing splicing (data not shown), comparable with the levels seen in the SR protein-depleted extract (see Fig. 1B). Extracts from one-cell embryos can also be rescued but show slightly lower activity after supplementation (data not shown). These results are consistent with the idea that the splicing defect in two- to four-cell-stage extracts is due to hyperphosphorylation of early-stage SR proteins.
Figure 4.
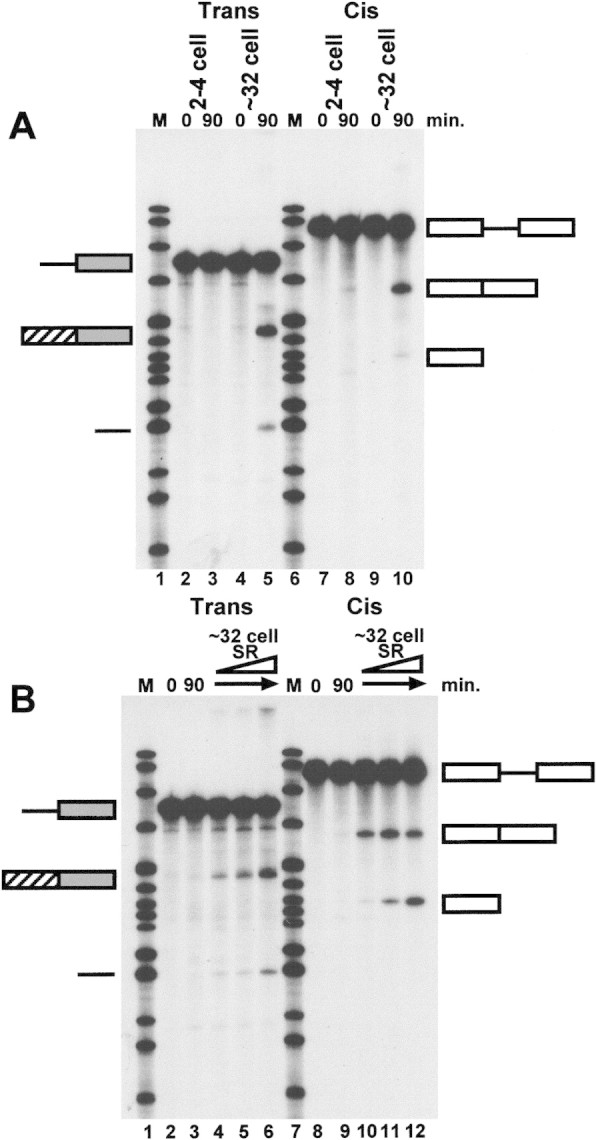
Inactive, early (2- to 4-cell-stage) extracts can be made splicing competent on addition of later-stage SR proteins. (A) Comparison of trans- and cis-splicing activity in 2- to 4- and ∼32-cell-stage whole cell extracts. (B) Two- to 4-cell-stage whole cell extracts can efficiently perform trans- and cis-splicing if supplemented with ∼32-cell-stage SR proteins. Splicing constructs are represented schematically as in Fig. 1B.
Discussion
We have shown that the state of phosphorylation of SR proteins changes during early development in nematodes. Initially, in embryos prior to major ZGA (Cleavinger et al. 1989; Spicher et al. 1994), SR proteins exist in a hyperphosphorylated form. Concurrent with major ZGA, the SR proteins undergo partial dephosphorylation, reaching an intermediate state of phosphorylation by the four- to eight-cell stage. These changes in phosphorylation directly correlate with the activity of SR proteins in both in vitro trans- and cis-splicing assays in an SR protein-depleted whole cell extract. SR proteins prepared from early embryos (prior to the 4- to 8-cell stage) are inefficient at rescuing splicing, whereas SR proteins prepared from later stages (∼32-cell embryos) are able to promote both trans- and cis-splicing. In addition, we have demonstrated that the splicing defect in pre-ZGA whole cell extracts can be overcome by the addition of SR proteins prepared from embryos that have developed beyond ZGA. Thus, we have determined that the state of phosphorylation of SR proteins in a developing embryo can determine the overall splicing activity at discrete stages of the developmental program.
Dramatic alterations in the state of phosphorylation of a cell’s total complement of SR proteins has been observed in several cases. As cells progress through the cell cycle, immunofluorescence staining has shown that the signal observed with mAb104 becomes more intense during mitosis (Roth et al. 1990). In addition, SR proteins prepared from in vivo 32P-labeled HeLa cells arrested at metaphase are three- to fivefold more phosphorylated than the same factors purified from interphase cells (Gui et al. 1994a). The SR protein kinase activity of HeLa cell extracts prepared from these same arrested stages shows a similar pattern, with metaphase extracts able to phosphorylate SR protein targets three- to fivefold more than other phases (Gui et al. 1994a). Recently, large-scale dephosphorylation of SR proteins was observed in late adenovirus-infected HeLa cells (Kanopka et al. 1998). In this case the virus encodes a phosphatase regulator, E4-ORF4, that can promote dephosphorylation of the endogenous HeLa cell SR proteins. Here, SR proteins at an active level of phosphorylation are further dephosphorylated to the point that they exhibit reduced activity in both splicing enhancement and splicing repression assays (Kanopka et al. 1998). Thus, it appears that any major deviation from an active, intermediate level of SR protein phosphorylation, whether it involves hyperphosphorylation (i.e., metaphase of the cell cycle or early development prior to ZGA) or hypophosphorylation (i.e., late in adenovirus infection) can lead to a decrease in SR protein activity.
In addition to the loss of splicing activity that hyperphosphorylated SR proteins exhibit, they also are altered with respect to their subcellular localization. Upon increases in either SRPK1 (Gui et al. 1994a), or Clk/Sty (Colwill et al. 1996b), nuclear speckles become diffuse. In addition, hyperphosphorylation of certain shuttling SR proteins can lead to their accumulation in the cytoplasm (Cáceres et al. 1998). Preliminary results suggest that the changes in SR protein phosphorylation that we have documented here correlate with changes in their subcellular localization as nuclear, speckled distribution is only observed beyond the four- to eight-cell stage (J.R. Sanford and J.P. Bruzik, unpubl.).
Dynamic changes in phosphorylation appear to be important for spliceosomal components as well as for the carboxy-terminal domain (CTD) of RNA polymerase II (Pol II). At the level of the individual splicing reaction, dephosphorylation of ASF/SF2 was demonstrated to be required for the transition from spliceosomal complexes to the first transesterification (Cao et al. 1997). In addition, it was shown recently that the non-RS domain-containing splicing factor, spliceosome-associated protein 155, is phosphorylated at or immediately after the first transesterification (Wang et al. 1998). During individual transcription reactions, high levels of Pol II CTD phosphorylation are required for elongation, whereas a decrease in phosphorylation is necessary for termination (for review, see Greenblatt 1997). In addition, changes in phosphorylation of the Pol II CTD have been reported during early development (Bellier et al. 1997) as is the case here with SR proteins. Finally, both SR proteins and the Pol II CTD can serve as substrates for multiple kinases. In the case of the CTD, there is temporal regulation of phosphorylation. The Srb10 kinase acts as a negative regulator of Pol II prior to the formation of the initiation complex, whereas the TFIIH kinase (Kin28) exerts a positive effect on transcription by phosphorylating the Pol II CTD while it is bound to a promoter, allowing for subsequent elongation (Hengartner et al. 1998). In the case of SR proteins, the potential for different roles of phosphorylation by SRPK1 and Clk/Sty has been suggested (Colwill et al. 1996a). SRPK1 might have a more global role in the control of general splicing activity, whereas Clk/Sty activity might manifest itself in the process of alternative splice site selection (Colwill et al. 1996a). Thus, in addition to the increasing number of identified interactions between components of the transcriptional and RNA processing machineries, there appears to be an extensive utilization of reversible protein phosphorylation to regulate both processes.
In summary, we have shown that the state of phosphorylation of SR proteins functionally changes during early development in nematodes from an inactive, hyperphosphorylated form to an active intermediate level of phosphorylation. The activities of both whole cell extracts and SR proteins prepared at different early stages are consistent with the same window of trans- and cis-splicing activation. Thus, our data suggest that the control of SR protein phosphorylation is likely to be a means by which splicing is activated coordinate with the onset of gene expression during early development.
Materials and methods
Protein purification
SR proteins were purified with ammonium sulfate/magnesium precipitation as described previously (Zahler et al. 1992), except for the following modification. To purify SR proteins from one- and two- to four-cell A. lumbricoides embryos, ammonium sulfate was added to exactly 93.5% saturation following the standard first-step precipitation. SR proteins from ∼32-cell-stage were quantified by the Bradford assay (Bio-Rad). Although the concentration of developmentally staged SR protein preparations varied, the relative ratios of 1-, 2- to 4-, and ∼32-cell-stage SR proteins were normalized by Coomassie staining as well as by 32P incorporation in dephosphorylation/rephosphorylation assays (see below). These ratios were maintained for all subsequent assays of activity and phosphorylation state.
GST-tagged SRPK1 in the pAcG2T Baculovirus Transfer Vector (Pharmingen, provided by X.-D. Fu, University of California, San Diego, CA) was used to construct a recombinant baculovirus. The fusion protein was expressed in SF9 cells. GST–SRPK1 was affinity purified with glutathione–Sepharose 4B following the manufacturer’s instructions (Pharmacia). The unit definition was determined as described previously (Gui et al. 1994b).
Western blot analysis of A. lumbricoides SR proteins
SR proteins purified from 1-, 2- to 4-, and ∼32-cell A. lumbricoides embryos were separated by 12% SDS-PAGE and transferred to nitrocellulose (Immobilon NC, Millipore). The membrane was then probed with the SR protein-specific mAb104 (Roth et al. 1990) and detected with the BM Chemiluminescence blotting system (anti-mouse IgG/IgM POD-conjugated secondary antibody, Boehringer Mannheim). For two-dimensional gel analysis, 8 μg of SR proteins purified from 1-, 2- to 4-, and ∼32-cell stages were separated on Immobiline Drystrip gels with a linear 3–10 pH gradient (Pharmacia) and then on ExelGel SDS 8%–18% acrylamide gradient precast gels (Pharmacia). Following two-dimensional gel electrophoresis, SR proteins were transferred and probed as described above. SR proteins were visualized, following incubation with anti-mouse immunoglobulin–biotin-conjugated secondary antibody (Boehringer Mannheim), by biotinylated alkaline phosphatase/streptavidin complex (BioRad) and detected by colorimetric assay (Promega).
In vitro kinase reactions
In vitro kinase assays of 1-, 2- to 4-, and ∼32-cell SR proteins (800 ng) were performed as described previously (Gui et al. 1994a). For dephosphorylation reactions (10 μl), SR proteins (800 ng) were incubated with protein phosphatase 1γ (0.64 units, GIBCO-BRL), in kinase buffer (50 mm Tris-HCl at pH 7.5, 10 mm MgCl2, 1 mm DTT) for 60 min at 37°C. The reactions were then stopped by boiling for 5 min and placed immediately on ice. Rephosphorylation of the dephosphorylated SR proteins was performed by adding 10 μl of kinase mixture (1 unit of GST–SRPK1, 50 mm Tris-HCl at pH 7.5, 10 mm MgCl2, 1 mm DTT, 2 mm ATP, 2 μCi of [γ32P]ATP) and incubating for 15 min at room temperature. The proteins were then resolved by 12% SDS-PAGE and visualized by autoradiography.
Preparation of extracts and in vitro splicing assays
Trans-splicing acceptor RNA was described previously (Hannon et al. 1990), as was the cis-splicing substrate (Hannon et al. 1991). Pre-mRNA substrates were transcribed in vitro as described previously (Hannon et al. 1990). Whole cell extracts were prepared as described (Hannon et al. 1990) from 1-, 2- to 4-, and ∼32-cell A. lumbricoides embryos. The activity of developmentally staged SR proteins (1 μg) was assayed in SR protein-depleted whole cell extract (Sanford and Bruzik 1999). All in vitro splicing assays with either developmentally staged or SR protein-depleted whole cell extracts were performed as described previously (Hannon et al. 1990).
Acknowledgments
We thank X.-D. Fu for both purified SRPK1 and the SRPK1 expression vector, S. Nesich for overexpression of SRPK1, T. Nilsen for splicing constructs, and X.-D. Fu, T. Maniatis, T. Nilsen, F. Rottman, J. Steitz, J.A. Wise, and members of the Bruzik laboratory for comments on the manuscript. This research was supported by Burroughs Wellcome Fund New Investigator Award in Molecular Parasitology (no. 0523 to J.P.B.) and by National Institutes of Health grant GM-54204 (J.P.B.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jxb83@po.cwru.edu; FAX (216) 368-3033.
References
- Bellier S, Chastant S, Adenot P, Vincent M, Renard JP, Bensaude O. Nuclear translocation and carboxy-terminal domain phosphorylation of RNA polymerase II delineate the two phases of zygotic gene activation in mammalian embryos. EMBO J. 1997;16:6250–6262. doi: 10.1093/emboj/16.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzik JP. Splicing glue: A role for SR proteins in trans splicing? Microb Pathog. 1996;21:149–155. doi: 10.1006/mpat.1996.0050. [DOI] [PubMed] [Google Scholar]
- Bruzik JP, Maniatis T. Enhancer-dependent interaction between 5′ and 3′ splice sites in trans. Proc Natl Acad Sci. 1995;92:7056–7059. doi: 10.1073/pnas.92.15.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres JF, Krainer AR. Mammalian pre-mRNA splicing factors. In: Krainer AR, editor. Eukaryotic mRNA processing. New York, NY: Oxford University Press; 1997. pp. 174–212. [Google Scholar]
- Cáceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes & Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Jamison SF, Garcia-Blanco MA. Both phosphorylation and dephosphorylation of ASF/SF2 are required for pre-mRNA splicing in vitro. RNA. 1997;3:1456–1467. [PMC free article] [PubMed] [Google Scholar]
- Cardinali B, Cohen PTW, Lamond AI. Protein phosphatase 1 can modulate alternative 5′ splice site selection in a HeLa splicing extract. FEBS Lett. 1994;352:276–280. doi: 10.1016/0014-5793(94)00973-2. [DOI] [PubMed] [Google Scholar]
- Chiara MD, Reed R. A two-step mechanism for 5′ and 3′ splice-site pairing. Nature. 1995;375:510–513. doi: 10.1038/375510a0. [DOI] [PubMed] [Google Scholar]
- Cleavinger PJ, McDowell JW, Bennett KL. Transcription in nematodes: Early Ascarisembryos are transcriptionally active. Dev Biol. 1989;133:600–604. doi: 10.1016/0012-1606(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Colwill K, Feng LL, Yeakley JM, Gish GD, Cáceres JF, Pawson T, Fu X-D. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for Serine/Arginine-rich splicing factors. J Biol Chem. 1996a;271:24569–24575. doi: 10.1074/jbc.271.40.24569. [DOI] [PubMed] [Google Scholar]
- Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, Duncan PI. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 1996b;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- Du C, McGuffin ME, Dauwalder B, Rabinow L, Mattox W. Protein phosphorylation plays an essential role in the regulation of alternative splicing and sex determination in Drosophila. Mol Cell. 1998;2:741–750. doi: 10.1016/s1097-2765(00)80289-0. [DOI] [PubMed] [Google Scholar]
- Graveley BR, Maniatis T. Arginine/serine-rich domains of SR proteins can function as activators of pre-mRNA splicing. Mol Cell. 1998;1:765–771. doi: 10.1016/s1097-2765(00)80076-3. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- Gui J-F, Lane WS, Fu X-D. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994a;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- Gui J-F, Tronchére H, Chandler SD, Fu X-D. Purification and characterization of a kinase specific for the serine and arginine-rich pre-mRNA splicing factors. Proc Natl Acad Sci. 1994b;91:10824–10828. doi: 10.1073/pnas.91.23.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ, Maroney PA, Denker JA, Nilsen TW. Trans splicing of nematode pre-messenger RNA in vitro. Cell. 1990;61:1247–1255. doi: 10.1016/0092-8674(90)90689-c. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Maroney PA, Nilsen TW. U small nuclear ribonucleoprotein requirements for nematode cis and trans splicing in vitro. J Biol Chem. 1991;266:22792–22795. [PubMed] [Google Scholar]
- Hengartner CJ, Myer VE, Liao S-M, Wilson CJ, Koh SS, Young RA. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- Kanopka A, Mühlemann O, Akusjärvi G. Inhibition by SR proteins of splicing of a regulated adenovirus pre-mRNA. Nature. 1996;381:535–538. doi: 10.1038/381535a0. [DOI] [PubMed] [Google Scholar]
- Kanopka A, Mühlemann O, Peterson-Mahrt S, Estmer C, Öhrmalm C, Akusjärvi G. Regulation of adenovirus alternative RNA splicing by dephosphorylation of SR proteins. Nature. 1998;393:185–187. doi: 10.1038/30277. [DOI] [PubMed] [Google Scholar]
- Kohtz JD, Jamison SF, Will CL, Zuo P, Lührmann R, Garcia-Blanco MA, Manley JL. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature. 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- Manley JL, Tacke R. SR proteins and splicing control. Genes & Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- Mermoud JE, Cohen P, Lamond AI. Ser/Thr-specific protein phosphatases are required for both catalytic steps of pre-mRNA splicing. Nucleic Acids Res. 1992;20:5263–5269. doi: 10.1093/nar/20.20.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermoud JE, Calvio C, Lamond AI. Uncovering the role of ser/thr protein phosphorylation in nuclear pre-mRNA splicing. Adv Protein Phosphatases. 1994a;8:99–118. [Google Scholar]
- Mermoud JE, Cohen PTW, Lamond AI. Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J. 1994b;13:5679–5688. doi: 10.1002/j.1460-2075.1994.tb06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T, Spector DL. The cellular organization of gene expression. Curr Opin Cell Biol. 1998;10:323–331. doi: 10.1016/s0955-0674(98)80007-0. [DOI] [PubMed] [Google Scholar]
- Reed R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr Opin Genet Dev. 1996;6:215–220. doi: 10.1016/s0959-437x(96)80053-0. [DOI] [PubMed] [Google Scholar]
- Roth MB, Murphy C, Gall JG. A monoclonal antibody that recognizes a phosphorylated epitope stains lampbrush chromosome loops and small granules in the amphibian germinal vesicle. J Cell Biol. 1990;111:2217–2223. doi: 10.1083/jcb.111.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, J.R. and J.P. Bruzik. 1999. SR proteins are required for nematode trans-splicing in vitro. RNA in press. [DOI] [PMC free article] [PubMed]
- Spicher A, Etter A, Bernard V, Tobler H, Müller F. Extremely stable transcripts may compensate for the elimination of the gene fert-1 from all Ascaris lumbricoidessomatic cells. Dev Biol. 1994;164:72–86. doi: 10.1006/dbio.1994.1181. [DOI] [PubMed] [Google Scholar]
- Stark JM, Bazett-Jones DP, Herfort M, Roth MB. SR proteins are sufficient for exon bridging across an intron. Proc Natl Acad Sci. 1998;95:2163–2168. doi: 10.1073/pnas.95.5.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke R, Manley JL. The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities. EMBO J. 1995;14:3540–3551. doi: 10.1002/j.1460-2075.1995.tb07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J, Daugeron M-C, Cathala G, Brunel C, Jeanteur P. Adenosine phosphorothioates (ATPαS and ATPγS) differentially affect the two steps of mammalian pre-mRNA splicing. J Biol Chem. 1992;267:4322–4326. [PubMed] [Google Scholar]
- Tazi J, Kornstädt U, Rossi F, Jeanteur P, Cathala G, Brunel C, Lührmann R. Thiophosphorylation of U1-70K protein inhibits pre-mRNA splicing. Nature. 1993;363:283–286. doi: 10.1038/363283a0. [DOI] [PubMed] [Google Scholar]
- Wang C, Chua K, Seghezzi W, Lees E, Gozani O, Reed R. Phosphorylation of spliceosomal protein SAP 155 coupled with splicing catalysis. Genes & Dev. 1998;12:1409–1414. doi: 10.1101/gad.12.10.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CL, Lührmann R. Protein functions in pre-mRNA splicing. Curr Opin Cell Biol. 1997;9:320–328. doi: 10.1016/s0955-0674(97)80003-8. [DOI] [PubMed] [Google Scholar]
- Wu JY, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- Xiao S-H, Manley JL. Phosphorylation of the ASF/SF2 RS domain affects both protein–protein and protein–RNA interactions and is necessary for splicing. Genes & Dev. 1997;11:334–344. doi: 10.1101/gad.11.3.334. [DOI] [PubMed] [Google Scholar]
- ————— Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J. 1998;17:6359–6367. doi: 10.1093/emboj/17.21.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler AM, Lane WS, Stolk JA, Roth MB. SR proteins: A conserved family of pre-mRNA splicing factors. Genes & Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]


