Abstract
Background:
Prefrontal cortex deficits have been consistently demonstrated in schizophrenia. The orbitofrontal lobe (OFL), a critical component of the prefrontal cortex, subserves social and neuro-cognitive functions. While these functional impairments are established in schizophrenia, the OFL volume deficits have not been well studied, especially in antipsychotic-naïve patients.
Aim:
To study OFL volume deficits in antipsychotic-naïve schizophrenia patients in comparison with matched healthy controls using high-resolution 3-tesla (3T) magnetic resonance imaging (MRI).
Materials and Methods:
Fourteen antipsychotic-naïve schizophrenia patients (DSM-IV) and 14 age-, sex-, handedness- and education-matched healthy controls were scanned using 3T MRI. Psychopathology was assessed in the patient group using the scale for assessment of negative symptoms and the scale for assessment of positive symptoms (SAPS). The OFL volume was measured using Region of Interest (ROI)-based manual morphometry technique, with good inter-rater reliability (intra-class correlation coefficient = 0.98).
Results:
Total OFL volume was significantly smaller in schizophrenia patients (43.3 ± 9.6 mL) in comparison with healthy controls (52.1 ± 12.2 mL) after controlling for the potential confounding effects of age, sex and intracranial volume (F = 5.3, P = .03). Duration of untreated psychosis did not correlate significantly with OFL volumes. There was a trend towards significant negative correlation between the left and total OFL volumes and SAPS scores (r = –0.49, P = .06).
Conclusion:
OFL volume deficits might underlie the pathogenesis of schizophrenia symptoms with possible neuro-developmental origins.
Keywords: Antipsychotic-naïve, orbitofrontal lobe, schizophrenia
INTRODUCTION
Prefrontal cortical deficits have been consistently demonstrated in schizophrenia in various neuroimaging studies, and these studies support the neuro-developmental hypothesis of schizophrenia.[1,2] However, the relation of individual components of the prefrontal cortex to schizophrenia has not been well studied. The orbitofrontal cortex is one of the important parts of the prefrontal cortex, along with the dorsolateral prefrontal and anterior cingulate cortex; however, its structure and functions are poorly understood. Deficits in orbitofrontal cortex volumes have been demonstrated in schizophrenia patients,[3] and this volume deficit is associated with severity of negative symptoms.[4]
Functional neuroanatomy of orbitofrontal cortex
Studies across species have demonstrated that the orbitofrontal cortex is phylogenetically the most developed in primates, and this distinguishes primates from nonprimate species. This brain area is also among the last to mature. Hence the orbitofrontal cortex is an important area of the prefrontal cortex, linked to human evolution.[5] The orbitofrontal cortex occupies the ventral area of the frontal part of the brain. It is defined as that part of the prefrontal cortex that receives projections from the magnocellular medial nucleus of the mediodorsal thalamus. Classically it is that area which occupies areas 10, 11, 47 as described by Broadmann.[6] Functionally this area has been described to include parts of the medial prefrontal and anterior cingulate cortex and is called the orbito- medial prefrontal cortex (OMPFC).[7] Its main functions include sensory integration, reward processing, decision making, reward prediction and subjective hedonic processing. A meta-analysis[8] of neuroimaging data showed that the medial orbitofrontal cortex is related to monitoring, learning and memory of reward value of reinforcers, whereas the lateral orbitofrontal cortex activity is related to evaluation of punishers. A posterior-anterior distinction was also found, with more complex or abstract reinforcers being represented more anteriorly. Aberrant salience to stimuli has been shown to underlie the pathogenesis of schizophrenia, and salience discrimination has been described to be an important function of the orbitofrontal cortex.
Hence the orbitofrontal cortex, which serves important social and neuro-cognitive functions, is an important brain region that needs to be studied in schizophrenia patients. However, the interpretation of the results of earlier studies is limited by the following methodological factors: 1) Most of these studies have been done on treated patients of schizophrenia, and antipsychotic exposure is known to effect brain structure and function. 2) The studies have used voxel-based morphometry and automated parcellation techniques for volumetric assessment. The ROI-based manual morphometry is generally considered more reliable for brain volumetric assessments.[9] 3) Magnetic resonance imaging (MRI) acquisition in earlier studies has been done using a 1.5-tesla scanner. Images thus obtained would have a lower signal-to-noise ratio and hence poorer structural differentiation and a lesser contrast between gray and white matter. The orbitofrontal cortex has been described to face technical limitations while imaging, in the form of geometric distortions and susceptibility artifacts due to its close proximity to air-filled sinuses.[10] Hence better structural differentiation using advance 3T scanner would be crucial to volumetric assessments of the orbitofrontal lobe (OFL). Hence further imaging studies in schizophrenia need to address these methodological issues to enhance our understanding of the neurobiology of schizophrenia.
In this study, we aimed to examine orbitofrontal lobe (OFL) volume deficits in antipsychotic-naïve schizophrenia patients in comparison with healthy controls, using ROI-based manual morphometry technique on images obtained using a 3T MRI scanner. We hypothesized that the patient group would have smaller OFL volumes and there would be a negative correlation between the OFL volumes and severity of psychopathology.
MATERIALS AND METHODS
Subjects
Subjects of the study comprised of 14 antipsychotic- naïve schizo phrenia patients and 14 age-, sex-, education- and handedness-matched healthy control subjects. Patients meeting the DSM-IV diagnostic criteria for schizophrenia were recruited from the clinical services of the National Institute of Mental Health and Neurosciences, Bangalore, India. The patients were in the age range of 18-45 years, with no previous exposure to antipsychotics. They were assessed independently by 2 qualified psychiatrists, and diagnosis was ascertained by applying the Mini International Neuropsychiatric Interview-Plus (MINI-Plus) and through independent clinical interview. Patients with any substance dependence in the last 6 months (except nicotine) and those with comorbid medical or neurological illness were excluded from the study. The healthy control subjects were recruited by ‘word of mouth’ from the hospital staff and their friends/relatives. They were screened using MINI-Plus and comprehensive mental status examination, to rule out psychiatric disorder. None of the controls had family history of psychiatric disorder in first-degree relatives. Written informed consent to participate in the study was obtained from all subjects. The study was approved by the institute's ethics committee.
Psychopathology in the patient group was assessed using the Scale for Assessment of Negative Symptoms (SANS) and Scale for Assessment of Positive Symptoms (SAPS), with good inter-rater reliability (the intra-class correlation coefficients for SANS and SAPS were 0.92 and 0.98, respectively).
Scanning protocol
MRI was done with 3.0-T scanner. T1-weighted images (with capability for three-dimensional reconstruction) were acquired using the following parameters: TR = 8.1 msec, TE = 3.7 msec, nutation angle = 8 degrees, FOV = 256 mm, slice thickness = 1 mm without interslice gap, NEX = 1, matrix = 256 × 256. The images were transferred onto a personal computer (PC) platform. They were stored with coded identification.
Region of interest
In the present study, the region of interest, the orbitofrontal lobe (OFL) (both right and left sides), was measured using the software ‘Medical Image Processing and Visualization’ (MIPAV). This software is in the public domain and can be downloaded from the internet (http://mipav.cit.nih.gov/). The ROI was marked using computer-mouse-controlled pointer as per the following landmarks.[2] The ROI marking was done in the sagittal MRI sections. The mid-sagittal section was identified as the slice where the cerebral aqueduct was clearly visible. Markings were begun from the slice before and after the mid-sagittal section. The superior margin in all the slices was marked by a line drawn horizontally from the anterior commissure to the anterior cortical margin (AC line). This AC line distinguished the OFL from the dorsolateral prefrontal lobe [Figure 1]. The posterior boundary in the medial slices was marked from the anterior tip of the corpus callosum and inferior cortical margin at the first appearance of the head of the caudate [Figure 2]. In the lateral slices, the posterior boundary was marked by the peri-insular sulcus [Figure 3]. The OFL was marked similarly on both left and right sides, and individual volumes were computed in milliliters (mL). Good inter-rater reliability was established. (OFL volumes of 5 images were measured independently by 2 raters — intra-class correlation coefficient was 0.98.) Subsequently all ROI measurements were done on coded images with the rater being blind to the status.
Figure 1.
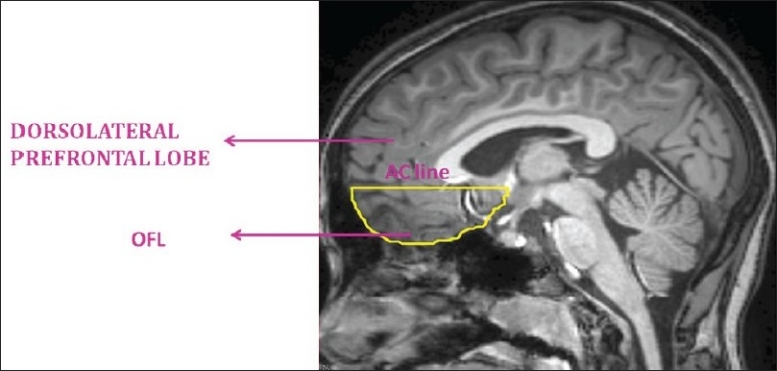
Anatomical landmarks of orbitofrontal lobe: Anterior cortical line demarcating orbitofrontal lobe (OFL) from dorsolateral prefrontal lobe
Figure 2.
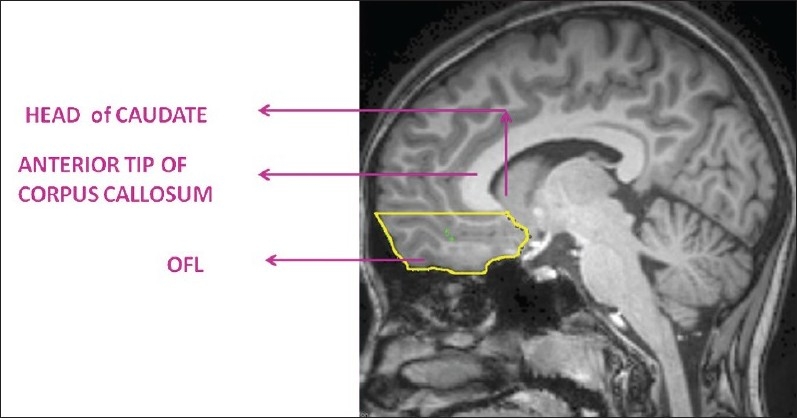
Anatomical landmarks of orbitofrontal lobe (OFL) in medial sagittal sections
Figure 3.
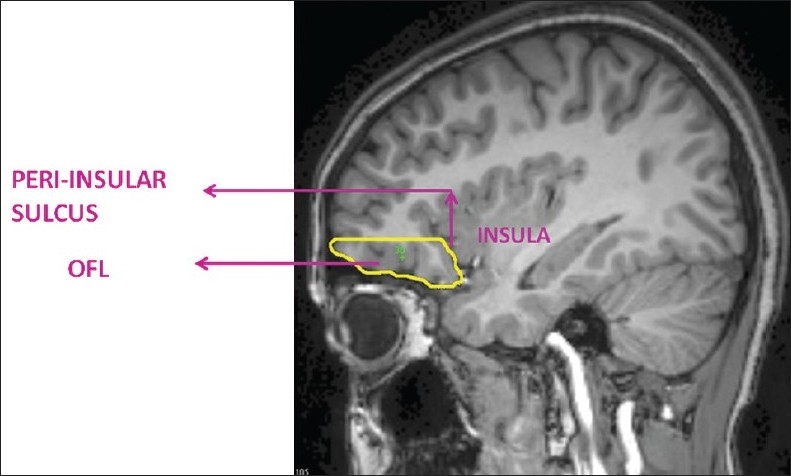
Anatomical landmarks of orbitofrontal lobe (OFL) in lateral sagittal sections
Statistical analysis
The statistical analysis was performed using the statistical package for social sciences-13.0. Independent sample t test and chi-square test were used to analyze the socio-demographic and clinical data. Paired samples t test was used to look for any differences between right and left OFL volumes in patients and control subjects. The effect of the status (schizophrenia patients versus healthy controls) on the total OFL volume was examined using the analysis of covariance (ANCOVA) with measures to control for the potential confounding effects of age, sex and intracranial volume. The statistical significance was set at P < .05 (two-tailed).
RESULTS
Socio-demographic and clinical variables
The patient group and healthy control group were matched on age and had a sex ratio of (M: F = 9:5). The educational status was recorded as a categorical variable as follows: (Patients: Controls — illiterate, 5:2; primary school, 3:2; middle school, 4:7; high school, 0:3; and graduate, 2:0). There was also no significant difference in the educational status between the two groups (χ2 = 2.76, P = .6). The patient group had a mean duration of untreated psychosis of 27.6 ± 28.7 months [Table 1].
Table 1.
Comparison of socio-demographic and clinical variables between patients and healthy controls
Orbitofrontal lobe volume analysis
There was no significant difference between right versus left OFL in both patient and control groups on paired samplest test [patient group — right OFL: 22.1 ± 5.6, left OFL: 21.2 ± 4.3, t = 1.1, P = .3; and control group — right OFL: 26.1 ± 6.7, left OFL: 25.9 ± 6.0, t = 0.3, P = .7]. On ANCOVA, the main effect of status was significant for total OFL volume [Table 2, Figure 4] after controlling for potential confounding effects of age, sex and intracranial volume — with the patients having significantly smaller volume than healthy controls. On Pearson's correlation analysis, there was a trend toward significant negative correlation between SAPS scores and left OFL volume (r = -0.49, P = .06) and total OFL volume (r = -0.44, P = .09); however, SANS scores did not show any such trend. There was no significant correlation between the OFL volumes and duration of untreated psychosis.
Table 2.
Comparison of orbitofrontal lobe volumes between patients and healthy controls
Figure 4.
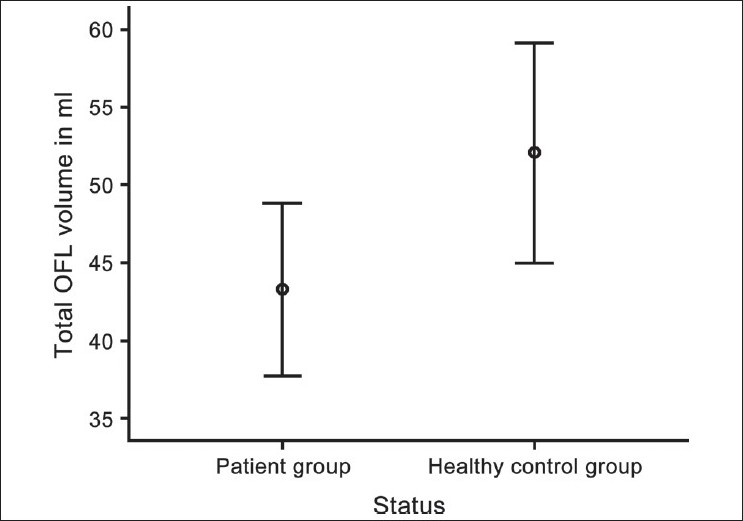
Error bars showing comparison of total orbitofrontal lobe (OFL) volume (mL) between patient and control groups
DISCUSSION
To the best of our knowledge, this is the first study to use high-resolution 3T MRI to examine the OFL volume deficits in antipsychotic-naïve schizophrenia patients in comparison with matched healthy controls. The results of this study demonstrate a significant deficit in total OFL volume in antipsychotic-naïve schizophrenia patients in comparison with matched healthy controls. There was also a trend towards significant negative correlation between the left and total OFL volumes and SAPS score. There was no significant correlation between duration of untreated psychosis and OFL volume.
Orbitofrontal lobe deficits and schizophrenia
The findings of the study replicate the results of an earlier 1.5-tesla MRI study on antipsychotic-naïve schizophrenia from India, which also demonstrated similar volume and thickness deficits in bilateral OFL.[4] The presence of these deficits in antipsychotic-naïve state along with the lack of correlation of OFL volume deficit with duration of untreated psychosis indicates that this volume deficit might underlie the pathogenesis of schizophrenia symptoms with possible neuro-developmental origins. This is supported by findings of other studies which have demonstrated OFL volume deficits in first-episode subjects and ultrahigh-risk subjects of schizophrenia.[11,12]
Our study also showed a trend towards significant negative correlation between left and total OFL volumes and SAPS scores. Earlier studies have shown a significant negative correlation of thickness of left medial orbitofrontal cortex to SANS scores.[4] Other studies have shown an association of OFL volumes with various other clinical aspects of schizophrenia, such as poor functioning,[12] poor insight[13] and violent behaviors.[14] However, no study to date has established clear association of OFL volume with positive symptoms. Possibly, analysis of a larger sample of patients would give a better understanding of the relation of OFL volume to positive symptoms of schizophrenia.
One of the major functions of the OFL includes facial emotional processing, through its cortico-limbic connections with amygdala and anterior cingulate. Deficits in facial emotional processing have been consistently demonstrated to be a core feature of schizophrenia,[15,16] and abnormal activations in the OFL have been implicated in functional neuroimaging studies on emotion recognition tasks.[17] We have recently reported beneficial effects of risperidone on improving facial emotion recognition deficits in a short-term follow-up study.[16] Interestingly, dopamine has been described to modulate emotional processing in cortico-limbic circuits involving the orbitofrontal cortex, and this could have useful clinical implications.[18] In addition, the OFL is also described to subserve various functions such as reward processing, decision making and reward prediction;[8] deficits in which have been demonstrated in schizophrenia. Hence further structural and functional neuroimaging studies involving the OFL could give useful insights to our understanding of the neurobiology of schizophrenia.
Methodological issues
Some of the methodological advantages of this study are that 1) the patient group consisted of antipsychotic-naïve schizophrenia patients; 2) diagnosis was reliably ascertained by independent assessments by two qualified psychiatrists using MINI-Plus and clinical interview; 3) psychopathology was comprehensively assessed using SAPS and SANS, with good inter-rater reliability; 4) 3T MRI scans were obtained; 5) volumetric assessments were done using ROI-based manual morphometry on coded images with rater being blind to the status and good inter-rater reliability being established.
CONCLUSION
The results of this study demonstrate significant total OFL volume deficits in antipsychotic-naïve schizophrenia and the possible association of OFL volume with positive symptoms. These volume deficits might underlie the pathogenesis of schizophrenia symptoms with possible neuro-developmental origins. Further structural and functional neuroimaging studies in larger samples of patients could give us better insights into the role of orbitofrontal cortex deficits in the pathogenesis of schizophrenia.
ACKNOWLEDGMENT
The authors thank the patients and their relatives for their valuable time given for study assessments. This study was supported by the Department of Biotechnology (DBT), Government of India research grant to Dr. G. Venkatasubramanian under the Innovative Young Biotechnologist Award (IYBA) scheme. The authors acknowledge the support provided by the facilities of Computational NeuroScience Laboratory (CNS Lab), National Institute of Mental Health and Neurosciences, Bangalore, India.
Footnotes
Source of Support: Department of Biotechnology (DBT), Government of India Research Grant to Dr. G. Venkatasubramanian under the Innovative Young Biotechnologist Award (IYBA) scheme.
Conflict of Interest: None.
REFERENCES
- 1.Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Res. 2008;164:215–22. doi: 10.1016/j.pscychresns.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–8. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- 3.Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pfluger MO, Stieglitz RD, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–14. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2008;117:420–31. doi: 10.1111/j.1600-0447.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 5.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 6.Pandya DN, Yeterian EH. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351:1423–32. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- 7.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 8.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: A comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res. 2005;74:135–47. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Wilson JL, Jenkinson M, de Araujo I, Kringelbach ML, Rolls ET, Jezzard P. Fast, fully automated global and local magnetic field optimization for fMRI of the human brain. Neuroimage. 2002;17:967–76. [PubMed] [Google Scholar]
- 11.Witthaus H, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Gallinat J, et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2009;173:163–9. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kasparek T, Prikryl R, Schwarz D, Kucerova H, Marecek R, Mikl M, et al. Gray matter morphology and the level of functioning in one-year follow-up of first-episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1438–46. doi: 10.1016/j.pnpbp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar AP, et al. Prefrontal cortex and insight in schizophrenia: A volumetric MRI study. Schizophr Res. 2007;89:22–34. doi: 10.1016/j.schres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Naudts K, Hodgins S. Schizophrenia and violence: A search for neurobiological correlates. Curr Opin Psychiatry. 2006;19:533–8. doi: 10.1097/01.yco.0000238484.12023.aa. [DOI] [PubMed] [Google Scholar]
- 15.Mandal MK, Pandey R, Prasad AB. Facial expressions of emotions and schizophrenia: A review. Schizophr Bull. 1998;24:399–412. doi: 10.1093/oxfordjournals.schbul.a033335. [DOI] [PubMed] [Google Scholar]
- 16.Behere RV, Venkatasubramanian G, Arasappa R, Reddy N, Gangadhar BN. Effect of risperidone on emotion recognition deficits in antipsychotic-naive schizophrenia: A short-term follow-up study. Schizophr Res. 2009;113:72–6. doi: 10.1016/j.schres.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr Bullin press. 2009 doi: 10.1093/schbul/sbn192. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clin Neuropharmacol. 2005;28:228–37. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]


