Abstract
Background:
As per Frith's neuro-cognitive model, inferior parietal lobule (IPL) is implicated in the pathogenesis of Schneiderian first-rank symptoms (FRS) in schizophrenia. The specific role of IPL structural abnormalities in the pathogenesis of FRS is yet to be ascertained.
Materials and Methods:
Using 3-tesla MRI scanner, this first-time study examined antipsychotic-naïve schizophrenia patients ( n = 28) (patients with FRS [FRS +]: N = 14, M: F = 7:7; and patients without FRS [FRS-]: N = 14, M: F = 7:7) in comparison with sex-, handedness-, education- and socioeconomic status-matched healthy controls (n = 14, M: F = 7:7). The volume of IPL was measured using a three-dimensional, interactive, semi-automated analysis, with good inter-rater reliability.
Results:
FRS + patients showed significant volume deficit in right IPL in comparison with healthy controls (F = 4.0; P=.028) after controlling for the potential confounding effects of age, sex and intracranial volume.
Conclusions:
Right IPL volume deficit in FRS+patients adds further support to the Frith's model of FRS in schizophrenia.
Keywords: Inferior parietal lobule, schizophrenia, Schneiderian first-rank symptoms
INTRODUCTION
First-rank symptoms (FRS) in schizophrenia, described first by Kurt Schneider, are a set of experiences and beliefs that are characterized by the striking breach of self versus non-self boundaries.[1] Though there has been lack of consistent support for the diagnostic significance of FRS in schizophrenia, these symptoms have critically influenced the conceptualization of this disorder.[2,3]
For over 40 years after Schneider proposed first-rank symptoms, there was little investigation in this area.[4] A seminal study by Mellor gave an exhaustive list of 11 FRS and detected a prevalence of 72%.[1] Further studies for the next two and half decades were mainly focused on studying the prevalence and specificity of FRS in schizophrenia and predictability of outcome based on the symptomatology.[5,6] In Indian schizophrenia patients, a study on FRS showed a prevalence of 53.3% in patients with schizophrenia; and the rest, in affective and reactive psychoses; and also a statistically significant higher association of occurrence of FRS with positive family history of schizophrenia.[4]
Consequent upon the support offered by these studies towards the significance of FRS in diagnosing schizophrenia, various theoretical formulations have been put forth to understand the genesis of these intriguing phenomena. One of the most widely cited and influential of these is the neuro-cognitive model proposed by Christopher Frith.[7] Frith et al. have explained the cognitive and the neuroanatomical basis for the delusion of control, one of the Schneiderian FRS.[7] The central tenet of Frith's model emphasizes upon the importance of inferior parietal lobule (IPL) in the genesis of FRS. Various lines of studies support the role of IPL in processing endogenous sensory data; optimal processing of these endogenous sensory data is critical in differentiating self versus non-self. Hence IPL deficits can potentially lead to aberrations in mis-attributing self-generated movements to an external agent.
In healthy individuals, the interactions between the anterior frontal areas and the posterior parietal areas are reciprocal in the sense that high activity in the frontal region goes with reduced activity in the posterior regions. Normally, signals arising in prefrontal cortex, where actions are initiated, inhibit activity in posterior regions, where the sensory consequences of actions are received. This relationship is not observed in patients with schizophrenia, leading to abnormal independent activities in the frontal and the parietal regions suggesting lack of connectivity. Some of the striking FRS might be associated with deficits in IPL. It is noteworthy that parietal cortical aberrations are given increasing importance.[8]
While IPL volume deficits in schizophrenia have been documented by few studies,[9–11] the specific role of IPL structural abnormalities in the pathogenesis of FRS is yet to be ascertained. The existing functional imaging studies have consistently implicated IPL abnormalities to underlie FRS in schizophrenia.[12–14] However, the structural brain abnormalities related to FRS have received sparse attention. Till date, only one study that has examined the morphological changes in schizophrenia patients with FRS did not reveal any significant IPL volume deficits[15] ; however, the study is critically limited, especially because it has examined schizophrenia patients who have been exposed to long-term antipsychotic treatment. Increasingly, studies have indicated treatment with antipsychotics can lead to brain volume changes.[16] Hence it is possible that the observations of presence or absence of structural abnormalities in schizophrenia could have been influenced by varying durations of antipsychotic treatment. Consequently, the importance of examining antipsychotic-naïve schizophrenia patients to avoid the potential confounding effect of neuroleptic treatment has been emphasized.[17]
In this first-time 3T MRI study, we examined antipsychotic-naïve schizophrenia patients (n = 28) [14 patients with FRS and 14 patients without FRS] in comparison with sex-, handedness-, education- and SES-matched healthy control subjects (n = 14) using a three-dimensional, interactive, semiautomated analysis, with good inter-rater reliability. We hypothesized that antipsychotic-naïve schizophrenia patients with FRS will have significantly lesser volume of IPL than the schizophrenia patients without FRS and healthy controls.
MATERIALS AND METHODS
Subjects
The study sample consisted of 28 antipsychotic-naïve schizophrenia patients (14 patients with FRS, constituting FRS + group; and 14 patients without FRS, constituting FRS- group) and 14 healthy controls. Patients meeting the DSM-IV[18] diagnostic criteria for schizophrenia were recruited from the clinical services of the National Institute of Mental Health and Neurosciences, Bangalore, India. Diagnosis was established by applying Mini International Neuropsychiatric Interview-Plus (MINI-Plus),[19] and it was confirmed by another experienced psychiatrist through independent clinical interview. The patients were not exposed to any antipsychotic drug before or at the time of assessments. They had neither history of medical illness nor comorbid psychiatric illness, including substance dependence.
The psychopathology was assessed by scale for the assessment of positive symptoms (SAPS)[20] and scale for the assessment of negative symptoms (SANS),[21] with good inter-rater reliability as assessed by the intra-class correlation coefficients greater than 0.9. The SAPS score (mean ± SD) of the patients was 31.0 ± 16.1, and the SANS score was 69.3 ± 31.0. Schneiderian FRS were assessed as per the definitions of Mellor[1] ; the status of FRS was ascertained independently by two qualified psychiatrists.
Healthy controls who volunteered for participation in the study were recruited from consenting individuals through ‘word of mouth.’ The absence of any psychiatric diagnosis was ascertained by applying MINI-Plus.[19] They had neither any history of medical illness nor that of substance dependence. There was no family history of psychiatric illness, including alcohol dependence syndrome, in their first-degree relatives. All 42 subjects were right handed. Written informed consent was taken from all subjects before assessment. Approval from the ethics committee of the institute was obtained.
Scanning protocol
MRI was done with 3.0-tesla scanner. T1-weighted images (with capability for three-dimensional reconstruction) were acquired using the following parameters: TR = 8.1 msec, TE = 3.7 msec, nutation angle = 8 degrees, FOV = 256 mm, slice thickness = 1 mm without interslice gap, NEX = 1, matrix = 256 x 256. The images were transferred onto a personal computer (PC) platform. They were stored with coded identification.
Region of interest
In the present study, the region of interest, the IPL (both right and left sides), was measured using the software ‘Multitracer’ (www.loni.ulca.edu). Using this software, the desired structure was outlined and measured by the rater using the computer-mouse-controlled pointer [Figures 1 and 2]. The following are the details of the IPL tracing guidelines[22] : The area was marked in each consecutive coronal slice using the intrinsic anatomical landmarks. Superior margin was marked by intraparietal sulcus (InPS), only for the anterior part until its medial segment appeared; and thereafter, by the InPS laterally and the parieto-occipital fissure medially, for the posterior part. Inferior margin was marked by lateral sulcus for the anterior part until it disappeared; and thereafter, by the superior temporal sulcus (STS), for the most posterior part. Anteriorly, the relevant portion of the posterior border for the postcentral gyrus; and posteriorly, the coronal level where the horizontal posterior segment of STS disappeared were taken as landmarks. The intracranial volume, which was used as a covariate in statistical analyses to control for the potential confounding effect of the global brain size, was automatically computed using established methods.[23]
Figure 1.
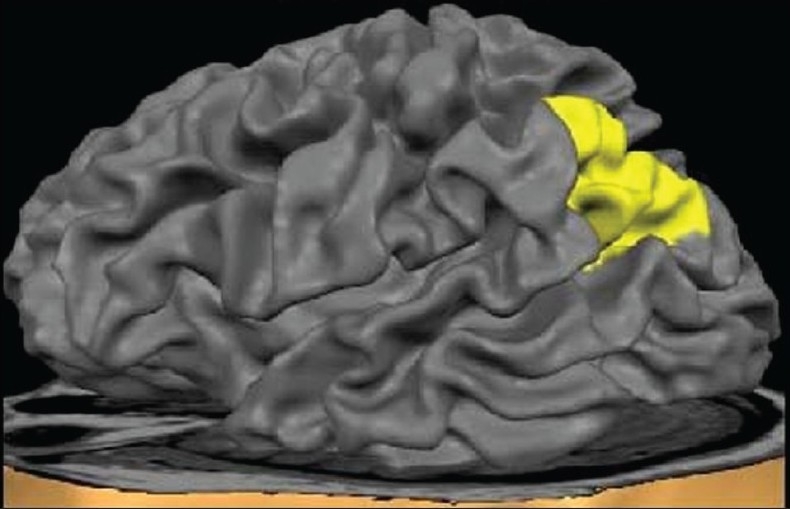
Surface view of the inferior parietal lobule
Figure 2.
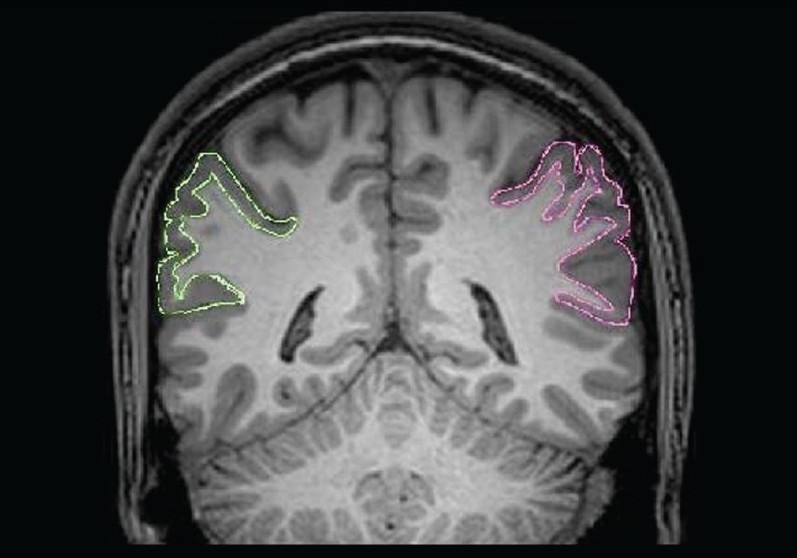
Tracing of the inferior parietal lobule (right side in pink color and left side in green color) in a coronal section
The first author was initially trained by another psychiatrist with experience in neuroimaging research (GVS). For inter-rater reliability, 5 coded images were measured by 2 raters. There was good inter-rater reliability for both right and left IPL volumes (Cronbach's alpha > 0.8). Subsequently, all the measurements were performed by the first author, who was blind to the subjects’ identities.
Statistical analysis
The statistical analysis was performed using the statistical package for social sciences-13.0. Analysis of variance (ANOVA), analysis of covariance (ANCOVA) and chi-square test were used to analyze the clinical data. The effect of the diagnostic status (schizophrenia patient with FRS [FRS+], without FRS [FRS–] or healthy control) on the IPL volumes was examined using the ANCOVA with measures to control for the potential confounding effects of age, sex and intracranial volume.
RESULTS
Socio-demographic profile
The analysis of socio-demographic profile showed that the mean age of the patients with FRS (31.2 ± 7.0 years) was higher than that of those without FRS (26 ± 5.2 years) and healthy controls (25.2 ± 6.7 years) (F = 3.7; P = .034) [Table 1]. But the potential confounding effect of age was controlled using ANCOVA [Table 2]. The educational status of the two groups did not differ significantly (P = .8). Also, the socioeconomic status as assessed by the monthly family income did not differ significantly among the three groups (P = .3).
Table 1.
Comparison of socio-demographic details and psychopathology between first-rank symptoms+, first-rank symptoms –, and healthy controls
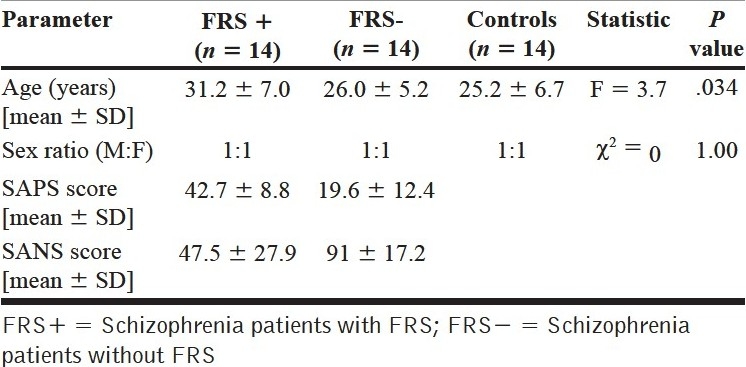
Table 2.
Volume of inferior parietal lobule in first-rank symptoms+, first-rank symptoms – patients and healthy controls
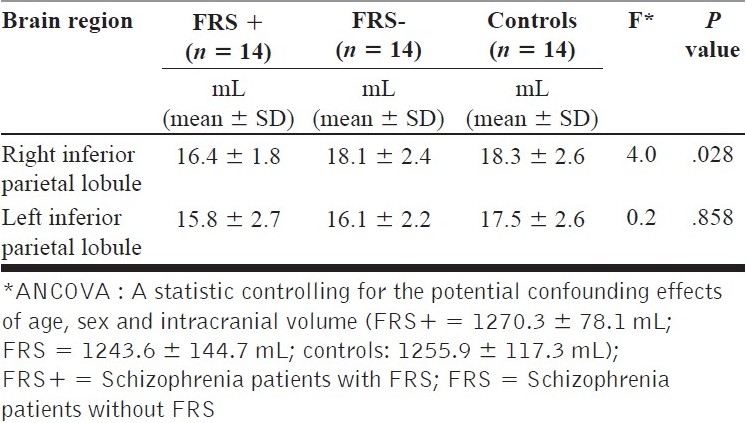
Inferior parietal lobule volume analyses
ANCOVA tests revealed significant effect of FRS status on the right IPL volume (but not on the left IPL volume) [Table 2 and Figure 3]; post hoc analysis revealed schizophrenia patients with FRS had significantly larger deficit in right IPL volume in comparison with healthy controls (P = .015). However, schizophrenia patients without FRS did not differ significantly from healthy controls.
Figure 3.
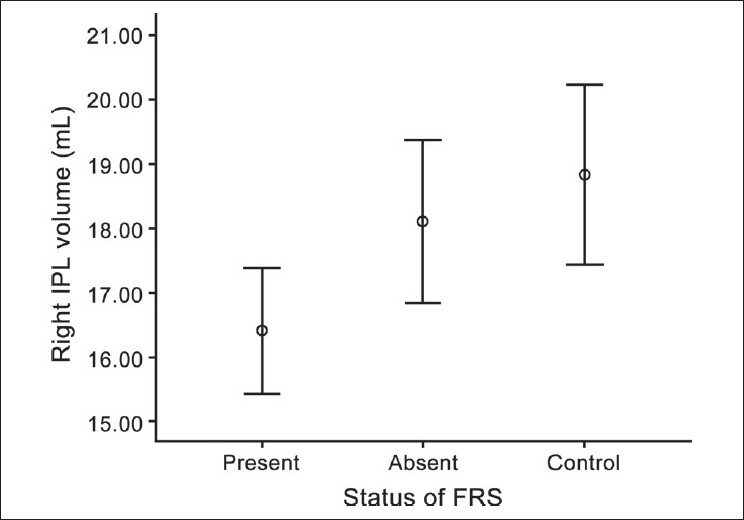
Error bar showing significant volume deficit in right inferior parietal lobule in schizophrenia patients with first-rank symptoms
DISCUSSION
To the best of our knowledge, this is the first study to analyze the volume deficits in antipsychotic-naïve schizophrenia patients with and without FRS using 3-tesla MRI scans. Our study results showed that the volume of the right inferior parietal lobule was significantly smaller in patients with FRS in comparison with healthy controls. We did not find any statistically significant difference in the volume of the left inferior parietal lobule between patients with FRS and healthy controls.
Inferior parietal lobule
The human parietal lobe has traditionally fallen into the category of ‘heteromodal association cortex.’[24] Parietal lobe has also been implicated to have role in perception, attention, working memory, motor planning and control, mental calculation, language, self-awareness and emotion.[8] Inferior parietal lobule (IPL) is one of the last areas of the human brain to mature. IPL is not found in monkeys; and even in apes, it is present only in the rudimentary form.[25] It is among the most highly lateralized areas of the brain. Inferior parietal lobule shows sexual dimorphism, with the left IPL being larger in males than in females, which may be associated with some of the cognitive differences between the sexes.[10] This gives a serious implication that all the studies focusing on this region should have matched for the sex differences for statistical analysis. In our study, we have matched for sex, handedness and education. Given the evolutionary recentness of this brain region, our study finding may potentially be considered to offer indirect support to the evolutionary theories of schizophrenia.[26]
Inferior parietal lobule and schizophrenia
Previous studies that have looked into the volume changes have found volume deficits in inferior parietal lobule (IPL).[9–11] Our study findings are consistent with the findings of previous studies that inferior parietal lobule volume deficits are found in schizophrenia patients. But none of these studies have looked into the differences between FRS+ and FRS– patients.
Frith et al. have speculated involvement of the parietal lobe in the origin of delusion of control, one of the FRS.[7] The model moves from an explanation of motor behavior to an explanation of cognitive experience. Across these different levels (motor to perception and cognition), the problem in schizophrenia is the same: A failure in basic self-monitoring processes. This self-monitoring process has been hypothesized to involve IPL (especially the right IPL), which is critical in determining the match between the efferent neural command (which is generated by the brain) and the re-afferent feedback (which is received from the periphery) [Figure 4]. A match between the efferent and re-afferent is important to infer the ‘agency,’ i.e., the sense of the action being initiated by oneself. Importantly, this sense of ‘agency’ is impaired in schizophrenia. This could be attributed to IPL volume deficits. Our study finding of structural deficit in right IPL is in support of this hypothesis. Moreover, this finding is in tune with the findings from previous functional imaging studies.[12–14]
Figure 4.
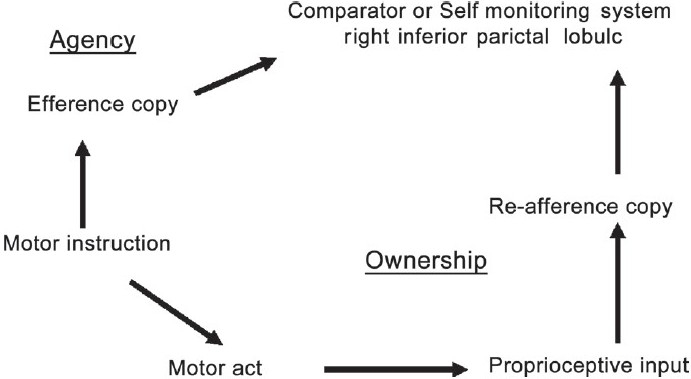
Frith's neuro-cognitive model for first-rank symptoms in schizophrenia
Methodological issues
This is the first study to examine antipsychotic-naïve schizophrenia patients for neuroanatomical correlates of FRS in schizophrenia using 3T MRI scans. Some of the other methodological advantages of the study include the following: 1) antipsychotic-naïve status of the patients during the assessments; 2) MINI-Plus to establish the diagnosis of the patients; 3) Independent confirmation of the diagnosis by an experienced psychiatrist; 4) reliable methodology - good inter-rater reliability for IPL volume measurements; 5) sex-, education-, handedness- and socioeconomic status-matched controls; 6) use of 1-mm MRI slices with no interslice gap; and 7) first-time use of 3-tesla MRI scan.
Potential clinical implications
Elucidating neural basis for clinically observable FRS emphasizes the need for careful clinical examination for the same. In addition to the preexisting data on the biological differences between FRS+and FRS- patients,[4,26] our study findings offer further support for the clinical importance of FRS in identifying subtypes of schizophrenia. Such an approach can potentially lead to better understanding of the brain on the basis of these intriguing symptoms and possibly better treatment strategies.
CONCLUSIONS
In summary, this study states that inferior parietal lobule volume is significantly lesser in schizophrenia patients with FRS in comparison with schizophrenia patients without FRS and healthy controls. Thus it supports the Frith's neuro-cognitive model of FRS in schizophrenia patients. Further studies with larger samples of patients, looking into the relationship of IPL volume deficits with prefrontal and temporal brain regions, as well as the differential effect of subtypes of FRS, are warranted for better understanding of the basis of the phenomena.
ACKNOWLEDGMENT
The authors thank the patients and their relatives for their valuable time given for study assessments. This study was supported by the Department of Biotechnology (DBT), Government of India research grant to Dr. G. Venkata subramanian under the Innovative Young Biotechnologist Award (IYBA) scheme. The authors acknowledge the support provided by the facilities of Computational NeuroScience Laboratory (CNS Lab), National Institute of Mental Health and Neurosciences, Bangalore, India.
Footnotes
Source of Support: Nil,
Conflict of Interest: None.
REFERENCES
- 1.Mellor CS. First rank symptoms of schizophrenia. I) The frequency in schizophrenics on admission to hospital. II) Differences between individual first rank symptoms. Br J Psychiatry. 1970;117:15–23. [PubMed] [Google Scholar]
- 2.Goldman D, Hien DA, Haas GL, Sweeney JA, Frances AJ. Bizarre delusions and DSM-III-R schizophrenia. Am J Psychiatry. 1992;149:494–9. doi: 10.1176/ajp.149.4.494. [DOI] [PubMed] [Google Scholar]
- 3.Peralta V, Cuesta MJ. Diagnostic significance of Schneider's first-rank symptoms in schizophrenia. Comparative study between schizophrenic and non-schizophrenic psychotic disorders. Br J Psychiatry. 1999;174:243–8. doi: 10.1192/bjp.174.3.243. [DOI] [PubMed] [Google Scholar]
- 4.Raguram R, Kapur RL. A study of first rank symptoms of Schneider in functional psychoses. Indian J Psychiatry. 1985;27:111–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Koehler K, Guth W, Grimm G. First-rank symptoms of schizophrenia in Schneider-oriented German centers. Arch Gen Psychiatry. 1977;34:810–3. doi: 10.1001/archpsyc.1977.01770190072007. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter WT, Jr, Strauss JS. Cross-cultural evaluation of Schneider's first-rank symptoms of schizophrenia: A report from the International Pilot Study of Schizophrenia. Am J Psychiatry. 1974;131:682–7. doi: 10.1176/ajp.131.6.682. [DOI] [PubMed] [Google Scholar]
- 7.Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: Abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31:357–63. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 8.Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97:215–25. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, et al. Morphometric assessment of the heteromodal association cortex in schizophrenia. Am J Psychiatry. 2004;161:322–31. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- 10.Frederikse M, Lu A, Aylward E, Barta P, Sharma T, Pearlson G. Sex differences in inferior parietal lobule volume in schizophrenia. Am J Psychiatry. 2000;157:422–7. doi: 10.1176/appi.ajp.157.3.422. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–47. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 12.Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120:1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- 13.Franck N, O’Leary DS, Flaum M, Hichwa RD, Andreasen NC. Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:277–82. doi: 10.1176/jnp.14.3.277. [DOI] [PubMed] [Google Scholar]
- 14.Ganesan V, Hunter MD, Spence SA. Schneiderian first-rank symptoms and right parietal hyperactivation: A replication using FMRI. Am J Psychiatry. 2005;162:1545. doi: 10.1176/appi.ajp.162.8.1545. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Zhou SY, Hagino H, Niu L, Takahashi T, Kawasaki Y, et al. Morphological brain changes associated with Schneider's first-rank symptoms in schizophrenia: A MRI study. Psychol Med. 2005;35:549–60. doi: 10.1017/s0033291704003885. [DOI] [PubMed] [Google Scholar]
- 16.Scherk H, Falkai P. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19:145–50. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- 17.Garver DL, Holcomb JA, Christensen JD. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol Psychiatry. 2005;58:62–6. doi: 10.1016/j.biopsych.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.4th ed. American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–23. [PubMed] [Google Scholar]
- 20.Andreasen NC. University of Iowa; 1984. The Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- 21.Andreasen NC. University of Iowa; 1983. The Scale for the Assessment of Negative Symptoms (SANS) [PubMed] [Google Scholar]
- 22.Zhou SY, Suzuki M, Takahashi T, Hagino H, Kawasaki Y, Matsui M, et al. Parietal lobe volume deficits in schizophrenia spectrum disorders. Schizophr Res. 2007;89:35–48. doi: 10.1016/j.schres.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RS, Dolan RJ. Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry. 2002;159:1497–505. doi: 10.1176/appi.ajp.159.9.1497. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Tork I. Neuroanatomical nomenclature. Trends Neurosci. 1990;13:169. doi: 10.1016/0166-2236(90)90041-8. [DOI] [PubMed] [Google Scholar]
- 25.Geschwind N. Disconnexion syndromes in animals and man. I Brain. 1965;88:237–94. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 26.Crow TJ. Schizophrenia as the price that homo sapiens pays for language: A resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000;31:118–29. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]


