Abstract
A 40-year-old male presented with acutely oncoming neuropsychiatric symptoms in the form of extrapyramidal disturbances, personality change, behavior changes and urinary incontinence. One month prior to this symptomatology, he had been found unconscious in his closed room where a coal fire was burning overnight. He recovered completely in two days with supportive therapy. Magnetic resonance imaging during this presentation showed bilateral white matter changes suggestive of demyelination. Tc99m-ECD SPECT study revealed hypoperfusion in bilateral frontal region. He was managed conservatively and eventually made a nearly complete recovery. Repeat SPECT study revealed marked improvement in tracer uptake correlating with his improvement. Delayed neuropsychiatric syndrome after acute carbonmonoxide poisoning is a rare and distinct entity. This case report discusses the various aspects of this disease entity.
Keywords: Carbonmonoxide poisoning, demyelination
INTRODUCTION
Carbonmonoxide (CO) intoxication can occur following inhalation in a closed space from coal used for cooking or from exhaust of the vehicles. A high incidence of 3% has been reported from Korea because of coal being used as a major domestic fuel.[1] Symptoms of CO poisoning can follow an acute or a chronic exposure. Delayed neuropsychiatric syndrome following acute CO intoxication constitutes a rare and a distinct entity.
CASE REPORT
A 40-year-old male from hilly areas of Himachal Pradesh, India presented with acutely oncoming symptoms of slowness of activities, decreased speech output, disorientation, confusion, emotional lability , urinary incontinence, increased appetite and disturbed sleep of two weeks duration. One month prior to these symptoms he had been found in an unconscious state by his friends, in his closed, unventilated room where he had been sleeping overnight with a coal fire burning. He was managed with fluids and oxygenation at a local village hospital and made an uneventful recovery within 48h. He remained well thereafter until two weeks back when the above mentioned neuropsychiatric symptoms developed acutely. Examination revealed an MMSE score of 19/30. Detailed psychometry revealed a verbal IQ of 70, a performance IQ of 69 and a memory function at 14th percentile of normal. He had features of frontal lobe dysfunction in form of apathy, easy distractability, perseveration and bradyphrenia and prominent extrapyramidal signs (rigidity, stooped posture, decreased arm swing and a positive glabellar tap). Release reflexes in form of palmar grasp, palmomental reflex, pout and snout reflex were present. There was no evidence of cranial nerve dysfunction, motor weakness, sensory disturbances or cerebellar involvement. Electroencephalography (EEG) revealed a bifrontal delta range slowing. Cranial magnetic resonance imaging (MRI) showed hyperintensities, chiefly in the bilateral frontal white matter and centrum semiovale [Figures 1a, b]. Single photon emission computed tomography (SPECT) study performed using technetium ethylene cysteine diethylester (Tc99m-ECD), revealed irregularly reduced tracer uptake in deep frontal (L>R) and thalamic regions [Figures 2a, b]. Considering the clinical and imaging features, a diagnosis of delayed neuropsychiatric syndrome following acute CO poisoning was kept. He was treated symptomatically with dopaminergic therapy and closely observed in the hospital for two weeks. There was no further deterioration and there was improvement in extrapyramidal symptoms and signs. On follow-up two months later, there had been a dramatic improvement in his clinical picture. Follow-up cognitive assessment at four months revealed a MMSE score of 28/30, a verbal IQ of 91, performance IQ of 96 and a memory at 60thpercentile. Repeat ECD-SPECT scan [Figures 2c, d] showed a marked improvement in tracer uptake in frontal and thalamic regions correlating with his clinical improvement. At one-year follow-up, he is almost normal with only minimal verbal slowing and no other neuropsychiatric deficits.
Figure 1.
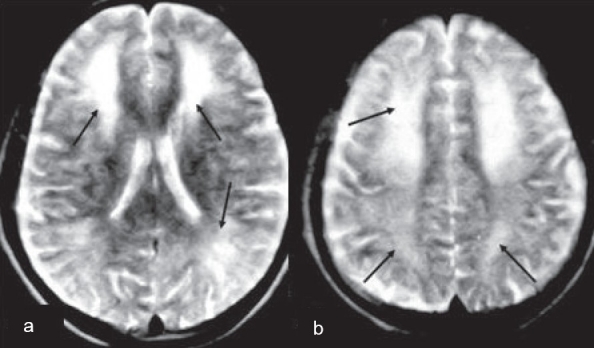
Cranial MRI axial T2W images showing multiple white matter hyper intensities (arrows) in (a) bilateral frontal and pareitooccipital regions and (b) centrumsemiovale
Figure 2.
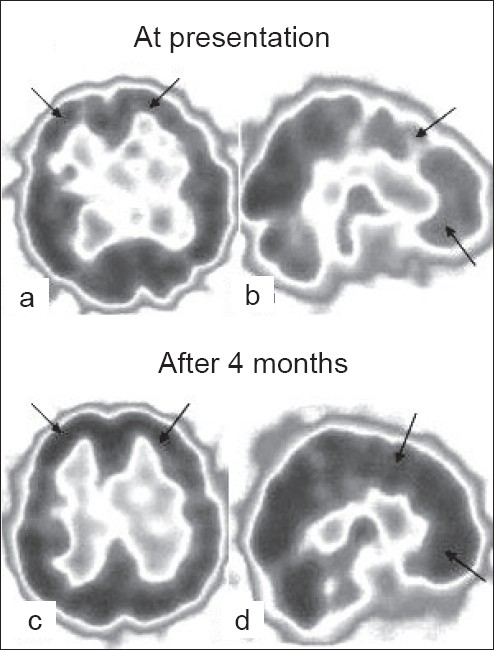
Tc99m-ECD SPECT. Initial presentation (a, b) shows reduced tracer uptake in bifrontal regions (arrows; less dark region indicates poor perfusion). At 4 months follow-up (c, d), shows marked improvement in perfusion (dark areas indicates normal perfusion)
DISCUSSION
1% CO inhalation is fatal in about half an hour during rest and even earlier during exercise. Low levels of carboxy-hemoglobin (COHb) leads to headache and dizziness and high levels result in coma. Acute exposure to CO results in headache, nausea, vomiting, confusion, lethargy and coma.[2] Dementia and psychosis can be the permanent sequelae.[3,4] Delayed neurological symptoms occur in about 3% (range 0.2 to 40%) of CO poisoning cases, more frequent in the elderly and those with a prolonged coma after exposure (12-48h).[1,2,4] Initially, following the acute exposure there is a complete recovery. After a pseudo-recovery period ranging from 2-40 days (average three weeks), rapid deterioration develops, with memory disturbances, disorientation, hypokinesia, urinary incontinence, psychotic behaviour, personality changes, apathy, anxiety, emotional liability, hyperventilation and autonomic dysregulation.[1,2,5] Examination usually reveals signs of frontal lobe dysfunction, extrapyramidal features and release reflexes.[2,5] EEG reveals delta range slowing in about 57% of patients.[1,2] Computerized tomography (CT) scan may be normal in half or may show hypodensities in basal ganglia and frontal white matter. MRI usually reveals globus pallidal hyperintensities in half of the cases and white matter changes especially in frontal areas in most of the patients.[2] SPECT scan shows decreased flow in frontal areas although variable changes have been described.[2,3] In the index case, tracer uptake improved with clinical improvement on follow up.
CO binds heme with an affinity 250 times that of oxygen, resulting in rapid hypoxemia. Other mechanisms of toxicity include a decrease in cardiac output and direct histotoxic effect of CO by binding mitochondrial Cyt P450 and myoglobin.[2,6] Hypoxia, hypotension with resultant decreased cerebral perfusion; metabolic acidosis and free radical formation causes neuronal damage. In the acute stage, damage chiefly occurs in basal ganglia, whereas in delayed toxicity, the cerebral white matter is predominantly affected. Oligodendroglial injury leading to demyelination, slow repair of metabolic systems and decrease in aryl sulphatase activity are thought to be responsible for the delayed effects.[6] Pathologic examination reveals patchy demyelination of rostral white matter supporting the hypothesis. Since oxygen can displace CO from hemoglobin, hyperbaric oxygen at 3 atmospheres for 5 cycles of 90 min each is advocated as a treatment for acute exposure.[2] Whether it prevents delayed symptoms is not proven, but it does decrease the duration of coma and acute mortality.[2,4] In delayed toxicity, steroids, aspirin, hyperbaric oxygen have been used previously without success. Symptomatic treatment with dopaminergic agents is of help in improving parkinsonian symptoms.[2,5,7] Poor outcome is seen in patients who have had cardiorespiratory arrest or have evidence of persisting basal ganglia hypodensities on CT scan and MRI hyperintensities in pallidum or white matter.[8] About 60-75% of patients with delayed symptoms have been reported to recover fully within one year and about 15% may suffer from persisting dementia and parkinsonism.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433–5. doi: 10.1001/archneur.1983.04050070063016. [DOI] [PubMed] [Google Scholar]
- 2.Roos RA. Neurological complications of carbon monoxide intoxication. In: Vinken PJ, Bruyn GW, editors. Hand Book of Clinical Neurology. Amsterdam: Elsevier Science B.V; 1994. pp. 31–8. [Google Scholar]
- 3.Min SK. A brain syndrome associated with delayed neuropsychiatric sequelae following acute carbon monoxide intoxication. Acta Psychiatr Scand. 1986;73:80–6. doi: 10.1111/j.1600-0447.1986.tb02671.x. [DOI] [PubMed] [Google Scholar]
- 4.Late sequelae of carbon monoxide poisoning. Lancet. 1984;2:637–8. [PubMed] [Google Scholar]
- 5.Schwartz A, Hennerici M, Wegener OH. Delayed choreoathetosis following acute carbon monoxide poisoning. Neurology. 1985;35:98–9. doi: 10.1212/wnl.35.1.98. [DOI] [PubMed] [Google Scholar]
- 6.Gottfried JA, Mayer SA, Shungu DC, Chang Y, Duyn JH. Delayed posthypoxic demyelination.Association with arylsulfatase A deficiency and lactic acidosis on proton MR spectroscopy. Neurology. 1997;49:1400–4. doi: 10.1212/wnl.49.5.1400. [DOI] [PubMed] [Google Scholar]
- 7.Tach F, De Rewek J. The use of bromocriptine in Parkinsonism after carbon monoxide poisoning. Clin Neurol Neurosurg. 1987;89:275–9. doi: 10.1016/s0303-8467(87)80030-6. [DOI] [PubMed] [Google Scholar]
- 8.Noorkool DM, Kirkpatrick JN. Treatment of acute carbonmonoxide poisoning with hyperbaric oxygen: A review of 115 cases. Ann Emerg Med. 1985;14:1168–71. doi: 10.1016/s0196-0644(85)81023-4. [DOI] [PubMed] [Google Scholar]


