Abstract
Objective
To investigate whether obesity and cardiovascular risk factors are associated with psoriasis in children and adolescents.
Study design
For this population-based, cross-sectional study, measured weight and height, laboratory data, and psoriasis diagnoses were extracted from electronic medical records of 710,949 patients 2–19 y enrolled in an integrated health plan. Weight class was assigned based on body mass index-for-age.
Results
The OR for psoriasis was 0.68, 1.00, 1.31, 1.39, and 1.78 (95%CI 1.49–2.14) for underweight, normal weight, overweight, moderately obese and extreme obese children, respectively (p for trend<0.001). The OR for psoriasis treated systemic therapy or phototherapy as indicator of severe or widespread psoriasis was 0.00, 1.00, 2.78, 2.93, and 4.19 (95%CI 1.81–9.68) for underweight, normal weight, overweight, moderately obese and extreme obese children, respectively (p for trend<0.003). In adolescents, mean total cholesterol, LDL cholesterol, triglycerides and ALT were significantly higher in children with psoriasis compared with children without psoriasis after adjustment for BMI.
Conclusion
Overweight and obesity are associated with higher odds of psoriasis in youths. Independent of body weight, adolescent patients with psoriasis have higher blood lipids. These data suggest that pediatricians and dermatologists should screen youths with psoriasis for cardiovascular disease risk factors.
Keywords: obesity, body weight, childhood, psoriasis, cardiovascular risk factors
Psoriasis is a chronic inflammatory disease of the skin which often starts early in life and affects 2% of the general population and 0.7% to 1.2% of children [1, 2]. Psoriasis in adults has been consistently shown to predict morbidity, particularly diabetes [3], hypertension [3], myocardial infarction [4], stroke [5], and mortality [6]. In children, the rate of obesity has more than tripled in the past 30 years [7] with more and more children becoming extremely obese [8, 9]. Like psoriasis, obesity is a condition characterized by chronic low-level inflammation in adults [10] and children [11]. The association between obesity and psoriasis has been well described in adults [12–14], including one prospective study suggesting that obesity is a risk factor for adult-onset psoriasis [15]. However, concerns of residual-confounding and lack of causality have been raised by others [16].
One study has examined the association between obesity and psoriasis in children and found obesity to be 1.7 times higher in frequency in children with than without psoriasis [2]. Results from that study also suggest that children with psoriasis have a higher prevalence of dyslipidemia and hypertension [2]. However, information on the degree of obesity is lacking in that study and the magnitude of the association cannot be quantified. Given the association of psoriasis with mortality and other metabolic disease risks, better understanding of the relationship between pediatric obesity and psoriasis may have implications for patient treatment and disease management strategies.
Therefore, we investigated the association between BMI, the degree of obesity and psoriasis in a population-based cross-sectional study of more than 710,000 racially/ethnically diverse children enrolled in an integrated prepaid health plan. We also evaluated the association of psoriasis and putative cardiovascular risk factors such as total cholesterol, LDL, HDL, triglycerides, and alanine aminotransferase (ALT) in children.
Methods
Participants of this study were enrolled in an integrated prepaid health plan of Kaiser Permanente Southern California (KPSC) which provided comprehensive health care to over 3.3 million patients as of July 2010. Members received their care in medical offices and hospitals owned by KPSC throughout the seven-county region. All health care providers have access to the KP health record system in which all medical information is stored.
For this cross-sectional study, we used data on children enrolled in the KPSC Children’s Health Study which is described in detail elsewhere [9]. Briefly, we identified 816,204 children 2 through 19 years of age who were members of KPSC in 2007–2008 and had at least one medical visit during this study period. To eliminate pregnancy-related weight gain, we then excluded pregnant adolescents. After the exclusion of pregnant members (n=6,475), 809,729 patients were eligible for participation in the present cross-sectional study. Of these patients, 710,949 (87.8 % of eligible patients) had at least one valid body weight and height available in the electronic health record in 2007–2008 and were included in the final study cohort. The study protocol was reviewed and approved by the Institutional Review Board of KPSC.
Patients with prevalent psoriasis (n=2,119) were identified by extracting International Classification of Disease (ICD-9) code 696.1 from electronic health records from all inpatient and outpatient encounters since enrollment into the health plan. Details of the chart review process are described elsewhere [17]. To control for undercoding of psoriasis, additional searches were performed to identify potentially un-coded patients with psoriasis using a comprehensive search for prescription of medications consistent with psoriasis (n=154) and patients with a diagnosis of psoriatic arthritis (ICD-9 code 696.0, n=23). A board-certified dermatologist and trained research staff validated all psoriasis diagnoses by confirming diagnosis codes for psoriasis from physician’s notes in the electronic health record as follows: (1) Cases in which the diagnosis was made by a primary care provider, who were younger than 5 years of age, had a diagnosis of psoriatic arthritis, or were prescribed psoriasis-specific medications, were reviewed by a dermatologist (n=1,082); (2) Potential psoriasis cases identified by ICD-9 code older than 5 years of age and with a diagnosis made at least once by a dermatologist and (n=1,037) were reviewed by trained research staff. Terms used for confirmation included “silvery, flaky, papulosquamous, red, papules, plaques” in the following body locations: “knees, elbows”. Additionally, the description of the location of the rash, family history, and physical findings were used to confirm or reject the diagnosis of psoriasis. Any unclear or questionable records reviewed by research staff were additionally referred to a dermatologist for further review. The diagnosis of 1,350 children was confirmed as prevalent psoriasis. Out of these 1,350 confirmed cases, 90% (n=1,234) were diagnosed by a dermatologist in the electronic medical record.
As an indicator of severe or widespread psoriasis, we identified patients (n = 53) who received any treatment with traditional systemic medications (acitretin, cyclosporine, methotrexate), other less commonly used systemic medications (azathioprine, mycophenolate mofetil, hydroxyurea), biologic medications (TNF-α inhibitors, T-cell inhibitors, or interleukin-12/23 inhibitors) or had received phototherapy (UVB or PUVA) [18].
Information from electronic health records was utilized to extract body weight and height data. Only encounters with weight and height measurement from the same day were selected. BMI was calculated as weight (kilograms) divided by the square of the height (meters). For each year 2007 and 2008, the median BMI-for-age of all encounters for a patient from the most recent available year was used for this analysis. Based on a validation study including 15,000 patients with 45,980 medical encounters, the estimated error rate in body weight and height data was <0.4% [19].
Definitions for overweight and obesity in children and adolescents are based on the sex-specific BMI-for-age growth charts developed by the CDC and World Health Organization definitions for overweight and obesity in adults [20–22]. Children were categorized as underweight (BMI-forage <5th percentile), normal weight (BMI-for-age ≥ 5th and < 85th percentile), overweight (BMI-for-age ≥85th percentile or a BMI ≥25 kg/m2 and BMI-for-age <95th percentile or a BMI <30 kg/m2), moderately obese (BMI-for age ≥95th percentile or a BMI ≥30 kg/m2 and BMI-for age <1.2 × 95th percentile or a BMI <35 kg/m2), and extremely obese (BMI-for age ≥1.2 × 95th percentile or a BMI ≥35 kg/m2).
Race and ethnicity information was obtained from health plan administrative records and birth certificates. We categorized race/ethnicity as non-Hispanic White, Hispanic White, Black (regardless of ethnicity), Asian or Pacific Islander, other or multiple race/ethnicity, and unknown due to missing information (52.5%). A validation study compared health plan administrative records and birth certificate records of 325,810 children. The positive predictive value (PPV) for Hispanic ethnicity was 95.6%. The PPV for White, Black, Asian/Pacific Islander, American Indian/Alaskan Native, multiple and other was 89.3%, 86.6%, 73.8%, 18.2%, 51.8% and 1.2%, respectively.
For unknown race and ethnicity information, administrative records were supplemented by an imputation algorithm based on surname lists and address information derived from the U.S. Census Bureau [23]. Hispanic ethnicity and Asian race were assigned based on surnames. For Blacks and non-Hispanic Whites, the child’s home address was used to link racial/ethnic information from the U.S. Census Bureau. Race/ethnicity was hierarchically assigned using probability cut-offs of >50% for Asian surname, >50% for Hispanic surname, >75% for Black race from geocoding if probability for Asian surname was <50% (Hispanic Blacks are assigned to Black race for this study), and White race >45% from geocoding if no other assignment could be made before. The specificity and PPV were >98% for all races/ethnicities [9].
We used Medi-Cal status as an indicator for low socioeconomic status. Medi-Cal is the California state-subsidized program providing health care coverage for more than six million low-income children and families as well as elderly, blind, or disabled individuals.
According to KPSC preventive screening guidelines, all children with a BMI-for-age ≥95th percentile or ≥85th percentile with additional potential risk factors such as familial hypertension should undergo screening for dyslipidemia and abnormal liver enzymes. Typically, blood screening is recommended for children pubertal age or older. For the present study, information on total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and alanine aminotransferase (ALT) was extracted from electronic health records of children ≥12 years with a BMI-for-age ≥85th percentile (n=133,270).
Statistical analysis
Each child was assigned to a specific age group (2–5, 6–11, or 12–19 years) according to the child’s mid-year age. Differences in the distribution of basic demographics across groups defined by weight class were assessed with the chi-square test.
Multiple logistic regression models were generated to estimate odds ratios (OR) and their 95% confidence intervals (CI) for psoriasis vs. weight class (underweight, normal weight (reference), overweight, moderate obesity, extreme obesity), sex, age and race/ethnicity. Logistic regression models, stratified by sex, race/ethnicity, and age group (2–5 years, 6–11 years, and 12–19 years) were used to test the association between psoriasis and weight class, adjusted for medical center (low vs. high number of patients with psoriasis) and Medi-Cal status (yes/no), as well as sex, age, and race/ethnicity where appropriate. Tests of linear trend across weight categories were conducted by modeling the exposure as a single continuous variable in the multivariate model, the coefficient for which was evaluated using a Wald test. The association between psoriasis and continuous cardiovascular risk factors was assessed with multiple linear regression models adjusted for age, sex, race/ethnicity, medical center, Medi-Cal status and BMI.
Among overweight and obese adolescents who met the criteria for preventive screening for cardiovascular risk factors (n=133,270), a substantial proportion of patients did not undergo screening and, thus, had missing data for total cholesterol (n= 73,884; 55.4%), LDL cholesterol (n= 89,519; 67.1%), HDL cholesterol (n= 84,685; 63.5%), triglycerides (n= 97,091; 72.9%) and ALT (n= 92,455; 69.4%). We compared adolescents with missing values for any of the above metabolic traits with those adolescents who did have data for differences in the distribution of demographic factors and/or proportion affected with psoriasis, compared with those with complete data with chi-square tests. Adolescents missing one or more lipid or ALT values were more likely to be male, white, or unknown race/ethnicity; they were also less likely to receive Medi-Cal covered services or to have psoriasis.
Because the analysis of only complete data may introduce bias in the estimation of parameters and reduce power to detect significant effects, we considered multiple imputation as an alternative method that allows individuals with incomplete data to be included in analyses [24]. Briefly, multiple imputations involve the replacement of missing values with values that have been sampled from their predictive distribution based on the observed data. Several values per observation are sampled, resulting in several simulated data sets. Standard regression analyses are then performed on each data set and parameter estimates combined to yield one final estimate, with standard errors calculated according to Rubin’s method, which takes into account the variability between data sets [24]. Thus, we performed multiple imputation of missing values using the Markov Chain Monte Carlo method, with posterior mode computed by Expectation-Maximization (EM) algorithm [25]. The means and standard deviations from available observations were used as the initial estimates for the EM algorithm. The association between psoriasis and continuous clinical measures was then assessed with multivariable linear regression models on existing and imputed data, adjusted for age, sex, race/ethnicity, Medi-Cal status and BMI. All analyses were conducted using SAS version 9.1 (Proc MI and PROC MIANALYZE, SAS Institute, Inc, Cary, NC) and PASW release 17.0 (SPSS Inc., Chicago, IL).
Results
The study population was racially and ethnically diverse. Patients who were overweight, moderately or extremely obese were more likely to be older (p<0.001), male sex (p<0.001), and Hispanic or Black (p<0.001), than those of normal weight (Table I). Underweight patients were more likely to be younger and Asian/PI (p<0.001).
Table 1.
Demographic characteristics of the study population according to weight class1.
| Under- weight (n=20,628) |
Normal weight (n=426,390) |
Over- weight (n=127,279) |
Moderately obese (n=91,258) |
Extremely obese (n=45,394) |
P-value | |
|---|---|---|---|---|---|---|
| Male (%) | 51.3 | 48.6 | 48.9 | 56.3 | 57.1 | <0.001 |
| Age group (%) | <0.001 | |||||
| 2–5 y | 37.5 | 27.3 | 18.7 | 19.9 | 9.3 | |
| 6–11 y | 24.9 | 27.6 | 29.0 | 35.3 | 33.9 | |
| 12–19 y | 37.6 | 45.1 | 52.4 | 44.7 | 56.8 | |
| Race/Ethnicity (%) | <0.001 | |||||
| Non-Hispanic White | 30.4 | 28.1 | 22.6 | 17.9 | 14.8 | |
| Hispanic White | 34.0 | 40.8 | 48.1 | 54.0 | 55.4 | |
| Black | 6.9 | 7.5 | 7.8 | 7.4 | 9.9 | |
| Asian/Pacific Islander | 12.5 | 7.8 | 5.6 | 4.9 | 3.7 | |
| Others | 3.7 | 3.4 | 3.2 | 4.0 | 3.7 | |
| Unknown | 12.6 | 12.4 | 12.6 | 11.8 | 12.6 | |
| Medi-Cal (%) | 10.4 | 11.5 | 12.0 | 13.8 | 13.2 | <0.001 |
Definition of weight class: underweight was defined BMI-for-age ≤ 5th percentile, overweight as BMI-for-age ≥ 85th percentile or a BMI ≥ 25 kg/m2, moderate obesity as ≥ 95th percentile or a BMI ≥ 30 kg/m2, and extreme obesity ≥1.2 × 95th percentile or a BMI ≥ 35 kg/m2.
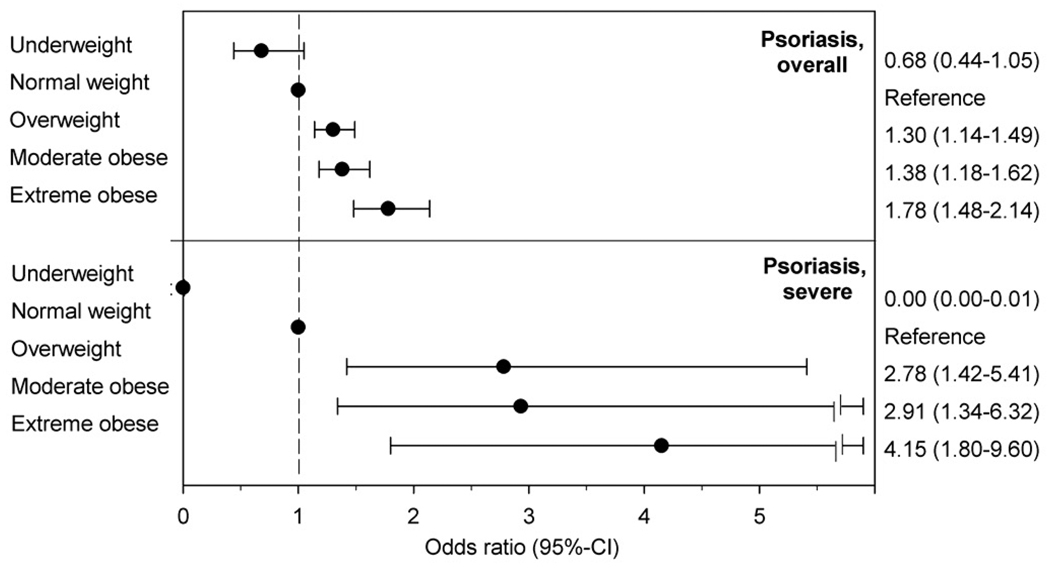
We identified 1,350 patients with prevalent psoriasis (19/10,000 patients). The crude ORs for psoriasis were 0.62 (95%-CI 0.40–0.95), 1.00, 1.38 (1.20–1.58), 1.33 (1.13–1.55), and 1.86 (1.55–2.24) for underweight, normal weight, overweight, moderately obese and extreme obese patients, respectively (p for trend<0.001). After adjusting for potential confounders, the ORs for psoriasis were 0.68 (95%-CI 0.44–1.06), 1.00, 1.31 (1.13–1.49), 1.39 (1.19–1.63), and 1.78 (1.49–2.14) for underweight, normal weight, overweight, moderately obese and extreme obese patients, respectively (p for trend<0.001, Figure 1). The association between weight class and psoriasis was comparable across groups defined by sex, age and race (interaction p ≥0.15 for all tests).
Figure 1.
Adjusted odds ratios for psoriasis and psoriasis with systemic therapy or phototherapy indicating severe or widespread psoriasis according to weight class.
A total of 53 patients with psoriasis received systemic therapy or phototherapy indicating severe or widespread psoriasis. The adjusted ORs for severe or widespread psoriasis were 0.00 (95%-CI 0.00–0.01), 1.00, 2.78 (1.43–5.42), 2.93 (1.35–6.37), and 4.19 (1.81–9.68) for underweight, normal weight, overweight, moderately obese and extreme obese children, respectively (p for trend<0.003).
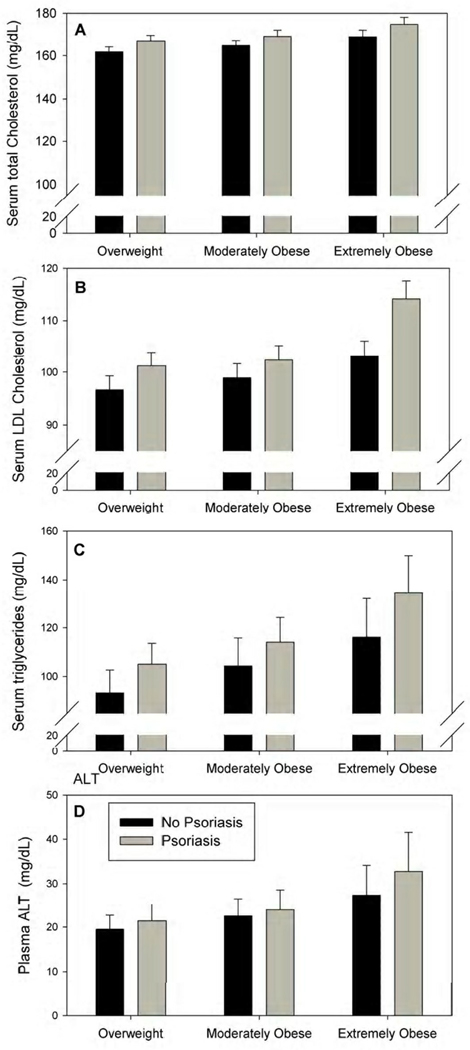
The associations between psoriasis and cardiovascular risk factors in overweight to extremely obese adolescents 12–19 years of age are presented in Table II. Among the 133,270 overweight to extremely obese adolescents included in this analysis, those with psoriasis had significantly higher mean total cholesterol (p=0.020), LDL cholesterol (p=0.007), triglycerides (p=0.014) and ALT (p=0.016) compared with those without psoriasis. Mean HDL cholesterol was not significantly different between patients with and without psoriasis. The difference in lipids and ALT between patients with and without psoriasis was marginally larger in extremely obese than in overweight subjects. The interaction between weight class and psoriasis, however, was not significant (0.30 < p < 0.68) (Figure 2).
Table 2.
Total cholesterol, LDL cholesterol, triglycerides and alanine aminotransferase (ALT) in overweight to obese adolescent patients 12–19 years of age (n=133,270) with and without psoriasis.
| Overweight and obese adolescents |
|||
|---|---|---|---|
| Without psoriasis |
With psoriasis | p-value1 | |
| n = 132,831 | n = 439 | ||
| Serum total Cholesterol (mg/dL)2 | 163.9 ± 3.6 | 169.6 ± 4.2 | 0.021 |
| Serum LDL Cholesterol (mg/dL)2 | 98.4 ± 3.5 | 103.9 ± 4.1 | 0.007 |
| Serum HDL Cholesterol (mg/dL)2 | 45.4 ± 2.8 | 45.3 ± 2.9 | 0.937 |
| Serum Triglycerides (mg/dL)2 | 100.6 ± 13.8 | 114.4 ± 16.4 | 0.015 |
| Plasma ALT (units/L)2 | 21.7 ± 5.9 | 25.1 ± 8.3 | 0.016 |
P-value for associations between weight class and psoriasis determined by multiple logistic regression, adjusted for age, sex, race/ethnicity, Medi-Cal. P-value for associations between psoriasis and continuous clinical measures determined by multiple linear regression, adjusted for age, sex, race/ethnicity, Medi-Cal and BMI.
Mean ± SE presented are adjusted for age, sex, race/ethnicity, Medi-Cal and BMI.
Figure 2.
Adjusted mean LDL in overweight to extremely obese adolescents 12–19 years of age with and without psoriasis, by weight class.
Mean total cholesterol (A), LDL cholesterol (B), triglycerides (C), and alanine aminotransferase (ALT, D) are adjusted for age, sex (male vs. female), race (non-Hispanic White, Hispanic White, Black, Asian, other, and unknown), Medi-Cal (yes vs. no) and BMI.
In a sensitivity analysis, we included only patients who were screened for dyslipidemia or elevated ALT (i.e. no imputed data were analyzed). The association between psoriasis and blood lipids/ALT was essentially unaltered.
Discussion
In this cross-sectional study, overweight and obesity was associated with a significantly higher frequency of psoriasis. One prospective study [26], one nested case-control study [27], several cross-sectional [14, 28–31], and case-control studies [32] in adults support the hypothesis that obesity is associated with a higher risk for psoriasis. Other studies, however, observed no association between obesity and psoriasis [33, 34] of which one [33] reported an association in a later update from the same data source [28]. In the Nurses’ Health Study, relative risks of adult onset psoriasis for overweight (BMI 25.0–29.9 kg/m2), moderately (BMI 30.0–34.9 kg/m2) and severely obese (BMI ≥ 35 kg/m2) individuals at baseline were 1.23 (95% CI 1.00–1.50), 1.73 (95% CI 1.15–1.91), and 2.69 (95% CI 2.12–3.40), respectively [26]. Comparable with the results from our study in a pediatric population, findings in adult populations suggest that overweight is already associated with a slightly higher risk of psoriasis.
Results from our study also suggest that the severity of psoriasis is associated with high body weight. Receiving systemic therapy or phototherapy indicating severe or widespread psoriasis was almost three times as likely to occur in moderately obese children, and more than four times as likely to occur in extremely obese children compared with normal weight children. The link between the degree of obesity and the severity of psoriasis may be explained by reports that obesity negatively impacts the effectiveness of psoriasis treatment [35]. The interpretation of our results, however, is limited by the small number of patients (n=53) with psoriasis who received systemic therapy or phototherapy indicating severe or widespread psoriasis, and the cross-sectional study design.
In our pediatric population, total cholesterol, LDL cholesterol, and triglycerides were higher in adolescents with psoriasis than in adolescents without psoriasis independent of obesity. Although the differences appear to be small, they are of clinical relevance. For example, the observed difference in total cholesterol translates to a change from the 50th percentile to almost the 70th percentile for total cholesterol in a 17-year old girl [36]. Previous studies in adult populations have linked psoriasis to the metabolic syndrome and its components dyslipidemia and disturbed glucose metabolism [29, 37]. Data on children, however, are scant. A diagnosis of dyslipidemia and hypertension was reported 2.15 (95% CI 1.65–2.80) and 1.89 (95% CI 1.47–2.67) more frequent in children with than in children without psoriasis, respectively [2].
Several mechanisms have been suggested to explain the association between obesity, cardiovascular risk factors, and psoriasis. First, low level chronic inflammation is characteristic of both psoriasis and obesity [38]. In the obese state, production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) is upregulated, which may contribute to a worsening of psoriasis. Chronic inflammation, subsequent insulin resistance, and disturbed free fatty acid metabolism [39] may also explain the high incidence of diabetes and metabolic syndrome in patients with psoriasis. Moreover, treatment of severe psoriasis by biologic agents that inhibit inflammatory processes has been shown to rapidly improve psoriasis [40] and remission of psoriasis has been reported after bariatric surgery [41]. These data suggest that obesity-related inflammatory processes may contribute to the severity of psoriasis.
Second, obesity after onset of psoriasis has been linked to a sedentary lifestyle in adults with psoriasis [14]. Adults with psoriasis were more likely to smoke and show other unhealthy lifestyle factors than adults without psoriasis; these lifestyle factors also predict psoriasis severity [14]. Interestingly, these behaviors are also often associated with obesity. Thus, although we can describe the coincidence of psoriasis and obesity, it is still unclear whether the biological processes that underlie obesity contribute to the development of psoriasis, or whether obesity and psoriasis simply share a common set of risk factors.
The cross sectional design of this study does not allow causal conclusions, and as such, the interpretability of our findings is limited. Because both exposure and outcome have been simultaneously assessed, no conclusions pertaining to causality can be made. Therefore, results on psoriasis and higher blood lipids and ALT measures must be interpreted with caution. Obesity, dyslipidemia, and elevated ALT are associated with chronic inflammation and cardiometabolic syndrome; obesity, dyslipidemia, and elevated ALT may be both consequence and contributor to the severity of symptoms in psoriatic patients.
To address the issue of potential underdiagnosis of psoriasis, we expanded our case identification beyond the standard approach of using diagnosis codes alone. We identified all cases with a diagnosis of psoriatic arthritis and/or a medication consistent with psoriasis. Electronic health records of all potential cases of psoriasis were manually reviewed. We cannot rule out potential underdiagnosis because patients with very mild psoriasis who do not seek medical attention may be missed. The prevalence of psoriasis is relatively low compared with other pediatric populations, which may be explained by underdiagnosis or by an improvement of skin lesions due to sunlight exposure in southern California, and consequently, potentially fewer patients seeking medical advice [42]. We can also not rule out misclassification as severe psoriasis because this classification was solely based on psoriasis treatment. Patients with severe psoriasis who do not undergo phototherapy or receive systemic treatment of psoriasis may be missed. However, misclassification due to underdiagnosis would most likely bias our results towards the null.
Because these screening tests are only performed in overweight or obese adolescent patients at risk for dyslipidemia and elevated liver enzymes, our analyses was limited to this subset of patients. The large number of missing blood lipid and ALT values required us to apply a multiple imputation method in order to avoid the potential bias in estimates of association and substantial loss of power that are inherent in a complete case analysis. For the present study, we assumed that blood lipid screening is more complete in older and/or extremely obese than in younger and/or overweight patients. Thus, analyses of only those observations with complete data may result in bias toward the null, and attenuate the association between blood lipids and psoriasis. Variables that are predictive of missing blood lipid data, such as age, weight class, sex, and race/ethnicity, were included in the imputation model because multiple imputation analyses will only avoid bias if enough variables predictive of the missing values are included in the imputation model [24]. We also performed sensitivity analyses including only patients who were screened for dyslipidemia or elevated ALT, i.e. no imputed data were analyzed with essentially unaltered results.
Despite these limitations, our study is a population-based approach. The extremely large multiethnic and diverse pediatric population has equal access to health care and lives throughout the seven-county region of southern California. Therefore, we can assume that our results are generalizable to other insured populations.
Acknowledgments
The authors gratefully thank Alexander Carruth and Monica Levitt for their technical support.
Funded by Kaiser Permanente Direct Community Benefit Funds. J.W. received research funding from Abbott Laboratories. S.J. received research grants from Merck and Beckmancoulter, and served as consultant for Merck without compensation.
List of abbreviations and acronyms
- BMI
Body Mass Index
- KPSC
Kaiser Permanente Southern California
- IRB
Internal Review Board
- ICD
International Classification of Disease
- BMI
Body Mass Index
- LDL
Low-Density Lipoprotein
- HDL
High-Density Lipoprotein
- ALT
Alanine Aminotransferase
- EM
Expectation-Maximization
- PPV
Positive predictive value
- TNF-α
Tumor Necrosis Factor Alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
References
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schafer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162:633–636. doi: 10.1111/j.1365-2133.2009.09593.x. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Kimball AB, Guerin A, Latremouille-Viau D, Yu AP, Gupta S, Bao Y, et al. Coronary heart disease and stroke risk in patients with psoriasis: retrospective analysis. Am J Med. 2010;123:350–357. doi: 10.1016/j.amjmed.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index for age by using the 2000 Centers for Disease Control and Prevention growth charts. AmJ ClinNutr. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 9.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157:26–31. doi: 10.1016/j.jpeds.2010.01.025. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 11.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes Rev. 2010;11:118–126. doi: 10.1111/j.1467-789X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 12.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J AmAcadDermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649–655. doi: 10.1111/j.1365-2133.2007.08068.x. [DOI] [PubMed] [Google Scholar]
- 14.Herron MD, Hinckley M, Hoffman MS, Papenfuss J, Hansen CB, Callis KP. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527–1534. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 15.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses' Health Study II. ArchInternMed. 2007;167:1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 16.Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. J Invest Dermatol. 2009;129:1601–1603. doi: 10.1038/jid.2009.55. [DOI] [PubMed] [Google Scholar]
- 17.Wu JJ, Porter AH, Black MH, Smith N, Jacobsen SJ, Koebnick C. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. doi: 10.1016/j.jaad.2010.09.005. in press. [DOI] [PubMed] [Google Scholar]
- 18.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 19.Smith N, Coleman KJ, Lawrence JM, Quinn VP, Getahun D, Reynolds K, et al. Body weight and height data in electronic medical records of children. Int J Pediatr Obes. 2010;5:237–242. doi: 10.3109/17477160903268308. [DOI] [PubMed] [Google Scholar]
- 20.World Health O. Technical Report Series 894: Obesity: Preventing and managing the global epidemic. Geneva: World Health Organization; 2000. ISBN 92-4-120894-5. [PubMed] [Google Scholar]
- 21.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index for age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 23.Bureau of the Census. Census 2000 surname list. Washington DC: 2009. http://wwwcensusgov/genealogy/www/freqnames2khtml. [Google Scholar]
- 24.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 26.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses' Health Study II. Arch Intern Med. 2007;167:1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 27.Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143:1559–1565. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 28.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216:152–155. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 29.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 30.McGowan JW, Pearce DJ, Chen J, Richmond D, Balkrishnan R, Feldman SR. The skinny on psoriasis and obesity. Arch Dermatol. 2005;141:1601–1602. doi: 10.1001/archderm.141.12.1601. [DOI] [PubMed] [Google Scholar]
- 31.Murray ML, Bergstresser PR, Adams-Huet B, Cohen JB. Relationship of psoriasis severity to obesity using same-gender siblings as controls for obesity. Clin Exp Dermatol. 2009;34:140–144. doi: 10.1111/j.1365-2230.2008.02791.x. [DOI] [PubMed] [Google Scholar]
- 32.Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61–67. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 33.Cohen AD, Gilutz H, Henkin Y, Zahger D, Shapiro J, Bonneh DY, et al. Psoriasis and the metabolic syndrome. Acta Derm Venereol. 2007;87:506–509. doi: 10.2340/00015555-0297. [DOI] [PubMed] [Google Scholar]
- 34.Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157:68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- 35.Naldi L, Addis A, Chimenti S, Giannetti A, Picardo M, Tomino C, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology. 2008;217:365–373. doi: 10.1159/000156599. [DOI] [PubMed] [Google Scholar]
- 36.Cook S, Auinger P, Huang TT. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr. 2009;155 S6:e15–e26. doi: 10.1016/j.jpeds.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008;20:416–422. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamminga EA, van der Lely AJ, Neumann HA, Thio HB. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768–773. doi: 10.1016/j.mehy.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 39.Kirk EP, Klein S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2009;11:761–765. doi: 10.1111/j.1559-4572.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–251. doi: 10.1056/NEJMoa066886. [DOI] [PubMed] [Google Scholar]
- 41.Higa-Sansone G, Szomstein S, Soto F, Brasecsco O, Cohen C, Rosenthal RJ. Psoriasis remission after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2004;14:1132–1134. doi: 10.1381/0960892041975569. [DOI] [PubMed] [Google Scholar]
- 42.Wu JJ, Black MH, Smith N, Porter AH, Jacobsen SJ, Koebnick C. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2010 doi: 10.1016/j.jaad.2010.09.005. in press. [DOI] [PubMed] [Google Scholar]