Abstract
Introduction:
Apart from the visual assessment, measurement of plasma hemoglobin in the supernatant from red cell units provides an objective measure of the extent of hemolysis during storage.
Study Design and Methods:
Packed red cells (N=50), 25 units each in triple (CPD-A1 and SAGM) and quadruple (CPD-A1 and ADSOL) blood bags were evaluated for plasma hemoglobin by the tetramethylbenzidiene (TMB) method on day 1, 7, 14, 21 and 28 of collection. The hemoglobin, hematocrit, MCV, LDH and potassium levels were also noted. Whole blood units (N=25) were used as controls.
Results:
Hemolysis increased in all the stored red cell units. Plasma hemoglobin increased significantly in the first week of storage. The hemolysis, LDH and potassium levels were found to be significantly higher in the red cell units harvested from the triple blood bags. However, on day 28 of storage, free hemoglobin in all the red cell units was much below the 0.8% hemolysis.
Conclusion:
Hemolysis of the red cells increases due to processing and during storage and is maximum during the first week. Adequate process control and proper storage facilities should be ensured to minimize the hemolysis of red cells during processing and storage.
Keywords: Component separation, red blood cell hemolysis, storage
Introduction
Sometimes a RBC unit exhibits clear red discoloration of its supernatant plasma. The action taken is rather subjective - some individuals issue such a product without hesitation whereas others might discard it due to visual impression of excessive hemolysis. Hemolysis of the red cells that occurs during component processing and storage of red cell units has serious clinical implications for the transfused patients. Detecting excessive hemolysis is important to minimize transfusion of bacterially contaminated RBC units. Also an elevated potassium and free hemoglobin itself may cause significant complications in some patients. The extent of hemolysis in blood components is an important indicator of cellular integrity and a quality parameter. Shortcomings in processing / storage of the red cell components and interventions like washing, irradiation and filtration of red cells for leukocyte depletion are typical situations where high plasma Hb levels can occur.
The extent of hemolysis can be estimated by:[1–4]
(i) Visual assessment, (ii) Spectrophotometric assays (iii) Photometeric method and (iv) Microplate technique.
In most blood banks, visual inspection of the sample tube segments and the blood unit is used as a quick and easy method to detect hemolysis in blood units.
The objective of this study was to investigate the impact of various RBC preparation procedures on the extent of hemolysis and to quantify the levels of plasma Hb in packed red cell units with two different additive solutions during storage.
Materials and Methods
Study design
Whole blood units were collected in a single blood bag (with CPDA-1) (Terumo Penpol Limited), triple blood bag (with SAGM) (Terumo Penpol Limited) and a top and bottom (TAB) blood bag system (with Adsol) (Baxter). After a holding period of at least 30 min, components were separated from the blood units collected in the triple and TAB blood bags. The whole blood collected in single blood bags was kept as control samples. Subsequently, all red cell units were stored under standard storage conditions at 4°C. Assessment for hemolysis was done using the 3,3’,5,5’ Tetramethyl Benzidine (TMB) method at varied storage intervals.
Blood collection and component separation
Whole blood was collected in each type of blood bag (N=25) from healthy blood donors. Appropriate preparation of donor arm was done to ensure minimum contamination during phlebotomy. Blood collection monitors were used to ensure collection of accurate blood volumes. The time required for collection of each unit was recorded. Blood components were separated from whole blood collected in triple and TAB blood bags after appropriate holding period.
In case of the TAB system (N=25), the whole blood units (450 ml) were centrifuged by a “hard spin” technique (3900 rpm, 8 min, 22 °C) in the Cryofuge 6000i Heraeus refrigerated centrifuge. The red cells were added into the Adsol solution and plasma was simultaneously collected in another transfer bag using the Optipress II Extractor (Baxter Healthcare Corp. Deerfield Illinois, USA). The buffy coat (BC) left behind was centrifuged after two to three hours by a “soft spin” technique (800 rpm, 5 min, 22°C) in Sorvall centrifuge to obtain the platelet concentrate.
The WB units collected in triple blood bag system (N=25) (450 ml) were centrifuged with a “soft spin” (1800 rpm, 8 min, 22°C) in Sorvall RC3C plus refrigerated Centrifuge. The platelet rich plasma (PRP) was separated and SAGM solution was added into the packed red cell bag. The PRP was subjected to a “hard spin” (4500 rpm, 15 min, 22°C) in a Sorvall refrigerated centrifuge to obtain the platelet concentrate and platelet poor plasma.
The WB units collected in single blood bags were kept as controls. All the red cell units were stored at 4°C in blood bank refrigerators with continuous computerized temperature monitoring.
Sampling technique
Samples were collected after gentle mixing by inversion, by a sterile sampling procedure in a laminar airflow cabinet. The blood bag tubing was uniformly and carefully stripped and refilled to collect representative samples. 5 ml of blood sample was collected from each unit and was centrifuged for 10 min at 1000 rpm. This sampling was performed immediately after blood collection (day 0) and after component separation (day 1) and weekly thereafter for four weeks.
In vitro measurements
The volumes were calculated by dividing the net weight of the red cell concentrates by their density. Total hemoglobin, hematocrit, red cell, leukocyte and platelet counts and the mean corpuscular volume were analyzed on the Coulter (Beckman Coulter AcT) hematology analyzer. The plasma Hb was measured using the TMB method with absorbance measured at 600 nm on a spectrophotometer against a deionized water blank.
Calculations were done using the 60 mg dl-1 Hb standard as follows:
![]()
Potassium and LDH were estimated using an Olympus AU 640 automated biochemistry analyzer.
On Day 28 of storage, a representative sample from units showing evidence of excessive hemolysis on visual inspection was submitted for microbiological analysis.
Calculation of percentage hemolysis
The percentage of hemolysis in a RBC unit was calculated as follows:
(100 - Hct) × plasma Hemoglobin (g dl-1) / Total Hb (g dl-1).
Statistical analysis
All the data was analyzed using SPSS (version 11.5) software for windows. The data was grouped according to the period of storage and the type of method used for component separation.
Paired samples statistics (T-test) was used to compare the various parameters in different groups at varied storage intervals.
Correlations between various parameters under study in the individual groups were analyzed with Pearsons correlations coefficient (PCC) and a PCC of < 0.05 was considered as statistically significant.
Repeated measures (RM) ANOVA on Ranks and Tukey's and Bonferroni's part-wise multiple comparison test was used to assess the changes in various parameters over storage period in the three groups.
Results
Effect of storage on hemolysis
Hemolysis increased in all stored RBC units with storage period (repeated measures ANOVA P< 0.001) [Table 1]. The plasma Hb level in the three groups was almost similar when measured before component separation (day 0). The level of hemolysis showed significant (P< 0.05) variation between the three groups when assessed after component separation (day 1).
Table 1.
Plasma Hb levels (mg dl -1) in CPDA-1 versus SAGM and Adsol RBC units
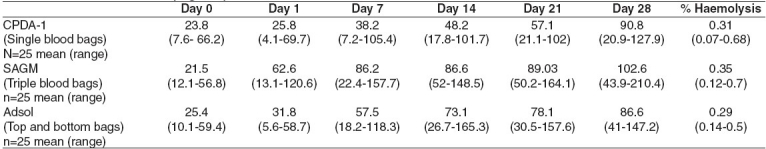
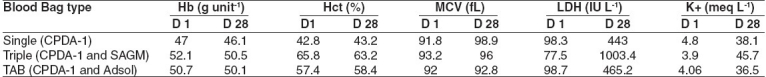
A significant (P< 0.05) increase in plasma Hb occurred in all the three groups during the first seven days of storage. However the hemolysis was much below the permissible level of 0.8% hemolysis in all the three groups when measured on day 28 of storage. The mean Hb content of all red cell units in the three groups was above 45 g unit -1 at day 28 of storage. The hematocrits of RBC units collected in TAB bags and harvested by buffy coat method were lower compared to the RBC units prepared by the PRP method. The LDH and K+ levels were found to be significantly higher (P< 0.05) in the RBC units collected in the triple blood bags (harvested using the PRP method) on day 28 of storage [Table 2].
Table 2.
Cellular content and biochemical parameters
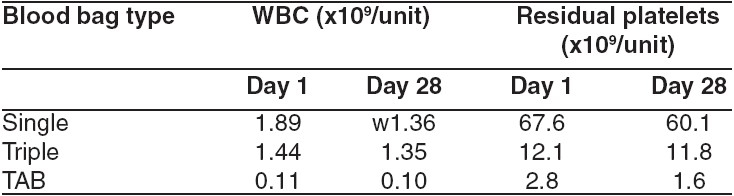
The residual contaminating leucocytes and platelets were lowest in the RBC units collected in TAB bags as compared to the other two groups [Table 3]. Sterility was confirmed by the absence of bacterial and fungal growth in units, which appeared hemolysed on visual examination on Day 28 of storage. 18 red cell units (24%) showed visual evidence of hemolysis on Day 28 of storage. These included 4 units (16%) each with CPDA-1 and Adsol while 10 units (40%) with SAGM. The plasma Hb of the above units ranged from 120-210 mg dl -1 but none of them exceeded the threshold of 0.8% hemolysis.
Table 3.
Residual Leukocyte and Platelet content
Discussion
RBC units are prepared predominantly as concentrates (packed red cells) re-suspended in additive solutions (SAGM and Adsol) at our center. Different type of blood bags, additive solutions and different preparation techniques are used for this purpose.
RBC hemolysis is known to occur during blood collection, processing, handling and storage within the transfusion service and also during transport to the patient's bedside. RBC hemolysis is an obvious marker of the failure of an RBC storage system and also bacterial contamination. Such hemolysis can be in the form of the rupture of whole cells or as the loss of microvesicles from the surface of the still intact cells. Leucoreduction, membrane stabilizers such as mannitol, citrate and hypotonic storage solutions can contribute to reduced hemolysis.
RBC units are inspected visually for evidence of hemolysis before being issued from the blood bank and also again before transfusion. However, currently there is no specific threshold for the level of hemolysis assessed by visual inspection of blood components before release for transfusion. The visual method is biased, inaccurate, misleading and results in overestimation of hemolysis in RBC units.[2] Grossly visible pink discoloration of plasma or red cell suspending medium occurs with plasma Hb levels as low as 25 mg dl-1 (≅0.09% hemolysis). The US Food and Drug Administration (FDA) has not established an official guideline for acceptable level of hemolysis in blood products for transfusion. However, the FDA has recommended a maximum of 1% hemolysis for deglycerolized RBCs and has approved and licensed additive solutions for long term storage of packed RBC units with less than 1% hemolysis at the end of storage period. The guidelines of the council of Europe for hemolysis in RBC products for transfusion are 0.8%. Janatpour et al.[2] have recently reported in their study that a subjective visual inspection would have resulted in discard of approximately 50% of units collected in CPDA-1 and 10% of units stored in Adsol. All the units showing evidence of hemolysis on visual examination in our study did have plasma Hb levels within the 0.8% hemolysis threshold. These units would have been discarded had we not objectively evaluated the extent of hemolysis by the TMB method. Spectrophotometric methods, such as the TMB method, have been the traditional “gold standard” for measurement of free Hb[5] and therefore this was our method of choice. In our study, we have used the TMB method to assess the level of plasma Hb in RBC concentrates prepared by two methods viz, the platelet rich plasma (PRP) method and the buffy coat (BC) method. We have simultaneously studied the storage properties of RBC units in two different additive solutions viz, SAGM and Adsol. We found that hemolysis increased in all RBC units over time as also reported by other investigators.[6,7]
However, on Day 28 of storage, the extent of hemolysis in all the RBC units was well below the permissible level of 0.8% hemolysis as stated in the Council of Europe guidelines. We opted to study the extent of hemolysis up to Day 28 of storage only since most of our RBC units get issued within this period.
The hemolysis in all the CPDA-1 units and those harvested by PRP method and stored in SAGM was higher compared to the RBC units collected in TAB bags and stored in Adsol.
We found that the RBC units in SAGM showed a higher level of hemolysis as compared to those in Adsol. Zimmermann et al. (2003) have also reported similar findings.[1] This increased level of hemolysis in SAGM units can be attributed to the preparatory technique i.e., the PRP method in which the additive solution (SAGM) is added to the packed red cells after separation of the PRP. Sudden exposure of RBCs to additive solutions in the blood storage bags may result in either damage or lysis of the more fragile populations of RBCs due to change in osmolality. Factors relating to preparative procedures, which could have caused haemolysis are large variation in centrifugation speeds, rapid re-suspension of packed red cells in additive solutions and variations in blood storage bag configurations or compositions. Hogman et al.[8] have reported that the type of storage containers can significantly affect RBC hemolysis during storage.
In our study, the containers used for storage were made of polyvinyl chloride (PVC) with di-(2-ethylhexyl) phthalate (DEHP) as plasticizer. DEHP has been shown to decrease the rate of hemolysis during storage. Polyvinyl chloride (PVC) plasticized with DEHP is the standard material used for RBC storage bags. An extractable plasticizer like DEHP improves RBC storage by reducing hemolysis and membrane loss by microvesiculation.[9,10] RBC units that are damaged during the preparative procedures and then stored in non-DEHP bags are more likely to hemolysed either during storage or further manipulation or handling. The difference in extent of hemolysis that we found in the three different blood bag types was not due to the type of plasticizer since DEHP was uniformly used in all three bag types. Zimmermann et al. (2003) have reported significantly lower hemolysis in RBC's in AS-1 (Adsol) compared to RBC's in SAGM. However the authors found that RBC concentrates containing SAGM from one single manufacturer had higher invitro hemolysis at the end of shelf life compared to all other manufacturers and therefore the increased hemolysis was not due to the kind of additive solution. However the method used for component preparation was uniform in all the above units i.e., buffy coat method, whereas we have compared the PRP and BC methods in our study. The above study also reports higher rates of invitro hemolysis in RBC units with low Hb content. We could not demonstrate any such correlation in our analysis. Improved red cell viability with Adsol causing only 0.3% hemolysis at 42 days as against 0.58% with other solutions like SAGM has been reported (Unpublished data). Studies conducted by various investigators to quantify the levels of plasma Hb in packed red cell units during storage showed significant increase in free Hb. At 26 days of storage, an unfiltered unit of packed RBC's in Adsol has plasma Hb level of 90.2 mg dl -1(range, 46.5 to 151.5 mg dl -1). In our study we recorded plasma Hb levels of 86.6 mg dl -1(41-147.2 mg dl 1) in non-leucoreduced packed RBC units stored in Adsol.
We found that the RBC units stored in Adsol (TAB blood bags and BC method) had minimum hemolysis (Mean = 0.29%, Range: 0.14% to 0.5%). Maximum hemolysis (mean-0.35%, range: 0.12% to 0.7%) was associated with RBC units stored in SAGM whereas the CPDA-1 RBC units showed mean 0.31% hemolysis (range: 0.07% to 0.68%). The additive solutions SAGM-2 and Adsol have been used popularly for extended storage of RBC units. The marginal increase in hemolysis in the SAGM units could be due to the higher content of Dextrose monohydrate IP (2200 mg vs 900 mg) and Mannitol (750 mg vs 525 mg) in Adsol compared to SAGM.
The above difference in the composition of the two additive solutions might be contributory to lysis in addition to the differences in the preparatory techniques (PRP Vs BC methods). Janatpour et al. have also reported higher level of hemolysis in CPDA-1 units compared to Adsol units.[2]
During storage leukocytes break down and release a number of chemicals and enzymes such as hydrogen peroxide and proteases. These proteases released by leucocytes during storage have been reported to cause RBC lysis during storage.[11]
Though none of the RBC units were leucoreduced, the units prepared by buffy coat method had lower WBC counts compared to the other two groups [Table 3]. This could be a significant factor attributing to the lower hemolysis in RBC units collected and stored in the TAB bags.
There are many variables in the preparation of blood components influencing the final quality. These include the duration and force of centrifugation, the separation procedure, the configuration of blood bag sets and composition of anticoagulants and storage solutions. Therefore there is no doubt that blood components from different manufacturers differ in parameters that characterize their quality.[12] The difference in the hemolysis levels in different blood bags highlighted in our study can be accounted for based on the above observation.
Our finding of decreased hemolysis in Adsol units can be explained in part by the observations made by Greenwalt et al. who have reported that RBCs stored in Adsol (AS1) shed significantly less vesicle membrane Cholesterol, phospholipids and protein and maintained better morphology scores compared to those stored in CPDA-1.[13] These changes occur mainly in the first ywo to three weeks of storage.[7]
There was no co-relation between the MCV and storage period or type of blood bag and additive solution. Nakao et al. have also reported similar findings.[14]
The temperature of the blood and components during storage or processing is a very important factor affecting hemolysis. The temperature greatly affects membrane deformability and therefore the stability of the membrane during processing. Such thermally damaged RBC's may be broken down during processing, centrifugation and separation of blood units into different components.
Zimmermann et al. have elucidated the possible influence of interruptions of optimal storage conditions for RBC components by everyday events in a hospital-based transfusion service like cross match testing and re-entry of issued components. The authors conclude that small rises in temperature during interruptions of standard storage conditions are probably not the only cause for the increased in vitro hemolysis in RBC units. We use the computerized central monitoring system (CMS) for monitoring temperature of all storage equipments. Besides online alarms, temperature readings at intervals as low as 10 min are archived from the CMS. Any rise or fall in the specified storage temperature for individual equipment is immediately detected by the CMS and prompt corrective action is taken whenever essential.
Conclusion
Although accurate evaluation of hemolysis in RBC units has relevance to transfusion recipient safety, it is also an important quality indicator of blood manufacturing processes. Hemolysis is a very important parameter for assessing the quality of stored RBC′s. Hemolysis of red cell units occurs during processing for component separation and also due to repeated handling during storage, issue and transport before transfusion to the patient. The extent of hemolysis however does not exceed the permissible threshold for hemolysis up to day 28 of storage. Visual assessment of hemolysis leads to inadvertent discard of precious RBC units and therefore routine quantitative analysis for hemolysis in a blood component production setting must be done using methods like TMB or Hemocue plasma Hb analyzer. Red cell units that are likely to have excess of hemolysis for e.g., units nearing their outdate period, over-collected or under-collected units, those returned after being issued should be subjected to quantitative analysis for extent of hemolysis before a decision to discard them is taken.
Acknowledgments
The authors acknowledge Mrs. Rohini Havaldar and Ms. Anagha Kakade - Clinical Research Secretariat, Tata Memorial Hospital, Mumbai for their contribution towards statistical analysis of the data. Dr. A. S. Raste and Mr. T. J. Matale, Dept. of Biochemistry, Tata Memorial Hospital, Mumbai for technical support in biochemical analysis.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Zimmermann R, Heidenreich D, Weisbach V, Zingsem J, Neidhardt B, Eckstein R. In vitro quality control of red blood cell concentrates outdated in clinical practice. Transfusion Clinique et Biologique. 2003;10:275–83. doi: 10.1016/s1246-7820(03)00032-6. [DOI] [PubMed] [Google Scholar]
- 2.Janatpour KA, Paglieroni TG, Crocker VL, Dubois DJ, Holland PV. Visual assessment of hemolysis in red cell units and segments can be deceptive. Transfusion. 2004;44:984–9. doi: 10.1111/j.1537-2995.2004.03315.x. [DOI] [PubMed] [Google Scholar]
- 3.Cookson P, Sutherland J, Cardigan RA. Simple spectrophotometric method for the quantification of residual haemoglobin in platelet concentrates. Vox Sanguinis. 2004;87:264–71. doi: 10.1111/j.1423-0410.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 4.Seghatchian J, Krailadsiri P, Beard M, Vickers M. Development of a Microplate method for the measurement of plasma haemoglobin and its use in monitoring the storage lesion of red cell components. Transfus Apher Sci. 2002;26:91–3. doi: 10.1016/s1473-0502(01)00139-2. [DOI] [PubMed] [Google Scholar]
- 5.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16:46–60. doi: 10.1053/tmrv.2002.29404. [DOI] [PubMed] [Google Scholar]
- 6.Gammon RR, Strayer SA, Avery NL, Mintz PD. Hemolysis during leukocyte- reduction filtration of stored red blood cells. Ann Clin Lab Sci. 2000;30:195–9. [PubMed] [Google Scholar]
- 7.Müller-Steinhardt M, Janetzko K, Kandler R, Flament J, Kirchner H, Klüter H. Impact of various red cell concentrate preparation methods on the efficiency of prestorage white cell filtration and on red cells during storage for 42 days. Transfusion. 1997;37:1137–42. doi: 10.1046/j.1537-2995.1997.37111298088042.x. [DOI] [PubMed] [Google Scholar]
- 8.Hogman CF, Eriksson L, Ericson A, Reppucci AJ. Storage of saline-adenine-glucose-mannitol-suspended red cells in a new plastic container:Polyvinyl chloride plasticized with butyryl-n-trihexyl - citrate. Transfusion. 1991;31:26–9. doi: 10.1046/j.1537-2995.1991.31191096180.x. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz B, Stryker MH, Waldman AA, Woods KR, Grass JD, Drago J. Stabilization of red blood cells by the plasticizer, diethyl hexylphthalate. Vox Sang. 1985;48:150–5. doi: 10.1111/j.1423-0410.1985.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 10.Estep TN, Pedersen RA, Miller TJ, Stupar KR. Characterization of erythrocyte quality during the refrigerated storage of whole blood containing di-(2-ethylhexyl) phthalate. Blood. 1984;64:1270–6. [PubMed] [Google Scholar]
- 11.Heaton WA, Holmes S, Smith K, Brecher ME, Pineda A, AuBuchon JP, et al. Effects of 3-5 log10 pre-storage leucocyte depletion on red cell storage and metabolism. Br J Hematol. 1994;87:363–8. doi: 10.1111/j.1365-2141.1994.tb04923.x. [DOI] [PubMed] [Google Scholar]
- 12.Heaton WA, Rebullla P, Pappalettera M, Dzik WH. A comparative analysis of different methods for routine blood component preparation. Transfus Med Rev. 1997;11:116–29. doi: 10.1053/tm.1997.0110116. [DOI] [PubMed] [Google Scholar]
- 13.Greenwalt TJ, Zehner S, Dumaswala UJ. Studies in Red blood cell preservation. 2. Comparison of vesicle formation, morphology and membrane lipids during storage in AS-1 and CPDA-1. Vox Sang. 1990;58:90–3. doi: 10.1111/j.1423-0410.1990.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakao M, Hoshino K, Nakao T. Constancy of cell volume during shape change of erythrocytes induced by increased ATP content. J Bioenerg Biomembr. 1981;13:307–16. doi: 10.1007/BF00743208. [DOI] [PubMed] [Google Scholar]


