Abstract
Background:
Autologous, and in some cases allogeneic, hemopoietic stem cells (HSC) are stored for varying periods of time prior to infusion. For periods of greater than 48 h, storage requires cryopreservation. It is essential to optimize cell storage and ensure the quality of the product for subsequent reinfusion.
Methods:
A number of important variables may affect the subsequent quality of infused HSC and therapeutic cells (TC). This review discusses these and also reviews the regulatory framework that now aims to ensure the quality of stem cells and TC for transplantation.
Results:
Important variables included cell concentration, temperature, interval from collection to cryopreservation, manipulations performed. They also included rate of freezing and whether controlled-rate freezing was employed. Parameters studied were type of cryoprotectant utilized [dimethyl sulphoxide (DMSO) is most commonly used, sometimes in combination with hydroxyethyl starch (HES)]; and storage conditions. It is also important to assess the quality of stored stem cells. Measurements employed included the total cell count (TNC), mononuclear cell count (MNC), CD34+ cells and colony-forming units - granulocyte macrophage (CFU-GM). Of these, TNC and CD34+ are the most useful. However, the best measure of the quality of stored stem cells is their subsequent engraftment. The quality systems used in stem cell laboratories are described in the guidance of the Joint Accreditation Committee of ISCT (Europe) and the EBMT (JACIE) and the EU Directive on Tissues and Cells plus its supporting commission directives. Inspections of facilities are carried out by the appropriate national agencies and JACIE.
Conclusion:
For high-quality storage of HSC and TC, processing facilities should use validated procedures that take into account critical variables. The quality of all products must be assessed before and after storage.
Keywords: Accreditation, cryopreservation, hemopoietic stem cells, regulatory authorities
It is necessary to store hemopoietic stem cells (HSC) prior to autologous stem cell transplantation (SCT) where these are collected from the transplant recipient prior to conditioning therapy. Most allografts are given straight after collection except:
When cord blood from either sibling or unrelated donors for SCT is collected and banked prior to the procedure and stored frozen until required
When it is impractical to harvest allogeneic stem cells on the day of transplant, e.g., donors geographically distant, very anxious or with commitments that make them unavailable on the day
In these cases, collections may be performed in advance and the cells stored prior to transplantation. A number of variables may have impact on the quality of HSC products for transplantation.[1] These include:
Contamination with mature blood cells; manipulations performed prior to storage, such as red cell depletion or separation of the buffy coat or mononuclear cell fractions
The cell concentration, temperature and length of storage for products stored in liquid state prior to infusion or cryopreservation
Cryopreservation variables – type of cryoprotectant used, rate of cooling and final storage temperature.
The speed of engraftment of stored HSC products varies according to the amount of pretreatment received by patients who donate cells for autologous reinfusion.[2] The source of stem cells is also important since it is known, for example, that cord blood stem cells engraft more slowly than those derived from the bone marrow or peripheral blood.[1] The number and quality of the stem cells collected and stored prior to administration are critical.
It was shown in mice that marrow cells cryopreserved using glycerol were able to reconstitute marrow function after irradiation.[3] There is extensive data in clinical transplantation confirming that, stored under appropriate conditions HSC can reconstitute hemopoiesis.
Liquid Storage
Most stem cell products are infused or processed shortly after collection but some, e.g., from registry donors, may have extended periods of transit – up to 24-48 h.[4,5] Autologous HSC products may sometimes have to be transported over long distances to processing centers.
It may be advantageous to maintain cells in the liquid state if storage is for <96 h, since cryopreservation and thawing cause loss of myeloid colony forming unit granulocyte macrophage (CPU-GM) progenitors.[1] HSC stored at 4°C show a progressive loss of nucleated cells, cell viability and CPU. Preti et al. showed that erythroid burst forming units (BPU-E) in liquid storage fell below numbers in frozen samples only after 5 days of storage; the difference for CPU-GM was not significant even after 9 days of storage.[6] In other reports, losses of CPU-GM varied from 61% after 72 h to only 39’6 after 96 h for bone marrow.[7,8] It is not known which temperature is optimal for liquid storage, although most reports describe storage at 4°C. Data from our laboratory indicates that storage at 2-6°C is not detrimental to stem cell recovery.[9] Products stored overnight at room temperature have lower pH when compared to products stored at 4°C, which could be damaging to HSC.[1] Probably the interaction of cell concentration, temperature and time of storage influences the quality of HSC.
Cryopreservation of HSC
The quality of cryopreserved HSC is dependant on:
The cell concentration
Temperature
Interval between collection and cryopreservation
Presence of mature blood cells
Manipulations prior to storage and cryopreservation
These factors likely interact. For example, it has been found that if cells are stored overnight at room temperature with cell concentration in excess of 200 × 109/L, there is a significant reduction of the viability of cells and of the recovery of CD34+ -cells and CPU-GM[10] (unpublished observations). Cells stored at 400-500 × 109/L but cryopreserved within 4 h show satisfactory recovery.[9] At the present time, the recommendation in use by the English National Blood Service is that cells not for immediate processing or infusion should be diluted to cell concentration <200 × 109/L. Cells in transit for >1 h should be stored at 4°C (16°C). HSC comprise a small portion (usually < 1%) of bone marrow, peripheral blood or tort {blood products}. Bone marrow, in particular, contains a range of mature blood cells and other noncellular material. These are not optimally preserved using techniques that result in good stem cell recovery. The presence of mature blood cells has three effects:
Granulocytes and platelets may clump and interfere with stem cell processing.
Red cells may lyse upon thawing and infusion, leading to renal failure.
Large cell numbers may require freezing in large volumes, leading to volume overload and cryoprotectant-related toxicity upon infusion.[1]
Stem cell cryopreservation may be optimized by processing to either a buffy coat or a mononuclear cell (MNC) preparation or CD34 selected cells.
Rate of Freezing
The main principle underlying successful cell cryopreservation is the prevention of ice crystal formation during cooling.[10] This is a primary cause of cell damage. If cells are cooled too quickly, intracellular ice crystals form, resulting in mechanical disruption of cells and their destruction. At slow rates of cooling, ice crystals form in the extracellular space, causing increased osmolality as free water is taken up. This causes cellular dehydration.[11] Glycerol and DMSO prevent dehydration by inhibiting the increased concentration of sodium that can occur during ice formation and by decreasing the amount of water absorbed into ice crystals at any given temperature. They are referred to as colligative cryoprotectants.[1] Controlled-rate freezing protocols aim to achieve a rate of cooling that minimizes intracellular ice crystal formation and protects against cellular dehydration. The optimal rate of cooling is influenced by the type of cryoprotectant and the cells being frozen. When the transition from liquid to solid state occurs, the latent heat of fusion is released and this causes a plateau to appear in the freezing curve. This should be kept as short as possible to minimize cell injury.[12]
Colligative cryoprotectants are most frequently used, and their optimal rates of cooling fall within a narrow range. Ma et al., using in vitro cultures, showed an optimal cooling rate of 1°C/ min for human HSC suspended in 10% DMSO.[13] Murine colony -forming unit spleen (CFU-S) were frozen in 12% glycerol, and no difference was found for pre-plateau cooling rates ranging from 0.8-4°C/min.[14] Rapid cooling rates have been associated with delayed engraftment for autologous marrow recipients.[15] Rowley et al. have reported that the rate of cooling can be increased to 10°C/min when HSC products have reached –40°C and that bags can be transferred to refrigerators when the temperature has reached –80°C.[1] A sensible recommendation is that for HSC frozen in 10% DMSO and a minimum of 10% plasma, the optimal freezing rate should be 1–2°C/min from 0°C to –40 or –80°C.[12]
Cryoprotectants
Dimethylsulfoxide (DMSO)
DMSO is a colligative agent that diffuses rapidly through the cell membrane. It has a half-life of 20 h and is metabolized to DMSO 2, which is excreted through the kidneys, whilst a small proportion of DMSO is reduced to dimethylsulfide (DMS) and excreted through the lungs,[1] accounting for the characteristic smell. A number of studies have determined that the best concentration for DMSO or glycerol for cryopreservation of HSC is 10%, although concentrations of 5% have been used successfully.[16] When DMSO was used in conjunction with hydroxyethyl starch (HES), CD34+ cell recovery increased from a mean of 12.2% to 85.4% as DMSO increased from 2.5% to 5%. Varying the concentration of HES in the presence of 5% DMSO did not affect CD34+ cell recovery.[17]
Hydroxyethyl starch (HES)
HES is a polymer containing chains of different molecular weights. It does not freely penetrate the cell and may work by forming a viscous shell over the cell surface, inhibiting the movement of water and preventing progressive cellular dehydration. HES is generally combined with cryoprotectants such as DMSO. Autologous bone marrow IISC frozen in a combination of 5% DMSO, 6% HES and 4% human serum albumin showed good CFU recovery and satisfactory engraftment of both bone marrow and peripheral blood-derived HSC.[18] In a study of 294 patients who received stem cells cryopreserved with DMSO + HES or DMSO alone, the time to an absolute neutrophil count (ANC) > 0.5 × 109/L and discontinuation of antibiotics was one day shorter for the combination.[19]
Protein
Plasma proteins have cryoprotectant effects, and their addition to cryoprotectant solutions improves HSC survival. Lymphocytes can be preserved in serum alone.[20] One report found that marrow HSC were better preserved in the presence of serum.[21] Progenitor cell survival rose from a mean of 41.1% to 64.8% with 15% serum and to a mean of 75.4% with 50% serum. Likewise, murine CFU--S recovery increased from 18.2% to 100.5% when 10% serum was added to 10% DMSO.[22] Most cryopreservation solutions incorporate either autologous plasma or human albumin solutions. Albumin avoids the marrow fat, cellular debris and anticoagulant contained in plasma derived from marrow collections.[1] Autologous plasma collected at the same time as stem cells on cell separators is a cleaner product than plasma derived from marrow collections.
Sugars
Many sugars function as cryoprotectants. In one report, 50% CFU-S survival was found when murine bone marrow cells were cryopreserved in 0.35M sucrose alone.[23] Other sugars such as glucose, manitol and sorbitol at concentrations >0.1M also have cryoprotectant properties[15] and may serve to stabilize the cell membrane during freezing or dehydration.
Conditions for Cryostorage
Following cryopreservation, cells may be stored in either the liquid or vapor phase of nitrogen at ≥130°C or at –80°C in mechanical freezers. Storage at lower temperatures prevents the progressive growth of ice crystals, which does not occur in pure water at temperatures below –130°C.[1] Halle et al. stored PBSC collections at –80°C in HAS 1%, HES 2.5% and DMSO 3.5% and recorded median recoveries of nucleated cells, CD34+ cells, CFU--GM and BFU-E of 60.8, 79.6, 35.6 and 32.6% respectively. After 7 weeks of storage (median), the median time to ANC > 0.5 × 109/L and platelets > 20 × 109/L was 11 days. This is a simple and inexpensive cryopreservation strategy.[24] Galmes et al. studied long-term storage at –80°C using 5% DMSO as the only cryoprotectant and found MNC viability declined to 32% after 31 months. Recovery of CFU-GM and BFU-E decreased to 50 and 43.5% respectively after 12 months and to 0 (for both) after 24 months. No differences in cell viability or recovery of clonogenic cells were observed between 5 and 10% DMSO. Hemopoietic reconstitution was rapid when cells stored for between 123 and 202 days were reinfused, and the authors conclude that –80°C with 5% DMSO is satisfactory for up to 6 months of storage.[16] Most laboratories store HSC below 130°C in liquid or vapor phase nitrogen.
The report of probable cross-contamination with hepatitis B virus in units of autologous HSC cryopreserved in liquid phase of nitrogen[25] prompted authorities in the UK to recommend storage in vapor phase. The same report also recommended that HSC components should be double-bagged and that donations positive for infectious disease markers should be stored separately.[26] Donations where the results of microbiological testing are not available should be stored in quarantine until the results are known.[27]
Authorities responsible for the accreditation and regulation of HSC transplantation recommend that cryopreservation facilities should be secure, with access granted only to trained and responsible individuals. They further recommend that the temperature of all cryopreserved components be monitored continuously, that a record is made of the temperature every 4 h and that an alarm should alert a trained member of staff if a storage device fails.[27] Laboratory staff should never work in liquid nitrogen cryopreservation facilities on their own; another member of staff should always be available. Moreover, the oxygen concentration should be monitored, and an alarm should sound if this falls below 18%.[28]
Length of Storage
PBSC stored for a mean of 33 days compared with bone marrow HSC stored for >10 years showed no loss of CFU and CD34– cells.[29] When cord bloods were cryopreserved for either 2-8 weeks or for 15 years, the MNC recovery, clonogenic potential and proliferative and cytotoxic responses against HLA antigens were maintained.[10] We have evaluated 13 HSC components (2 BM and 11 PB) stored for a period >1 year (range 12-65) and found that engraftment of neutrophils occurred at a mean of 16 days (range 11-49 days); furthermore, no differences were seen when compared to a matched cohort of HSC products cryopreserved for a period of < 1 year.[30]
Post-thaw Manipulations
The infusion of DMSO or clumped cells may cause adverse effects. In order to avoid loss of stem cells, most units infuse cryopreserved units within a few minutes of thawing. Some centers recommend that a microaggregate filter be used to avoid the infusion of debris[1] when thawed HSC components are administered, but viable HSC may be trapped in aggregated material and the overall stem cell content could be compromised. Systematic study of this issue has not been done. Currently, it is our recommendation that products should not be filtered. Cells may be washed after thawing to remove DMSO and cellular debris. Beaujean and colleagues reported a mean of 73.9 and 93.9% CFU-GM recovery after thaw-wash procedures with marrow and PBSC respectively.[31]
Cryoprotectant Toxicity
DMSO has a number of toxic effects; but fortunately, severe reactions are unusual. Rowley described the following types of reactions:
Anaphylaxis
Hypotension resulting from histamine-induced vasodilatation
Skin flushing, dyspnea, abdominal cramps, nausea and diarrhea – also attributed to histamine release
Cardiovascular effects: increased blood pressure, decreased heart rate, cardiac arrest, heart block
Headache, reversible encephalopathy
The other main side effects result from hemolysis of cryopreserved red cells, causing fever; chills; hemoglobinuria; and in severe cases, renal impairment.
Forced alkaline diuresis is generally unnecessary to prevent renal complications. Most centers routinely premeditate paracetamol, either alone or combined with antihistamines and corticosteroids.
Assessment of the quality of stored HSC
Product quality may be assessed as follows:
Cell counts: The total nucleated cell count (TNC) and MNC should be measured on a standard hematology analyzer and recorded for all products. SCT using doses of TNC greater than the median has been reported to improve the outcome of transplant.[32]
CD34 measurement: The CD34 dose of marrow or peripheral blood transplants may correlate with the clinical outcome. Some reports suggest that higher doses are associated with an increase in graft-versus-host disease, independent of the dosage of T cell co-administered,[33] whilst a large series, doses of CD34+ cells greater than the median decreased treatment-related mortality and the relapse rate.[34] The quality of the laboratory procedures used, e.g., CD34+ cell selection and cryopreservation, can he assessed by CD34, assays. CD34+, cells are measured by flow cytometry using `single-platform′ assays.[35]
CFU-GM colonies: These are measured by culture in semisolid media with relevant cytokines and scored at 10-14 days. In autologous SCT, correlation has been reported between CD34+ cells and CFU-GM in HSC collections. This is not the case in allogeneic SCT. We perform CFU-GM assay now on 5-10% of all procedures that involve significant cell manipulation, as a quality control procedure.
Engraftment: Restoration of hemopoiesis in lethally irradiated mice using cryopreserved HSC indicates that the process allows for recovery of functional cells.[3] A large number of studies have reported the clinical outcome of SCT with either autologous or allogeneic HSC. The majority of these patients engraft satisfactorily, which indicates that the cell storage gave acceptable numbers of HSC.[1] Twenty-two patients – with small cell lung cancer (18) and AML (4) – received autologous marrow held at 4°C for 34–54 h prior to infusion and engrafted >1.0 neutrophils on day 17 (mean) for lung cancer and 26-40 days for AML[7] patients. Allogeneic HSC may be cryopreserved prior to transplantation sometimes as a result of concerns over donor availability. Available reports indicate comparable engraft meat between cryopreserved and fresh stem cells.[4,36] Cord blood HSC engraft more slowly when compared to either marrow or peripheral blood HSC. [7–39] This is likely an effect of the cord blood stem cell dose rather than the cryopreservation process itself.
Regulatory Requirements in Hemopoietic Stem Cell Therapy
One of the first documents that set standards for stem cell processing was issued by the Department of Health in the United Kingdom in 1997. This followed an incident where cross-contamination with hepatitis B virus occurred amongst cryopreserved units of stem cells.[25] Documents relevant to SCT include:
Joint Accreditation Committee of ISCT (Europe) and EBMT (JACIE) Standards and Accreditation manual – 2nd edition, 2005[27]
EU Directive on Tissues and Cells 2004/23/EC[40]
EU Directive on Medicinal Products 2001/83/EC[41]
EU Directive on Clinical Trials 2001/20/EC[42]
Council of Europe (CoE) guidance on the safety and quality of organs, tissues and cells (2nd edition, 2004)[43]
EC proposals for a consultation on human tissue engineering and tissue-engineered products (TEP), 2005[44]
WHO: Consultation on regulatory requirements for human tissue and cell transplantation, 2005[45]
In addition, in the UK the collection, storage and use of tissues and cells for transplantation is regulated by the provisions of the Human Tissue Act.[46] The following sections describe briefly the challenges that SCT units face in order to comply with the current accreditation and regulatory requirements.
The EU directive for tissues and cells (EUDTC) is applicable to therapeutic HSC (hemopoietic and nonhemopoietic) from peripheral blood, bone marrow and cord blood. It also includes cellular therapies such as cytotoxic T lymphocytes and dendritic cells. The directive is organized into a number of articles, which describe and outline the requirements for staffing, facilities and testing. It states that work must take place within establishments that are either accredited or licensed or designated or authorized and that there must be a competent authority which should organize 2-yearly inspections. Other articles outline the requirements for:
Product traceability and unique identifiers
Import and export of cells
The reporting of adverse reactions and adverse events
The voluntary and unpaid nature of stem cell donation
The requirements for consent
Quality management systems (standard operating procedures [SOPs], guidelines. training records, reporting mechanisms and records)
A person responsible for the activities of the tissue establishment
Requirements for cell reception, quarantine, process control, validation and storage
-
Agreements with third parties - standards, agreed activities
Both the EUDTC and JACIE (see below) require that systems should be in place for the reporting of errors, accidents, severe adverse reactions, biological product deviations. At the present time, there is no agreed system for doing this. In the UK we are developing some proposals for a system of adverse event reporting following SCT. These are shown in Figure 1.
The EUDTC is supported by two Technical Directives. These have recently been published.[47,48]
Figure 1.
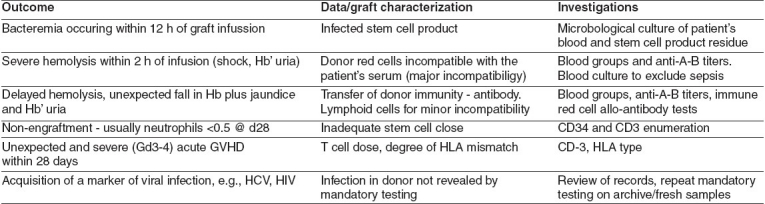
Severe adverse outcomes following stem cell graft infusion
JACIE
JACIE was established in 1999 and was based on the guidelines and manual introduced by the American Foundation for Accreditation of Cell Therapy (FACT).[49] It has two principal roles:
The accreditation of individual SCT centers. This is achieved through the process of inspection, review of documentation, production of reports and finally certification.
Information and education. The JACIE website provides a large amount of information to SCT centers.[27] It runs courses in quality management and for inspector training and provides sample documents.
JACIE provides standards, which are regularly updated. It has been agreed that in future there will be no differences between FACT and JACIE guidelines; so in future, these will be known as FACT-JACIE guidelines. A very detailed accreditation manual is produced. All procedures and documentation are now managed online. The standards stress the importance of documentation, training and competency. Like the EUDTC, they require that systems for duality management are in place. These should include SOPs, policies and procedures for validation. Performance should be monitored and this should include outcome review, audits and adverse event reporting. Prior to inspection, applicants register with JACIE and then complete a detailed checklist. A number of other documents such as the qualifications of staff in the program and a list of standard operating procedures are also required. There is usually one inspector per facility (clinical, collection, processing) and a pediatric inspector if the program conducts transplants in children. The inspectors then review the submitted documentation and conduct a visit lasting 1-2 days. All noncompliances are noted and reports are submitted to the JACIE office. These are returned to the applicant center, which is asked to address the deficiencies that have been highlighted. Following this, a level of compliance is assigned as follows:
No deficiencies. Full accreditation
Few minor deficiencies
Significant deficiencies but not requiring a reinspection
Significant deficiencies, usually systematic in nature, requiring focused reinspection
Significant deficiencies requiring a reinspection of the entire program
Non-accreditation
The aim of the process is that when the SCT center has made the agreed changes and submitted evidence to confirm that these changes have been made, it will progress to full accreditation. Reinspections are carried out every 3 years. The following significant deficiencies have been recorded in SCT programs (for details, see the JACIE website[27]):
Program not functioning as a single program (e.g., SOPs not uniform across different clinical sites)
Inpatient isolation facilities inadequate (shared rooms for autografts)
Engraftment data not monitored by the processing facility
No continuous temperature monitoring of freezers
Temperature not monitored during transport of HSC from laboratory to clinical unit
Inadequate quality management program
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Rowley SD. Hematopoietic stem cell processing and cryopreservation. J Clin Apher. 1992;7:132–4. doi: 10.1002/jca.2920070307. [DOI] [PubMed] [Google Scholar]
- 2.Humpe A, Riggert J, Veluneyer K, Troff C, Hicidemann W, Kohler M, et al. Comparison of CD34+ cell numbers and colony growth before and after cryopreservation of peripheral blood progenitor and stem cell harvests: Influence of prior chemotherapy. Transfusion. 1997;37:1050–7. doi: 10.1046/j.1537-2995.1997.371098016444.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrebee JW, Billen D, Urso IM, Lu WC, Thomas FD, Congdon CC. Preservation ref radiation recovery factor in frozen marrow. Blood. 1957;12:1096–100. [PubMed] [Google Scholar]
- 4.Lazarus HM, Pecora AL, Shea TC, Koc ON, White JM, Gabriel DA, et al. CD34+selection of hemtopoietic blood cell collections and auto transplantation in lymphoma: Overnight storage of cells at 4 degrees C does not affect outcome. Bone Marrow Transplant. 2000;25:559–66. doi: 10.1038/sj.bmt.1702175. [DOI] [PubMed] [Google Scholar]
- 5.Beaujeau F, Pico J, Norol F, Divine M, Le Forestier C, Duedari N. Characteristics of peripheral blood progenitor cells frozen alter 24 hours of storage. J Hematother. 1996;5:681–6. doi: 10.1089/scd.1.1996.5.681. [DOI] [PubMed] [Google Scholar]
- 6.Preti RA, Razis F, Ciavarella D, Fan Y, Kulups RE, Cook P, et al. Clinical and laboratory comparison study of refrigerated and cryopreserved bone marrow for transplantation. Bone Marrow Transplant. 1994;13:253–60. [PubMed] [Google Scholar]
- 7.Burnett AK, Tansey P, Hills C, Alcorn MJ, Sheehan T, McDonald GA, et al. Hematological reconstitution following high dose and supralethal chemo-radiotherapy using stored, none cryopreserved autolugous bone marrows. Br J Haematol. 1983;54:309–16. doi: 10.1111/j.1365-2141.1983.tb02100.x. [DOI] [PubMed] [Google Scholar]
- 8.Delforge A, Ronge-Collard E, Stryckmans P, Spira T, Malarme MA. Granuocyte-macrophage progenitor cell preservation at 4 degrees C. Br J Haematol. 1983;53:49–54. doi: 10.1111/j.1365-2141.1983.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 9.Guttridge MG, Sidders C, Booth-Davey E, Pamphilon D, Watt SM. Factors affecting volume reduction and red blood cell depletion of bone marrow on the COBE Spectra cell separator before haematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:175–81. doi: 10.1038/sj.bmt.1705420. [DOI] [PubMed] [Google Scholar]
- 10.Rowley SD, Bensinger WI, Gooley TA, Buckner CD. Effect of cell concentration on bone marrow and peripheral blood stern cell cryopreservation. Blood. 1994;83:2731–6. [PubMed] [Google Scholar]
- 11.Karow AM, Jr, Webb WR. Tissue freezing. A theory for injury and survival. Cryobiology. 1965;2:99–108. doi: 10.1016/s0011-2240(65)80094-3. [DOI] [PubMed] [Google Scholar]
- 12.Meagher RC, Hergiz MD. Techniques of harvesting and cryopreservation of stem cells. In: Haematology/Oncology Clinics of North America. In: Williams SF, editor. Philadelphia PA: WB Saunders; pp. 501–33. [PubMed] [Google Scholar]
- 13.Ma DD, Johnson LA, Chan PM, Biggs JC. Factors influencing myeloid stem cell (CFU-C) survival alter cryopreservation of human marrow and chronic granulocytic leukemia cells. Cryobiology. 1982;19:1–9. doi: 10.1016/0011-2240(82)90118-3. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JP, Passovoy M, Trobaugh RE. Karger: Stockholm, Sweden, Basel/New York; 1964. Studies on the effect of controlled rate cooling oar marrow viability. Proceedings of the 10th Congress elf the International Society for Blood Transfusion; pp. 656–61. [Google Scholar]
- 15.Willhite CC, Katz PI. Dismethyl sulfoxide. J Appl Toxicol. 1984;4:155–60. doi: 10.1002/jat.2550040308. [DOI] [PubMed] [Google Scholar]
- 16.Galmes A, Besalduch J, Bargay J, Matamoros N, Duran MA, Money M, et al. Cryopreservation of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide at -80 degrees C without rate controlled freezing. Transfusion. 1996;36:794–7. doi: 10.1046/j.1537-2995.1996.36996420755.x. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson C, Armitage WJ, Denning-Kendall PA, Nicol AJ, Bradley BA, Hows JM. Optimal cryopreservation of human umbilical cord blood. Bone Marrow Transplant. 1996;18:725–31. [PubMed] [Google Scholar]
- 18.Stiff PJ, Koester AR, Weidner MK, Dvorak K, Fisher RI. Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without controlled-rate freezing. Blood. 1987;70:974–8. [PubMed] [Google Scholar]
- 19.Rowdy SD, Feng Z, Chen L, Holmberg L, Heimfeld S, MacLeod B, et al. A randomized phase III clinical trial of autulogous blood stem cell transplantation comparing cryopreservation using dimethylsulfoxide versos dimethylsulfoxide with hydroxyethylstarch. Bone Marrow Transplant. 2003;31:1043–51. doi: 10.1038/sj.bmt.1704030. [DOI] [PubMed] [Google Scholar]
- 20.Knight SC, Farrant J, McGaran LE. Storage of human lymphocytes by freezing in serum alone. Cryobiology. 1977;14:112–5. doi: 10.1016/0011-2240(77)90129-8. [DOI] [PubMed] [Google Scholar]
- 21.Ragab AH, Gilkerson E, Myers M. Factors in lire cryopreservation of bone marrow cells front children with acute lymphocytic leukemia. Cryobiology. 1977;14:125–34. doi: 10.1016/0011-2240(77)90132-8. [DOI] [PubMed] [Google Scholar]
- 22.Grilli G, Porcellini A, Lucarclli G. Role of serum oar cryopreservation and subsequent viability of mouse bone marrow hemopoietic stem cells. Cryobiology. 1980;17:516–20. doi: 10.1016/0011-2240(80)90063-2. [DOI] [PubMed] [Google Scholar]
- 23.Leibo SP, Farrant J, Mazur P, Hanna MG, Jr, Smith LH. Effects of freezing on marrow stern cells suspensions: Interactions of cooling and warming rates in the presence of PVP, sucrose or glycerol. Cryobioloy. 1970;6:315–32. doi: 10.1016/s0011-2240(70)80086-4. [DOI] [PubMed] [Google Scholar]
- 24.Halle P, Tournilhac O, Knopinska-Posluszny W, Kanold J, Gembara P, Boiret N, et al. Uncontrolled-rate freezing and storage at 80 degrees C, with only 3.5 percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion. 2001;41:667–73. doi: 10.1046/j.1537-2995.2001.41050667.x. [DOI] [PubMed] [Google Scholar]
- 25.Tedder RS, Zuckerman MA, Goldstone AH, Hawkins AE, Fielding A, Briggs FM, et al. Hepatitis B transmission from contaminated cryopreservation tank. Lancet. 1995;346:137–40. doi: 10.1016/s0140-6736(95)91207-x. [DOI] [PubMed] [Google Scholar]
- 26.Guidance Notes on the Processing. Storage and Issue of Bone Marrow and Blood Stem Cells. NHS Executive. HSG. 97(19) Available from: http://www.dh.gov.uk/publicationandstatistics/lett ersandcirculars/healthserviceguidelines/healthserviceguidelinesarticles/fs/en?content-id -4018321andchk-crq/w3 . [Google Scholar]
- 27.Joint Accreditation Committ ee of ISCT (Europe) and EBMT (JACIE Standards and Accreditation manual. 2nd edition. 2005. Available from: http://www.jacie.org .
- 28.UK: 2005. EH4O “Occupational exposure limits” Health and safety executive. [Google Scholar]
- 29.Donnenberg AD, Kocit FK, Griffin DL, Stanczak HM, Kiss JE, Carlos TM, et al. Viability of cryopreserved bone marrow progenitor cells stored for more than a decade. Cytotherapy. 2002;4:157–63. doi: 10.1080/146532402317381866. [DOI] [PubMed] [Google Scholar]
- 30.Pauson R. Personal communication. 2007 [Google Scholar]
- 31.Beaujean F, Harmann O, Kuentz M, Le Forestier C, Divine M, Duedari N. A simple, efficient washing procedure for cryopreserved human hematopoietic stem cells prior to reinfusion. Bone Marrow Transplant. 1991;8:291–4. [PubMed] [Google Scholar]
- 32.Sierra J, Stoves B, Hansen JA, Bjerke JW, Martin PJ, Petersdorf EW, et al. Transplantation of marrow cells from unrelated donors for treatment of high risk acute leukemia: The effect of leukemia burden donor HLA-matching and marrow cell dose. Blood. 1997;89:4226–35. [PubMed] [Google Scholar]
- 33.Urbano-Ispizua A, Rozman C, Pimentel P, Solano C, de la Rubia J, Brunet S, et al. Risk factors for acute graft-versus-host disease in patients undergoing transplantation with CD34+ selected blood cells from HLA-identical siblings. Blond. 2002;100:724–7. doi: 10.1182/blood-2001-11-0057. [DOI] [PubMed] [Google Scholar]
- 34.Ringden O, Barret AJ, Zhang MJ, Loberiza FR, Bolwell BJ, Cairo MS, et al. Decreased treatment failure in recipients of HLA -identical bone marrow or peripheral blood stem cell transplants with high CD34 cell closes. Br J Haematol. 2003;121:874–85. doi: 10.1046/j.1365-2141.2003.04364.x. [DOI] [PubMed] [Google Scholar]
- 35.Gratama JW, Kraan J, Keeney M, Sutherland DR, Granger V, Barrett D. Validation of the single platform ISHAGE method tot CD34(+) hematopoietic stem and progenitor cell enumeration in an international multicenter study. Cyotherapy. 2003;5:55–65. doi: 10.1080/14653240310000083. [DOI] [PubMed] [Google Scholar]
- 36.Stockschlader M, Kruger W, Kroschke G, Zeller W, Hoffknecht M, Loliger C, et al. Use of cryopreserved bone marrow in allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;15:569–72. [PubMed] [Google Scholar]
- 37.Laughlin MJ, Barker J, Bamback B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical cord blood from unrelated donors. N Engl J Med. 2001;344:1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 38.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: Results of a matched-pair analysis. Blood. 2001;97:2957–61. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 39.Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants far children with acute leukemia. Blood. 2001;97:2962–71. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 40.EU Directive on Tissues and Cells 2004/23/EC. Available from: http://europa.eu.int/smartapi/cgi/sga_doc?smartapi!celexapilprocl!CELEYnumdocartdlgenandnumdoc=3 2004L0023andmodel=guichett .
- 41.EU Directive on Medicinal Products 200l/83/EC. Available from: http://www.europa.eu.int/eur-lex/pri/en/oj/dat/2001/1_311/1_31120011128en00670128.pdf .
- 42.EU Directive Clinical Trials 2001/20/EC. Available from: http://www.europa.cu.iut/eur-lex/pri/en/of/dat/2001/1/121/1/12120010501en003-40044.pdf .
- 43.Guidance on the Safety and Quality of Organs. 2nd ed. Council of Europe Publishing; 2004. Council of Europe (CoE.) ISBN 92 871 5517 8. Available from: htt p://www.bsbmt.org/docs/EU_tissues_and_cells_directive_v3.pdf . [Google Scholar]
- 44.EC Proposals for a Consultation on Human Tissue Engineering and Tissue Engineered products (TEPs) 2005. This includes somatic cell therapy products. Available from: http://www.pluirtnacos.eudra.org/F2/advtherapies/docs/consultationpaperadvancedtherapies2005-may-04.pdf .
- 45.WHO: Consultation on Regulatory Requirements for Human Tissue and Cell Transplantation [Google Scholar]
- 46.Human Tissue Act. Available from: http://www.opsi.gov.uk/acts/acts2004/20040030.htm .
- 47.EU Commission Directive 2006/17/EC. Available from: http://europa.eu.int/eurlex/lex/LexUriServ/site/en/oi/2006/I_038/I_03820060209en00400052.pdf .
- 48.European Commission Directive 2006/86/EC. Available from: http://eur lex.europa.eu/LexUriServ/site/en/oi/20061/I_294/I_29420061025en00320050pdf.
- 49.FACT. Available from: http://www.factwebsite.org/


