Abstract
By 2003, 97% autologous transplants and 65% of allogeneic transplants in Europe used mobilised peripheral blood stem cells (PBSC). Soon after their introduction in the early 1990's, PBSC were associated with faster haemopoietic recovery, fewer transfusions and antibiotic usage, and a shorter hospital stay. Furthermore, ease and convenience of PBSC collection made them more appealing than BM harvests. Improved survival has hitherto been demonstrated in patients with high risk AML and CML. However, the advantages of PBSC come at a price of a higher incidence of extensive chronic GVHD. In order to be present in the blood, stem cells undergo the process of “mobilisation” from their bone marrow habitat. Mobilisation, and its reciprocal process – homing – are regulated by a complex network of molecules on the surface of stem cells and stromal cells, and enzymes and cytokines released from granulocytes and osteoclasts. Knowledge of these mechanisms is beginning to be exploited for clinical purposes. In current practice, stem cell are mobilised by use of chemotherapy in conjunction with haemopoietic growth factors (HGF), or with HGF alone. Granulocyte colony stimulating factor has emerged as the single most important mobilising agent, due to its efficacy and a relative paucity of serious side effects. Over a decade of use in healthy donors has resulted in vast experience of optimal dosing and administration, and safety matters. PBSC harvesting can be performed on a variety of cell separators. Apheresis procedures are nowadays routine, but it is important to be well versed in the possible complications in order to avoid harm to the patient or donor. To ensure efficient collection, harvesting must begin when sufficient stem cells have been mobilised. A rapid, reliable, standardized blood test is essential to decide when to begin harvesting; currently, blood CD34+ cell counting by flow cytometry fulfils these criteria. Blood CD34+ cell counts strongly correlate with the apheresis yields. These are, in turn, predictive of the speed of haemopoietic recovery after transplantation, which has helped establish the adequate cell dose for transplantation. Following collection, PBSC may be transfused unmanipulated, processed to select specific cell subtypes, or stored for future use. Cryopreservation techniques allow long term storage of stem cells without significant loss of viability. Increasingly demanding calls for safety led to introduction of vapour phase storage, separate storage of infected material, and mandatory quality control measures at all stages of the cryopreservation process and subsequent thawing and transfusion. At the same time, safety of the personnel working in stem cell processing and storage laboratories is safeguarded by a set of regulations devised to minimize the risk of infection, injury or hypoxia. Requirements for quality and safety have been shaped into a number of documents and directives in Europe and USA, emphasising the importance of product traceability, reporting of adverse reactions, quality management systems (standard operating procedures, guidelines, training records, reporting mechanisms and records), requirements for cell reception, quarantine, process control, validation and storage. Establishments that collect, process and store stem cells must be accredited or licensed by appropriate national or international authorities on a regular basis. These regulatory measures have recently become law across the European Union.
Keywords: Autologous transplant, allogeneic transplant, peripheral blood stem cells, haemopoietic growth factor
By 2003, 97% of autologous transplants and 65% of allogeneic transplants in Europe used mobilized peripheral blood stem cells (PBSC). Soon after their introduction in the early 1990s, PBSC were associated with faster hemopoietic recovery, fewer transfusions and antibiotic usage and a shorter hospital stay. Furthermore, ease and convenience of PBSC collection made them more appealing than BM harvests. Improved survival has hitherto been demonstrated in patients with high risk AML and CML. However, the advantages of PBSC come at a price of a higher incidence of extensive chronic GVHD.
In order to be present in the blood, stem cells undergo the process of ‘mobilization’ from their bone marrow habitat. Mobilization and its reciprocal process – homing – are regulated by a complex network of molecules on the surface of stem cells and stromal cells and enzymes and cytokines released from granulocytes and osteoclasts. Knowledge of these mechanisms is beginning to be exploited for clinical purposes. In current practice, stem cells are mobilized by use of chemotherapy in conjunction with hemopoietic growth factors (HGF) or with HGF alone. Granulocyte colony stimulating factor has emerged as the single most important mobilizing agent, due to its efficacy and a relative paucity of serious side effects. Over a decade of use in healthy donors has resulted in vast experience of optimal dosing and administration and safety matters.
PBSC harvesting can be performed on a variety of cell separators. Apheresis procedures are nowadays routine, but it is important to be well versed with the possible complications in order to avoid harm to the patient or donor. To ensure efficient collection, harvesting must begin when sufficient stem cells have been mobilized. A rapid, reliable, standardized blood test is essential to decide when to begin harvesting; currently, blood CD34+ cell counting by flow cytometry fulfils these criteria. Blood CD34+ cell counts strongly correlate with the apheresis yields. These are, in turn, predictive of the speed of hemopoietic recovery after transplantation, which has helped establish the adequate cell dose for transplantation.
Following collection, PBSC may be transfused unmanipulated, processed to select specific cell subtypes or stored for future use. Cryopreservation techniques allow long-term storage of stem cells without significant loss of viability. Increasingly demanding calls for safety led to introduction of vapor phase storage, separate storage of infected material and mandatory quality control measures at all stages of the cryopreservation process and subsequent thawing and transfusion. At the same time, safety of the personnel working in stem cell processing and storage laboratories is safeguarded by a set of regulations devised to minimize the risk of infection, injury or hypoxia. Requirements for quality and safety have been shaped into a number of documents and directives in Europe and the United States, emphasizing the importance of product traceability, reporting of adverse reactions, quality management systems (standard operating procedures, guidelines, training records, reporting mechanisms and records), requirements for cell reception, quarantine, process control, validation and storage. Establishments that collect, process and store stem cells must be accredited or licensed by appropriate national or international authorities on a regular basis. These regulatory measures have recently become law across the European Union.
The use of mobilized blood cells for transplantation started in the 1980s and within 10-15 years resulted in an almost complete switch from bone marrow (BM) to peripheral blood as the preferred source of hemopoiesis-regenerating cells.[1] From 1991 to 1997, the proportion of autologous peripheral blood stem cell transplants (PBSCT) increased from 15% to over 90%; in 2003, 97% of autologous transplants in Europe were derived from peripheral blood. First allogeneic PBSCT were reported in 1993; a decade later, they accounted for 65% of allogeneic transplants in Europe.[2] What do peripheral blood stem cells owe this success to?
Firstly, hemopoietic recovery is faster with PBSC than with bone marrow cells. In a randomized study of autologous transplantation in Hodgkin's and non-Hodgkin lymphoma, Shmitz et al.[3] showed a reduction in neutrophil recovery from 14 to 11 days and the reduction in time to a platelet count of 20 × 109/L from 23 to 16 days. In the allogeneic PBSCT setting, Couban et al.[4] found that the recovery of granulocytes and platelets was 4 and 6 days faster respectively than after BM transplants. PBSCT is also associated with a faster immune reconstitution, probably due to a large number of memory T cells in PBSC grafts.[5] As a result of faster hematological recovery, PBSCT are associated with fewer transfusions and antibiotic usage and shorter hospital stay. These advantages and the absence of theatre charges offset the costs of PBSC mobilization and collection.[6] In addition to being more economical, the ease of PBSC collection makes it much more convenient for the donor, a fact with doubtlessly positive impact on donor recruitment.
Notwithstanding the advantages, the effect of PBSCT on patient survival is less certain: superiority of PBSC has been convincingly demonstrated only in patients with high risk AML and CML.[7] The incidence of acute graft vs. host disease (aGVHD) after PBSC and BM transplantation is comparable. However, the incidence of extensive chronic graft vs.host disease (cGVHD) is higher in PBSCT patients,[8] with a relative risk of 1.66 (P= 0.001) found in a recent meta-analysis.[9] The higher risk of chronic GVHD with PBSC may make them a less attractive option in children.[10]
In order to be collected in sufficient numbers from the blood, stem cells have to undergo the process of ‘mobilization’ from their usual bone marrow habitat. Subsequent collection by apheresis is referred to as ‘harvesting.’ Depending on the circumstances, PBSC may be infused to the patient or stored until needed. All these processes have become subject to increasingly strict regulation intended to ensure that good manufacturing procedures are followed and that the end product is safe and reliable. In the following chapters, we will describe the processes involved in stem cell collection, processing and storage; address the issues relevant to patients and donors; and discuss the current regulatory measures.
Mobilization of Hemopoietic Stem Cells
Mechanisms of mobilization
HSCs circulate in the blood at very low levels; Richman et al.[11] made the seminal observation of a large number of circulating hemopoietic progenitors following chemotherapy. Subsequently, a number of stimuli was found to induce mobilization and was used in clinical practice. However, mechanisms underlying HSC mobilization and homing have only recently begun to be unravelled. Papayannopoulou[12] found that the VLA4 integrin, present on the surface of stem cells, plays a critical role: antibodies to VLA4 blocked the adhesion to its ligand on the stromal cell, VCAM-1 (CD106), causing HSC mobilization. Another ‘retention signal’ consists of binding of the stromal stem cell factor (SCF) to its receptor on the stem cells, c-kit (CD117). More recently, the stromal cell derived factor (SDF-1) and its receptor, CXCR4, emerged as the key regulators of stem cell homing and mobilization.[13] In addition, degranulation of neutrophils and activation of osteoclasts release Interleukin-8 and proteases CD26, MMP-9, cathepsin G and elastase, which cleave critical bonds that keep HSC in contact with the microenvironment; however, transgenic mice deficient in MMP-9, elastase and cathepsin G and CD26 mobilize HSC normally in response to granulocyte colony stimulating factor (GCSF), questioning the major role of these enzymes in HSC egress.[14] The apparent redundancy of HSC mobilization pathways may be explained by the recent findings suggestive of a ‘flexible hierarchy’ in which various pathways ‘cooperate’ so that in the steady state bone marrow cells, the action of VLA4/VCAM-1 predominates, whereas the SDF-1/CXCR4 pathway becomes dominant upon cytokine stimulation.[15] Better understanding of mechanisms of mobilization has allowed the procurement of HSC for clinical purposes to involve specific targets. AMD3100, a CXCR4 antagonist, has been shown to be a potent HSC mobilizing agent and is currently undergoing clinical trials.[16]
Why do PBSC grafts result in faster hemopoietic regeneration? Firstly, they contain more committed hemopoietic progenitors than the bone marrow. At King's College Hospital, the average yield of CD34+ cells in PBSC collections from GCSF-mobilized healthy donors is 6.98 × 106/kg recipient weight (87% obtained by a single apheresis), compared with 2.56 × 106/kg in bone marrow harvests obtained from a similar donor population. There are also significant biological differences between the CD34+ cells originating from the bone marrow and those mobilized and retrieved from the blood. Cell cycling and DNA synthesis activity are greater in the bone marrow cells; conversely, some differentiation-blocking factors and apoptosis-driving genes have higher expression in PBSC.[17] How exactly these differences impact the speed of hemopoietic recovery is not presently known.
Stem cell mobilization in clinical practice
Early mobilizing regimes consisted of cytotoxic agents, such as high dose cyclophosphamide (4-7 g/m2), on their own. Though effective, such regimes had significant toxicity and frequently required admission to hospital.[18,19] Introduction of the granulocyte colony stimulating factor (GCSF) and the Granulocyte-Macrophage colony stimulating factor (GM-CSF) into clinical practice in the late 1980s was followed by the discovery that these agents can – a) augment the mobilizing effects of chemotherapy, b) act as mobilizing agents on their own. The efficacy of GCSF in HSC mobilization led to its use in healthy donors and paved the way for allogeneic PBSC transplantation.
The choice of a mobilizing regime depends on the clinical circumstances. If chemotherapy is required for disease control and an autologous PBSC transplant planned at a later stage, a number of chemotherapy regimes coupled with GCSF are adequate for both purposes. If PBSC collections are planned after several courses of intermediate-intensity cytotoxic therapy, which itself may not cause sufficient HSC mobilization (e.g., C-VAMP regime for multiple myeloma), it is preferable to use GCSF on its own, 3-4 weeks after completion of chemotherapy. Use of chemotherapy for HSC mobilization has the additional advantage of ‘ in vivo purging’ of the graft. Addition of the anti-CD20 antibody Rituximab to standard mobilizing chemotherapy/HGF regimes resulted in significant B cell reduction in the graft, as assessed by PCR methodology.[20] Some of the chemotherapy/HGF combinations suitable for HSC mobilization are shown in Table 1. One of them, CAIP a, developed at King's College Hospital in London, is a potent salvage therapy for Hodgkin's disease and non-Hodgkin lymphoma and a powerful mobilizing regime.
Table 1.
Chemotherapy mobilisation regimens
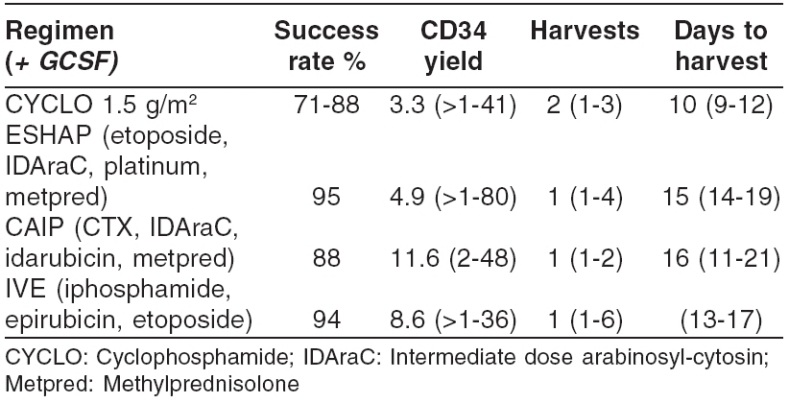
The dose of GCSF to use with chemotherapy for HSC mobilization is a matter of controversy. Demirer et al.[21] collected more CD34+ cells following chemotherapy with 16 µg/kg GCSF rather than 8 µg/kg, but the clinical benefits appeared small. In routine practice, 5 µg/kg GCSF is used, often rounded to the nearest vial, starting 1-7 days after chemotherapy; higher doses may be preferred in anticipation of poor yields. The use of pegfilgrastim, a long-acting GCSF preparation, with chemotherapy has been reported to be more convenient yet adequate for HSC mobilization in patients with myeloma.[22]
Ten to 15% of candidates for autologous transplantation fail to mobilize sufficient hemopoietic cells with standard mobilizing regimes and are referred to as ‘poor mobilizers.’ It is important to predict poor mobilization, in order to 1) avoid unnecessary procedures and 2) use potentially more effective mobilization regimes. Apart from having failed a recent mobilization procedure, poor mobilization may be predicted by using scoring systems based on history of chemotherapy and/or radiation exposure, age and platelet counts.[23–25] One such system,[24] which assigns a score to each cytotoxic agent administered to the patient, has been independently validated.[26] However, scoring systems based solely on the extent of previous treatment, apart from using arbitrary values, ignore the effects of drug dose and intensity an individual susceptibility to cytotoxic damage. An individualized method for prediction of poor mobilization is the ‘GCSF test.’[27] The method uses the CD34+ cell (or CFU-GM) response to a single dose of GCSF to predict the magnitude of mobilization in response to standard regimes [Figure 1].
Figure 1.
CD34 yield [106] in relation to CD34 blood concentration
Current options to mobilize ‘poor mobilizers’ include high-dose GCSF (>16 µg/kg for 4-5 days, subcutaneously), after a 2-4 week ‘holiday’ to allow marrow recovery. Weaver et al.[28] successfully mobilized 25 and 32% patients who had failed previous mobilization using GCSF 16-20 µg/kg or 30-32 µg/kg respectively. Alternatively, it is worth considering the addition of another HGF: both stem cell factor (SCF)[29,30] and GM-CSF[31] augment the mobilizing effects of GCSF. Premedication with antihistaminics reduces the rate of allergic reactions to SCF, which is still unavailable in the UK, although it has been licensed elsewhere. Conceivably, the combination of GCSF and AMD3100[32] will supersede other regimes. Preliminary data suggest that the two agents are also effective in poor mobilizers.[33] Other strategies to procure critical CD34+ counts include bone marrow harvesting (with the proviso that poor HSC mobilization predicts a poor quality bone marrow harvest[34]) and large volume leukapheresis.
Stem cell mobilization in donors
Granulocyte colony stimulating factor has emerged as the gold standard of HSC mobilization, due to its efficacy and the dearth of serious side effects. Therapeutic dose range is wide and HSC mobilization activity is dose dependent.[28,35] The standard dose for mobilization is 10 µg/kg/day, given subcutaneously for 4-5 days. The target of 4 × 106/kg CD34+ cells is achieved in 68-87% of healthy donors with a single apheresis, with less than 5% donors requiring more than two aphereses.[36,37] Using a higher dose, e.g., 16 µg/kg (2 × 8 µg/kg/day),or splitting the single daily dose into two, given 8-12 h apart, increases the CD34+ cell yield.[38,39] However, the cost-effectiveness of this approach in healthy subjects is questionable. When using a higher dose, e.g., 16-20 µg/kg, administration of the drug as two daily injections becomes necessary because of the volume of the drug injected.
Though ‘weight for weight’ comparison suggested that glycosylated GCSF is a better mobilizing agent than the nonglycosylated one,[40] a comparison of bioequivalent doses of the two forms showed little difference.[41]
Subcutaneous injection of GCSF is followed by a fall in leukocytes, with the peak of HSC mobilization occurring 3-6 h later. For this reason, it is advisable to delay apheresis until at least 2 h after the injection. For practical purposes, if the apheresis is planned for the morning, GCSF is usually given the previous evening. The long-acting, pegylated GCSF (Pegfilgrastim, AMGEN) administered as a single 12 mg dose has resulted in adequate CD34+ cell yields.[42] If this approach becomes standard, timing of GCSF administration relative to the apheresis may cease to be important.
GCSF administration frequently causes bone and muscle pain, fatigue and headache, affecting 50-80% of users. These side effects ordinarily resolve shortly after the drug is stopped. In 1-3% of donors, severe side effects required discontinuation of treatment.[43] Rare side effects reported included vascular incidents, anaphylaxis, and acute enlargement and even rupture of the spleen. Exacerbation of inflammatory conditions such as iritis or rheumatoid arthritis has also been reported.[44]
About 1-5% of donors receiving standard 4-5 day GCSF regime develop significant leukocytosis (WBC > 80 × 109/L).[42] In such cases, it is common practice to halve the next GCSF dose if subsequent harvests are required. Mild to moderate thrombocytopenia is common following apheresis, but it is occasionally caused by GCSF itself. One of us (AM) has witnessed severe acute thrombocytopenia (platelet count 8 × 109sub/L) in a patient with a normal platelet count prior to mobilization with 10 µg/kg GCSF. Thrombocytopenia resolved after GCSF was stopped, but the patient received 2 units of platelets to cover apheresis procedures.
At the time of writing of this review (March 2006), three deaths had been reported after PBSC donation: one was due to a crisis precipitated by GCSF given to an asymptomatic woman with hemoglobin SC disease.[45] The other two deaths were due to stroke and cardiac arrest. There has been one report of acute leukemia arising in a 61-year-old woman, 14 months after she donated PBSC for her brother.[46] If this reflects the true incidence of leukemia after GCSF administration for HSC mobilization, it may not be higher than the risk of leukemia in random population.
The age of donors has also increased, following the increase in patients’ age due to reduced-intensity transplantation. We have collected HSC from 15 donors over the age of 60 years (median 64 years, range 61-72 years), mobilized with GCSF 10 µg/kg prior to undergoing 1-2 PBSC harvests. No serious adverse events were encountered; however, the mean pre-apheresis platelet count was 227 ± 49 × 109/L falling to the mean of 77 ± 26 after one apheresis. In 3/11 donors the platelet count fell < 50 × 109/L after one apheresis. Four donors had a second procedure, and all had platelet counts below 50 × 109/L immediately afterwards. HSC mobilization and collection is feasible in elderly donors, but caution is required due to comorbid conditions, medication and risk of thrombocytopenia.
When to Harvest and how much is Required?
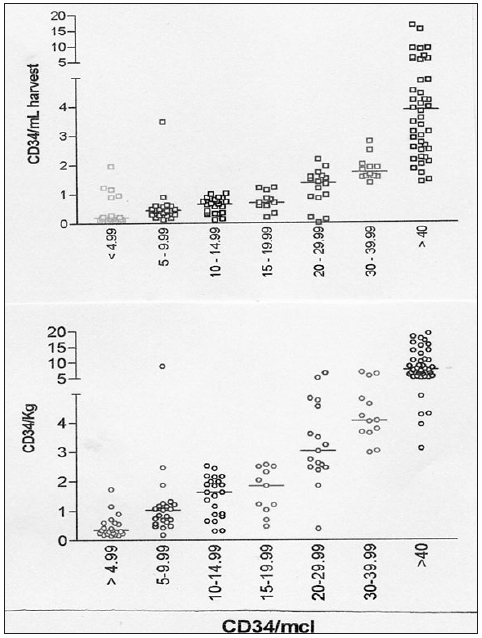
In order to ensure efficient HSC collection, it is important to begin harvesting when sufficient HSC have been mobilized. This is less of a problem in healthy donors, where HSC mobilization is by and large adequate, if of variable magnitude or when using regimes with predictable HSC mobilization kinetics. For example, with GCSF mobilization, peak CD34+ cell counts occur on days 5 or 6 (day 1 being the day of the first injection) and decline rapidly thereafter. Mobilization kinetics is less predictable if chemotherapy is used to mobilize HSC in previously treated patients. In this situation, a rapid and reliable blood test is required to decide whether to begin or defer harvesting. In the early days of PBSC transplantation, the rise of WBC from their postchemotherapy nadir to 5-10 × 109/L heralded HSC mobilization. Some centers, including ours, used automated counter parameters, such as the Sysmex SE9500 counter HPC[47] or ‘immature myeloid precursors,’ as convenient and reasonably accurate surrogate marker of HSC mobilization. Nevertheless, standardized blood CD34+ cell counting by flow cytometry superseded these and other methods, providing a rapid (about 1 h) estimate of HSC mobilization. Mohle et al.[48] found a strong linear correlation between the blood CD34+ cell count and the yield of CD34+ cells in the apheresis product. Moreover, they showed that 1 × 106/kg CD34+ cells were collected in less than 5% harvests done with the blood CD34+ cells of < 10 µL. Conversely, if blood CD34+ cell count was 30-100/µL, collection of 2.5 × 106/kg CD34+ cells by apheresis was achievable in a single procedure. For patient with 10-20 CD34+ cells /mL blood, large volume leukapheresis may help reduce the number of days of harvesting to a minimum [Figure 2].
Figure 2.
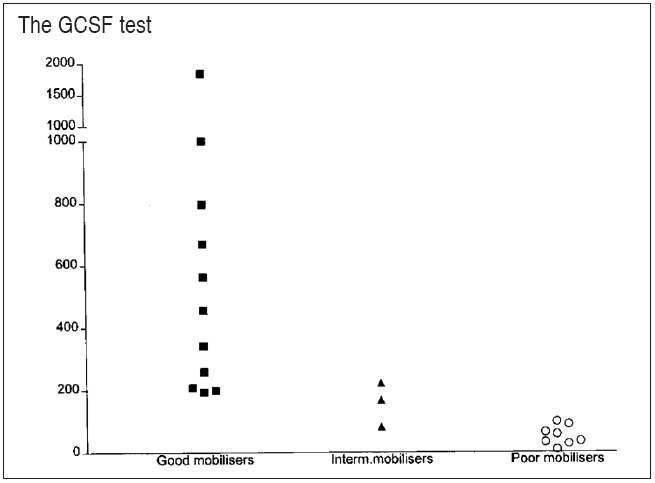
The ΔCFU-GM (colony-forming unit granulocyte-macrophage) after granulocyte colony-stimulating factor test injection in good, intermediate, and poor mobilizers. Y-axis = CFU-GM/mL blood
Numerous studies have demonstrated the dependence of hematological regeneration after PBSCT on the cell dose infused. To achieve adequate neutrophil and platelet counts within 10-14 days after autologous PBSCT, at least 2 × 106/kg CD34+ cells are required – preferably 5 ´ 106/kg as this dose permits prompt engraftment.[19,23] The minimal safe dose is 1 × 106/kg CD34+ cells. However, up to 50% of patients receiving this dose have delayed platelet recovery. In recipients of allogeneic transplant, Singhal et al.[49] reported higher transplant-related mortality in patients who had less than 2 ´ 106/kg CD34+ cells infused. Zaucha et al.[50] found a weak correlation between CD34+ cell dose and neutrophil and platelet recovery in sibling transplants but warned that doses above 8 ´ 106/kg CD34+ cells may be associated with a higher incidence of cGVHD (hazard ratio = 2.3, 95% CI 1.4–3.7, P= 0.001). Higher CD34+ cell dose is required when transplantation is performed across the HLA barrier, e.g., in haploidentical transplants.
Hemopoietic Stem Cell Harvesting
The procedure to be used in patients/donors undergoing HSC mobilization and apheresis should be prescribed by the physician in charge. The prescription must specify the type of venous access, choice of anticoagulant, the target cell dose, changes in concomitant medication and transfusion requirements before or after apheresis. When the blood CD34+ cell count becomes available, even if the apheresis procedure has commenced, the endpoints (time or total blood volume processed) may be adjusted to meet individual requirements.
Efficient separation of mononuclear cells, the fraction that contains hemopoietic stem cells and progenitors, is possible with all currently available cell separators. Continuous flow devices are more efficient than the discontinuous-flow ones. Among the former, there are small and generally clinically insignificant differences in CD34+ cell collection efficiency,[51,52] but the differences in product volume, platelet count or hematocrit or procedure time may be relevant in particular settings. Similarly, logistical differences, e.g., in anticoagulant-to-blood ratio, may be important when processing large volumes of blood. Performance may vary if different software is used with the same device; for example, the version 6 program on COBE Spectra has a smaller product volume and lower platelet contamination than version 4.7, which makes it more suitable for collections in donors or in thrombocytopenic patients.[53]
Whereas processing 2-2.5 total blood volume (TBV) generally yields sufficient CD34+ cell counts for an uneventful transplant, in some circumstances it is desirable to process more than 3 TBV. This ‘large volume leukapheresis’ (LVL) takes advantage of the fact that the blood CD34+ cell yields and their collection efficiencies remain fairly constant over the 6 TBV processed.[54] This implies that CD34+ cells must be recruited into the blood during the procedure, but the exact mechanisms of this recruitment have not been elucidated.[55] Bolan et al.[56] evaluated CD34 cell kinetics, yields and donor experiences during a single LVL, compared to two consecutive standard apheresis procedures. Though total CD34+ cell yields were similar, LVL donors spent less time in the clinic and with the venous catheter in place; in addition, LVL resulted in higher end-procedure platelet counts than two consecutive standard procedures.
PBSC apheresis is usually associated with few procedure-related adverse events, with the notable exception of citrate toxicity, which occurs in 45% of patients, manifesting itself initially as tingling and numbness of lips and fingers and progressing occasionally to nausea, muscle cramps, seizures and tetany. The first measure to alleviate the symptoms is to reduce the blood flow, allowing less citrate into the system, or stop the procedure for a while. Chewable calcium tablets are often useful and intravenous calcium is seldom needed. However, when larger blood volumes are processed using faster blood flows, heparin may be substituted for citrate, with extra citrate added into the collection bag to prevent clotting and platelet clumping.
Hypotension is a relatively uncommon side effect, but caution should be exerted in patients taking beta adrenergic blockers and angiotensin-convertase inhibitors. It is advisable to omit the dose of these drugs in the hours preceding apheresis.
Thrombocytopenia was reported in 22% of healthy donors who had two apheresis procedures.[57] If a patient has a platelet count of < 30 × 109/L prior to PBSC harvest, we transfuse a unit of platelets and check the platelet count again after procedure. For obvious reasons, use of aspirin is discouraged. For the safety of the donors who fly from far away to donate, it is advisable not to plan the return flight on the first post-apheresis day.
Venous access is of paramount importance in PBSC harvesting. PBSC donors rarely require a venous line, but many patients may do so. Tunneled double- or triple-lumen lines are generally not suitable for apheresis, due to narrow lumen and walls that collapse on suction; specially designed catheters may be necessary to use. Most serious adverse events during apheresis are related to the use of venous catheters. This includes thrombosis, infection, bleeding or pneumothorax.[57] The latter complication is eliminated if temporary femoral catheters are used.
Cell Processing
In addition to hemopoietic repopulating cells, PBSC collections contain lymphocytes, monocytes and a small percentage of granulocytes and myeloid precursors. The red cell content is generally under 10 ml per collection and the product volume is 70-250 ml. Therefore, PBSC are an already processed product, ready to transfuse or process for freezing. However, they can also be a starting material for further manipulation with the aim to enrich, expand or remove a particular subpopulation of cells.
Cell processing in autologous transplants
Among strategies that have been used to improve outcomes after autologous hemopoietic cell transplantation, removal of tumor cells (‘purging’) has been extensively studied. Gene marking experiments have demonstrated that the disease relapse can originate from the residual tumor cells in the graft,[58] providing scientific basis for purging. Negative selection strategies, designed to remove tumor cells from the graft, sparing hemopoietic stem cells, use monoclonal antibodies coupled with magnetic beads, toxins or complement or pharmacological agents.[59] Positive selection utilizes immunomagnetic beads coupled with antibodies to achieve CD34+ cell purification with simultaneous removal of non-CD34+ cells, including residual tumor cells and lymphocytes. Current methods of CD34+ cell enrichment achieve a mean product purity of 98-99% with a consequent 3-5 log tumor cell and lymphocyte reduction. However, CD34+ cell recovery is about 50-60%, which necessitates high starting cell counts in order to have sufficient CD34+ cells for a safe transplant. With comparable cell counts, time to hemopoietic recovery after autologous transplantation with purified CD34+ cells is not different to that after unmanipulated PBSC,[60] but lymphoid reconstitution may be delayed.
Evidence for a clinical benefit of using purging methods in autologous transplantation is not yet compelling. Gribben et al.[61] reported that successful B-cell purging translated into prolonged survival in follicular lymphoma. However, a randomized study in multiple myeloma[62] showed comparable disease-free and overall survival in recipients of CD34+ cell purified and unselected HSC.
Allogeneic transplantation
As the concept of graft-versus-tumor (GVT) effect crystallized as the principal beneficial effect of allogeneic transplantation, attempts were increased to segregate GVT from the graft-versus-host disease. A major question is whether the cells responsible for the GVT are the same T lymphocytes and/or NK cells that are responsible for the GVHD. Depletion of CD8+ lymphocytes from donor lymphocytes resulted in abrogating GVHD effect without compromising the disease response, suggesting that these two effects can be separated.[63]
More recently, the role of regulatory T cells in tolerance induction has been acknowledged. Methods for isolation of CD4+ CD25high T cells are developed for use to protect from GVHD after allogeneic transplantation.[64]
Lack of definitive methods for selective removal of the cells responsible for the GVHD effect and the association of global T cell depletion with increased risk of relapse have diminished the enthusiasm for cell selection in allogeneic transplantation. Exceptions are situations where the risk of GVHD is exceptionally high, such as transplantation from haploidentical donors. The CD34+ cell selection of three to four PBSC harvests, followed by a second method of T cell depletion, yields a graft that contains minimal T cell count but sufficient CD34 cells for uneventful hemopoietic regeneration. Encouraging clinical results have been reported in resistant and refractory acute leukemia.[65] It has been postulated that the GVT effect is provided by the donor NK cells not inhibited by the HLA class I molecules of the recipient. Early reports have been published of purified NK-cell infusions given to consolidate engraftment after haploidentical stem cell transplantation.[66]
Conclusions
Mobilized peripheral blood swiftly became the preferred source of hemopoietic stem cells for transplantation. Future challenges will include targeting molecules crucial for stem cell-stromal contact and stem cell retention; more successful mobilization of ‘poor mobilizers’; and even more efficient stem cell collection in healthy subjects. Whereas the use of CD34+ cell selection has a limited role in clinical practice, it may become a necessary step for subsequent cell expansion; lymphocyte subsets separated from the blood are already being subjected to trials in the allogeneic transplant setting, with the hope to achieve graft-versus-tumor effect without a graft-versus-host disease.
We are witnessing a proliferation of safety and quality control regulations which puts an onerous task on the transplant establishments, but it is expected to guarantee the safety of the process and be of benefit to the patients.
References
- 1.To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–58. [PubMed] [Google Scholar]
- 2.Gratwohl A, Baldomero H, Schmid O, Horisberger B, Bargetzi M, Urbano-Ispizua A. Change in stem cell source for haematopoietic cell transplantation in Europe: A report of the EBMT activity survey in 2003. Bone Marrow Transplant. 2005;36:575–90. doi: 10.1038/sj.bmt.1705104. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomized trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone marrow transplantation in lymphoma patients. Lancet. 1996;347:353. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 4.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–31. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 5.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775–9. [PubMed] [Google Scholar]
- 6.Smith TJ, Hillner BE, Schmitz N, Linch DC, Dreger P, Goldstone AH, et al. Economic analysis of a randomized clinical trial to compare filgrastim-mobilized peripheral-blood progenitor-cell transplantation and autologous bone marrow transplantation in patients with Hodgkin's and non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:5–10. doi: 10.1200/JCO.1997.15.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. A prospective, randomised trial of transplantation of marrow vs.peripheral blood from HLA-identical siblings treated for haematological malignancies. N Engl J Med. 2001;344:175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–7. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 9.Cutler C, Giri S, Jeyapaln S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral blood stem cell and bone marrow transplantation: A meta analysis. J Clin Oncol. 2001;19:3685–91. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Horowitz MM, Klein JP, Champlin RE, Loberiza FR, Jr, Ringden O, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: The Histocompatibility and Alternate Stem Cell Source Working Committee of the Internati onal Bone Marrow Transplant Registry. J Clin Oncol. 2004;22:4872–80. doi: 10.1200/JCO.2004.02.189. [DOI] [PubMed] [Google Scholar]
- 11.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031–9. [PubMed] [Google Scholar]
- 12.Papayannopoulou T. Mechanisms of stem-/progenitor-cell mobilization: The anti-VLA4 paradigm. Semin Hematol. 2000;37:11–8. doi: 10.1016/s0037-1963(00)90084-2. [DOI] [PubMed] [Google Scholar]
- 13.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m (null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 14.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, et al. Characterization of hematopoietic progenitor mobilisation in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 15.Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107:79–86. doi: 10.1182/blood-2005-05-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 17.Steidl U, Kronenwett R, Rohr UP, Fenk R, Kliszewski S, Maercker C, et al. Gene expression profiling identifies significant differences between the molecular phenotypes of bone marrow-derived and circulating human CD34+ hematopoietic stem cells. Blood. 2002;99:2037–44. doi: 10.1182/blood.v99.6.2037. [DOI] [PubMed] [Google Scholar]
- 18.To LB, Shepperd KM, Haylock DN, Dyson PG, Charles P, Thorp DL, et al. Single high doses of cyclophosphamide enable the collection of high numbers of hemopoietic stem cells from the peripheral blood. Exp Hematol. 1990;18:442–7. [PubMed] [Google Scholar]
- 19.Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L, et al. Peripheral blood stem cell transplants for multiple myeloma: Identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85:588–96. [PubMed] [Google Scholar]
- 20.Lazzarino M, Arcaini L, Bernasconi P, Alessandrino EP, Gargantini L, Cairoli R, et al. A sequence of immuno-chemotherapy with Rituximab, mobilization of in vivo purged stem cells, high-dose chemotherapy and autotransplant is an effective and non-toxic treatment for advanced follicular and mantle cell lymphoma. Br J Haematol. 2002;116:229–35. doi: 10.1046/j.1365-2141.2002.03256.x. [DOI] [PubMed] [Google Scholar]
- 21.Demirer T, Ayli M, Ozcan M, Gunel N, Haznedar R, Dagli M, et al. Mobilization of peripheral blood stem cells with chemotherapy and recombinant human granulocyte colony-stimulating factor (rhG-CSF): A randomized evaluation of different doses of rhG-CSF. Br J Haematol. 2002;116:468–74. doi: 10.1046/j.1365-2141.2002.03264.x. [DOI] [PubMed] [Google Scholar]
- 22.Steidl U, Fenk R, Bruns I, Neumann F, Kondakci M, Hoyer B, et al. Successful transplantation of peripheral blood stem cells mobilized by chemotherapy and a single dose of pegylated G-CSF in patient with multiple myeloma. Bone Marrow Transplant. 2005;35:33–6. doi: 10.1038/sj.bmt.1704702. [DOI] [PubMed] [Google Scholar]
- 23.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collection in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9. [PubMed] [Google Scholar]
- 24.Drake M, Ranaghan L, Morris TC, Nolan L, Desai ZR, Irvine AE, et al. Analysis of the effect of prior therapy on progenitor cell yield: Use of a chemotherapy scoring system. Br J Haematol. 1997;98:745–9. doi: 10.1046/j.1365-2141.1997.2743091.x. [DOI] [PubMed] [Google Scholar]
- 25.Morris CL, Siegel E, Barlogie B, Cottler-Fox M, Lin P, Fassas A, et al. Mobilization of CD34+ cells in elderly patient with multiple myeloma: Influence of age, prior therapy, platelet count and mobilization regime. Br J Haematol. 2003;120:413–23. doi: 10.1046/j.1365-2141.2003.04107.x. [DOI] [PubMed] [Google Scholar]
- 26.Clare RE, Brammer CG. Previous treatment predicts the efficiency of blood progenitor cell mobilisation: Validation of a chemotherapy scoring system. Bone Marrow Transplant. 1998;22:859–63. doi: 10.1038/sj.bmt.1701461. [DOI] [PubMed] [Google Scholar]
- 27.Mijovic A, Pagliuca A, Mufti GJ. The “G-CSF test”: The response to a single dose of granulocyte colony-stimulating factor predicts mobilization of hemopoietic progenitors in patients with hematologic malignancies. Exp Hematol. 1999;27:1204–9. doi: 10.1016/s0301-472x(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 28.Weaver CH, Tauer K, Zhen B, Schwartzberg LS, Hazelton B, Weaver Z, et al. Second attempts at mobilization of peripheral blood stem cells in patients with initial low CD34+ cell yields. J Hematother. 1998;7:241–9. doi: 10.1089/scd.1.1998.7.241. [DOI] [PubMed] [Google Scholar]
- 29.Mijovic A, Russell N, Clark RE, Morris TC, Browne P, Crown J, et al. Ancestim associated with Filgrastim and/or chemotherapy can improve blood progenitor yields in patients who previously failed mobilization. Bone Marrow Transplant. 2005;35:1019. doi: 10.1038/sj.bmt.1704950. [DOI] [PubMed] [Google Scholar]
- 30.Horsfall MJ, Hui CH, To LB, Begley CG, Basser RL, Simmons PJ. Combination of stem cell factor and granulocyte colony-stimulating factor mobilizes the highest number of primitive haemopoietic progenitors as shown by pre-colony-forming unit assay. Br J Haematol. 2000;109:751–8. doi: 10.1046/j.1365-2141.2000.02108.x. [DOI] [PubMed] [Google Scholar]
- 31.Ho A, Young D, Maruyama M, Corringham RE, Mason JR, Thompson P, et al. Pluripotent and lineage-committed CD34+ subsets in leukapheresis products mobilized by G-CSF, GM-CSF vs a combination of both. Exp Hematol. 1996;24:1460–8. [PubMed] [Google Scholar]
- 32.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarthy JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–74. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 33.Fassas A, Cottler-Fox M, Calandra G, Tricot G. ASCO annual meeting. 2004;23:6643a. [Google Scholar]
- 34.Watts MJ, Sullivan AM, Leverett D, Peniket AJ, Perry AR, Williams CD, et al. Back-up bone marrow is frequently ineffective in patients with poor peripheral blood stem cell mobilization. J Clin Oncol. 1998;16:1554–60. doi: 10.1200/JCO.1998.16.4.1554. [DOI] [PubMed] [Google Scholar]
- 35.Stroncek DF, Clay ME, Petzoldt ML, Smith J, Jaszcz W, Oldham FB, et al. Treatment of normal individuals with granulocyte colony stimulating factor: Donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601–10. doi: 10.1046/j.1537-2995.1996.36796323059.x. [DOI] [PubMed] [Google Scholar]
- 36.Anderlini P, Donato M, Lauppe MJ. Peripheral blood progenitor cell apheresis: 4-year results of the MD Anderson collection protocol in normal donors. Transfusion. 1998;38:95S. [Google Scholar]
- 37.Mijovic A. Unpublished data. [Google Scholar]
- 38.Kroger N, Renges H, Sonnenberg S, Kruger W, Gutensohn K, Dielschneider T, et al. Stem cell mobilisation with 16 microg/kg vs 10 microg/kg of G-CSF for allogeneic transplantation in healthy donors. Bone Marrow Transplant. 2002;29:727–30. doi: 10.1038/sj.bmt.1703509. [DOI] [PubMed] [Google Scholar]
- 39.de la Rubia J, Arbona C, de Arriba F, del Canizo C, Brunet S, Zamora C, et al. Analysis of factors associated with low peripheral blood progenitor cell collection in normal donors. Transfusion. 2002;42:4–9. doi: 10.1046/j.1537-2995.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 40.Hoglund M, Smedmyr B, Bengtsson M, Totterman TH, Cour-Chabernaud U, Yver A, et al. Mobilisation of CD34+ cells by glycosylated and non-glycosylated GSCF in healthy volunteers – A comparative study. Eur J Haematol. 1997;59:177–83. doi: 10.1111/j.1600-0609.1997.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 41.De Arriba F, Lozano ML, Ortuno F, Heras I, Moraleda JM, Vicente V. Prospective randomized study comparing the efficacy if bioequivalent doses of glycosylated and nonglycosylated rG-CSF for mobilizing peripheral blood progenitor cells. Br J Haematol. 1998;96:418–20. doi: 10.1046/j.1365-2141.1997.d01-2029.x. [DOI] [PubMed] [Google Scholar]
- 42.Kroschinsky F, Holig K, Poppe-Theide K, Zimmer K, Ordemann R, Bleschshmidt M, et al. Single dose pegfilgrastim for the mobilisation of allogeneic CD34+ peripheral blood progenitor cells in healthy family and unrelated donors. Hematologica. 2005;12:1665–71. [PubMed] [Google Scholar]
- 43.Anderlini P, Korbling M, Dale D, Gratwohl A, Schmitz N, Stroncek D, et al. Allogeneic blood stem cell transplantation:considerations for donors. Blood. 1997;90:903–8. [PubMed] [Google Scholar]
- 44.Korbling M, Anderlini P. Peripheral blood stem cells versus bone marrow allotransplantation: Does the source of hematopoietic cells matter? Blood. 2001;98:2900–8. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 45.Adler BK, Salzman DE, Carabasi MH, Vaughan WP, Reddy VV, Prchal JT. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood. 2001;97:3313–4. doi: 10.1182/blood.v97.10.3313. [DOI] [PubMed] [Google Scholar]
- 46.Makita K, Ohta K, Mugitani A, Hagihara K, Ohta T, Yamane T, et al. Acute myelogenous leukemia in a donor after granulocyte colony-stimulating factor-primed peripheral blood stem cell harvest. Bone Marrow Transplant. 2004;33:661–5. doi: 10.1038/sj.bmt.1704394. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Leisenring W, Fritschle W, Heimfeld S, Shulman H, Bensinger WI, et al. Enumeration of HPC in mobilized peripheral blood with the Sysmex SE9500 predicts final CD34+ cell yield in the apheresis collection. Bone Marrow Transplant. 2000;25:1157–64. doi: 10.1038/sj.bmt.1702406. [DOI] [PubMed] [Google Scholar]
- 48.Mohle R, Murea S, Pforsich M, Witt B, Haas R. Estimation of the progenitor cell yield in a leukapheresis product by previous measurement of CD34+ cells in the peripheral blood. Vox Sang. 1996;71:90–6. doi: 10.1046/j.1423-0410.1996.7120090.x. [DOI] [PubMed] [Google Scholar]
- 49.Singhal S, Powles R, Treleaven J, Kulkarni S, Sirohi B, Horton C, et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2000;26:489–96. doi: 10.1038/sj.bmt.1702542. [DOI] [PubMed] [Google Scholar]
- 50.Zaucha JM, Gooley T, Bensinger WI, Heimfeld S, Chauncey TR, Zaucha R, et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98:3221–7. doi: 10.1182/blood.v98.12.3221. [DOI] [PubMed] [Google Scholar]
- 51.Jeanne M, Bouzgarrou R, Lafarge X, Fizet D, Dazey B, Vezon G, et al. Comparison of CD34+ cell collection on the CS-3000+ and Amicus blood cell separators. Transfusion. 2003;43:1423–7. doi: 10.1046/j.1537-2995.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 52.Valbonesi M, Pollicardo N, Carlier P, Florio G, Ruzzenenti MR, Pungolino E, et al. PBSC collection from G-CSF primed donors. Transfus Sci. 1996;17:619–27. [PubMed] [Google Scholar]
- 53.Ravagnani F, Siena S, De Reys M, Di Nicola M, Notti P, Giardini R, et al. Improved collection of mobilized CD34+ hematopoietic progenitor cells by a novel automated leukapheresis system. Transfusion. 1999;39:48–55. doi: 10.1046/j.1537-2995.1999.39199116894.x. [DOI] [PubMed] [Google Scholar]
- 54.Cassens U, Momkvist PH, Zuehlsdorf M, Mohr M, Kienast J, Berdel W, et al. Kinetics of standardized large volume leukapheresis in patients do not show a recruitment phenomenon of peripheral blood progenitor cells. Bone Marrow Transplant. 2001;28:13–20. doi: 10.1038/sj.bmt.1703082. [DOI] [PubMed] [Google Scholar]
- 55.Ford CD, Greenwood J, Strupp A, Lehman CM. Change in CD34+ cell concentration during peripheral blood progenitor cell collection: Effects on collection efficiency and efficacy. Transfusion. 2002;42:904–11. doi: 10.1046/j.1537-2995.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- 56.Bolan CD, Carter CS, Wesley RA, Yau YY, Barrett AJ, Childs RW, et al. Prospective evaluation of cell kinetics, yields and donor experiences during a single large-volume apheresis versus two small volume consecutive day collections of allogeneic peripheral blood stem cells. Br J Haematol. 2003;120:801–7. doi: 10.1046/j.1365-2141.2003.04157.x. [DOI] [PubMed] [Google Scholar]
- 57.Stroncek DF, Confer DL, Leitman SF. Peripheral blood progenitor cells for HPC transplants involving unrelated donors. Transfusion. 2000;40:731–41. doi: 10.1046/j.1537-2995.2000.40060731.x. [DOI] [PubMed] [Google Scholar]
- 58.Brenner MK, Rill DR, Moen RC, Krance RA, Mirro J, Jr, Anderson WF, et al. Gene-marking to trace origin of relapse after autologous bone marrow transplantation. Lancet. 1993;341:85–6. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- 59.Champlin R. Purging: The separation of normal from malignant cells for autologous transplantation. Transfusion. 1996;36:910–8. doi: 10.1046/j.1537-2995.1996.361097017179.x. [DOI] [PubMed] [Google Scholar]
- 60.Kvalheim G, Wang MY, Pharo A, Holte H, Jacobsen E, Beiske K, et al. Purging of tumour cells from leukapheresis products: Experimental and clinical aspects. J Hematother. 1996;5:427–36. doi: 10.1089/scd.1.1996.5.427. [DOI] [PubMed] [Google Scholar]
- 61.Gribben JG, Freedman AS, Neuberg D, et al. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med. 1991;28:1525–33. doi: 10.1056/NEJM199111283252201. [DOI] [PubMed] [Google Scholar]
- 62.Vescio R, Schiller G, Stewart AK, Ballester O, Noga S, Rugo H, et al. Multicenter phase III trial to evaluate CD34(+) selected versus unselected autologous peripheral blood progenitor cell transplantation in multiple myeloma. Blood. 1999;93:1858–68. [PubMed] [Google Scholar]
- 63.Alyea EP, Soiffer RJ, Canning C, Neuberg D, Schlossman R, Pickett C, et al. Toxicity and efficacy of defined doses of CD4+ donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998;91:3671–80. [PubMed] [Google Scholar]
- 64.Hoffman P, Edinger M. CD4+ CD25+ regulatory T cells and graft-versus-host disease. Semin Hematol. 2006;43:62–9. doi: 10.1053/j.seminhematol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 66.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]


