Abstract
BACKGROUND:
Estrogen receptor (ER) is a ligand-inducible transcription factor that mediates estrogen action in target tissue. Several common polymorphisms of the ERα gene have been reported to be associated with alterations in receptor expression in breast cancer.
MATERIALS AND METHODS:
A case-control study was designed to compare 250 breast cancer patients with 250 age-matched healthy controls. The frequency distribution of PvuII polymorphism in the ERα gene was assessed by PCR-RFLP method.
RESULTS:
The frequency of the PP genotype (35.3%) was increased significantly in breast cancer patients when compared to controls (19.8%), with a corresponding increase in P allele frequency (χ2= 16.4; P = 0.0003). The OR for genotypes PP vs. Pp was 1.989 (95% CI: 1.2708 to 3.113). Premenopausal women with breast cancer had an elevated frequency of the PP genotype (22.8%) as compared to postmenopausal women (16.8%). The frequency of the PP genotype was increased in patients positive for ER and HER-2/neu as compared to those with receptor-negative status. The pp and p allele frequencies were increased in progesterone-receptor-negative status. When stage of the disease was considered, both Pp and pp genotype frequencies were elevated in patients with advanced stage breast cancer. The frequency of the P allele and PP genotype frequencies tended to increase with increase in body mass index, whereas the Pp genotype frequency was elevated only in obese patients. The reverse was observed in the case of pp genotype frequency.
CONCLUSION:
The study thus highlighted the influence of ERα PvuII polymorphism on the development and progression of breast cancer.
Keywords: Body mass index, breast cancer, estrogen receptor, menopausal status, polymorphism
Introduction
Estrogen, a steroid hormone, has an essential role in the development and maintenance of female secondary sexual characters. It plays a crucial role in the pathogenesis and progression of breast cancer. The biological effects of estrogen, such as growth stimulation and differentiation of normal mammary tissue, is mediated primarily through high-affinity binding to ERs.[1] ERs are nuclear receptor proteins that have an estrogen-binding domain and a DNA-binding domain.[2] There are two types of ERs: ERα and ERβ. The ERα gene is localized on chromosome 6q25.1.[3] Estrogen-bound ERα acts like a transcription factor, which binds to estrogen response element (ERE) upstream of the target genes. The ERα is closely associated with breast cancer biology, especially in the development of tumors. ERβ gene is located on chromosome 14q22-24. ERβ regulates genes that function as tumor suppressors.[4]
The association of genetic polymorphisms in the ERα gene and the risk of disease, including breast cancer, have been a subject of increasing interest. Several DNA sequence variations in the ERα gene have been reported.[5] In particular, the PvuII polymorphism in ERα has been found to have a close association with breast cancer and spontaneous miscarriage.[5] Several studies have shown that among ERα genotypes assessed by PvuII restriction fragment-length polymorphism (RFLP), the PP genotype showed higher bone mineral density than the Pp and pp genotypes,[6] and adolescent boys with the PP genotype had greater body height than the others.[7] These findings may suggest that the local estrogenic action is more potent in those with a PP genotype than in those with the Pp and pp genotypes. This is also supported by the presence of an association between ERα gene polymorphisms and estrogen-dependent diseases, including endometriosis,[8] and the risk of premenopausal hysterectomy and onset of natural menopause.[9]
We studied a series of breast cancer cases and age-matched controls to determine whether PvuII polymorphism in the ERα gene influences the risk for development of breast cancer.
Materials and Methods
A group of 250 breast cancer patients, including 9 male breast cancer cases, were selected for the study. Healthy, age-matched women, without a family history of breast cancer or any other cancers, were selected to serve as the control group. Informed consent was taken from all the subjects selected for the study. Cases were chosen from the Nizam′s Institute of Medical Sciences after confirmed diagnosis. The diagnosis of breast cancer was established by pathological examination, mammography, FNAC, and biopsy. Epidemiological history, such as age at onset of breast cancer, diet, socioeconomic status, occupation, reproductive history, family history, and consanguinity was taken through a personal interview with the breast cancer patients, using a specific proforma. The patients were screened for receptor status of estrogen, progesterone and HER-2/neu through immunohistochemical assay. Clinical history such as size of the tumor, presence of axillary nodes, metastasis, stage and type of the breast cancer, chemotherapeutic drugs used, and prognosis of the disease was collected with the help of an oncologist.
ERα genotyping
Five milliliters of blood was collected in an EDTA vaccutainer from patients as well as controls. DNA was isolated[10] and used for amplification of intron 1 of the ERα gene by PCR, using specific oligonucleotide primers.[11] Each amplification reaction contained 0.1 μgm of DNA, 0.4 μM of each primer, 200 μM of each of the four deoxyribonucleotides, and 1 U of Taq polymerase. PCR was performed through 30 cycles with the following steps: denaturation at 94°C for 30 s, annealing at 55°C for 1 min, and extension at 72°C for 90 s. The PCR product was a 1.3 Kbp fragment. After amplification, the PCR product was digested overnight with 10 U of PvuII restriction endonuclease (New England Biolabs) at 37°C and genotyped on 2% agarose gel. The sizes of the bands were estimated using a 100 bp ladder. The genotyping was done on the basis of the presence or absence of the PvuII restriction site, as follows: PP 1300, Pp 1300,850,450, and pp 850,450.
Statistical analysis
The results were analyzed using appropriate statistical tests. Odds ratios were estimated to calculate the relative risk for each genotype to develop disease. Differences in genotype frequency distribution between disease and control groups were estimated using the 2 × 2 χ2 and the χ2 test for heterogeneity.
Results
Two hundred and fifty breast cancer patients and healthy controls were analyzed for genotype distribution of PvuII polymorphism of the ERα gene. The mean age at diagnosis of breast cancer in the present sample was 47.6 years. The genotype distribution was studied with respect to risk confounding factors, such as menopausal status, body mass index, hormone receptor status (estrogen receptor, progesterone receptor, and HER-2/neu status), and stage of the tumor.
Table 1 shows the genotype frequency distribution of PvuII polymorphism of the ERα gene in both breast cancer patients and controls. The frequency of the PP genotype (35.3%) was increased significantly in breast cancer as compared to controls (19.8%), with a corresponding increase in P allele frequency (χ2 = 16.4; P = 0.0003). The OR for genotype PP vs. Pp was 1.989 (95% CI: 1.2708 to 3.113). Premenopausal women had elevated frequencies of the PP genotype (22.8%) as compared to postmenopausal women (16.8%) [Table 2]. The frequency of the PP genotype was elevated in patients positive for ER and HER-2/neu as compared to those who were negative for the same [Tables 3 and 5], whereas the pp genotype and p allele frequencies were increased in patients with progesterone receptor-negative status [Table 4]. When the stage of the disease was considered, both Pp and pp genotype frequencies were found to be elevated in advanced stage breast cancer [Table 6].
Table 1.
Genotype distribution of PvuII polymorphism of ERa gene in breast cancer patients and controls
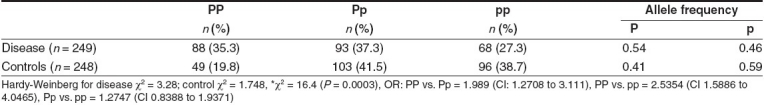
Table 2.
PvuII polymorphism of ERα gene and menopausal status of breast cancer patients
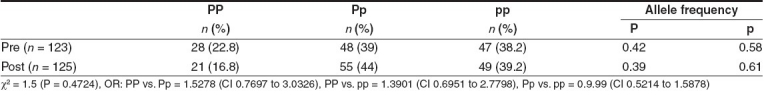
Table 3.
PvuII polymorphism of ERα gene and estrogen receptor status in breast cancer patients
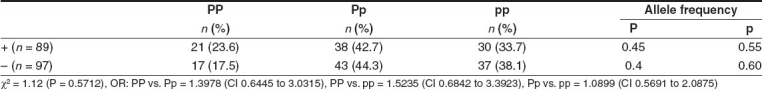
Table 5.
PvuII polymorphism of ERα gene and HER-2/neu status in breast cancer patients
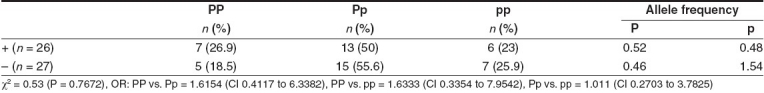
Table 4.
PvuII polymorphism of ERα gene and progesterone receptor status in breast cancer patients
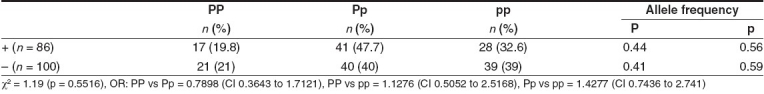
Table 6.
PvuII polymorphism of ERα gene and stage of breast cancer
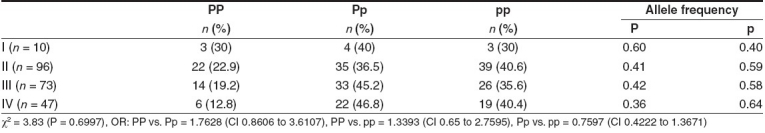
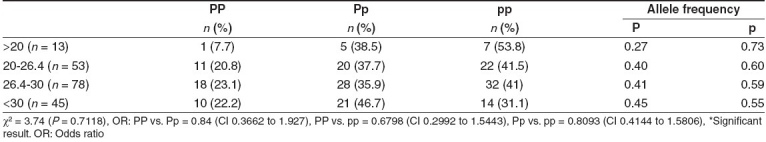
The P allele and the PP genotype frequencies tended to increase with increase in body mass index, whereas the Pp genotype frequency was elevated only in obese patients. The reverse was observed in the case of the frequency of the pp genotype [Table 7].
Table 7.
PvuII polymorphism of ERa gene and BMI of breast cancer patients
Discussion
The present study attempted to evaluate the role of ERα polymorphism in the development of breast cancer. The ER gene comprises more than 140 kb and has 8 exons and 5 functional domains, designated A/B-F. The PvuII RFLP site was found in the first intron, with a point mutation (T-C) in the recognition sequence CAGCTA responsible for the P allele.[12] PvuII polymorphism is the most studied in several diseases, including breast cancer,[13] endometrial cancer,[14] Alzheimer′s disease,[15] endometriosis,[8] and also with increased bone mineral density.[7]
The PP genotype was significantly elevated in breast cancer patients as compared to controls, suggesting that this genotype confers a risk for the development of breast cancer. However, a number of studies have failed to show an association between PvuII polymorphism in the ERα gene and breast cancer,[5,13] though some studies have shown an association with the p allele.[16] The possible explanation for the association is that the local estrogenic action is more potent in the presence of P allele, which might confer the risk to develop breast cancer. Estrogen is known to induce cell proliferation, and prolonged exposure to environmental xenoestrogens is associated with breast cancer.[7]
It is unclear how the anonymous intronic polymorphism of the ERα gene influences its protein function. Some studies have postulated that the ERα gene polymorphism may influence its action as a modulator of the ligand estrogen.[6] Some introns contain regulatory sequences such as enhancers, i.e., binding sites for elements that regulate the level of gene expression, and thus also affect protein synthesis.[17] The intronic polymorphism may be in linkage disequilibrium with exon alteration, which affects ERα protein function. The PvuII polymorphism in the ERα gene may be linked with the alteration of another unidentified gene adjacent to the ERα gene, which might increase breast cancer risk.[6] Intronic changes in gene sequences may have an impact on the expression of other genes by influencing the transcription/stability of mRNA of these genes.[18]
Recent studies suggested that PvuII polymorphism might affect the splicing of ERα mRNA, resulting in the alteration of protein expression. The P allele has a potential binding site for myeloblastosis (myb) transcription factor that, in the presence of B-myb, is capable of augmenting in vitro transcription of a downstream reporter construct 10 fold. Thus, in some settings, the presence of the P allele might amplify ERα transcription. Because B-myb expression is itself responsive to estrogen activation, it may contribute to a signal-amplifying system, producing augmented responses to estrogen in those cell types that commonly express B-myb or related transcription factors.[19]
There was an elevation in the frequency of the PP genotype in premenopausal patients as compared to postmenopausal cases, which supports further the role of the PP genotype, with strong estrogenic action in breast cancer development. This is also supported by the finding that the PP genotype has a higher risk for premenopausal hysterectomy (for menorrhagia and fibroids) and earlier onset of menopause than the Pp and pp genotypes.[9]
PP genotype frequency was increased in patients with ER and HER-2/neu positive status, which are the risk conferring factors. The frequencies of the pp genotype as well as of the p allele were increased in progesterone-receptor-negative status, which is similar to the Shanghai breast cancer study.[16]
When the size and stage of the disease were considered, Pp and pp genotype frequencies were found to be increased in patients with large tumor size and advanced stage of the disease; there was a corresponding elevation of p allele frequency in advanced stage disease, suggesting that the presence of the p allele might confer a risk for an aggressive form of the disease. It is possible that individuals with the p allele have a lower expression of the ERα receptor or lower estrogen affinity and are, therefore, not controlled by endocrine therapy, resulting in greater tumor aggressiveness and poor prognosis.[20] The probability of estrogen-independent ER function (non-genomic pathway) leading to poor response and rapid progression cannot be ruled out.
The P allele frequency was elevated in overweight and obese patients. Being fatty, breast tissue can absorb and accumulate the end products of xenobiotics and xenoestrogens. Further, the distribution of adipose tissue through endocrine and paracrine effects was mediated by the activation of ER. Estrogen exposure will increase breast cancer incidence and proliferation.[20] Hence, overweight and obesity might independently predispose women to breast cancer, which gets confounded by the ERα polymorphism status.
In conclusion, our data suggests an influence of PvuII polymorphism of the ERα gene in the development of breast cancer.
Acknowledgments
This work was supported by University Grants Commission, New Delhi, and the Nizam′s Institute of Medical Sciences, Hyderabad.
Footnotes
Source of Support: University Grants Commission, New Delhi
Conflict of Interest: None declared.
References
- 1.Roodi N, Bailey LR, Kao WY, Verrier CS, Yee CJ, Dupont WD, et al. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. J Natl Cancer Inst. 1995;87:446–51. doi: 10.1093/jnci/87.6.446. [DOI] [PubMed] [Google Scholar]
- 2.Rayter Z. Steroid receptors in breast cancer. Br J Surg. 1991;78:528–35. doi: 10.1002/bjs.1800780506. [DOI] [PubMed] [Google Scholar]
- 3.Menasce LP, White GR, Harrison CJ, Boyle JM. Locolization of the estrogen receptor locus (ESR) to chromosome 6q25.1 by FISH and a simple post FISH banding technique. Genomic. 1993;17:263–5. doi: 10.1006/geno.1993.1320. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi SI, Eguch E, Tanimoto K, Yoshida T, Omoto Y, Inoue A, et al. The expression and function of estrogen receptor α and β in human breast cancer and its clinical application. Endocr Relat Cancer. 2000;10:193–202. doi: 10.1677/erc.0.0100193. [DOI] [PubMed] [Google Scholar]
- 5.Hill SM, Fuqua SA, Chamness GC, Greene GL, McGuire WL. Estrogen receptor expression in human breast cancer associated with an estrogen receptor gene restriction fragment length polymorphism. Cancer Res. 1989;49:145–8. [PubMed] [Google Scholar]
- 6.Kobayashi S, Inoue S, Hosoi T, Ouchi Y, Shiraki M, Orimo H. Association of bone mineral density with polymorphism of the estrogen receptor gene. J Bone Miner Res. 1996;11:306–11. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- 7.Lorentzon M, Lorentzon R, Backstrom T, Nordstrom P. Estrogen receptor gene polymorphism but not estradiol levels, is related to bone density in healthy adolescent boys: A cross sectional study. J Clin Endocrinol Metb. 1999;84:4597–601. doi: 10.1210/jcem.84.12.6238. [DOI] [PubMed] [Google Scholar]
- 8.Georgious I, Syrrou M, Bouba I, Dalkalitsis N, Paschopoulos M, Navrozoglou I, et al. Association of estrogen receptor gene polymorphisms with endometriousis. Fertil Seril. 1999;72:164–6. doi: 10.1016/s0015-0282(99)00198-3. [DOI] [PubMed] [Google Scholar]
- 9.Weel AE, Uitterlinden AG, Westendorp IC, Burger H, Schuit SC, Hofman A, et al. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. Clin Endocrinol Metab. 1999;84:3146–50. doi: 10.1210/jcem.84.9.5981. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood RFLP studies. Nucleic Acid Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becherini L, Gennari L, Masi L, Mansani R, Massart F, Morelli A, et al. Evidence of linkage disequilibrium between polymorphisms in the human estrogen receptor alpha gene and their relationship to bone mass variation in post menopausal Italian women. Hum Mol Genet. 2000;9:2043–50. doi: 10.1093/hmg/9.13.2043. [DOI] [PubMed] [Google Scholar]
- 12.Yaich L, Dupont WD, Cavener D, Parl FF. Analysis of the PvuII restriction fragment-length polymorphism and exon stricture of the estrogen receptor gene in breast cancer and peripheral blood. Cancer Res. 1992;52:77–83. [PubMed] [Google Scholar]
- 13.Parl FF, Cavener DR, Dupont WD. Genomic DNA analysis of the estrogen receptor gene in breast cancer. Breast Cancer Res Treat. 1989;14:57–64. doi: 10.1007/BF01805976. [DOI] [PubMed] [Google Scholar]
- 14.Weiderpass E, Persson I, Melhus H, Wedren S, Kindmark A, Baron JA. Estrogen receptor a polymorphisms and endometrial cancer risk. Carcinigenesis (Lond) 2000;21:623–7. doi: 10.1093/carcin/21.4.623. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama H, Toji H, Harrington CR, Sasaki K, Izumi Y, Ohnuma T, et al. Lack of an association of estrogen receptor α gene polymorphisms and transcriptional activity with Alzheimer′s disease. Arch Neurol. 2000;57:236–40. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- 16.Cai Q, Shu XO, Jin F, Dai Q, Wen W, Cheng JR, et al. Genetic polymorphisms in the estrogen receptor a gene and risk of breast cancer: Result from Shanghai Breast cancer study. Cancer Epidemiol Biomarker Prev. 2003;12:853–9. [PubMed] [Google Scholar]
- 17.Laurie CC, Stan LF. The effect of an intronic polymorphism on alcohol dehydrogenase expression in Drosophila Melanogaster. Genetics. 1994;138:379–85. doi: 10.1093/genetics/138.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goessl C, Plaschke J, Pistrius S, Hahn M, Frank S, Hampl M, et al. An intronic germline transition in the HNPCC GENE Hmsh2 is associated with sporadic colorectal cancer. Eur J Cancer. 1997;33:1869–74. doi: 10.1016/s0959-8049(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 19.Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Hawkins GA, et al. Common estrogen receptor polymorphism augment effects of hormone replacement therapy on E-selection but not C-reactive protein. Circulation. 2000;105:1879–82. doi: 10.1161/01.cir.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- 20.Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006;11:1–8. doi: 10.1634/theoncologist.11-1-1. [DOI] [PubMed] [Google Scholar]


