Abstract
Regulatory T cells (Tregs) are critical for maintenance of peripheral tolerance via suppression of T-cell responses, and absence of Tregs results in autoimmunity. The role of aberrations in the Treg pool for the development of systemic lupus erythamatosus (SLE, lupus) remains uncertain. Treg-mediated generation of adenosine, dependent on the ectonucleotidase CD39, is an important mechanism for suppression of T-cell responses. We tested whether decreases in numbers of Tregs, and specifically CD39-expressing Tregs, are associated with human lupus. We studied 15 SLE patients, 6 patients with rheumatoid arthritis (RA) and 24 healthy controls. Treg phenotypic markers, including CD39 expression, were studied by flow cytometry. Varying numbers of sorted Tregs cells were co-cultured with responder T (Tresp) cells, with proliferation assessed by 3H-thymidine incorporation. The proportion of Tregs as defined by Foxp3+ CD25+high CD127−/low was similar in lupus and control populations. CD39-expressing Tregs comprised 37 ± 13% of the Treg population in healthy controls and 36 ± 21% in lupus subjects using nonsteroidal immunosuppressants to control active disease, but was nearly absent in 5 of 6 lupus subjects with minimally active disease. In contrast to healthy controls and lupus subjects without the CD39 defect, in SLE subjects with the CD39 defect, adenosine-dependent Treg-mediated suppression was nearly absent. These results suggest that functional defects in Tregs, rather than reduced Treg numbers, are important for the loss of peripheral tolerance in lupus. Presentation of this defect may serve as a biomarker for untreated disease.
Keywords: Autoimmunity, Human Lupus, Regulatory T cells, NTPDase CD39
1. INTRODUCTION
Regulatory T cells (Tregs) are critical mediators of peripheral tolerance to self antigens, as well as to some tolerogenic foreign antigens. During T cell development in the thymus, both autoreactive T cells and T cells with insufficient recognition of self-major histocompatibility antigen (MHC) molecules are deleted, resulting in central tolerance. However, some autoreactive T cells are not deleted, but rather attain a regulatory phenotype in which these Tregs, if activated, inhibit antigen-stimulated activation of nearby T cells [1, 2]. Induction of the forkhead transcription factor Foxp3 is critical in establishing this regulatory phenotype. These ‘natural’ Tregs were first phenotypically characterized as a CD4+ CD25+hi T cell population capable of inhibiting T cell proliferation. Other markers of these natural Tregs include CTLA-4, GITR (glucocorticoid-induced TNF receptor-related protein), and low-level expression of IL-7Rα, all of whose genes are targets of Foxp3 [3, 4]. However, expression of these molecules, including Foxp3 (or downregulation of expression in the case of IL-7Rα) is also observed for activated T cells [5–7].
Recently, adenosine produced by Tregs has been implicated as a soluble mediator of Treg suppression. Murine Foxp3+ CD25+hi Tregs express CD39 and CD73 [8–11], which mediate extracellular metabolism of ATP/ADP and AMP, respectively, into adenosine. Adenosine and the adenosine 2A receptor (A2AR), a GS-coupled GPCR, are critical in the down-regulation of inflammatory responses in general [12, 13] and T cell responses in specific [14]. Expression of CD39 in human Foxp3+ CD25+hi Tregs was also confirmed, and reduced numbers of CD25+hi CD39+ Tregs correlated better with multiple sclerosis than did numbers of Tregs defined solely by CD25+hi expression [9]. Mandapathil et al. confirmed that adenosine generated by CD39-expressing Tregs significantly contributes to Treg-mediated suppression of T cell profileration in humans [15].
The role of Tregs in SLE remains uncertain. It was initially reported that the proportions of Tregs (defined by CD4+ CD25+hi) were lower in the SLE population in general or only in those with active/flaring SLE and weakly correlated with active disease severity [16–18]. Others groups reported that higher levels of Foxp3 were associated with active disease [3] or with recent corticosteroid therapy [19] or both [20]. Recently two groups reported that Treg proportions were similar to that in controls, but their suppressive capacity is defective in a subset of SLE cases [21, 22].
Herein we demonstrate that CD39-expressing Tregs are nearly absent in lupus patients with minimally active disease, and the absence of CD39 is associated with reduced adenosine-mediated suppression of mitogen-stimulated T cells. Presentation of this defect may serve as a biomarker for presence of disease, and early intervention for bypassing this defect may help to alleviate disease progression.
2. METHODS
2.1. STUDY SUBJECTS
SLE subjects (age range 25–61 years) were either selected from a previously studied cohort [23, 24], restricted to those with minimally active disease, or were recruited from an academic rheumatology clinic (mostly patients with currently active disease) (Table I). All SLE subjects met diagnostic criteria as described by American College of Rheumatology [25]. SLE Disease Activity Index (SLEDAI) was quantified semi-annually or during a flare (Table I). Patients were classified as minimally active or active based on SLEDAI. Twenty four healthy participants (age range 25–55 years) who had no history of autoimmune disease (healthy controls,) and rheumatoid arthritis subjects (age range 37–68 years) were included. Informed consent was obtained from each subject, as approved by the Institutional Review Board of Wake Forest University Health Sciences.
Table I.
SLE patient characterization.
| SLE populationa | SLEDAIb | dsDNA | Cytoxan | Pred | Plaq | MTX | Imuran | Cellcept | NSISc | CD39+d |
|---|---|---|---|---|---|---|---|---|---|---|
| Minimally active | 4 | 11 | N | N | Y | N | N | N | N | 0.5 |
| 0 | 21 | N | Y | Y | N | N | N | N | 1.5 | |
| 0 | 10.2 | N | N | N | N | N | N | N | 1.0 | |
| 4 | 11 | N | N | Y | N | N | N | N | 3.7 | |
| 0 | 12 | N | N | Y | N | N | N | N | 3.8 | |
| 0 | 5 | N | N | N | Y | N | N | Y(24) | 50.5 | |
| Active | 18 | 29.6 | N | Y | Y | N | N | N | Y(12) | 12.0 |
| 5 | 50 | N | Y | Y | N | N | Y | Y(36) | 39.0 | |
| 14 | 11 | N | Y | Y | Y | N | N | Y(08) | 50.1 | |
| 10 | 15.6 | N | N | Y | N | Y | N | Y(48) | 20.0 | |
| 8 | 50 | Y | Y | Y | N | N | N | Y(01) | 22.7 | |
| 6 | 12 | N | Y | Y | N | N | Y | Y(NA) | 70.0 |
SLE patients recruited from our established cohort or from new patients evaluated in clinic
Abbreviations: SLEDAI (SLE disease activity index), Pred (Prednisone), Plaq (Plaquenil), MTX (methotrexate), NSIS (Nonsteroidal immunosuppressant)
NSIS = Cytoxan, methotrexate (MTX), Imuran, or Cellcept; the duration (in months) of NSIS treatment is indicated in parenthesis. NA: Information not available.
% CD39-expressing cells in CD4+ Foxp3+ Treg population
The study was designed by Dr. Khan in consultation with Drs. Loza and O’Rourke. Data were collected and analyzed by Drs. Khan, Loza, Anderson and Wood. All authors have full access to the data and vouch for the accuracy and completeness of the data and analyses. The first draft of manuscript was written by Dr. Loza, and the final content of the manuscript was developed collaboratively by all authors.
2.2. CELL ISOLATION
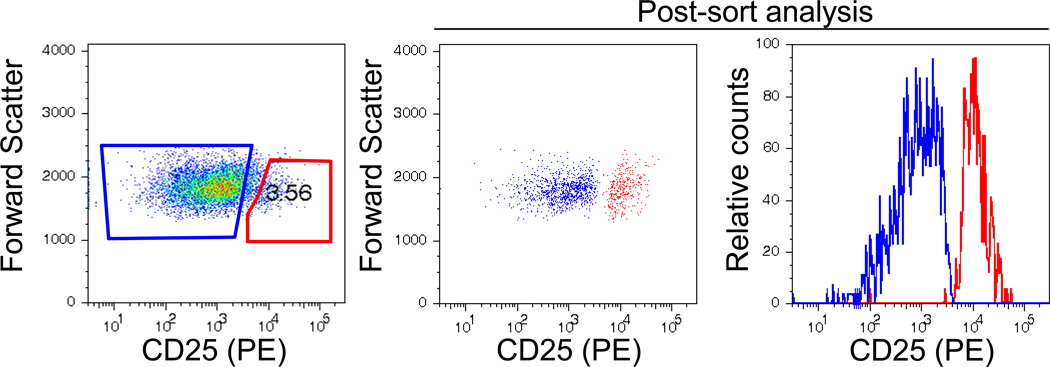
All subjects were required to refrain from intake of caffeine-related products in the morning prior to donation. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation of whole blood obtained from SLE and healthy control donors. CD4+ T cells were isolated and enriched by negative selection from the PBMC using the high gradient magnetic cell separation system Midi MACS (Miltenyi Biotec, Auburn, CA) [26] with ≥ 96% of purified cells expressing CD3 and CD4. CD4+ T cells expressing high levels of CD25 (CD25+high) and having low forward scatter characteristics (FSlow) were purified by flow cytometric sorting of anti-CD25 (PE)-labeled cells. CD25 non-expressor (CD25−) cells were collected simultaneously.
2.3. FLOW CYTOMETRY
Freshly isolated PBMC were surface stained with monoclonal antibody (mAb) to CD3 (Pacific Blue, BioLegend), CD4 (APC-AlexaFluor750, eBioscience), CD25 (PE-Cy5 or APC, Caltag-Invitrogen), CD127 (AlexaFluor647, BioLegend), and either CD39 (PE, AbD Serotec) or CD69 (PE, BioLegend). Intracellular Foxp3 (AlexaFluor488 labeled, clone, BioLegend) was then stained according to the methods provided by the manufacturer. Immunofluorescence was analyzed on FACS Canto II flow cytometer (Becton-Dickinson), and data analyzed using FlowJo software (v7, TreeStar).
2.4. Treg SUPPRESSION ASSAY
Suppressive activity of Tregs were evaluated by co-culturing 0 or 25,000 “responder” CD4+ T cells (CD25−) with 0, 12,500, or 25,000 Tregs (CD25+hi FSlo). Responder and Treg populations obtained simultaneously from the same donor were used for the co-cultures, as described in Methods: Cell Isolation section above. The cells were activated with 500 ng/ml PHA-L and 1 µg/ml anti-CD28 mAb, with or without added 300nM xanthine amine congener (XAC), an adenosine receptor antagonist [27]. On day 4 of culture, 1 µCi tritiated-thymidine deoxyribonucleoside (3H-TdR) was added to each well. After 20-h, cells were frozen at −20°C until harvesting of DNA onto glass microfiber filters. Proliferation of cells was assessed by degree of 3H-TdR incorporation into DNA, measured by β-liquid scintillography.
Treg –mediated suppression of T cell proliferation was calculated as: 100% × [1 − (Tresp:Treg − Treg)/(Tresp − Treg)], with background counts subtracted first from each parameter (Tresp:Treg, cpm from cultures with both responder CD25− T cells and CD25+ Tregs, with or without XAC; Treg, cpm from cultures with CD25+ Tregs only, without XAC; Tresp, cpm from cultures with CD25− responder T cells only, without XAC). Reversal of suppression by XAC was calculated as: 100% × [1 − (% suppression with XAC) / (% suppression without XAC)].
2.5. STATISTICAL ANALYSIS
Normality of data distributions was evaluated using the D’Agostino and Pearson Omnibus normality test. Statistical differences were evaluated using either ANOVA (for comparisons among 3 or more populations) or student’s t-tests (with Welch correction when variances significantly different) for normally distributed data sets. Significance of correlations was evaluated by linear correlation analyses with alternative hypothesis of a non-zero slope. All analyses were performed using GraphPad software (v4).
3. RESULTS
3.1. Tregs AND CD39 EXPRESSION
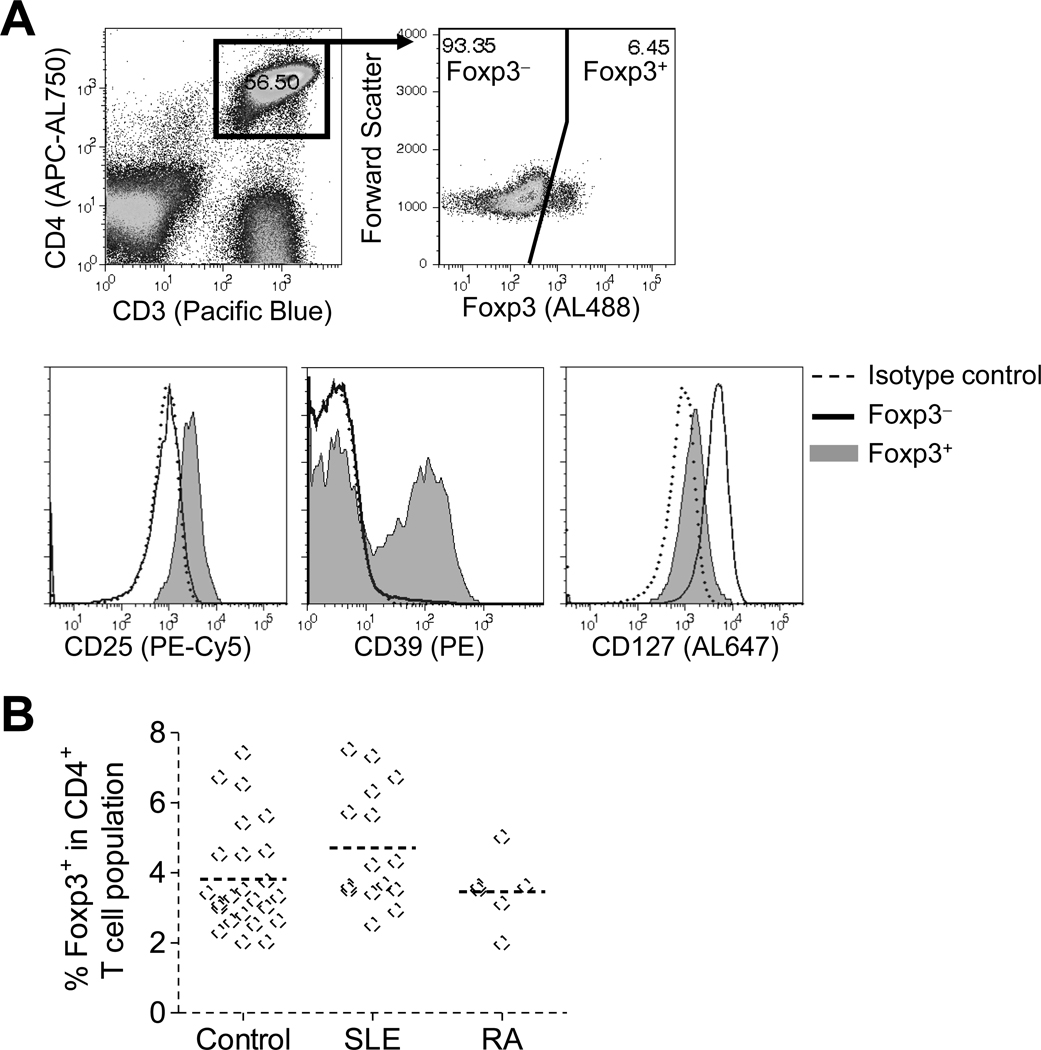
It remains unclear whether the proportions of Tregs in peripheral blood T cells are reduced in SLE subjects. To stringently identify Tregs in freshly isolated PBMC, multi-marker phenotyping was performed using immunofluorescence-flow cytometry analysis. Foxp3 expression was analyzed in gated CD3+ CD4+ T cells (within the lymphocyte population defined by forward and light scatter characteristics) (Fig. 1A top, healthy control; Fig. 2A,B top, SLE patient). Analyzing Foxp3 expression vs. forward scatter (FS) allows clearer resolution of Foxp3− and Foxp3+ populations, as Tregs in fresh PBMC have low forward scatter. To ensure that Tregs were faithfully gated, high expression of CD25 and low expression of CD127 (IL-7Rα) [3] in Foxp3+ compared to Foxp3− cells were simultaneously analyzed (Fig. 1A bottom, healthy control; Fig. 2C, SLE patient). Using this method, the proportion of Foxp3+ cells in CD4+ T cell populations was analyzed in fresh PBL from healthy control, (n=24), SLE (n=15, including patients with active and minimally active disease), and rheumatoid arthritis (n=6) subjects (Fig. 1B). The mean proportion of Foxp3+ cells was similar among the subject populations (3.8 ± 1.5%, controls; 4.7 ± 1.7%, SLE; and 3.5 ± 1.0%, rheumatoid arthritis).
Fig. 1. Treg phenotyping in freshly isolated PBMC.
Freshly isolated PBMC were stained for the indicated surface markers and Foxp3 and then analyzed by flow cytometry. Foxp3 expression was analyzed in gated CD3+ CD4+ lymphocytes, and expression of CD25, CD39, and CD127 analyzed within gated Foxp3− (solid line) and Foxp3+ (shaded region) populations. Isotype control stainings (---). (A) Representative of results of PBMC for a healthy control subject. (B) The percent Foxp3+ cells in the CD4+ T cell population is indicated for each healthy control, SLE, and RA subject tested. P > 0.05 for all comparisons among disease groups.
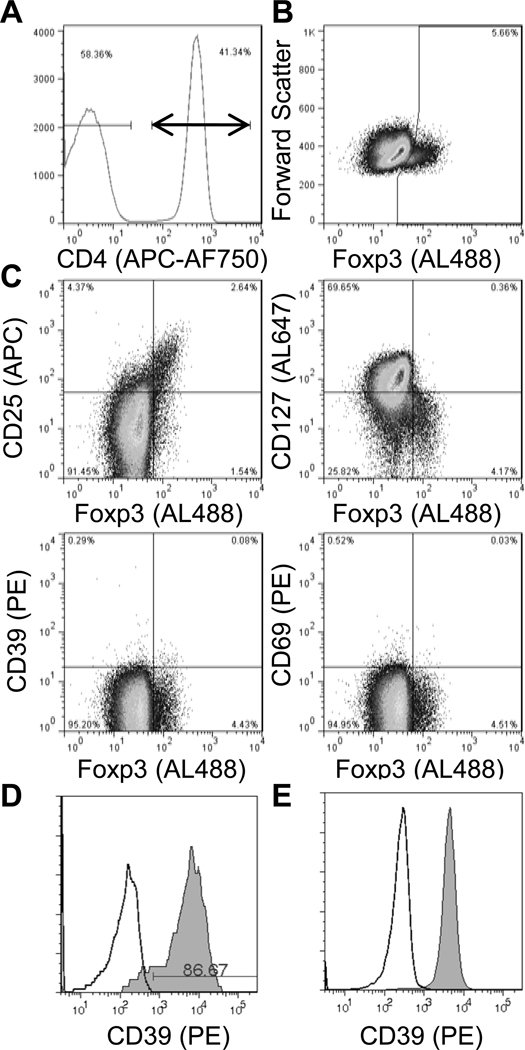
Fig. 2. Lack of CD39 expression in Treg cells from SLE patient.
Freshly isolated PBMC were stained for the indicated surface markers and Foxp3 and then analyzed by flow cytometry. Within gated CD3+ CD4+ lymphocytes (A), Foxp3 expression was analyzed vs. forward scatter (B), or CD25, CD127, CD39, and CD69 (C). Gates were drawn based on isotype control staining (not shown). CD39 expression (shaded; isotype control, open) is shown for gated CD19+ B lymphocytes (D) and monocytes (E).
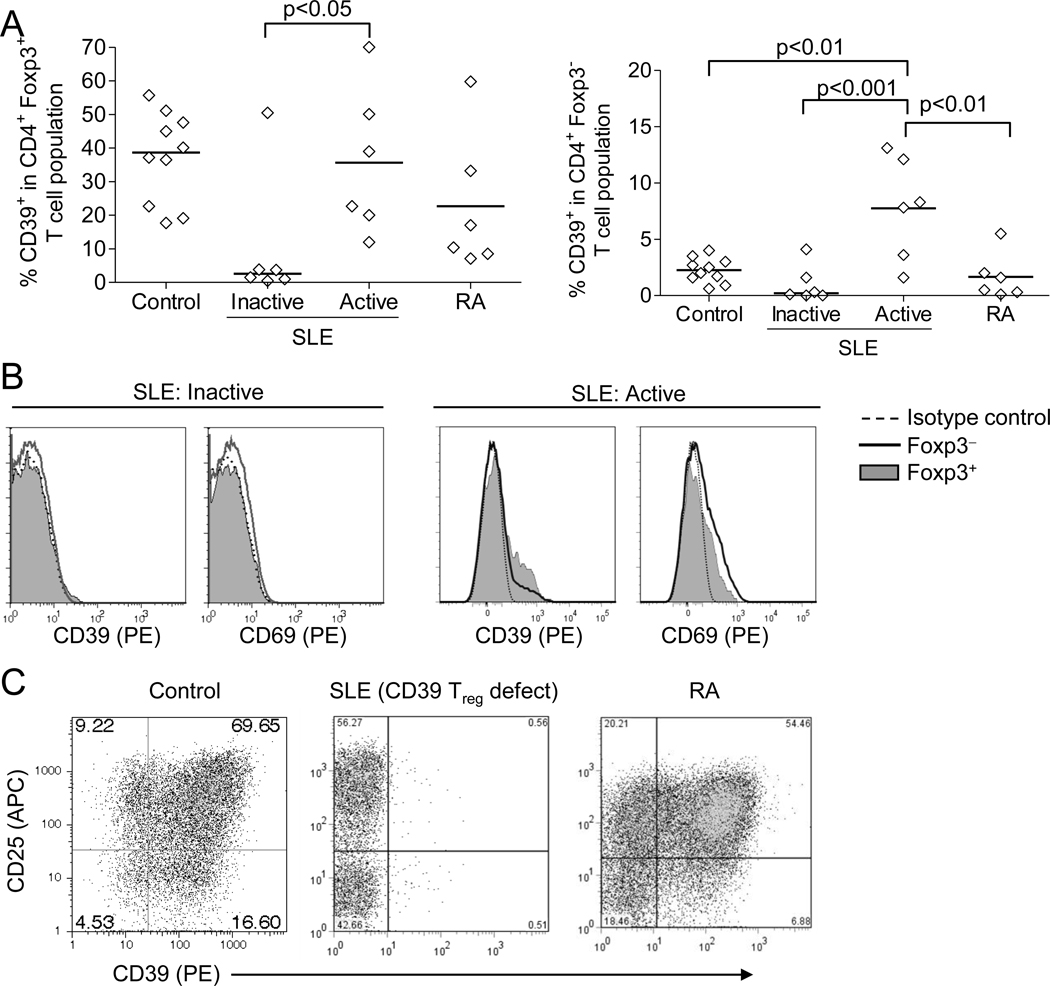
Confirming recent reports, among T cell subsets CD39 expression was present almost exclusively in the Foxp3+ Treg subset from all control subjects tested, with 37 ± 13% of Tregs expressing CD39, compared to 2.2 ± 1.1% in the non-Treg population (Fig. 1A bottom, summarized in Fig. 3A). In contrast, CD39 expression was nearly absent in Tregs from 5 of 6 SLE subjects with minimally active disease who were specifically recruited from our previously established cohort (SLEDAI = 1.3 ± 2.1) (Fig. 2C and Fig. 3B, representative SLE patients with minimally active disease; summarized in Fig. 3A). Importantly, the CD39 defect was observed in the same SLE subjects at several time points (not shown). During the study, additional subjects were recruited from our rheumatology clinic, most of whom had active disease (SLEDAI = 10.0 ± 5.2). Interestingly, this new cohort exhibited CD39 expression within their Treg population to a level similar to that observed in healthy controls (summarized in Fig. 3A, representative patient in Fig. 3B). CD39 expression in Tregs from rheumatoid arthritis subjects was similar to that for control subjects (Fig 3A). The defect in expression of CD39 in the SLE subjects was restricted to Tregs, as normal CD39 expression was observed in nearly 100% of B cells and monocytes from healthy controls and SLE subjects, regardless of T-cell CD39 defect (not shown; representative SLE patient with T-cell CD39 defect, Fig. 2D, E).
Fig. 3. Defective expression of CD39 in inactive SLE.
A. The proportion of CD39+ cells in Tregs (CD4+ Foxp3+ T cells, left) and non-Tregs (CD4+ Foxp3− T cells, right) were evaluated as described in Methods. SLE patients with minimally active disease (SLEDAI<4, ‘inactive’) (and patients with active disease (SLEDAI>4) were separated in the analysis. Significance of differences is shown among groups. B. Representative CD39 and CD69 expression in Foxp3+ and Foxp3− CD4+ T cell populations from SLE subjects with inactive and active disease. C. Induction of CD39 and CD25 expression in sorted CD25− CD4+ T cells from representative healthy control, SLE (demonstrating CD39 Treg defect), and RA subjects, after 6-d culture with CD3+CD28 mAb and IL-2.
T cells from the second, active-disease cohort demonstrated evidence of current T cell responses, indicated by expression of CD69, both in non-Tregs (Foxp3−) and cells with Treg phenotype (Foxp3+ FSlow) (Fig. 3B). It is possible that CD69-expressing Foxp3+ cells may be either activated non-Tregs, activated Tregs, or newly induced Tregs. Because the Foxp3+ gating was restricted to cells demonstrating relatively low forward light scatter profile, these cells are likely not activated non-Tregs, which would present a relatively high forward scatter profile. These results demonstrate that SLE patients, restricted to those with minimally active disease, present with defective expression of CD39 by Tregs.
3.2. INDUCED CD39 EXPRESSION IN T CELLS
CD39 is also reported to be inducible upon CD3-mediated stimulation of T cells. CD39 induction was indeed inducible in sorted CD4+ CD25− T cells from control and rheumatoid arthritis subjects after stimulation via CD3 and CD28, coinciding with induced expression of CD25 (Fig. 3C). However, CD39 induction in CD4+ CD25− T cells from SLE subjects who demonstrated lack of CD39 expression in Tregs was minimal, despite normal induction of CD25 expression. In SLE subjects with normal Treg-CD39 expression, in vivo expression of CD39 was evident on non-Tregs as well, in the absence of ex vivo stimulation, consistent with concomitant expression of CD69 (Fig. 3B). These results indicate that the CD39 defect observed in a subset of SLE subjects with minimally active disease is a pan-T cell defect and not limited to the Treg subset.
3.3. CD39 DEFECT, DISEASE ACTIVITY, AND MEDICATION USAGE
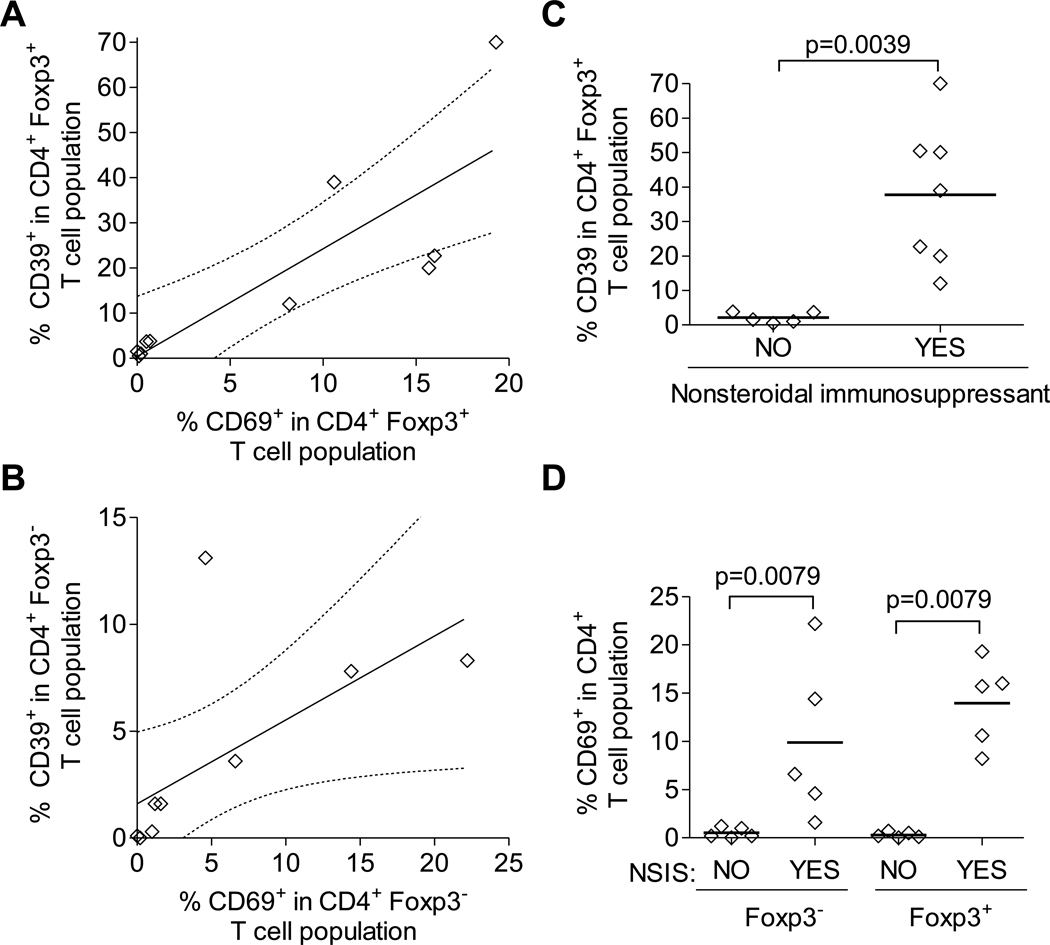
Disease activity and medication usage were explored for correlations with Treg expression of CD39. The mean SLEDAI for the SLE subjects with <4% CD39+ cells in the Treg population (1.6 ± 2.2) was significantly lower (p=0.024) than the mean value for those with >12% (8.0 ± 6.1). However, there was not a significant linear relationship between SLEDAI and % CD39+ cells in the Treg population. Supporting the association of active disease and CD39 expression was the observation that expression of CD39 significantly correlated with CD69 expression, in both Treg (r=0.83, p=0.0028) and non-Treg (r=0.64, p=0.046) (Fig. 4A and B) populations. Associations for current use of SLE-control medications and CD39 expression were also considered. SLE subjects were grouped based on their current usage of nonsteroidal immunosuppressants (NSIS, including cyclophosphamide, azathioprine, mycophenolate mofetil, and methotrexate) to control active disease. Current use of NSIS was strongly associated with expression of CD39 in Tregs (p=0.0039), as well as expression of CD69 in both non-Tregs and Tregs (Fig. 4C and D) Importantly, the only SLE subject with minimally active disease (as assessed by SLEDAI) who demonstrated Treg expression of CD39 was indeed currently taking an NSIS (methotrexate, over previous 2 years). Collectively, these results suggest that either active disease triggers CD39 expression in Tregs (and non-Tregs) or that NSISs are capable of reversing the defect in CD39 expression by both Tregs and non-Tregs.
Fig. 4. Association of CD39 expression with T cell activation and medication usage.
Correlation of proportion of CD39+ cells with CD69+ cells in (A) CD4+ Foxp3+ Treg populations and (B) CD4+ Foxp3− non-Treg populations among SLE patients (active and minimally active disease). Expression of CD39 (C) and CD69 (D) in Foxp3+ Tregs (C,D) and Foxp3− non-Tregs from SLE patients (active and minimally active disease), stratified by usage of nonsteroidal immunosuppressants (NSIS).
3.4. CD39 DEFECT AND Treg-MEDIATED SUPPRESSION
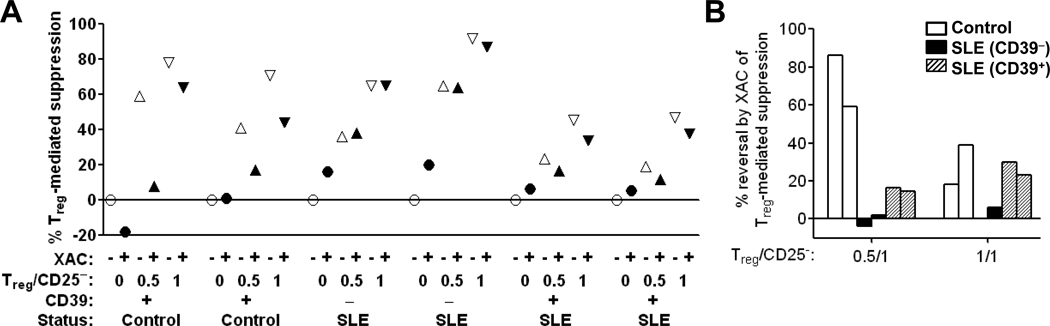
The role of adenosine in Treg-mediated suppression in healthy controls and the biological significance of the defect in Treg CD39 expression in the subset of SLE subjects was demonstrated by standard “Treg suppression” assays. Purified CD4+ T cells were cell sorted for CD25+hi cells (Tregs) and CD25− cells (responder T cells) (Fig. 5) and then co-cultured in the presence or absence of XAC, a pan-adenosine receptor antagonist [28] (Fig. 6). Addition of Tregs suppressed PHA+CD28-stimulated proliferation of responder cells in healthy subjects (n=2) (Fig. 6A). A similar level of Treg-mediated suppression was observed in SLE subjects presenting the CD39 defect (n=2, minimally active disease) (Fig. 6A). In healthy subjects, blocking adenosine receptors with XAC returned proliferation almost to original levels or reversed suppression by 20 – 40% when Tregs were at a 0.5:1 and 1:1 ratio, respectively, with responder cells (Fig. 6A, filled vs. open symbols; summarized in Fig. 6B). Importantly, in the SLE subjects with the CD39 defect, adenosine receptor antagonism did not reverse the residual Treg-mediated suppression (Fig. 6A, filled vs. open symbols; summarized in Fig. 6B). Suppression assays were also performed in SLE subjects without the CD39 defect (n = 2, active disease). Treg-mediated suppression was lower in these subjects compared to SLE subjects with the CD39 defect and healthy subjects (Fig. 6A). In SLE subjects without the CD39 defect, XAC reversed the observed Treg-mediated suppression to a lesser extent (15 – 16% reversal at 0.5:1 Treg: CD25− ratio) and similar extent (23 – 30% reversal at 0.5:1 Treg: CD25− ratio) compared to healthy controls (Fig. 6A, filled vs. open symbols; summarized in Fig. 6B). These results confirm the hypothesized role of adenosine generation in human Treg-mediated suppression in healthy subjects and that adenosine-mediated suppression by Tregs is defective in SLE patients presenting with the CD39 defect. However, because of the limited sample sizes, conclusions cannot be drawn regarding differences among controls and SLE patients with and without the CD39 defect.
Fig. 5. Treg cell sorting.
CD4+ T cells were isolated from PBMC by magnetic cell separation, and then labeled with CD25-PE mAb. CD25+ and CD25− lymphocyte populations were sorted by FACS. (A) Pre-sort gates for CD25+ (red, Treg) and CD25− (blue, Tresp) populations are shown. (B) Post-sort purity analysis of CD25− (blue) and CD25+ (red) sorted populations. The post-sort CD25+hi population had 99.1% purity. Representative results from 1 of 6 subjects.
Fig. 6. Adenosine-dependent Treg-mediated suppression of T cell proliferation.
Purified CD4+ CD25+hi (Treg) and CD4+ CD25− (responder) T cells (both populations obtained simultaneously from the same donor) were co-cultured 5-d at indicated ratio (Treg/CD25−) in the presence of PHA and CD28 mAb, with (filled symbols) or without (open symbols) XAC, as detailed in Methods. 3H-TdR was added for the final 20-h of culture, and incorporation of 3H-TdR into DNA of proliferating cells assessed by β-scintillography of DNA obtained from cellular lysates. Normal (+) or defective (−) Treg expression of CD39 in SLE subjects is indicated. (A) Percent suppression of 3H-TdR incorporation in cultures of CD4+ CD25− responder cells with Tregs relative to that observed CD4+ CD25− responder cells alone without added Tregs or XAC, calculated as described in Methods. (B) % reversal of Treg-mediated suppression in cultures with XAC, relative to those without XAC, calculated as described in Methods. Results for 2 healthy control subjects (white bars) and 2 SLE subjects demonstrating the CD39 expression defect (black bars), and 2 SLE subjects without the CD39 defect (hatched bars) are shown.
4. DISCUSSION
We demonstrate in current study that T-cell expression of the ATP/ADP-metabolizing ectoenzyme CD39 is defective in a subset of lupus subjects, restricted to participants with minimally active disease (based on SLEDAI and, objectively, on lack of CD69 expression) and not currently using nonsteroidal immunosuppressants to control active disease. This defect is apparent in freshly isolated Tregs. Importantly, non-regulatory T cells are also defective in their capacity to upregulate expression of CD39 upon CD3-mediated stimulation specifically in this population. CD39 expression was normal in all SLE participants who were currently taking NSIS, including one of whom had a reported SLEDAI of zero. In addition to being an indicator of minimally active disease (in the absence of NSIS use), the CD39 defect demonstrated a functional impact on T cell regulation. Unlike in T cells obtained from healthy controls, blocking the adenosine receptor failed to reverse observed suppression. An interpretation is that adenosine was not available to mediate suppression in SLE subjects with the CD39 defect. Because of the limited sample size of the Treg suppression assays, whether there are absolute differences in extent of Treg-mediated suppression among the groups cannot be clearly established from these experiments.
Considerable CD39/adenosine-independent suppression was still observed in the SLE patients presenting with the CD39 T-cell defect. Despite substantial reversal of suppression upon adenosine receptor antagonism in healthy controls, residual suppression was still observed though not to the degree observed in the SLE patients with the CD39 defect. These results suggest that adenosine contributes to, but is not absolutely sufficient, for efficient Treg-mediated suppression. Numerous mechanisms of suppression have been proposed for Tregs, including: secreted or membrane-bound TGF-β and IL-10; secreted IL-35 and PGE2; and competition for APC engagement (CTLA-4 and LAG-3 on Tregs competing for CD80/CD86 and MHC class II, respectively, on antigen-presenting cells). Whether these other mechanisms of suppression are defective in SLE, their overlap with the T-cell CD39 defect, and impact of SLE therapeutics on these mechanisms will be important for the understanding of T- cell and Treg functions in SLE patients.
Currently, disease activity in SLE is routinely assessed using cumbersome composite disease activity indices: SLE Disease Activity Index (SLEDAI), Systemic Lupus Activity Measurement (SLAM), European Consensus Lupus Activity Measure (ECLAM), and British Isles Lupus Assessment Group (BILAG). To add to this inherent complexity of the disease, the prompt diagnosis and effective management of this disease remains a great challenge to clinicians. T cell subsets are considered critical in the pathogenesis of this disease [22, 29–34]. Thus our discovery of a defect in CD39 expression on Tregs represents a viable potential as a predictor/biomarker of human lupus and objective indicator of disease activity.
It seems paradoxical that CD39 expression would be suppressed in inactive disease but not in active disease. However, whether CD39 expression (on both Tregs and non-Tregs) is induced as a result of the flare in disease activity or as a result of NSIS therapy needs to be resolved. Based on the case study of the one SLE subject who had minimally active disease and taking NSIS, but who did not have the CD39 defect, it is possible that NSIS therapy rather than active disease per se is associated with normal CD39 expression. Longitudinal studies for assessing changes in CD39 expression in the same subject from inactive disease to the start of flare/active disease and during stabilization of flares with medications will be needed to answer this important question. If NSIS do induce expression of CD39, this would provide a mechanism for these drugs to suppress uncontrolled T-cell responses by returning normal suppressive capacity of Tregs, as well as the potential suppressive potential of CD3-stimulated CD39 expression on non-regulatory T cells (e.g., autocrine suppression).
Alternatively, inducible CD39 expression, on Tregs or T cells in general, during the active phase of disease may contribute to the pathophysiology of SLE, with downmodulation during inactive disease being an as-yet appreciated negative feedback mechanism. In support of this possibility, a murine model has been reported that CD39 can have pro-tumorigenic effects by scavenging extracellular ATP, thus relieving anti-proliferative effects of ATP on tumor cells [35]. Associations of increased CD39+ Tregs in human cancer patients have also been reported [36]. Up-regulation of CD39 expression is a normal physiological response to antigenic stimulation, being observed in T cells from healthy controls. Indeed, Tregs in SLE patients without the CD39 defect also had concomitant expression of the activation antigen CD69. However, T cells from SLE patients with the CD39 defect failed to similarly upregulate CD39 expression upon CD3-mediated stimulation despite upregulation of CD25 expression, suggesting that factors beyond simple activation of T cells would be required to restore CD39 expression.
Because the CD39 defect was presented by all SLE subjects with minimally active disease not currently using NSISs, the CD39 defect may be useful as a biomarker for early detection of disease, before onset of clinical symptoms (active disease). Family-based studies for familial aggregation of the defect and prospective follow-up of pre-pubescent family members (comparing those who demonstrate the defect in the absence of clinically apparent disease and those who do not) would confirm whether this defect is a primary mediator of disease onset and whether it is a useful predictor of disease onset. Novel interventions (other than NSISs) capable of restoring CD39 expression, but with more limited deleterious side effects, could be proven extremely valuable should the CD39 defect be determined to be a primary effector of disease progression.
CONCLUSIONS
In summary, we have demonstrated that CD39 expression is minimal in SLE patients with mild disease. Our results of suppression assay are indicative of the critical involvement of adenosine-dependent Treg-mediated suppression. Thus, the CD39-defect would likely to be a causative in disease progression, for which pharmacologic interventions may be developed to help prevent or delay the clinical pathology associated with SLE.
HIGHLIGHTS.
>Adenosinergic pathway mediated by CD39 is critical in regulatory T (Tregs) cell function. >We tested whether decreased numbers of Tregs, and specifically CD39-expressing Tregs, are associated with human lupus. > CD39 defect observed in SLE with minimally active disease is a pan-T cell defect and not limited to the Treg subset. > The defect in CD39 expression by both Tregs and non-Tregs is not observed in patients with non-steroidal therapy. > The CD39 defect may be useful as a biomarker for early detection of disease, before onset of clinical symptoms.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Irene Olorenshaw, BSN, RN for recruiting the patients included in the performance of this study, and to all cohort participants for making this study possible. We also like to thank Dr. Raymond Penn for the critical review of the manuscript.
This work is supported by National Institutes of Health Grants R01 AR 39501 and R21 AI070897 (IUK) and the General Clinical Research Center of the Wake Forest University School of Medicine Grant M01-RR07122 (IUK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflict of interest.
REFERENCES
- 1.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 2.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- 5.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 8.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 10.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 12.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 13.Sitkovsky MV, Ohta A. The 'danger' sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 15.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Wang LC, Lin YT, Yang YH, Lin DT, Chiang BL. Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology. 2006;117:280–286. doi: 10.1111/j.1365-2567.2005.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellor-Pita S, Citores MJ, Castejon R, Tutor-Ureta P, Yebra-Bango M, Andreu JL, Vargas JA. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:553–554. doi: 10.1136/ard.2005.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Nochy D, Debre P, Piette JC, Gorochov G. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 19.Suarez A, Lopez P, Gomez J, Gutierrez C. Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis. 2006;65:1512–1517. doi: 10.1136/ard.2005.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 21.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, Cubillas-Tejeda AC, Gonzalez-Amaro R. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 23.Kammer GM. High prevalence of T cell type I protein kinase A deficiency in systemic lupus erythematosus. Arthritis Rheum. 1999;42:1458–1465. doi: 10.1002/1529-0131(199907)42:7<1458::AID-ANR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Laxminarayana D, Khan IU, Mishra N, Olorenshaw I, Tasken K, Kammer GM. Diminished levels of protein kinase A RI alpha and RI beta transcripts and proteins in systemic lupus erythematosus T lymphocytes. J Immunol. 1999;162:5639–5648. [PubMed] [Google Scholar]
- 25.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 26.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 27.Antonysamy MA, Moticka EJ, Ramkumar V. Adenosine acts as an endogenous modulator of IL-2-dependent proliferation of cytotoxic T lymphocytes. J Immunol. 1995;155:2813–2821. [PubMed] [Google Scholar]
- 28.Jacobson KA, Ukena D, Kirk KL, Daly JW. [3H]xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: an antagonist radioligand for adenosine receptors. Proc Natl Acad Sci U S A. 1986;83:4089–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bermas BL, Petri M, Goldman D, Mittleman B, Miller MW, Stocks NI, Via CS, Shearer GM. T helper cell dysfunction in systemic lupus erythematosus (SLE): relation to disease activity. J Clin Immunol. 1994;14:169–177. doi: 10.1007/BF01533366. [DOI] [PubMed] [Google Scholar]
- 30.Crow MK. Enhancement of the impaired autologous mixed leukocyte reaction in patients with systemic lupus erythematosus. J Clin Invest. 1985;76:807–815. doi: 10.1172/JCI112038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan IU, Laxminarayana D, Kammer GM. Protein kinase A RI beta subunit deficiency in lupus T lymphocytes: bypassing a block in RI beta translation reconstitutes protein kinase A activity and augments IL-2 production. J Immunol. 2001;166:7600–7605. doi: 10.4049/jimmunol.166.12.7600. [DOI] [PubMed] [Google Scholar]
- 32.Tsokos GC, Kammer GM. Molecular aberrations in human systemic lupus erythematosus. Mol Med Today. 2000;6:418–424. doi: 10.1016/s1357-4310(00)01798-6. [DOI] [PubMed] [Google Scholar]
- 33.Via CS, Tsokos GC, Bermas B, Clerici M, Shearer GM. T cell-antigen-presenting cell interactions in human systemic lupus erythematosus. Evidence for heterogeneous expression of multiple defects. J Immunol. 1993;151:3914–3922. [PubMed] [Google Scholar]
- 34.Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, Fiehn C, Ho AD, Lorenz HM. Reduced CD4+,CD25− T cell sensitivity to the suppressive function of CD4+,CD25high,CD127 −/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 35.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 Directly Promotes Tumor Cell Growth by Scavenging Extracellular Adenosine Triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]