Abstract
Context:
This mini-review describes the toxic effects of vanadium pentoxide inhalation principally in the workplace and associated complications with breathing and respiration. Although there are some material safety data sheets available detailing the handling, hazards and toxicity of vanadium pentoxide, there are only two reviews listed in PubMed detailing its toxicity.
Aim:
To collate information on the consequences of occupational inhalation exposure of vanadium pentoxide on physiological function and wellbeing.
Materials and Methods:
The criteria used in the current mini-review for selecting articles were adopted from proposed criteria in The International Classification of Functioning, Disability and Health. Articles were classified from an acute and chronic exposure and toxicity thrust.
Results:
The lungs are the principal route through which vanadium pentoxide enters the body. It can injure the lungs and bronchial airways possibly involving acute chemical pneumonotis, pulmonary edema and/or acute tracheobronchitis. It may adversely influence cardiac autonomic function. It stimulates the secretion of cytokines and chemokines by hepatocytes and disrupts mitochondria function. It disrupts the permeability of the epithelium and promotes access of inflammatory mediators to the underlying neuronal tissue causing injury and neuronal death. When renal brush border membrane vesicles are exposed to vanadium pentoxide, there is a time-dependent inhibition of citrate uptake and Na+ K+ ATPase in the membrane possibly contributing to nephrotoxicity. Exposure results in necrosis of spermatogonium, spermatocytes and Sertoli cells contributing to male infertility.
Conclusion:
Vanadium pentoxide certainly has adverse effects on the health and the well-being and measures need to be taken to prevent hazardous exposure of the like.
Keywords: Breathing, dust, exposure, fumes, occupation, respiration, vanadium pentoxide
INTRODUCTION
This mini-review describes the toxic effects of vanadium pentoxide inhalation principally in the workplace and associated complications with breathing and respiration.[1] Vanadium is a by-product of oil-burning furnaces when vanadium pentoxide (MR 181.88) is deposited in the flues. It is an odorless gas.[2] Inhalation is the principal route of entry into the body and may result acutely in severe pneumonitis with associated mucus membrane irritation and gastrointestinal disturbances. Ambient vanadium pentoxide dust produces irritation of the eyes, nose and throat.[3] Over long periods, inhalation may potentiate chronic bronchitis, eczematous skin lesions, fine tremors of the extremities and greenish discoloration of the tongue.[1] As it has a rapid renal clearance, it may be monitored in urine specimens to determine exposure to vanadium pentoxide (American Conference of Governmental Industrial Hygienists, ACGIH and British Education Index, BEI) 50 μg.g-1 creatinine for an end-of-shift, end-of-workweek sample.[1] Most absorbed vanadium is excreted in the urine within one day after a long-term moderate exposure to vanadium dust.[4] During its measurement vanadium oxide is sampled onto a PVC filter in a Higgins and Dewell cyclone for subsequent analysis using X-ray powder diffraction.[1] The workplace exposure limit for vanadium pentoxide is according to the Health and Safety Executive (HSE), 0.05 mg.m-3/8h.[1] MSDS[2] details airborne exposure limits of 0.5 mg.m-3 (ceiling) for vanadium respirable dust and 0.1 mg.m-3 (ceiling) for vanadium fumes.
Studies in laboratory rats have shown that one acute inhalation study available reported an LC67 of 1440 mg/m3 following a 1-h exposure of rats to vanadium pentoxide dust.[5] Oral studies in rats and mice demonstrate greater toxicity of vanadium as oxidation state increases. The lungs are a significant site of entry of vanadium in the case of community exposure. The distribution pattern of particles and the solubility of vanadium compounds, as well as alveolar and mucociliar clearance, are important factors that determine the rate of absorption in the respiratory tract.[6] Vanadium accumulates in the human lung with age, reaching approximately 6.5 μg/g in the over-65 year's age group.[7] The metal is deposited in the lung in relatively insoluble forms. Absorption of cationic vanadium is low, not exceeding 0.1-1%.[8] Skin is probably a minor route of absorption in man.[9] Vanadium lung exposure studies in rats appear to reach a steady state in low exposure groups of 0.5 mg/m3.[10] The retention rate of vanadium in rats aged 18 months was 13-15%.[10]
Absorbed vanadium is transported mainly in the plasma, bound to transferrin. The average value for the distribution of blood vanadium between plasma and cells in rats after an intravenous injection of 0.9-30 μg vanadium per kg was found to be 9: 1.[11] Vanadium is widely distributed in body tissues; principle organs of vanadium retention are kidneys, liver, testicles, spleen and bones. A major fraction of vanadium from cellular vanadium was found retained in nuclei.[12] In pregnant rats the injected vanadium was found in the fetus.[13]
Vanadium is excreted mainly in the urine, but also in the faces. Bile is probably not an important pathway for excretion into the faces, but the existence of alternative routes for excretion into the gut (salivary excretion or direct transfer across the intestinal wall) has been suggested.[11] When 4.5-9.0 mg vanadium as diammonium oxytetravanadate was fed daily to 16 elderly persons, urinary excretion, although quite variable, amounted to a mean of 5.2% of the amount ingested.[14] A study of pre-incubation of renal brush border membrane vesicles with vanadium pentoxide demonstrated a time-dependent inhibition of citrate uptake thereof, contributing to the nephrotoxicity observed in vanadium exposure.[15]
Studies describing the workplace exposure to and emission sources of vanadium pentoxide are limited. Fuel-oil ash is a significant source of exposure to boilermakers.[16] During coal combustion, flue gases may contain dangerous levels of vanadium pentoxide.[17] In kilns, especially with inadequate ventilation, exposure to vanadium can be critical.[18] In industrial concerns, airborne monitoring is principally designed to detect vanadium rather than vanadium pentoxide.[19] The US National Institute of Occupational Safety and Health[20] and the US Occupational Safety and Health Administration[21] have published methods that are suitable for measuring vanadium and vanadium compounds in workplace air. Both are generic methods for metals and metalloids in which samples are collected by drawing air through a membrane filter mounted in a cassette-type filter holder, dissolved in acid on a hotplate and analyzed by inductively coupled plasma - atomic emission spectrometry. For both methods, the lower limit of the working range is approximately 0.005 mg/m3 for a 500 L air sample, although these methods are not widely available.[19]
The biological monitoring of vanadium is widely used. Urine testing, in preference for plasma testing, assumes that vanadium is excreted with a half-life of 15-40h.[22] Pre- and post-shift urine vanadium levels measured at the beginning and the end of a working week will, therefore, give a measure of daily absorption and accumulated dose from exposures over the preceding days.[19]
Environmental monitoring of vanadium employs various methods for the analysis of air samples of vanadium in air, surface waters and biota. For example, flameless AAS[23] gives a detection limit of 1 ng/ml in air, corresponding to an absolute sensitivity of 0.1 ng vanadium. ICP-AES has a working range of 5-2000 μg/m3 for a 500-litre air sample.[20]
Although there are some material safety data sheets available detailing the handling, hazards and toxicity of vanadium pentoxide,[2,24] there are only two reviews listed in PubMed detailing its toxicity.[25,26] The aim of this article therefore was to collate information on the consequences of occupational inhalation exposure of vanadium pentoxide on physiological function and wellbeing. An attempt to classify the like according to functionality of certain selected organ systems was decided.
MATERIALS AND METHODS
The criteria used in the current mini-review for selecting articles to be included were both theoretically and practically motivated and adopted from proposed criteria in The International Classification of Functioning, Disability and Health - ICF.[27] These criteria were as follows:
Articles were chosen only with internationally recognized impact factors greater than 0.10.
Articles were chosen based upon the impact of lifestyle, stress and/or environmental factor/s predisposing vanadium pentoxide exposure.
Criteria for selection of literature used included yes-no responses to: the appropriateness of methodology; adequacy of subject numbers; specificity of sex and/or age of subjects; and statistically significant response rates to survey questionnaires. Due consideration was given to a comparative effect between acute and chronic exposure to vanadium pentoxide. Additionally, it was noted whether the studies occurred in laboratory animal or human subjects.
The timeframe used was principally 1990-2007 inclusive, although articles of extreme importance from earlier decades were used where appropriate.
A multi-factorial overview of the factors eschewed concerning vanadium pentoxide exposure was elucidated. It was presumed that collective articles detailing known factors of usage were not necessarily correlated with functionality and health.
Compilation of materials for the mini-review started with published literature or easily accessible academic research.
The articles were accessible from on-line sources - PubMed and Medline (abstracts of journal articles); Cochrane (reviews of clinical evidence); Clinical Trials (reports); Dissertation.com (summaries of journal articles); and Occupational Health and Safety Information Services (full-text guidance material).
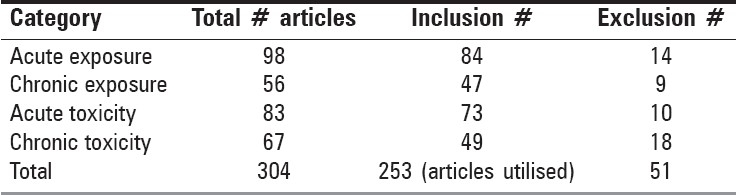
Articles were categorized according to their focus on acute or chronic exposure and toxicity, respectively [Table 1].
Table 1.
Selection results for articles of vanadium pentoxide exposure and toxicity
RESULTS
From the data elucidated in Table 1, the differences between total articles chosen in the acute exposure versus other categories, was 42.9% (chronic exposure), 15.3% (acute toxicity) and 31.6% (chronic toxicity). Using similar determinations, included articles were 44.1, 13.1 and 41.7%, respectively. The excluded articles were judged via chronic toxicity comparison with the other categories giving differences of 22.2% (acute exposure), 50% (chronic exposure) and 44.4% (acute toxicity).
Lungs: Vanadium pentoxide is regarded as a less soluble form of vanadium and is therefore eliminated from the lungs at a slow rate.[28] Inhalation of vanadium pentoxide can injure the lungs and bronchial airways,[2] possibly involving acute chemical pneumonotis, pulmonary edema and/or acute tracheobronchitis.[25] Symptoms include irritation and inflammation of the mucus membranes, nasal passages and pharynx.[2] Clinical complications include a persistent cough, shortness of breath, bronchiolar constriction, tightness of the chest and a pseudo-asthmatic inflammation.[2] In a study of 40 plant workers previously free of lung disease and exposed to vanadium pentoxide, 12 had bronchial hyper-reactivity and symptoms of asthma.[29] Vanadate acts directly on human bronchial smooth muscle promoting the release of Ca2+ from intracellular store via the production of inositol phosphate second messengers and inhibition of Ca2+-ATPase and causing spasms.[30] Occasionally pulmonary edema and/or pneumonia may result with fatal consequences.[2] First aid measures following inhalation include removing the patient into fresh air and applying artificial respiration if breathing has expired. Oxygen is needed if breathing is labored and it is essential to seek medical attention.[2] Vanadium pentoxide dust may be a potential mutagen via induced chromosomal aberrations in man[31] and hamsters.[32] Alveolar/bronchiolar neoplasms developed in male rats exposed to 0.5 and 2 mg.m-13 vanadium pentoxide, with only a marginal increase thereof in female rats exposed to 0.5 mg.m-13.[33] There were associated increase in inflammation, fibrosis and alveolar and bronchiolar hyperplasia/metaplasia and squamous metaplasia in both male and female rats.[33] In an investigation of cynomolgus monkeys exposed to vanadium pentoxide dust for six hours per day, five days per week for 26 weeks, it demonstrated that airway obstruction accompanied a significant influx of inflammatory cells into alveolar tissue.[34] In an earlier study, it was suggested that vanadium-induced pulmonary inflammatory changes involving polymorphonuclear leukocytes may play an important role in air-flow limitation.[35] Exposure to vanadium may cause a metal fume fever-like syndrome associated with neutrophilic alveolitis associated with marked neutrophilia.[36] Respiratory tract inflammation following inhalation of vanadium particles is characterized by abundant neurophilia initiated by alveolar macrophages and release of proinflammatory cytokines.[37] Short-term, repeated inhalation of occupationally relevant levels of vanadium by rats results in pulmonary immunocompetence via cytokine production and pulmonary macrophage induction.[38] Wang et al.,[39] describes the mechanism of multiple reactive oxygen species induced by vanadium involves activation of an NADPH oxidase complex and the mitochondrial electron transport chain, with hydrogen peroxide playing a major role in lung inflammation and apoptosis. There may be a pathological pattern within the lung which may be associated with the pattern and/or extent of vanadium deposition.[40] Its cumulative effect in lung tissue possibly contributes to the development lung cancer. Indeed, in lung tissue excised from post-mortem investigations, the mean vanadium concentrations of 1.36 ± 0.08 (sd) (1990s) were higher than 1.04 ± 0.05 in the 1960s.[41] The free-radical redox cycling of vanadium in the rat lung involving vanadium (V) undergoing one-electron redox cycling in the rat lung biomembranes and re-oxidation of vanadium (IV) initiating lipid peroxidation, possibly contribute to pulmonary toxicity.[42]
Circulatory system: Chronically, exposure to airborne metals including vanadium may result in alterations in cardiac autonomic function.[43] Vanadium induces thrombocytosis and may be associated with various thromboembolic diseases.[44] Acute studies of vanadium pentoxide inhalation on the heart in experimental animals revealed that there was myocardial vascular congestion was observed, with focal perivascular haemorrhages.[6] Studies in humans has revealed palpitation of the heart, high incidence of extrasystoles, changes in the blood picture (anemia, leukopenia, punctatebasophilia of the erythrocytes) and reduced levels of cholesterol in the blood.[45] Limited studies have suggested a positive correlation between vanadium inhalation in urban air and mortality from cardiac failure, despite an absence of lifestyle determination.[46]
Liver: Acutely, vanadium is a potent inhibitor of many enzymes, while it stimulates adenylate cyclase. It has been shown to inhibit cholesterol biosynthesis and lower plasma cholesterol levels. Vanadium can also directly influence glucose metabolism in vitro and may play a role in its regulation. Lipid peroxidation of rat liver microsomes and mitochondria was induced by sulphite and accelerated by the presence of vanadium compounds.[6] Severe acute exposure (tens of mg/m3) is responsible for systemic effects. Most frequent findings in animal experiments were in the liver, kidneys, gonads and the nervous, hematological and cardiovascular systems.[45] Chronically, histopathological changes observed in the liver following the higher level of inhalation exposure (27 μg/m3 for 70 days) included central vein congestion with scattered small hemorrhages and granular degeneration of hepatocytes.[6]
Vanadium concentrations of 10 μg/g dry weight (dw) in the liver is associated with mortality in ducks acutely exposed to sodium metavanadate.[47] Chronic exposure to increasing dietary concentrations of sodium metavanadate (38.5-2651 ppm) over 67 days resulted in vanadium accumulation in the liver of 25.2 μg/g dwt.[47] It is unlikely though that such concentrations would have been achieved via inhalation. Vanadium pentoxide exposure stimulates the secretion of cytokines and chemokines by hepatocytes and associated hepatotoxicity.[48] Significant amounts of vanadate accumulate in the intermembrane space of the liver mitochondria and the detoxification mechanism of reduction of vanadate is inadequate enough to prevent damaging actions on hepatic mitochondria.[49] In an old study, vanadium pentoxide retarded the development of vascular pathomorphology, supported cholesterol metabolism and activated oxidoreductive processes.[50]
CNS: Severe acute exposure to vanadium pentoxide has major patho-physioloogical manifestations on the nervous system.[6] Inhalation thereof produces a time-dependent loss of dendritic spines, necrotic-like cell death and considerable alterations of the hippocampus CA1 neurophile, all associated with spatial memory impairment.[51] Additionally, there is a decrease in the number of tyrosine hydroxylase immunreactive neurones in the substatia nigra pars compacta.[52] Within the ependymal epithelium, cilia loss, cell sloughing and cell layer detachment occur after vanadium pentoxide inhalation.[53] The damage results in disrupted permeability of the epithelium and promotes access of inflammatory mediators to the underlying neuronal tissue causing injury and neuronal death.[53] In humans, severe chronic exposure results in general symptomatology including nervous disturbances, neurasthenic or vegetative symptoms.[6]
Kidneys: Severe acute exposure (tens of mg/m3) is responsible for aberrations in renal function.[6] Vanadium concentrations of 25 μg/g dwt. in the kidneys are associated with mortality in ducks acutely exposed to sodium metavanadate.[47] Chronic exposure to increasing dietary concentrations of sodium metavanadate (38.5-2651 ppm) over 67 days resulted in vanadium accumulation in the kidneys of 13.6 μg/g dw.[47] It is unlikely though that such concentrations would have been achieved via inhalation. When renal brush border membrane vesicles are exposed to vanadium pentoxide, there is a time-dependent inhibition of citrate uptake in the membrane possibly contributing to nephrotoxicity.[15] In studies on dog and human kidney enzymes, vanadium may inhibit the Na+K+ATPase, a process independent of enzyme protein concentrations.[54] The authors suggested that vanadium inhibits the ATPase at the site activated by Na+ and ATP protects the enzyme by either binding vanadium or by competing for a mutual receptor on the enzyme. Chronically in experimental animals, histopathological changes observed in the kidneys following the higher level of inhalation exposure (27 μg/m3 for 70 days) included marked granular degeneration of the epithelium of the convoluted tubules. Dose-dependent histological changes, included corticomedullary microhaemorrhagic foci in the kidneys.[55]
Chronically, absorbed vanadium in either pentavalent or tetravalent states is distributed mainly to the bone (around 10-25% of the administered dose three days after administration) and to a lesser extent to the kidney (about 4%).[56] Distribution studies in which rats received a total of approximately 224 and 415 mg vanadium pentoxide/kg in drinking-water over a period of 1 and 2 months indicated that the vanadium content (assessed in 13 specific tissues) was greatest in the kidneys, spleen, tibia and testes.[57]
Testicles: Chronic ingestion of vanadium may have significant consequences for infertility by damaging spermatogenesis. Studies in mice have demonstrated that inhalation of vanadium pentoxide results in necrosis of spermatogonium, spermatocytes and Sertoli cells.[58] Vanadium accumulates in the testes and attenuates the percentage of gamma-tubulin in all analysed testicular cells, suggesting changes in microtubules used in cell division.[59] Vanadium also induces DNA damage.[60] Leydig cells may not be affected by vanadium pentoxide as testosterone levels remain unchanged.[61]
DISCUSSION
This mini-review has contributed to a brief synthesis of the literature which is currently rather scattered in nature into a compact format. Its main thrust was from both an acute and chronic exposure and toxicity angle. Vanadium pentoxide has adverse effects on health and well-being and measures need to be taken to prevent hazardous exposure of the like. Medical monitoring of workers exposed to the dust or fumes; workplace monitoring and measurement of ambient concentrations; dealing with contaminated attire and establishing personal hygienic procedures; dealing with emergency spills of dust; enforcing protocols for emergencies and hazardous waste management; and the use of inspected respiratory and personal protective equipment, are all essential in reducing toxic exposure.
Vanadium pentoxide exposure (acutely and chronically) in both experimental animal and human studies indicates a systemic patho-physiological and pathological influence on cell metabolism and tissue function. Therefore procedures need to be implemented in environments which potentially expose workers to vanadium pentoxide including influences on respiratory function and appropriate quality guidelines enforced. The lungs are likely to accumulate more vanadium particles than elsewhere particularly from polluted air. The lowest observed-adverse-effect level for acute exposure can be considered to be 60 μg vanadium per m3. Indeed, chronic exposure emanates as slight changes in the upper respiratory tract, with irritation, coughing and injection of pharynx, to more serious effects such as chronic bronchitis and pneumonitis. Persons suffering from lung problems including asthma and cystic fibrosis would need to ensure that they take adequate measures to prevent vanadium irritation of the mucosae. Obviously, however, a systemic assessment via renal and liver function tests needs to be completed in order to make an accurate clinical assessment of the influence of vanadium on body function and ultimately the efficient maintenance of homeostasis. More research is required to establish and add to the limited existing knowledge on the toxicokinetic and toxicological database on vanadium pentoxide. Indeed, there is limited understanding of the potential for dermal absorption and the potential long term effects as a result of sequestration in body tissues such as bone.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aw TC, Gardiner K, Harrington JM. In: Pocket consultant. Occupational Health. 5th ed. Oxford: Blackwell Publishing; 2007. Chapter 5. Occupational toxicology; pp. 71–114. [Google Scholar]
- 2.Vanadium pentoxide. Phillipsburg, New Jersey: Environmental Health and Safety; 2005. MSDS; p. 5. [Google Scholar]
- 3.Hauser R, Elreedy S, Hoppin JA, Christiani DC. Upper airway response in workers exposed to fuel oil ash:nasal lavage analysis. Occup Environ Med. 1995;52:353–8. doi: 10.1136/oem.52.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiviluoto M, Pyy L, Pakarinen A. Serum and urinary vanadium of workers processing vanadium. Int Arch Occup Envir Health. 1981;48:251–6. doi: 10.1007/BF00405612. [DOI] [PubMed] [Google Scholar]
- 5.Support: Dust inhalation toxicity studies with cover letter dated 081992. Washington, DC: US Environmental Protection Agency, Office of Toxic Substances; 1992. US EPA. Doc #89-940000275. [Google Scholar]
- 6.WHO Regional Office for Europe. Denmark: Copenhagen; 2000. WHO. Vanadium. Chapter 6.12; p. 9. [Google Scholar]
- 7.Waters MD. Toxicology of vanadium. In: Goyer RA, Mehlman MA, editors. Advances in modern toxicology. Vol. 2. New York: Wiley; 1977. pp. 147–89. Toxicology of trace elements. [Google Scholar]
- 8.Davies DJ, Bennett BG. University of London Monitoring Assessment and Research Centre. London: 1983. Exposure commitment assessments of environmental pollutants., London; MARC Report No; p. 3. MARC Report No 30. [Google Scholar]
- 9.Scientific and technical assessment report on vanadium. Washington, DC: 1977. US Environmental Protection Agency, Report No. EPA-600/6-77-00. [Google Scholar]
- 10.Dill JA, Lee KM, Mellinger KH, Bates DJ, Burka LT, Roycroft JH. Lung deposition and clearance of inhaled vanadium pentoxide in chronically exposed F344 rats and B6C3F1 mice. Toxicol Sci. 2004;77:6–18. doi: 10.1093/toxsci/kfh005. [DOI] [PubMed] [Google Scholar]
- 11.Sabbioni E, Marafante E, Rade J, Gregotti C, Di Nucci A, Manzo L. Biliary excretion of vanadium in rats. Toxicol Eur Res. 1981;3:93–8. [PubMed] [Google Scholar]
- 12.Sabbioni E, Marafante E, Amantini L, Ubertalli L, Birattari C. Similarity in metabolic patterns of different chemical species of vanadium in the rat. Bioinorg Chem. 1978;8:503–15. doi: 10.1016/0006-3061(78)80004-0. [DOI] [PubMed] [Google Scholar]
- 13.Soremark R, Ullberg S, Appelgren LE. Autoradiographic localization of V-48-labelled vanadium pentoxide (V2O in developing teeth and bones of rats. Acta Odontol Scand. 1962;20:225–32. doi: 10.3109/00016356209019771. [DOI] [PubMed] [Google Scholar]
- 14.Tipton IH, Stewart PL, Dickson J. Patterns of elemental excretion in long term balance studies. Health Phys. 1969;16:455–62. doi: 10.1097/00004032-196904000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Kusaka Y, Akino H, Kanamaru H, Okada K. Direct effects of vanadium on citrate uptake by rat renal brush border membrane vesicles (BBMV) Ind Health. 2002;40:278–81. doi: 10.2486/indhealth.40.278. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Woodin MA, Smith TJ, Herrick RF, Williams PL, Hauser R, et al. Exposure to fuel-oil ash and welding emissions during the overhaul of an oil-fired boiler. J Occup Environ Hyg. 2005;2:435–43. doi: 10.1080/15459620591034529. [DOI] [PubMed] [Google Scholar]
- 17.Lee CW, Srivastava RK, Ghorishi SB, Hastings TW, Stevens FM. Investigation of selective catalytic reduction impact on mercury speciation under simulated NOx emission control conditions. J Air Waste Manag Assoc. 2004;54:1560–6. doi: 10.1080/10473289.2004.10471009. [DOI] [PubMed] [Google Scholar]
- 18.Hirtle B, Teschke K, van Netten C, Brauer M. Kiln emissions and potters’ exposures. Am Ind Hyg Assoc J. 1998;59:706–14. doi: 10.1080/15428119891010884. [DOI] [PubMed] [Google Scholar]
- 19.Costigan M, Cary R, Dobson S. Vanadium pentoxide and other inorganic vanadium compounds. Stuggart: Concise International Chemical Association Document 29, Wissenschaftliche Verlagsgesellschaft mbH. 2001:54. [Google Scholar]
- 20.In: NIOSH manual of analytical methods. 4th ed. Washington DC: National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication; 1994. NIOSH. Method 7300 Elements (ICP) and Method 7504 Vanadium oxides; pp. 94–113. NTIS PB 85-17901. [Google Scholar]
- 21.In:Analytical methods manual. 2nd ed. Washington, DC: US Department of Labour, Occupational Safety and Health Administration; 1991. OSHA. Method ID-125G Metal and metalloid particulates in workplace atmospheres (ICP analysis) and Method ID 185 Confirmation of vanadium pentoxide in workplace atmospheres. [Google Scholar]
- 22.Sabbioni E, Moroni M. A study on vanadium in workers from oil-powered fire plants. Luxembourg, Commission of European Communities (EU Report -EN) 1983 [Google Scholar]
- 23.National Institute for Occupational Safety and Health: Document No. 77-222. Washington, DC: 1977. NIOSH. Occupational exposure to vanadium; p. 142. [Google Scholar]
- 24.Occupational safety and health guidelines for vanadium pentoxide dust. Washington, DC: Occupational Safety and Health Administration; 2007. OSHA; p. 10. [Google Scholar]
- 25.Nemery B. Metal toxicity and the respiratory tract. Eur Respir J. 1990;3:202–19. [PubMed] [Google Scholar]
- 26.Altamirano-Lozano M. Genotoxic effects of vanadium compounds. Invest Clin. 1998;39:39–47. [PubMed] [Google Scholar]
- 27.International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2001. p. 11. [Google Scholar]
- 28.Sharma RP, Flora SJ, Drown DB, Oberg SG. Persistence of vanadium compounds in lungs after intratracheal instillation in rats. Toxicol Ind Health. 1987;3:321–9. doi: 10.1177/074823378700300304. [DOI] [PubMed] [Google Scholar]
- 29.Irsigler GB, Visser PJ, Spangenberg PA. Asthma and chemical bronchitis in vanadium plant workers. Am J Ind Med. 1999;35:366–74. doi: 10.1002/(sici)1097-0274(199904)35:4<366::aid-ajim7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Cortijo J, Villagrasa V, Marti-Cabrera M, Villar V, Moreau J, Advenier C, et al. The spasmogenic effects of vanadate in human isolated bronchus. Br J Pharmacol. 1997;121:1339–49. doi: 10.1038/sj.bjp.0701277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roldán RE, Altamirano LM. Chromosomal aberrations, sister-chromatid exchanges, cell-cycle kinetics and satellite associations in human lymphocyte cultures exposed to vanadium pentoxide. Mutat Res. 1990;245:61–5. doi: 10.1016/0165-7992(90)90001-z. [DOI] [PubMed] [Google Scholar]
- 32.Zhong BZ, Gu ZW, Wallace WE, Whong WZ, Ong T. Genotoxicity of vanadium pentoxide in Chinese hamster V79 cells. Mutat Res. 1994;321:35–42. doi: 10.1016/0165-1218(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 33.Ress NB, Chou BJ, Renne RA, Dill JA, Miller RA, Roycroft JH, et al. Carcinogenicity of inhaled vanadium pentoxide in F344/N rats and B6C3F1 mice. Toxicol Sci. 2003;74:287–96. doi: 10.1093/toxsci/kfg136. [DOI] [PubMed] [Google Scholar]
- 34.Knecht EA, Moorman WJ, Clark JC, Delon Hull R, Biagini RE, Lynch DW, et al. Pulmonary reactivity to vanadium pentoxide following subchronic inhalation exposure in a non-human primate animal model. J Appl Toxicol. 2006;12:427–34. doi: 10.1002/jat.2550120611. [DOI] [PubMed] [Google Scholar]
- 35.Knecht EA, Moorman WJ, Clark JC, Lynch DW, Lewis TR. Pulmonary effect of acute vanadium pentoxide inhalation in monkeys. Am Rev Respir Dis. 1985;132:1181–5. doi: 10.1164/arrd.1985.132.6.1181. [DOI] [PubMed] [Google Scholar]
- 36.Vandenplas O, Binard-Van Caugh F, Gregoire J, Brumagne A, Larbanois A. Fever and neutrophilic alveolitis caused by a vanadium based catalyst. Occup Environ Med. 2002;59:785–7. doi: 10.1136/oem.59.11.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabowski GM, Paulauskis JD, Godleski JJ. Mediating phosphorylation events in the vanadium-induced respiratory burst of alveolar macrophages. Toxicol Appl Pharmacol. 1999;156:170–8. doi: 10.1006/taap.1999.8642. [DOI] [PubMed] [Google Scholar]
- 38.Cohen MD, Becker S, Devlin R, Schlesinger RB, Zelikoff JT. Effects of vanadium upon poly: C-induced responses in rat lung and alveolar macrophages. J Toxicol Environ Health. 1997;51:591–608. doi: 10.1080/00984109708984046. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Medan D, Mercer R, Overmiller D, Leornard S, Castranova V, et al. Vanadium-induced apoptosis and pulmonary inflammation in mice: Role of reactive oxygen species. J Cell Physiol. 2003;195:99–107. doi: 10.1002/jcp.10232. [DOI] [PubMed] [Google Scholar]
- 40.Dill JA, Lee KM, Mellinger KH, Bates DJ, Burka LT, Roycroft JH. Lung deposition and clearance of inhaled vanadium pentoxide in chronically exposed F344 rats and B6C3F1 mice. Toxicol Sci. 2004;77:6–18. doi: 10.1093/toxsci/kfh005. [DOI] [PubMed] [Google Scholar]
- 41.Fortoul TI, Quan-Torres A, Sánchez I, López IE, Bizarro P, Mendoza ML, et al. Vanadium in ambient air: Concentrations in lung tissue from autopsies of Mexico City residents in the 1960s and 1990s. Arch Environ Health. 2002;57:446–9. doi: 10.1080/00039890209601436. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinski L, Myczkowski JZ, Kulkarni AP. Toxic effects of long-term intratracheal administration of vanadium pentoxide in rats. Arch Environ Contam Toxicol. 1991;20:295–8. doi: 10.1007/BF01064393. [DOI] [PubMed] [Google Scholar]
- 43.Magari SR, Schwartz J, Williams PL, Hauser R, Smith TJ, Christiani DC. The association of particulate air metal concentrations with heart rate variability. Environ Health Perspect. 2002;110:875–80. doi: 10.1289/ehp.02110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Villalva A, Fortoul TI, Avila-Costa MR, Piñón-Zarate G, Rodriguez-Laraa V, Martínez-Levy G, et al. Thrombocytosis induced in mice after subacute and subchronic V205 inhalation. Toxicol Ind Health. 2006;22:113–6. doi: 10.1191/0748233706th250oa. [DOI] [PubMed] [Google Scholar]
- 45.Vouk VB. Vanadium. In: Friberg L, Nordberg GR, Vouk VB, editors. Handbook on the Toxicology of Metals. New York: Elsevier North Holland; 1979. pp. 659–674. [Google Scholar]
- 46.Hickey RJ, Schoff EP, Clelland RC. Relationship between air pollution and certain chronic disease death rates: Multivariate statistical studies. Arch Environ Health. 1967;15:728–38. doi: 10.1080/00039896.1967.10664990. [DOI] [PubMed] [Google Scholar]
- 47.Rattner BA, McKernan MA, Eisenreich KM, Link WA, Olsen GH, Hoffman DJ, et al. Toxicity and hazard of vanadium to mallard ducks (Anas platyrhynchos) and Canada geese (Branta Canadensis) Toxicol Environ Health A. 2006;69:331–51. doi: 10.1080/15287390500398265. [DOI] [PubMed] [Google Scholar]
- 48.Dong W, Simeonova PP, Gallucci R, Matheson J, Flood L, Wang S, et al. Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol Appl Pharmacol. 1998;151:359–66. doi: 10.1006/taap.1998.8481. [DOI] [PubMed] [Google Scholar]
- 49.Zychlinski L, Byczkowski JZ. Inhibitory effects of vanadium pentoxide on respiration of rat live rmitochondria. Arch Environ Contam Toxicol. 1990;19:138–42. doi: 10.1007/BF01059822. [DOI] [PubMed] [Google Scholar]
- 50.Ivanov VN. Effect of vanadium on tissue respiration in organs and on cholesterol metabolism in guinea pigs with experimental atherosclerosis. Cor Vasa. 1975;17:75–80. [PubMed] [Google Scholar]
- 51.Avila-Costa MR, Fortoul TI, Niño-Cabrera G, Colín-Barenque L, Bizarro-Nevares P, Gutiérrez-Valdez AL, et al. Hippocampal cell alterations induced by the inhalation of vanadium pentoxide (V9O) promote memory deterioration. Neurotoxicity. 2006;27:1007–12. doi: 10.1016/j.neuro.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Avila-Costa MR, Montiel Flores E, Colín-Barenque L, Ordoñez JL, Gutiérrez AL, Niño-Cabrera G, et al. Nigrostriatal modifications after vanadium inhalation: An immunocytochemical and cytological approach. Neurochem Res. 2004;29:1365–9. doi: 10.1023/b:nere.0000026398.86113.7d. [DOI] [PubMed] [Google Scholar]
- 53.Avila-Costa MR, Colín-Barenque L, Zepeda-Rodríquez A, Antuna SB, Saldivar OL, Espejel-Maya G, et al. Ependymal epithelium disruption after vanadium pentoxide inhalation. A mice experimental model. Neurosci Lett. 2005;381:21–5. doi: 10.1016/j.neulet.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 54.Nechay BR, Saunders JP. Inhibition by vanadium of sodium and potassium dependent adenosinetriphosphate derived from animal and human tissues. J Environ Pathol Toxicol. 1978;2:247–62. [PubMed] [Google Scholar]
- 55.Domingo JL, Llobet JM, Tomas JM, Corbella J. Short-term toxicity studies of vanadium in rats. J Appl Toxicol. 1985;5:418–21. doi: 10.1002/jat.2550050616. [DOI] [PubMed] [Google Scholar]
- 56.Sabbioni E, Pozzi G, Devos S, Pintar A, Casella L, Fischbach M. The intensity of vanadium(V)-induced cytotoxicity and morphological transformation in BALB/3T3 cells is dependent on glutathione-mediated bioreduction to vanadium(IV) Carcinogenesis. 1993;14:2565–8. doi: 10.1093/carcin/14.12.2565. [DOI] [PubMed] [Google Scholar]
- 57.Kucera J, Simkova M, Lener J, Mravcova A, Kinova L, Penev I. Vanadium determination in rat tissues and biological reference materials by neutron activation analysis. J Radioanal Nucl Chem. 1990;141:49–59. [Google Scholar]
- 58.Fortoul TI, Bizarro-Nevares P, Acevedo-Nava S, Piñón-Zárate G, Rodriguez-Lara V, Colin-Barenque L, et al. Ultrastructural findings in murine seminiferous tubules as a consequence of subchronic vanadium pentoxide inhalation. Reprod Toxicol. 2007;23:588–92. doi: 10.1016/j.reprotox.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Mussali-Galante P, Rodriguez-Lara V, Hernández-Tellez B, Avila-Costa MR, Colin-Barenque L, Bizarro-Nevares P, et al. Inhaled vanadium pentoxide decrease gamma-tubulin of mouse testes at different exposure times. Toxicol Ind Health. 2005;21:215–22. doi: 10.1191/0748233705th232oa. [DOI] [PubMed] [Google Scholar]
- 60.Altamirano-Lozano M, Alvarez-Barrera L, Basurto-Alcántara F, Valverde M, Rojas E. Reprotoxic and genotoxic studies of vanadium pentoxide in male mice. Teratog Carcinog Mutagen. 1996;16:7–17. doi: 10.1002/(SICI)1520-6866(1996)16:1<7::AID-TCM2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Yang Z, Zhang T. Toxicity of vanadium to Leydig cells in vitro. Hua Xi Yi Ke Da Xue Xue Bao. 1995;26:433–5. [PubMed] [Google Scholar]


