Abstract
The sense of taste is critical for human life. It informs the body about the quality of food that will be potentially ingested and stimulates metabolic processes that prepare the alimentary canal for digestion. Steady progress is being made towards understanding the early biochemical and molecular events underlying taste transduction (for a review, Breslin and Spector, 20081). However, progress to date has largely resulted from animal models. Yet, since marked differences in receptor specificity and receptor density vary among species, human taste transduction will only be understood by using human taste tissue. Here we describe a biopsy technique to collect human fungiform papillae, visible as rounded pink anterior structures, about 0.5 mm in diameter that contain taste buds. These biopsied papillae are used for several purposes including the isolation of viable taste bud cells, in situ hybridization, immunohistochemistry and, through techniques of molecular biology, the identification of taste-specific novel proteins.
Protocol
1. Introduction and General Considerations
The general procedure involves local anesthesia of a small (1 cm2) area of the dorsal surface of the anterior tongue, followed by removal of a number (6-8) of fungiform (taste) papillae from that anesthetized area using a curved spring micro-scissors (McPherson-Vannas type #SR 5603, Roboz, Rockville, MD). The removed fungiform papillae are approximately 0.25 mm3 each. There is very little non-papillae (taste) epithelial tissue removed along with each papilla. New papillae form in this area after 3 to 5 weeks and regain functioning taste buds (Spielman and Brand, unpublished observations). There is no noticeable effect on taste or flavor of food after removal of this small amount of taste material. From studies that have counted individual taste buds in the adult human tongue, it can be estimated that the human tongue contains approximately 5000 taste buds. Our procedure removes at most 20 to 25 of these taste buds (on average, ~ 3.5 to 4 taste buds per fungiform papilla in human)2, or about 0.4% of the total. In addition there is no pain associated with the surgical loss of this tissue. One feels, of course, slight pain at the injection site only, as the needle enters the tongue. After anesthesia has worn off, there is occasionally mild discomfort for less than a day, but this discomfort is much less than what one experiences after accidentally "biting" ones tongue.
Over the past several years we have developed an alternative procedure that does not include anesthesia. The technique for tissue removal is identical for both. The only difference in the procedure is that the surgeon is no longer confined to a 1 cm2 diameter area where the anesthesia was addressed. Rather s/he can choose any site on the dorsal surface of the tongue where prominent papillae appear. The total number of removed papillae are limited to 6-8 in both techniques, with or without anesthesia If performed properly, the discomfort of removing 6-8 fungiform taste papillae is considered less of an issue when compared with the pain associated with the administering of local anesthesia A general survey of individuals who underwent both procedures, i.e., with and without anesthesia, showed that an overwhelming majority of subjects would dispense without local anesthesia in all future biopsies. The surgeon who developed this technique (AIS) and several colleagues taught by the author who performed biopsies without anesthesia, have tried it on themselves and testify that the discomfort is temporary and is considered less than that of biting one's tongue. Finally, dozens of individuals who agreed to be biopsied were asked if they would be willing to try removal of one papilla without anesthesia and if they judge it to be too painful they would undergo anesthesia, have all agreed to continue with the removal of papillae without anesthesia.
The entire biopsy should not take longer than 5-10 minutes. If anesthesia is employed another 2-3 minutes is added. Biopsy is typically carried out in the morning hours. Before biopsy, volunteers are instructed to eat a light meal two hours before the scheduled start time. At biopsy, written informed consent is obtained. Exclusion criteria at the time of biopsy include those with serious chronic illness, whether controlled by medication or not (e.g., any gastrointestinal diseases, neurodegenerative diseases, cancer, blood dyscrasias, allergy to the local anesthetics, etc.) those with heart rate greater than 100 or less than 60; systolic blood pressure greater than 145 or less than 100; diastolic blood pressure greater than 90 or less than 60, and the opinion of the surgeon that the subject can participate in the study.
Finally, it is important to consider that the biopsy procedure should be carried out in the same facility where tissue will be processed for examination since tissue integrity is rapidly lost as we have often noted by the declining quality of mRNA. To minimize these problems the tongue tissue should be processed as quickly as possible.
2. Subject Preparation
The subject is seated in a dental chair or a seat with a headrest.
Check heart rate and blood pressure at the time of biopsy. Should the subject fall outside of the range of blood pressure, heart rate or oral competence, the surgeon recommends to the subject that he/she make immediate appointment to see a physician.
Inspect the oral cavity for general tissue health, with attention to indicators of xerostomia, geographic tongue or any tongue lesions. Any subject showing signs of oral disease, including tongue lesions or xerostomia are excluded from the study. Instruct the subject to rinse the mouth with water to remove all debris from the oral cavity. If necessary the tongue is cleaned with a disposable tongue scrapper to remove plaque or debris, which could be carried into the tongue during injection of the anesthesia. The surgeon uses sterile gauze pads in a gloved hand to hold the anterior ¼ of the tongue as it extends from the oral cavity.
If anesthesia is used, a 1cc Tuberculin syringe with a 28 gauge x ½' needle containing 0.25 mL lidocaine (2%) with epinephrine (1:100,000) is slowly injected into one site just below the dorsal surface of the anterior portion of the tongue. The site of anesthesia is distal to the collection site to avoid interference by the lidocaine with subsequent studies. For comparison, the amount of lidocaine injected (0.25 mL) is about one quarter of the amount normally given for nerve block in dental procedures (0.8 mL to 1 mL). After insertion of the needle but before injection of the anesthetic, aspiration ensures that no blood vessel is struck. This precaution is taken even though it is anatomically unlikely that a large vessel will be struck close to the dorsal surface. The only arteries on the dorsal surface, the dorsal lingual arteries are small blood vessels primarily located more posterior to the anterior third, where the tissue harvesting occurs. The larger lingual arteries and the lingual veins run on the ventral surface of the tongue, far removed from the dorsal area receiving anesthetic 3. The 1 cm anesthetized area appears to be slightly pale compared with the surrounding tissue. This is due to the swelling of the tissue as it compresses the small blood vessels. It is also due to the vasoconstrictive effect of the epinephrine. After about one minute, a probe is used to test the area for numbness. If the area is not numb, a second injection (0.1 to 0.2 mL) may be given.
3. Tongue Papillae Removal Technique
The anterior quarter of the tongue of the subject is wrapped into sterile gauze (Figure 1) and held in the left hand of the surgeon (if performing the biopsy with the right hand). One corner of the gauze is left open to allow the surgeon to wipe the tongue free of saliva. The tongue must be held firmly, but, of course, not so as to cause unnecessary discomfort. The tip of the index finger of the left hand is positioned under the tongue directly under the site where the biopsy is performed. By pushing the index finger upward, the tongue becomes stretched over the tip of the finger making the papillae stick out for easy biopsy.
Criteria for choosing papillae for biopsy: Taste papillae are easily distinguishable from the surrounding filliform papillae by their shape and size. Fungiform papillae are round and somewhat isolated from the surrounding. In their immediate vicinity there is a circular space devoid of filliform papillae (Figure 2A, blue arrows). This space is about 1 mm in diameter. Another distinguishing feature of the fungiform papillae is an extended capillary arborization visible on top of most fungiform papillae of young subjects. Although arborization makes locating fungiform papillae easier, they are not necessarily good predictors of the existence of taste buds in that papilla. As a rule one starts harvesting toward the front end and moves further away from the tip of the tongue to avoid any capillary bleeding from obscuring the site of the next tissue collection.
Once a good papilla has been identified, a spring micro-scissors (McPherson-Vannas, curved, sharp cutting edge 5 mm, comb, tip width 0.2 mm overall length 3" (RS-5603, Roboz) is used to clip it off. To effectively use the micro surgical scissors one should not use the tip of the blade for cutting; rather, as far inside the blade as possible (Figure 1, enlarged image and Figure 3), close to the point of intersection with the opposite blade. Removal of the papilla is achieved with one cutting move of the scissors (Figure 1). The further inside the open blades the targeted papilla appears, the more effective is the cutting. Care should be taken that the overall position of the scissors must be parallel with the dorsal surface of the tongue.
Sometimes the tongue becomes dry as the surgeon is trying to identify a papilla for removal. Once dry, the ability of the operator to clearly see which is a fungiform papilla becomes more challenging and makes sliding of the blades over the papilla very difficult. An overly dry tongue usually makes the metal blade stick to the tongue making the delicate surgical move less controllable. Letting the subject close his/her mouth and have saliva come in contact with the surface of the tongue helps the process. Overly wet tongue is not good either. Using the corner of the gauze that one uses to hold the tongue helps in this process.
Any capillary bleeding that does occur is treated by blotting with sterile gauze. Bleeding usually stops within a few minutes and does not interfere with additional tissue harvesting. Because the papillae extend above the epithelial surface of the tongue, and only the upper 3/4 of a fungiform papilla is removed (Figure 2B), that part of the fungiform showing taste buds, little bleeding is expected.
Using a small Dumont forceps (Pattern type), the removed papillae are placed in chilled basic Ringer buffer ( 130 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na-Pyruvate, 20 mM HEPES-Na, pH 7.2; osmolality of ~ 305 milli osmols) for subsequent manipulations . It is imperative to NEVER pinch the papilla between the blades of a forceps. The tissue is very fragile and should be handled with extreme care.
After removal of the papillae, the subject remains in the chair for ten to fifteen minutes. Each subject is told not to bite on their anesthetized tongue, but to keep their tongue at rest. Subjects should be visually monitored by the attending surgeon. Anesthesia is expected to wear off in approximately 30-60 minutes. Because the site of papillae removal may be slightly sore after the biopsy, they are instructed to be aware of spicy or hot (temperature) food, carbonated drinks or drinks that are either extremely cold or hot. A further indication is given to subjects that any slight reddish coloration of saliva is normal and will cease upon dissolution of the clot.
Subjects are seen 30-40 days following removal of the papillae for evaluation of the site where the papillae were removed. By this time taste papillae should have completely regenerated.
5. Representative Results
Tissue collected can be used for immunohistochemistry, RT-PCR, in situ hybridization, calcium imaging, patch clamping and tissue culture. When performed correctly the appearance of the papillae are healthy looking, white, about 0.5-1 mm in diameter (Figure 2B).
Papillae collected for immunohistochemistry are processed based on existing and published protocols 4. A typical taste bud from a paraformaldehide-fixed taste papilla is shown in Figure 4A.
Biopsied papillae can also be enzymatically treated5 to achieve dissociation of taste bud cells. After the enzymatic dissociation, cells can be maintained for up to 4 hours in a humidified Petri dish at 4C. In this state they can be used for single cell PCR (Figure 4B), Ca-imaging, etc.
Cells show a variety of shapes, many being slender and bipolar (Figure 5A and 5B) as one would expect of a taste bud cell. When collecting cells or performing Ca-imaging, after about 20 minutes under a microscope, the cells begin to develop apoptotic blebs (Figure 6A and 6B, arrows) and many of them become round. Using a technique developed in our lab, cells were picked up individually using a patch-pipette, as shown in Figure 5A and 5B.
As mentioned above, papillae begin to regrow after 5 to 8 weeks. Figure 7A demonstrates the appearance of a tongue where 8 fungiform papillae were removed from left side a volunteer's tongue using no anesthesia. Notice 8 slight reddish spots, where the papillae were snipped off. No bleeding is observed after surgery. The present image was taken 10 minutes after the biopsy. Figure 7B is an image of the tongue of the same subject seen 40 days after the biopsy. Notice all papillae have regrown. To determine if the regenerated papillae are functional we asked our volunteer to agree to a second biopsy involving the exact same papillae. To correctly identify the appropriate papillae we were planning to re-harvest, we generated a grid (Figure 7C) that fits the 8 papillae that were removed in the first place and superimposed it over the tongue of the same volunteer after 40 days post initial biopsy. Aided by the contour of the tongue and the distance from the margin to the grid, once identified, we have removed a few of the same exact papillae and processed them for immunohistochemistry. Figure 4A is one of these papillae. Notice the outline of a taste bud (in yellow) and the immunopositive staining for Phospholipase Cβ2 (PLCβ2), a type 2 taste cell transduction-associated enzyme. These data demonstrate that using the biopsy procedure as described herein, the fungiform papillae regenerate sufficiently by 40 days to have also in place likely functional taste buds.
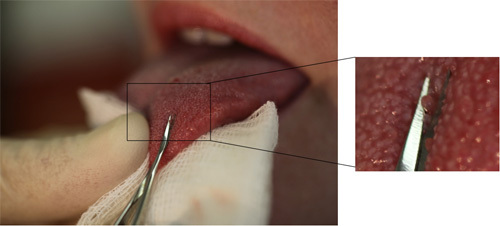 Figure 1. The anterior quarter of the tongue of the subject is wrapped into sterile gauze and held in the left hand of the surgeon. Tongue must be held firmly. The enlarged image on the right shows the proper position of the scissors and the position of the papilla in relation to the blade.
Figure 1. The anterior quarter of the tongue of the subject is wrapped into sterile gauze and held in the left hand of the surgeon. Tongue must be held firmly. The enlarged image on the right shows the proper position of the scissors and the position of the papilla in relation to the blade.
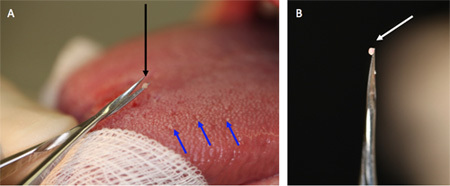 Figure 2. (A) Fungiform papillae are shown with blue arrows. These are round structures somewhat isolated from its surrounding and in their immediate vicinity there is a circular space devoid of filliform papillae. (B) Fungiform taste papilla on the tip of the spring micro-scissors seconds after it has been removed.
Figure 2. (A) Fungiform papillae are shown with blue arrows. These are round structures somewhat isolated from its surrounding and in their immediate vicinity there is a circular space devoid of filliform papillae. (B) Fungiform taste papilla on the tip of the spring micro-scissors seconds after it has been removed.
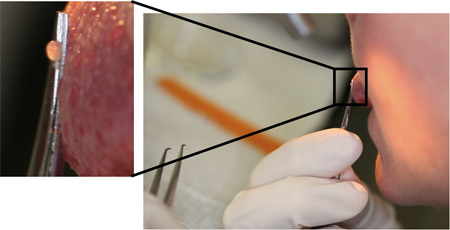 Figure 3. Self biopsy of a fungiform papilla using a mirror and no anesthesia. The inset illustrates the overall position of the scissors, which must be parallel with the dorsal surface of the tongue. If done properly and atraumatically, anesthesia is forgone by virtually all volunteers.
Figure 3. Self biopsy of a fungiform papilla using a mirror and no anesthesia. The inset illustrates the overall position of the scissors, which must be parallel with the dorsal surface of the tongue. If done properly and atraumatically, anesthesia is forgone by virtually all volunteers.
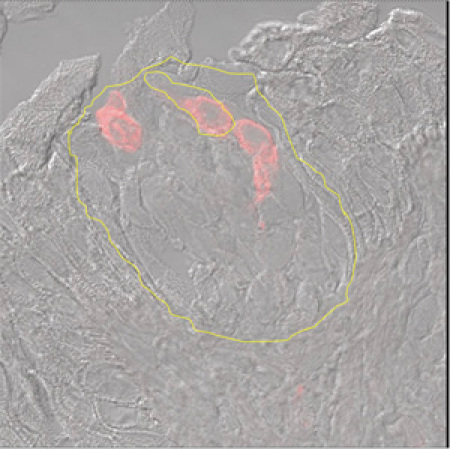 Figure 4 A. Immunohistochemistry of a taste bud. A fungiform papilla visualized by Nomarski optics using antibodies to Phospholipase β2 and tagged with Red Fluorescent Protein. The outline of the taste bud and a few labeled taste cells are shown in yellow.
Figure 4 A. Immunohistochemistry of a taste bud. A fungiform papilla visualized by Nomarski optics using antibodies to Phospholipase β2 and tagged with Red Fluorescent Protein. The outline of the taste bud and a few labeled taste cells are shown in yellow.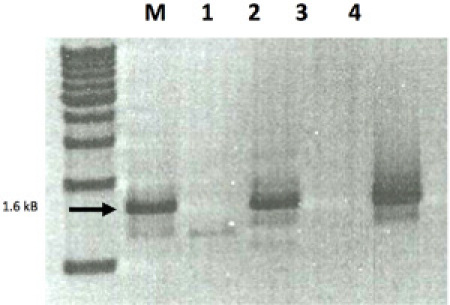 B. RT-PCR of a candidate sour taste receptor, Acid Sensing Ion Channel 1 (ASIC1). M= marker. Lanes 1 through 4 were single cell RT-PCR for ASIC1 from four different volunteers. After collection, fungiform papillae were enzymatically dissociated, cells individually picked up and expression of ASIC1 were done. Lanes 2 and 4 had normal expression of ASIC1.
B. RT-PCR of a candidate sour taste receptor, Acid Sensing Ion Channel 1 (ASIC1). M= marker. Lanes 1 through 4 were single cell RT-PCR for ASIC1 from four different volunteers. After collection, fungiform papillae were enzymatically dissociated, cells individually picked up and expression of ASIC1 were done. Lanes 2 and 4 had normal expression of ASIC1.
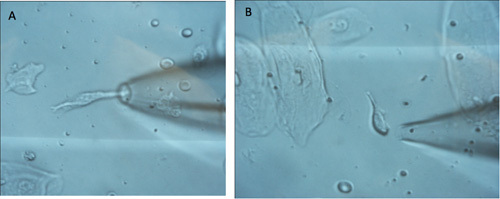 Figure 5. A. Image of single taste cells being picked up using a patch pipette. A large epithelial cell is visible in the lower left corner (5A). Two red blood cells are also visible above the patch pipette and behind the glass barrel. The taste cell is approximately 30-50 microns long and about 6 micron wide. For reference, a red blood cell is about 7-8 microns in diameter. B. shows another taste receptor cell surrounded by four epithelial cells. The taste cell looks crisp.
Figure 5. A. Image of single taste cells being picked up using a patch pipette. A large epithelial cell is visible in the lower left corner (5A). Two red blood cells are also visible above the patch pipette and behind the glass barrel. The taste cell is approximately 30-50 microns long and about 6 micron wide. For reference, a red blood cell is about 7-8 microns in diameter. B. shows another taste receptor cell surrounded by four epithelial cells. The taste cell looks crisp.
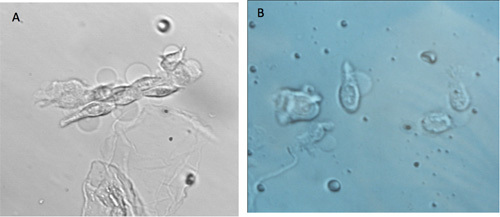 Figure 6. A. Images of cells that started to develop apoptotic blebs (ghost like round blebs attached to the taste cell, arrows). B. Cells are no longer harvested when their appearance changes as shown here.
Figure 6. A. Images of cells that started to develop apoptotic blebs (ghost like round blebs attached to the taste cell, arrows). B. Cells are no longer harvested when their appearance changes as shown here.
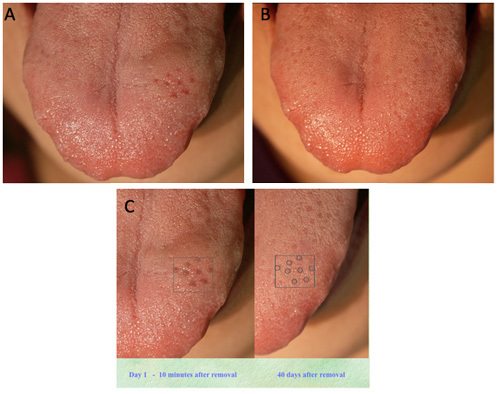 Figure 7. A. Ten minutes after removal of 8 taste papillae using no anesthesia. The surgical sites are visible as slightly reddish spots. No bleeding is noticed.B. The same tongue 40 days after biopsy. Notice all papillae regenerated. To help identify the removed papillae as regrown, a grid was created with the site on the right side picture and shifted over the regenerated papillae on the left side image. C. The regenerated papillae appeared in the exact same spot. Harvesting papillae again from this group followed by immunohistochemistry demonstrated that the regenerated papillae contained functional taste cells (See Figure 4A).
Figure 7. A. Ten minutes after removal of 8 taste papillae using no anesthesia. The surgical sites are visible as slightly reddish spots. No bleeding is noticed.B. The same tongue 40 days after biopsy. Notice all papillae regenerated. To help identify the removed papillae as regrown, a grid was created with the site on the right side picture and shifted over the regenerated papillae on the left side image. C. The regenerated papillae appeared in the exact same spot. Harvesting papillae again from this group followed by immunohistochemistry demonstrated that the regenerated papillae contained functional taste cells (See Figure 4A).
Discussion
One notable advantage to using human cells is their specificity. The procedure described here in detail provides tongue tissue for isolation of taste receptor cells from humans. These taste cells can be used for molecular studies, seeking to discover molecules important for transduction of taste information, and for calcium imaging studies that monitor taste cell activity to stimuli. Additionally, tongue tissue provided by this procedure can be analyzed with various techniques such as immunohistochemistry, in situ hybridization, and allows capturing individual cells for molecular procedures such as Polymerase Chain Reaction (PCR), including Real Time Quantitative Single Cell PCR. Because this procedure can be performed on individuals whose sense of taste can be psychophysically evaluated prior to biopsy, it is possible to get a clear picture of the molecular details responsible for taste abnormalities (for example, see Huque et al. 2009). This biopsy procedure allows production of a viable and metabolically intact single cell preparation enriched in cells from taste buds and thus the ability to perform patch clamp recordings on these cells. Using this technique, future studies could not only increase our understanding of taste transduction processes unique to man but also decipher molecular mechanisms underlying taste abnormalities in disease or medication-induced states and develop regimens to treat these abnormalities.
Finally, the results of our observations strongly indicate that the presence of "holes," often noted on fungiform papillae close to arborizations and generally considered to be taste bud pores, are, in fact, not good predictors of the existence of taste buds. Just what the nature of these holes is, or what functions they may perform, we do not know.
In 8 years since we started this procedure, we have repeatedly collected from several dozen volunteers over 4000 taste papillae. Two adverse incidence, both associated with the local anesthesia led to a temporary swelling of the tongue, but resolved within the next 48 hours without adverse effects. If done properly, under sterile and atraumatic conditions, this method is safe and effective.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by NIH R21 DC03969-01 (to JGB). We thank Dr. Fritz Lischka for capturing single taste bud cells, Dr. Tauf Huque for performing the RT-PCR shown in Figure 4B and Mr. D. Bayley and Ms. S. Alarcon for technical assistance. M. Yanina Pepino is a fellow supported by a NIDAT32 DA07313 Washington University School of Medicine, St. Louis, MO (Cottler, L.B. PI).
References
- Breslin PA, Spector AC. Mammalian taste perception. Curr Biol. 2008;26(4):R148–R155. doi: 10.1016/j.cub.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Miller I. Anatomy of the peripheral taste system. In: Doty RI, Dekker M, editors. Handbook of Olfaction and Gustation. New York: Marcel Dekker; 1995. pp. 521–547. [Google Scholar]
- Gray's Anatomy: The Anatomical Basis of Medicine and Surgery. 38th Edition. New York: Churchill Livingstone; 1995. Figure 12.67. [Google Scholar]
- Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman A, Feldman I, S R, Mackler Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One. 2009;4(10):e7347–e7347. doi: 10.1371/journal.pone.0007347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Mody I, Salter MW, Spielman AI. Patch-clamping of taste cells in mouse. In: Spielman AI, Brand JG, editors. Experimental Cell Biology of Taste and Olfaction. Boca Raton, FL: CRC Press; 1995. pp. 329–332. [Google Scholar]


