Abstract
Introduction:
Black smokers are reported to have higher lung cancer rates and greater tobacco dependence at lower levels of cigarette consumption compared to non-Hispanic White smokers. We studied the relationship between cigarettes per day (CPD) and biomarkers of nicotine and carcinogen exposure in Black and White smokers.
Methods:
In 128 Black and White smokers, we measured plasma nicotine and its main proximate metabolite cotinine, urine nicotine equivalents, 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol (NNAL), and polycyclic aromatic hydrocarbon (PAH) metabolites.
Results:
The dose–response between CPD and nicotine equivalents, and NNAL and PAH was flat for Black but positive for White smokers (Race × CPD interaction, all ps < .05). Regression estimates for the Race × CPD interactions were 0.042 (95% CI 0.013–0.070), 0.054 (0.023–0.086), and 0.028 (0.004–0.052) for urine nicotine equivalents, NNAL, and PAHs, respectively. In contrast there was a strong correlation between nicotine equivalents and NNAL and PAH independent of race. Nicotine and carcinogen exposure per individual cigarette was inversely related to CPD. This inverse correlation was stronger in Black compared to White smokers and stronger in menthol compared to regular cigarette smokers (not mutually adjusted).
Conclusions:
Our data indicate that Blacks on average smoke cigarettes differently than White smokers such that CPD predicts smoke intake more poorly in Black than in White smokers.
Introduction
Blacks smoke on average fewer cigarettes per day (CPD) compared to non-Hispanic White smokers (Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009; Carabello et al., 1998). Nonetheless, several studies indicate that Black smokers have a higher level of dependence than White smokers, particularly at lower levels of smoking (Collins & Moolchan, 2006; Department of Health and Human Services, Public Health Service, 1998; Luo et al., 2008; Okuyemi, Faseru, Sanderson Cox, Bronars, & Ahluwalia, 2007). Black smokers are more likely to attempt to quit than White smokers but have lower quit ratios (percentage of lifetime smokers who have quit smoking; Department of Health and Human Services, 1998; Fu et al., 2008). In addition, Blacks have a higher risk for lung cancer compared to Whites, and this racial difference is most pronounced at lower levels of daily cigarette consumption (Haiman et al., 2006). Relative risks for smoking-induced lung cancer are higher in Blacks compared to Whites at 10 or fewer CPD (relative risk = 2.22; p < .01) and at 11–20 CPD (0.1.75; p < .001) but not at 30 or more CPD (1.22; ns).These observations suggest that the relationship between cigarette smoking and exposure to nicotine and other tobacco smoke toxins might differ between Blacks and Whites.
Among the many carcinogens in cigarette smoke, two classes have been particularly implicated in the development of lung cancer: the tobacco-specific nitrosamines, especially 4-(methylnitrosamino)-1-(3)pyridyl-1-butanone (NNK),and the polycyclic aromatic hydrocarbons (PAHs). NNK is metabolized in the body to 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol (NNAL), also a pulmonary carcinogen, which can be measured in the urine and which reflects NNK exposure (Hecht, 2003). Of note are two recent case–control studies among smokers in which NNAL concentration in the urine was significantly associated with the risk for lung cancer, with a dose-dependent effect (Church et al., 2009; Yuan et al., 2009). PAHs are a class of combustion products that include benzo(a)pyrene and other carcinogens that are present in combustion products including tobacco smoke (Hecht, 2003). Several PAH metabolites can be measured in urine and are believed to reflect exposure to the carcinogenic PAHs.
We have previously reported that on average Black smokers take in 30% more nicotine and therefore more tobacco smoke per individual cigarette smoked compared to White smokers (Perez-Stable, Herrera, Jacob, & Benowitz, 1998). That study did not however examine intake or exposure to nicotine across a range of CPD and did not examine carcinogen exposure. Given these observations of racial differences in the relationship between CPD, tobacco dependence, lung cancer risk, and racial differences in nicotine intake per cigarette, we hypothesized that the relationship between CPD and nicotine and carcinogen exposure differs in Black compared to White smokers and, in particular, that Black lighter smokers take in higher levels of nicotine and carcinogens compared to White lighter smokers.
In this article, we analyzed the relationship between CPD and biomarkers of nicotine and carcinogen exposure in Black and White smokers. To better understand the basis for racial differences in exposure in relation to CPD, we also analyzed exposure per individual cigarette smoked and how that exposure varied with CPD. To determine the predictive value of CPD compared to biomarkers of nicotine intake for carcinogen exposure, we performed cross-correlations among CPD and various biomarkers in the two racial groups.
Materials and Methods
Subjects
The subjects were 128 cigarette smokers who were recruited by newspaper advertisements and notices posted in local colleges, community centers, and other public places as well as on Craigslist. Subjects were required to be 18–65 years old, to be healthy, and to have smoked an average of 10 CPD or more for the past year or longer as ascertained by telephone screening. Subjects had to be self-identified non-Hispanic White or Black, with four grandparents of the same race. Exclusions included active medical problems; pregnancy; breast feeding; current alcohol or drug abuse; current use of smokeless tobacco, pipes, cigars, and nicotine medications; and regular use of medications other than vitamins, oral contraceptives, hormone replacements, or aspirin.
Procedures
Subjects were screened for eligibility by telephone. Eligible subjects were asked to come to the Clinical Research Center at San Francisco General Hospital Medical Center, where the study was explained and written consent obtained. Questionnaires were administered regarding health history, drug use history, and smoking and tobacco dependence measures, including the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Average cigarette consumption was taken as the average number of CPD in the 3 days prior to the study visit.
After completing the questionnaires, a blood sample was taken and urine collected. The time of smoking the last cigarette prior to blood and urine sampling was recorded. Plasma was assayed for concentrations of nicotine, cotinine, and trans-3 hydroxycotinine (3HC). In the early part of this study, blood was analyzed for carboxyhemoglobin. Later in the study, for technical reasons, expired-air carbon monoxide (CO) was substituted (Vitalograph Breath CO). The urine samples were analyzed for concentrations of creatinine, nicotine and its five major metabolites, NNAL, and metabolites of several PAHs. Subjects were compensated financially for participation. The study was approved by the Institutional Review Board at the University of California San Francisco.
Data from this study that focused on urine menthol in relation to biomarkers of exposure to nicotine and tobacco carcinogens have previously been published (Benowitz, Dains, Dempsey, Yu, & Jacob, 2010).
Analytical Chemistry
Plasma nicotine was measured by gas chromatography with nitrogen phosphorus detection using a capillary column (Jacob, Wilson, & Benowitz, 1981; Jacob, Yu, Wilson, & Benowitz, 1991). Plasma cotinine and 3HC were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS; Dempsey et al., 2004). Urine concentrations of nicotine and its metabolites cotinine, 3HC, and their respective glucuronide metabolites were measured by (LC-MS/MS), as described previously (Benowitz, Jacob, Fong, & Gupta, 1994; Dempsey et al., 2004). Urine concentrations of NNAL (free plus conjugated) and PAH metabolites, including 2-naphthol, 1,2 and 3+4 hydroxyphenanthrenes, 1-hydroxypyrene, and 2-hydroxyfluorene, were measured by LC-MS/MS (Jacob et al., 2008; Jacob, Wilson, & Benowitz, 2007). Details on quality control measures for the various assays are provided in the methods papers cited previously. Urine creatinine was measured in the San Francisco General Hospital clinical laboratory using a colorimetric assay.
Nicotine equivalents was determined as the molar sum of nicotine, cotinine, 3HC, and their glucuronide metabolites in urine corrected for creatinine concentration. When measured at steady state, the sum of these metabolites accounts for on average 80%–90% of a daily dose of nicotine (Feng et al., 2007). We have shown that nicotine equivalents measured in this way are highly correlated with daily intake of nicotine, as validated by administration of labeled nicotine in steady-state conditions (Benowitz, Dains, Dempsey, Havel, et al., 2010). We expressed total PAHs as the molar sum of all PAH metabolites.
Data Analysis
Comparison of demographic and smoking history characteristics and exposure to various tobacco smoke constituents in Black versus White smokers, men versus women smokers, and menthol versus regular cigarette smokers were performed by Wilcoxon’s rank-sum test. Where values were not normally distributed, geometric means values are presented. Testing hypotheses relating biomarkers of exposure to race, sex, and CPD was performed by multivariate regression. Several models were tested. One model included the exposure biomarker as a dependent variable, and race, sex, age, body mass index (BMI), and CPD as independent variables. To test the hypothesis that race modifies the relationship between CPD and exposure biomarkers, a Race × CPD interaction term was added in another model. Cigarettes per day for all analyses was based on the average number of cigarettes smoked over the 3 days preceding the research visit. In one set of models, CPD was analyzed as a continuous variable. In another set of models, CPD was analyzed as an ordinal variable of 0–9, 10–19, and 20 or more CPD based on the categories that were studied by Haiman et al. (2006), demonstrating different relative risk for lung cancer in Blacks versus Whites. In addition to the models using CPD as an independent variable, to determine if race modifies the relationship between nicotine intake and carcinogen exposure, additional models examined NNAL and PAH metabolite concentrations as dependent variables and urine nicotine equivalents rather than CPD as the independent variable, including a Race × Nicotine Equivalents interaction term.
To examine the intensity of smoking each individual cigarette as a function of CPD, we analyzed the correlation between various biomarker levels/CPD versus CPD. To compare the predictive value of CPD with a biomarker of nicotine intake (plasma cotinine or urine nicotine equivalents) for carcinogen exposure, we performed cross-correlations of biomarkers within the two racial groups.
Results
Demographics and Smoking History
Demographic, smoking, and alcohol consumption data for the subjects compared by race and sex are presented in Table 1. The average age of the subjects was 38.2 years; 42% were women. The subjects smoked an average of 17.2 CPD. On average, Black smokers were significantly older and had a higher BMI and fewer years of education. On average, Blacks smoked one fewer CPD than Whites, but this difference was not significant. Despite reporting during telephone screening that they smoked 10 or more CPD on average over the past year, 25% of Blacks and 16% of Whites smoked on average 10 or fewer CPD in the 3 days preceding the blood and urine sample. Blacks and Whites began smoking at similar ages on average, but Blacks had smoked for significantly longer (because they were older). The prevalence of menthol cigarette smoking and the average machine-determined nicotine and tar yields of cigarettes (ISO method) smoked were significantly higher in Blacks. The score and the time to first cigarette in the morning (another measure of dependence and also a component of the FTND) was similar between races.
Table 1.
Demographic Comparisons by Sex and Race (25%–75% quartile)
| Characteristic | Black |
White |
p Value | ||||
| All (N = 61) | Men (n = 37) | Women (n = 24) | All (N = 67) | Men (n = 37) | Women (n = 30) | ||
| Age (years) | 41.7 (36–49) | 43.3 (37.5–50) | 39.2 (28–44.8) | 35.0 (25–45) | 35.7 (25–47) | 34.1 (25–42.3) | .0009* |
| BMI | 28.7 (23.7–32.2) | 26.0 (22.6–27.6) | 32.8 (26.2–37.6) | 25.5 (22.2–28.0) | 25.2 (22.3–27.8) | 25.9 (22.1–30.7) | .002* |
| Years of education | 13.7 (12–14) | 13.9 (12–14.5) | 13.4 (12–14) | 14.6 (13–16) | 14.2 (12–15.5) | 15.0 (13.8–16) | .007* |
| CPD (mean over previous 3 days) | 17.2 (10–20) | 18.5 (10.3–20.7) | 15.3 (7.3–20) | 18.3 (13.7–20) | 19.9 (14.8–25.8) | 16.4 (12.5–20) | .07 |
| CPD category (%) | |||||||
| 1–10 | 24.6 | 11.5 | 13.1 | 16.4 | 7.5 | 8.9 | |
| 11–20 | 49.2 | 32.8 | 16.4 | 59.7 | 9.9 | 29.9 | |
| ≥20 | 26.2 | 16.4 | 9.8 | 23.9 | 17.9 | 6.0 | |
| Years smoked | 23.5 (16–30) | 24.7 (19–30) | 21.8 (12.3–29.5) | 18.4 (9–30) | 18.7 (9.5–30) | 18.0 (7–26.8) | .01* |
| Age of smoking start (years) | 16.5 (13.3–19) | 16.9 (14–19.8) | 16 (12.3–17) | 16.2 (13–18) | 16.4 (13–18) | 18 (13–18) | .6 |
| Menthol (%) | 70.5 | 56.8 | 91.7 | 25 | 32.4 | 16.7 | .0001* |
| FTC nicotine (mg) | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) | 1.2 (1.1–1.3) | 1.0 (0.8–1.2) | 1.1 (1.0–1.2) | 1.0 (0.8–1.1) | <.0001* |
| FTC tar (mg) | 16.5 (15–19) | 17.0 (15–19) | 15.8 (15.3–17.8) | 13.3 (10.3–16) | 13.9 (11.8–16) | 12.5 (10–15.3) | <.0001* |
| Alcohol intake (g/week) | 62.2 (0–78) | 84.9 (0–129) | 28.0 (0–45) | 85.3 (0–130) | 102.6 (0–158) | 63.8 (0–107) | .054 |
| FTND score | 3.9 (2–5.8) | 3.8 (2–6) | 4.1 (3–5) | 3.7 (2–5) | 3.7 (2.5–5) | 3.7 (2–5) | .4 |
| Time to first cigarette (min) | 25.6 (5–41) | 26.7 (5–45) | 24.0 (6.3–30) | 27.1 (10–30) | 25.4 (10–30) | 29.2 (8.8–45) | .4 |
Note. BMI = body mass index; CPD = cigarettes per day; FTND = Fagerström Test for Nicotine Dependence; FTC = U.S. Federal Trade Commission.
*Significant difference between racial groups by Wilcoxon’s test.
Biomarkers of Exposure
Table 2 presents data on mean values for CPD, expired-air CO, plasma nicotine and metabolites, and urine nicotine metabolites, NNAL, and PAHs, comparing Blacks and Whites, men and women, and menthol versus non-menthol cigarette smokers. The time from the last cigarette to blood sampling was significantly longer (by 27 min on average) in Blacks versus Whites and tended to be longer (25 min on average) in menthol versus regular cigarette smokers. Plasma cotinine/CPD was significantly higher in Blacks versus Whites. Plasma nicotine levels were significantly higher in regular compared to menthol cigarette smokers. Urine nicotine equivalents, total NNAL, 2-naphthol, and total PAH metabolites were significantly lower in Black compared to White smokers. Urine nicotine equivalents, 2-naphthol, and total PAHs were significantly higher in women compared to men and in regular compared to menthol cigarette smokers.
Table 2.
Cigarette Smoking and Biomarkers of Cigarette Smoke Exposure (M and 95% CI)
| Characteristic | All (N = 128), M (95% CI) | Race (n), Black (61)/White (67) | Sex (n), male (74)/female (54) | Cigarette type (n), regular (67)/menthol (60) |
| CPD | 17.8 (16.2–19.4) | 17.2 (14.3–20.1)/18.3 (16.6–20.0) | 19.2 (16.9–21.5)/15.9 (13.7–18.1) | 17.4 (15.4–19.4)/18.7 (15.8–21.7) |
| Expired-air CO (ppm) | 16.5 (N = 79) (14.1–18.9) | 15.7 (n = 41) (13–19)/17.3 (n = 38) (13.5–21) | 17.5 (n = 51) (14–21)/14.6 (n = 28) (11–18) | 18.2 (n = 39) (15–22)/14.8 (n = 40) (12–18) |
| Plasma (ng/ml) | ||||
| Nicotine | 6.8 (5.7–8.1) | 6.0 (4.7–7.8)/7.6 (6.0–9.6) | 7.2 (5.7–9.1)/6.2 (94.8–8.2) | 8.0 (6.3–10.1)*/5.7 (4.4–7.3) |
| Cotinine | 170.5 (150–193) | 178.8 (149–214)/163.5 (137–194) | 182.5 (155–215)/155.8 (129–189) | 176.3 (148–209)/164.6 (137–198) |
| Cotinine/CPD | 10.9 (9.5–12.6) | 12.5 (10.2–15.3)*/9.7 (8.0–11.7) | 10.6 (8.8–12.8)/11.3 (9.1–14.0) | 11.2 (9.3–13.7)/10.5 (8.6–12.9) |
| 3HC | 55.9 (48.8–64.1) | 54 (44–66)/578 (48–70) | 60 (22–72)/51 (42–63) | 60 (50–73)/51 (42–63) |
| 3HC/cotinine | 0.33 (0.30–0.36) | 0.30 (0.26–0.35)/0.35 (0.31–0.40) | 0.33 (0.29–0.37)/0.33 (0.28–0.38) | 0.34 (0.30–0.39)/0.31 (0.27–0.36) |
| Urine (pmol/mg creatinine) | ||||
| Nicotine | 687 (548–860) | 604 (438–834)/772.7 (563–1061) | 721 (536–971)/641 (450–914) | 768 (561–1051)/606 (438–840) |
| Cotinine | 1323 (1136–1542) | 1117 (880–1417)/1549 (1281–1872) | 1204 (986–1471)/1510 (1190–1916) | 1445 (1167–1790)/1199 (964–1493) |
| Nicotine equivalents | 3587 (3110–4138) | 45 (38–54)*/63 (53–73) | 51 (43–60)/58 (48–70) | 60 (50–70)/48 (40–58) |
| Total NNAL | 1.1 (0.9–1.2) | 0.9 (0.8–1.1)*/1.2 (1.0–1.5) | 1.0 (0.8–1.2)/1.2 (1.0–1.5) | 1.2 (1.0–1.5)/1.0 (0.8–1.1) |
| Sum of fluorenes | 15.7 (13.9–17.7) | 14.5(12.4–16.8)/16.9 (14.2–20.1) | 15.8 (13.7–18.3)/15.6 (12.7–19.0) | 17.0 (14.5–19.8)/14.4 (12.1–17.3) |
| Sum of phenanthrenes | 3.3 (3.0–3.7) | 3.2 (2.7–3.8)/3.5 (3.1–4.0) | 3.3 (2.9–3.8)/3.4 (2.8–4.1) | 3.4 (3.0–3.9)/3.3 (2.7–3.9) |
| 2-Naphthol | 75.0 (66.9–84.2) | 57 (50–66)**/96 (82–112.5) | 67 (58–78)*/87 (73–105) | 90 (77–105)*/61 (52–72) |
| 1-Hydroxy-pyrene | 1.0 (0.9–1.2) | 1.0 (0.8–1.3)/1.1 (0.9–1.2) | 1.0 (0.8–1.2)/1.2 (0.9–1.4) | 1.0 (0.9–1.2)/1.1 (0.8–1.3)) |
| Total PAHs | 97.8 (87.7–108.9) | 79 (69–90)**/120 (103–139.5) | 89 (77–102)*/111.5 (94–132) | 114 (99–132)*/82 (71–96) |
| Nicotine equivalent/CPD | 3.4 (3.0–3.9) | 3.2 (2.6–3.9)/3.7 (3.0–4.4) | 3.0 (2.5–3.6)*/4.2 (3.4–3–5.2) | 3.8 (3.1–4.6)/3.1 (2.5–3.8) |
| NNAL/CPD | 0.12 (0.11–0.14) | 0.13 (0.11–0.16)/0.12 (0.10–0.14) | 0.12 (0.10–0.14)/0.13 (0.11–0.16) | 0.13 (0.11–0.16)/0.12 (0.10–0.14) |
| Total PAH/CPD | 6.3 (5.5–7.1) | 5.5 (4.6–6.6)/7.0 (5.9–8.4) | 5.2 (4.4–6.1)*/8.0 (6.6–9.7) | 7.3 (6.1–8.6)*/5.3 (4.4–6.3) |
Note. 3HC = trans-3 hydroxycotinine; CPD = cigarettes per day; CO = carbon monoxide; NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; PAH = polycyclic aromatic hydrocarbon. CPD and expired-air CO are arithmetic means; others are geometric means. Bold values indicate statistically significant differences.
*p < .05; **p < .01, significant difference between the groups by Wilcoxon’s test.
Relationship Between CPD and Nicotine and Carcinogen Exposure
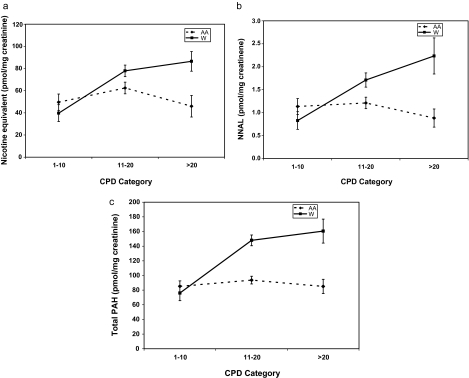
Figure 1 shows the relationship between CPD and urine nicotine equivalents, urine NNAL, and urine PAH metabolites in Blacks and Whites. In Whites, exposure to nicotine, NNAL, and PAHs increased with increasing CPD, but for Blacks, the CPD versus biomarker curves were generally flat. In Blacks, urine nicotine equivalents and NNAL were on average lower at less than 20 CPD compared to 11–20 CPD, but because of the variability, the shape of the curve is not significantly different from zero. Multivariate regression analyses revealed significant positive associations between CPD and urine nicotine equivalents, NNAL, and PAHs in Whites (all p < .002), but no significant associations for Blacks (Table 3). Significant CPD × Race interactions were observed for nicotine equivalents, NNAL, and total PAHs (all ps < .025). Thus, the slopes of the curves were significantly different for Black compared to White smokers. Significant interactions were also observed between race and CPD when CPD was assessed categorically (data not shown). There was not a significant effect for CPD or an interaction between CPD and race for expired-air CO.
Figure 1.
Relationship between cigarettes per day (CPD) and urine nicotine equivalents (a), CPD and urine total NNAL (b), and CPD and urine total PAH metabolites (c), comparing African American(AA) and White (W) smokers. The Race × CPD interaction was significant for all three biomarkers (p < .05). NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol.
Table 3.
Multiple Linear Regression Models of Predictors of Urine Nicotine Equivalents, Expired-Air Carbon Monoxide, Urine Total NNAL, and Urine Total PAH Metabolitesa
| Dependent variable | Model | Parameter | Estimate | p Value | 95% CI |
| Log urine NE (mmol/g creatinine) | 1 | White race | −0.502 | .099 | −1.100 |
| R2 = .19 | CPD for Black | 0.000 | .949 | −0.014 | |
| CPD for White | 0.042 | .001 | 0.017 | ||
| White × CPD interaction | 0.042 | .005 | 0.013 | ||
| Log expired-air CO (ppm) | 1 | White race | −0.451 | .271 | −1.260 |
| R2 = .10 | CPD for Black | −0.012 | .207 | −0.031 | |
| CPD for White | 0.025 | .171 | −0.011 | ||
| White × CPD interaction | 0.038 | .070 | −0.003 | ||
| 2 | White race | 0.952 | .148 | −0.346 | |
| R2 = .57 | Log NE for Black | 0.883 | <.0001 | 0.653 | |
| Log NE for White | 0.656 | <.0001 | 0.422 | ||
| White × Log NE interaction | −0.227 | .167 | −0.551 | ||
| Log NNAL (pmol/ mg creatinine) | 1 | White race | −0.631 | .061 | −1.292 |
| R2 = .22 | CPD for Black | −0.007 | .389 | −0.024 | |
| CPD for White | 0.047 | .001 | 0.020 | ||
| White × CPD interaction | 0.054 | .001 | 0.023 | ||
| 2 | White race | −0.712 | .204 | −1.816 | |
| R2 = .59 | Log NE for Black | 0.684 | <.0001 | 0.494 | |
| Log NE for White | 0.904 | <.0001 | 0.706 | ||
| White × Log NE interaction | 0.220 | .111 | −0.051 | ||
| PAH (pmol/mg creatinine) | 1 | White race | −0.079 | 0.755 | −0.577 |
| R2 = .28 | CPD for Black | 0.005 | .457 | −0.008 | |
| CPD for White | 0.033 | .002 | 0.012 | ||
| White × CPD interaction | 0.028 | .021 | 0.004 | ||
| 2 | White race | −0.508 | .190 | −1.271 | |
| R2 = .68 | Log NE for Black | 0.533 | <.0001 | 0.401 | |
| Log NE for White | 0.732 | <.0001 | 0.595 | ||
| White × Log NE interaction | 0.199 | .038 | 0.012 |
Note. CO = carbon monoxide; CPD = cigarettes per day; NE = nicotine equivalent; NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; PAH = polycyclic aromatic hydrocarbon.
All models were adjusted for sex, age, and body mass index.
Relationship Between Nicotine Intake and Carcinogen Exposure
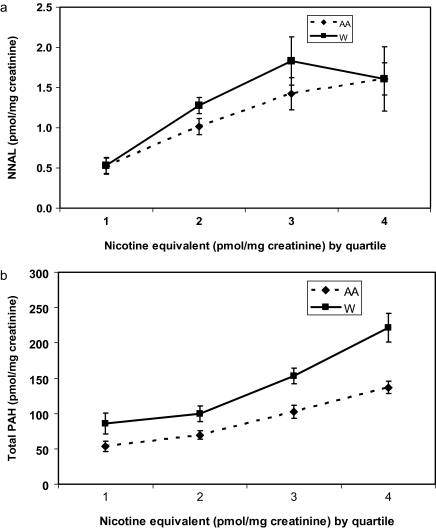
Figure 2 shows the relationship between urine nicotine equivalents and urine NNAL and urine PAH metabolites in Black and White smokers. In contrast to the racial difference observed in the CPD versus exposure curves, a positive relationship between urine nicotine equivalents and both carcinogen biomarker levels and expired-air CO (data for CO not shown) was seen both in Blacks and Whites. In multivariate analysis, urine nicotine equivalents were the strongest predictors of urine NNAL, PAH, and expired-air CO (all ps < .001). Multivariate regression analysis found no significant Urine Nicotine Equivalent × Race interaction between nicotine equivalents and urinary NNAL or expired-air CO, but there is a significant interaction for urine PAH metabolites (p = .012).
Figure 2.
Relationship between urine nicotine equivalents (by quartlile) and urine total NNAL (a) and urine total PAH metabolites (b), comparing African American (AA) and White (W) smokers. The Nicotine Equivalent × Race interactions were not significant. NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; PAH = polycyclic aromatic hydrocarbon.
Relationship Between CPD and Nicotine and Carcinogen Exposure per Individual Cigarette
Exposure to nicotine and carcinogens per individual cigarette increased as CPD decreased. There was a significant negative correlation between urine nicotine equivalents/CPD, NNAL/CPD, and total PAHs/CPD versus the number of CPD among all subjects. This was particularly marked at the very lowest level of cigarette consumption, where exposure per individual cigarette was very high. In general, the inverse correlations were stronger for Black compared to White smokers. Correlations with CPD were as follows: versus urine nicotine equivalents/CPD, Black r = −.54 (p < .01), White r = −.30 (p < .05); versus NNAL/CPD, Black r = −.48 (p < .01), White r = −.09 (ns); and versus PAHs/CPD, Black r = −.47 (p < .01), White r = −.34 (p < .05). The inverse correlations were similarly stronger for menthol compared to regular cigarette smokers. Correlations with CPD were as follows: versus urine nicotine equivalents/CPD, menthol r = −.54 (p < .01), regular r = −.42 (p < .01); versus NNAL/CPD, menthol r = −.46 (p < .01), regular r = −.16 (ns); and versus PAHs/CPD, menthol r = −.45 (p < .01), regular r = −.36 (p < .01).
Cross-Correlations Among CPD and Biomarkers of Exposure by Race
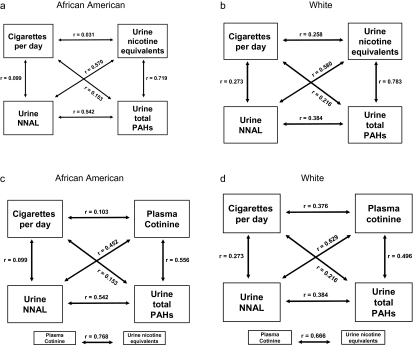
Figure 3 presents the correlation coefficients between CPD, urine nicotine equivalents, plasma cotinine, urine NNAL, and urine total PAHs for Black and for White smokers. Compared to White smokers, the correlations were weaker for Blacks for CPD versus nicotine equivalents (r = .031 vs. .258 in Black vs. White, respectively), plasma cotinine (r = .103 vs. .376), urine NNAL (r = .099 vs. .273), and urine PAHs (r = .153 vs. .216). In contrast, the correlations between nicotine equivalents or plasma cotinine and other biomarkers of exposure were generally strong and were similar in magnitude for both races. For urine nicotine equivalents, the correlations in Blacks versus Whites were as follows: urine NNAL, r = .570 versus .580; and urine PAHs, r = .719 versus 0.783. For plasma cotinine, the correlations were as follows: urine NNAL, r = .452 versus .629; and urine PAHs, r = .556 versus .496.
Figure 3.
Pearson correlation coefficients between cigarettes per day (CPD), urine nicotine equivalents, urine total 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol (NNAL), and urine total polycyclic aromatic hydrocarbon (PAH) metabolites in African American (a) and White smokers (b); and between CPD, plasma cotinine, urine total NNAL, and urine total PAH metabolites in African American (c) and White smokers (d). *p < .05; **p < .01.
Discussion
Main Observations
We make several observations in this study that may help to explain differences in smoking behavior in relation to lung cancer risk in Blacks compared to Whites. First, we find that the relationship between the number of cigarettes smoked per day versus daily intake of nicotine (as measured by nicotine equivalents in urine) and tobacco smoke carcinogens is relatively flat for Blacks, whereas there is a weak positive relationship for Whites. Second, we find a strong correlation in both Blacks and Whites between urine nicotine equivalents or plasma cotinine (reflecting systemic exposure to nicotine) and tobacco smoke carcinogens. Third, we find that the intake of nicotine and carcinogens per cigarette is inversely related to the number of cigarettes smoked per day, and this inverse relationship appears to be stronger for Black than for White smokers. Finally, our data support the use of a spot urine measurement of nicotine equivalents normalized for creatinine as a valid surrogate for exposure to tobacco smoke toxicants in smoking and health epidemiology studies.
Nicotine Equivalents as a Measure of Nicotine Intake
We have used the molar sum of nicotine and its five major metabolites normalized by creatinine as an indicator of daily intake of nicotine, termed “nicotine equivalents.” We have previously shown that the molar sum of these metabolites accounts for 85%–90% of the systemic dose of nicotine absorbed from transdermal nicotine, assessed by measuring nicotine clearance and nicotine plasma levels during patch use (Benowitz et al., 1994). Feng et al. (2007) determined that the molar sum of these metabolites represents on average 86% of a dose of nicotine, validated against the difference between nicotine inhaled and exhaled during cigarette smoking. We did not measure nicotine metabolites in 24-hr urine (the optimal period for sample collection), but collected a spot urine. We previously showed that the sum of nicotine metabolites corrected for creatinine in a spot urine is highly correlated with daily intake of nicotine, as validated by administration of labeled nicotine in steady-state conditions (Benowitz, Dains, Dempsey, Yu, et al., 2010). A high correlation between nicotine equivalents versus plasma cotinine, urine NNAL, and urine PAH metabolites in the present study supports this idea.
Racial Differences in CPD and per Individual Cigarette Exposure to Nicotine and Carcinogens
The findings of a flat relationship between CPD and nicotine or carcinogen exposure in Blacks suggest that Blacks smoke their cigarettes in a much different way according to how many cigarettes they smoke per day compared to Whites. In both races, we found that people who smoke fewer CPD smoke each cigarette more intensively, taking in higher levels of nicotine and carcinogens per cigarette than those who smoke more CPD. Based on the observation of a flat exposure curve for CPD versus biomarker levels, this effect appears to be stronger in Blacks such that the expected correlation between CPD and exposure to tobacco smoke constituents is substantially blunted.
A number of studies have found, as we have, that CPD is only modestly correlated with biomarkers of tobacco smoke exposure. Mustonen, Spencer, Hoskinson, Sachs, and Garvey (2005) found a weaker relationship between CPD and plasma cotinine in Black compared to White smokers, and also found an inverse relationship between plasma cotinine per CPD versus number of cigarettes smoked per day. Correlations between cigarettes smoked per day and plasma cotinine were reported to be 0.39 in a group of 700 Black light smokers (10 or fewer CPD) and 0.20 in another group of 600 heavier Black smokers (10 or more CPD; Ho et al., 2009). Both of these analyses were of smokers entered into smoking cessation trials. Joseph et al. (2005) found that in smokers of 15–45 CPD, correlations between CPD and total urine cotinine (cotinine plus glucuronide) were 0.426, between CPD and urine NNAL 0.478, and CPD versus 1-hydroxypyrene (a PAH metabolite) 0.126. As in our study, Joseph et al. found that the correlation between total cotinine and NNAL was much stronger than the correlation between CPD and NNAL. Carmella et al. (1995) reported in 61 smokers no significant correlation between CPD and urine total NNAL but did find a significant correlation between urine cotinine and urine NNAL (r = .58). No analysis of racial differences was performed in either the Joseph or the Carmella study.
Other researchers have shown that exposure to nicotine, as determined by plasma, saliva, or urine cotinine and urine NNAL, is not linearly related to CPD (Blackford et al., 2006; Carabello et al., 1998; Joseph et al., 2005). In these studies, nicotine and carcinogen exposures increased sharply in relation to CPD at lower levels of smoking and increased progressively less as cigarette consumption increases, with a peak exposure at about 15–20 CPD. Our data showing higher levels of chemical exposure per cigarette when smoking CPD support this type of relationship. Lubin, Caporaso, Hatsukami, Joseph, and Hecht (2007) reported a similar relationship between the urine NNAL/cotinine ratio versus urine cotinine. Melikian et al. (2007) found a significant inverse correlation between urine cotinine, NNAL, and 1-hydroxypyrene as a fraction of individualized smoking machine-estimated deliveries as daily smoke exposure decreased.
Thus, a number of studies demonstrate that people who smoke fewer CPD smoke their cigarettes much more intensively (i.e., greater intake of smoke constituents per cigarette) than heavier smokers. The reason for more intensive smoking when smoking fewer CPD is likely related to a desire to maximize nicotine intake to support dependence. In support of this idea is a report by Muscat, Stellman, Caraballo, and Richie (2009), showing that the level of dependence, as assessed by time to first cigarette, strongly influences plasma cotinine levels in relationship to CPD. Low-dependence smokers demonstrated a linear increase in cotinine with increasing CPD, whereas high-dependence smokers exhibited a generally flat relationship between cotinine and CPD. The latter pattern is similar to that seen in our Black smokers.
Our results support other findings that Black smokers have higher plasma cotinine levels per cigarette smoked compared to Whites (Carabello et al., 1998; Mustonen et al., 2005; Perez-Stable et al., 1998; Wagenknecht et al., 1990). Previously, we found that the average intake of nicotine per cigarette (as estimated using plasma cotinine levels combined with nicotine pharmacokinetic studies), presumably reflecting the intake of other tobacco smoke constituents, was 30% higher in Blacks compared to Whites (Perez-Stable et al., 1998). Richie et al. (1997) found that urine NNAL levels were higher in Black compared to White smokers.
However, in the present study, despite the higher plasma cotinine per individual cigarette smoked in Black smokers, exposure to nicotine and carcinogens per individual cigarette as assessed by urine biomarkers was similar or lower in Blacks compared to Whites. A key difference between prior studies and the present study may be that in prior studies, Blacks were smoking on average substantially fewer CPD than Whites, whereas in the present study, due to our selection criteria, Blacks and Whites were smoking roughly the same number of CPD. The Black subjects in our present study are not representative of the U.S. population, in which Blacks do on average smoke fewer CPD compared to Whites (Benowitz et al., 2009).
Relationship Between Nicotine Intake and Exposure to Tobacco Smoke Constituents
We found a strong relationship between nicotine exposure as indicated by urine nicotine equivalents or plasma cotinine and carcinogen exposure, with no difference between races. Similarly, Derby et al. (2009) found in a study of Native Hawaiian, White, and Japanese American smokers that racial differences were seen in the relationship between CPD and urine NNAL, but these racial differences were eliminated when the relationship between urine nicotine equivalents and NNAL was examined. Roethig et al. (2009) reported a strong correlation between urine nicotine equivalents and urine NNAL but did not compare the relationship between CPD and urine NNAL. Thus, our data and the data of other researchers indicate that NNK or PAH doses are proportional to nicotine intake and therefore proportional to smoke exposure, with no evidence of racial differences in that relationship.
Biomarkers of Nicotine Intake Compared to CPD in Relation to Carcinogen Exposure
Most smoking and health epidemiology studies have used CPD as a surrogate for exposure to tobacco smoke with its numerous toxic constituents. Many of these studies have found highly significant associations between CPD and disease risk. Our data and findings of other researchers indicate that CPD does not provide an accurate estimate of nicotine and carcinogen exposure, and we report for the first time that the reliability of this measure varies by race. That is, the use of CPD is a particularly poor measure of smoke exposure in Black smokers.
In contrast to CPD, the use of urine nicotine equivalents or plasma cotinine provides a good estimate of carcinogen exposure for both Blacks and Whites. For most comparisons, urine nicotine equivalents in a spot urine sample correlated more highly than plasma cotinine with carcinogens and would be the preferred biomarker for smoking and cancer studies, where feasible. This is of particular importance when trying to understand mechanisms of differences in disease risk in relation to tobacco smoke toxicant exposure between groups. Only by using exposure biomarkers can one determine whether differences in disease risk are due to different levels of exposure to tobacco smoke toxicants or due to different sensitivity to the disease-producing effects of these toxicants. Additionally, because biomarkers are more accurate indicators of exposure, the use of these would be expected to substantially increase the power of epidemiological studies of smoking and disease risk compared to the use of CPD.
Menthol and Biomarkers of Exposure
Plasma nicotine, urine 2-naphthol, urine total PAH, as well as urine total PAH normalized for CPD were all significantly lower in menthol compared to regular cigarette smokers. The significantly lower average plasma nicotine and trend toward lower expired-air CO levels in menthol compared to regular cigarette smokers can be explained, at least in part, by the longer time interval between smoking the last cigarette and time to blood sampling in the menthol cigarette smokers on the study day.
Two recent publications comparing smokers of regular versus menthol cigarettes reported no significant difference in exposure to tobacco smoke constituents, assessed by plasma and urine nicotine metabolites, urine NNAL, carboxyhemoglobin, and plasma thiocyanate (Heck, 2009; Muscat et al., 2009). The data from these two studies as well as the present study provide no support for the hypothesis that menthol results in greater overall exposure to NNK or PAHs in smokers.
However, we did observe that the slope of the inverse relationship between CPD and nicotine or carcinogen exposure was stronger for menthol compared with regular cigarette smokers. As most Blacks smoked menthol and most Whites smoked regular cigarettes, we cannot discriminate the effect of menthol from the effect of race. Menthol does have a cooling effect that can reduce the irritant quality of cigarette smoking. Therefore, menthol might facilitate deeper inhalation that occurs when people smoke fewer CPD and might explain why Black menthol smokers of fewer CPD can take in more cigarette smoke than White regular cigarette smokers who smoke similarly few CPD, contributing to the racial difference in the shape of the CPD versus tobacco smoke biomarker curves. This hypothesis needs to be examined in future studies.
Study Limitations
Our study represents one of the largest racial difference studies with extensive characterization of nicotine and carcinogen exposure. Limitations of our study include that our subjects were not a nationally representative sample. While the Black subjects in the present study smoked on average more CPD than the national average, cigarette consumption in White smokers was close to the national average. Since we tried to recruit smokers who typically smoked 10 or more CPD, we are not able to describe the shape of the CPD versus biomarker curves at low levels of cigarette consumption (five or fewer per day), the latter of which is common in Black smokers.
Another methodological issue is that some subjects smoked fewer CPD in the 3 days prior to the assessment than they reported smoking on average in the prior year. We assume that our biomarker assessment represents steady-state exposure in relation to the cigarettes actually smoked in the preceding 3 days. This is likely the case for nicotine metabolites and PAHs, which have relatively short half-lives but is not necessarily the case for NNAL, which has a much longer half-life (Carmella et al., 2009). However, we did find similar degrees of correlation between NNAL and PAHs with nicotine intake, suggesting that our assessments are not seriously biased.
Finally, prior research findings of higher cotinine levels normalized for CPD in Blacks has raised the question of whether Blacks are misreporting cigarette consumption compared to Whites. This has been of particular concern when Blacks report smoking fewer CPD than Whites. In our study, cigarette consumption was similar in Blacks and Whites. The possibility that Blacks misreport smoking more than White has also been suggested by studies of self-reported nonsmokers in which Blacks were more likely to have a plasma cotinine level consistent with active smoking (Caraballo, Giovino, Pechacek, & Mowery, 2001; Wagenknecht, Burke, Perkins, Haley, & Friedman, 1992). In contrast, another study found no evidence that Blacks report cigarette consumption differently than Whites (Sterling & Weinkam, 1989).
With respect to analyses of differences between menthol and non-menthol cigarette smoking, a limitation of our study is the imbalance between race and menthol smoking—70% among Blacks compared to 25% among Whites.
Conclusions
Our study furthers understanding of racial differences in the relationship between cigarettes smoked per day, tobacco addiction, and lung cancer risk in Blacks versus Whites. Haiman et al. (2006) found a large difference in lung cancer risk between Blacks compared to Whites among those smoking fewer than 10 CPD, but the difference in lung cancer risk decreased as cigarette consumption increased, and became nonsignificant at greater than 30 CPD. These data suggest that for Whites, the risk for lung cancer increases much more with an increasing number of CPD than is seen with Blacks. Our data showing a greater increase in carcinogen exposure with increasing CPD for Whites compared to Blacks are consistent with the shape of the dose–response curves in the Haiman lung cancer data. However, our study does not explain the higher risk for lung cancer among smokers of lower numbers of CPD in Black compared to White light smokers, as we found similar carcinogen exposure among the lightest smokers in both racial groups.
Funding
Supported by U.S. Public Health Service grants DA02277 and DA12393 from the National Institute on Drug Abuse and CA78603 from the National Cancer Institute, National Institutes of Health. Carried out in part at the General Clinical Research Center at San Francisco General Hospital Medical Center (NIH/NCRR UCSF-CTSI UL1 RR024131).
Declaration of Interests
NLB is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
Acknowledgments
The authors thank Dr. Mark Siegel and Miriam Cisternas for statistical advice; Dr. Faith Allen and Rebecca Cannon for data management and analysis; Sandra Tinetti for clinical coordination; Fredysha McDaniel, Lita Ramos, Minjiang Duan, and Janice Cheng for analytical chemistry activities; and Marc Olmsted for editorial assistance.
References
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology. 2009;169:236–248. doi: 10.1093/aje/kwn301. doi:10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., III Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiology & Biomarkers of Prevention. 2010;19(12):3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. doi:1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., III Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiology & Biomarkers of Prevention. 2010;19:1160–1166. doi: 10.1158/1055-9965.EPI-09-1303. doi:19/5/1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III, Fong I, Gupta S. Nicotine metabolic profile in man: Comparison of cigarette smoking and transdermal nicotine. Journal of Pharmacology & Experimental Therapeutics. 1994;268:296–303. [PubMed] [Google Scholar]
- Blackford AL, Yang G, Hernandez-Avila M, Przewozniak K, Zatonski W, Figueiredo V, et al. Cotinine concentration in smokers from different countries: Relationship with amount smoked and cigarette type. Cancer Epidemiology & Biomarkers of Prevention. 2006;15:1799–1804. doi: 10.1158/1055-9965.EPI-06-0427. doi:1055-9965.EPI-06-0427. [DOI] [PubMed] [Google Scholar]
- Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. American Journal of Epidemiology. 2001;153:807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- Carabello RS, Giovino GA, Pechacek TF, Mowery PD, Richter PA, Strauss WJ, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers. Journal of American Medical Association. 1998;280:135–139. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Akerkar SA, Richie JP, Jr., Hecht SS. Intraindividual and interindividual differences in metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiology & Biomarkers Prevention. 1995;4(6):635–642. [PubMed] [Google Scholar]
- Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chemical Research in Toxicology. 2009;22:734–741. doi: 10.1021/tx800479s. doi:10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church TR, Anderson KE, Caporaso NE, Geisser MS, Le CT, Zhang Y, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiology & Biomarkers of Prevention. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. doi:18/1/260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CC, Moolchan ET. Shorter time to first cigarette of the day in menthol adolescent cigarette smokers. Addiction Behavior. 2006;31:1460–1464. doi: 10.1016/j.addbeh.2005.10.001. doi:S0306-4603(05)00259-5. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Washington, DC: Government Printing Office; 1998. Tobacco use among U.S. racial/ethnic minority groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A report of the Surgeon General: USDHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Promotion, Office of Smoking and Health. [PubMed] [Google Scholar]
- Derby KS, Cuthrell K, Caberto C, Carmella S, Murphy SE, Hecht SS, et al. Exposure to the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers from 3 populations with different risks of lung cancer. International Journal of Cancer. 2009;125:2418–2424. doi: 10.1002/ijc.24585. doi:10.1002/ijc.24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Kapur S, Sarkar M, Muhammad R, Mendes P, Newland K, et al. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicology Letters. 2007;173:101–106. doi: 10.1016/j.toxlet.2007.06.016. doi:S0378-4274(07)00771-0. [DOI] [PubMed] [Google Scholar]
- Fu SS, Kodl MM, Joseph AM, Hatsukami DK, Johnson EO, Breslau N, et al. Racial/ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiology & Biomarkers of Prevention. 2008;17:1640–1647. doi: 10.1158/1055-9965.EPI-07-2726. doi:1055-9965.EPI-07-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. New England Journal of Medicine. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerstr. m Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature Reviews Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- Heck JD. Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiology & Biomarkers of Prevention. 2009;18:622–629. doi: 10.1158/1055-9965.EPI-08-0550. doi:1055-9965.EPI-08-0550. [DOI] [PubMed] [Google Scholar]
- Ho MK, Faseru B, Choi WS, Nollen NL, Mayo MS, Thomas JL, et al. Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiology & Biomarkers of Prevention. 2009;18:3426–3434. doi: 10.1158/1055-9965.EPI-09-0956. doi:18/12/3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, III, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Analytical Chemistry. 2008;80:8115–8121. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. Journal of Chromatography. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Analytical Chemistry. 2007;79:587–598. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: Absence of an isotope effect in the clearance of (S)-nicotine-3’,3’-d2 in humans. Biological Mass Spectrometry. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiology & Biomarkers of Prevention. 2005;14:2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. doi:14/12/2963. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Caporaso N, Hatsukami DK, Joseph AM, Hecht SS. The association of a tobacco-specific biomarker and cigarette consumption and its dependence on host characteristics. Cancer Epidemiology & Biomarkers of Prevention. 2007;16:1852–1857. doi: 10.1158/1055-9965.EPI-07-0018. doi:16/9/1852. [DOI] [PubMed] [Google Scholar]
- Luo Z, Alvarado GF, Hatsukami DK, Johnson EO, Bierut LJ, Breslau N. Race differences in nicotine dependence in the Collaborative Genetic Study of Nicotine Dependence (COGEND) Nicotine & Tobacco Research. 2008;10:1223–1230. doi: 10.1080/14622200802163266. doi:794985769. [DOI] [PubMed] [Google Scholar]
- Melikian AA, Djordjevic MV, Chen S, Richie J, Jr., Stellman SD. Effect of delivered dosage of cigarette smoke toxins on the levels of urinary biomarkers of exposure. Cancer Epidemiology & Biomarkers of Prevention. 2007;16(7):1408–1415. doi: 10.1158/1055-9965.EPI-06-1097. doi:16/7/1408. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie J, et al. Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiology & Biomarkers of Prevention. 2009;18:35–41. doi: 10.1158/1055-9965.EPI-08-0744. doi:18/1/35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Stellman SD, Caraballo RS, Richie JP., Jr. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiology & Biomarkers of Prevention. 2009;18:3415–3420. doi: 10.1158/1055-9965.EPI-09-0737. doi:18/12/3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen TK, Spencer SM, Hoskinson RA, Sachs DP, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine & Tobacco Research. 2005;7:581–590. doi: 10.1080/14622200500185199. doi:Q78290J1V1726638. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102:1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. doi:ADD2010. [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. Journal of the American Medical Association. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr., Carmella SG, Muscat JE, Scott DG, Akerkar SA, Hecht SS. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiology & Biomarkers of Prevention. 1997;6:783–790. [PubMed] [Google Scholar]
- Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine & Tobacco Research. 2009;11(10):1216–1225. doi: 10.1093/ntr/ntp126. doi:10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- Sterling TD, Weinkam JJ. Comparison of smoking-related risk factors among black and white males. American Journal of Industrial Medicine. 1989;15:319–333. doi: 10.1002/ajim.4700150307. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Burke GL, Perkins LL, Haley NJ, Friedman GD. Misclassification of smoking status in the CARDIA study: A comparison of self-report with serum cotinine levels. American Journal of Public Health. 1992;82:33–36. doi: 10.2105/ajph.82.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, et al. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults Study. American Journal of Public Health. 1990;80:1053–1056. doi: 10.2105/ajph.80.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Research. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. doi:0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]