Abstract
Introduction:
Long-term smokeless tobacco (ST) use is known to increase the risk for oropharyngeal cancer, heart attack, and stroke. Varenicline has recently been demonstrated to increase ST abstinence rates among Swedish snus users. We have conducted a pilot study to obtain preliminary evidence of efficacy of varenicline for the treatment of ST users in Midwestern United States.
Methods:
We conducted a randomized, placebo-controlled Phase II clinical trial to evaluate the potential efficacy of 12 weeks of varenicline for the treatment of ST users with an a priori decision rule that a 1-tailed p < .20 for the comparison of the primary endpoint was evidence to conclude that future studies were warranted. Subjects were followed for 6 months after randomization.
Results:
We randomized 76 subjects (38 varenicline and 38 placebo). Subjects were similar at baseline with a mean age of 41 years, and all were male. The biochemically confirmed point prevalence tobacco abstinence rates at end of treatment were 55.3% for varenicline and 42.1% for placebo (p = .126) and 47.4% and 31.6% (p = .080), respectively, at 6 months. Point prevalence ST abstinence rates at end of treatment for varenicline were 57.9% and 42.1% for placebo (p = .084) and 57.9% and 31.6% (p = .011), respectively, at 6 months. Varenicline was associated with significantly less craving compared with placebo. Varenicline was well tolerated with nausea and sleep disturbance being the most common side effects.
Conclusions:
Varenicline decreases craving and may be effective for increasing tobacco abstinence rates among ST users. Larger trials may be warranted to confirm these results.
Introduction
Smokeless tobacco (ST) is tobacco consumed orally and not burned. A variety of types of ST are consumed throughout the world. In the United States, the principal types of ST are chewing tobacco (cut tobacco leaves) and snuff (moist ground tobacco), and new ST products are being introduced by cigarette manufacturers (Rogers, Biener, & Clark, 2010). In 2008, 3.5% of the U.S. population ≥12 years of age reported past month use of ST (Substance Abuse and Mental Health Services Administration, 2009).
ST use leads to tobacco dependence and long-term use. Available literature suggests that adverse health consequences may vary by the type of ST used that is strongly associated with geography (i.e., United States, Sweden, and India; Critchley & Unal, 2003). ST consumed in the United States has been associated with significant adverse health consequences, such as oral cancer (Stockwell & Lyman, 1986) and cancer of the kidney (Goodman, Morgenstern, & Wynder, 1986; Muscat, Hoffmann, & Wynder, 1995), pancreas (Muscat, Stellman, Hoffmann, & Wynder, 1997), and digestive system (Henley, Thun, Connell, & Calle, 2005). ST use is also associated with death from coronary heart disease and stroke (Henley et al., 2005).
Given the adverse health consequences of ST use, the increasing promotion of ST as a potential harm-reduction strategy for cigarette smoking (McNeill, 2004; National Institutes of Health, 2006) and the fact that 64% of ST users report the desire to quit (Severson, 1992), the need exists to validate and disseminate effective behavioral and pharmacological therapies for ST users.
Varenicline is a selective nicotinic receptor partial agonist with specificity for the α4β2 nicotine acetylcholine receptor that has demonstrated remarkable efficacy for increasing long-term tobacco abstinence rates in cigarette smokers (Gonzales et al., 2006; Jorenby et al., 2006; Tonstad et al., 2006). Varenicline has recently been demonstrated to increase tobacco abstinence rates among Scandinavian snus users (Fagerstrom, Gilljam, Metcalfe, Tonstad, & Messig, 2010). In order to obtain preliminary evidence of efficacy of varenicline for the treatment of ST users in the United States, we conducted a randomized placebo-controlled clinical trial.
Methods
Study Design
This study was a randomized placebo-controlled clinical trial conducted at the Mayo Clinic in Rochester, MN, and Franciscan Skemp Medical Center in LaCrosse, WI. The Mayo Clinic Institutional Review Board (IRB) and Franciscan Skemp Medical Center IRB approved the protocol prior to recruitment. Subjects were randomized to varenicline or placebo for 12 weeks with follow-up 6 months after randomization. Enrollment took place between April 2009 and August 2010.
Study Population
Subjects were eligible to participate if they (1) were at least 18 years of age; (b) had used ST daily for the past twelve months; (c) identified ST as their primary tobacco product; and (d) had been provided with, understood, and signed the informed consent. Individuals were excluded from study participation if they (a) had used other behavioral or pharmacological tobacco cessation programs in the past thirty days, (b) had self-reported current, untreated depression, or a Beck Depression Inventory (BDI-II) Score of ≥20 (Arnau, Meagher, Norris, & Bramson, 2001; Beck, Steer, & Brown, 1996); (c) had, as defined by the Columbia-Suicide Severity Rating Scale (Posner, 2007; Posner, Oquendo, Gould, Stanley, & Davies, 2007), current nonspecific suicidal thoughts or have a lifetime history of a suicidal attempt defined as “potentially self-injurious act committed with at least some wish to die, as a result of act”; (d) had a history of psychosis or bipolar disorder; (e) were currently pregnant or lactating or of childbearing potential and not willing to use contraception; (f) had another member of their household already participating in this study; (g) were allergic to varenicline; or (h) had a medical history of (a) unstable angina, (b) myocardial infarction within the past three months, (c) cardiac dysrhythmia other than medication-controlled atrial fibrillation or paroxysmal supraventricular tachycardia, or (d) medically treated or untreated hypertension with blood pressure ≥200 systolic or ≥100 diastolic.
Screening and Recruitment
Potential subjects were screened by telephone. If potential subjects passed the phone screen, they were invited to attend an information session at which time the study was explained and informed consent was completed. Subjects who passed the initial screening returned for a baseline visit, which included a medical screening and physical examination and study randomization. Baseline measures included the Fagerström Test for Nicotine Dependence-Smokeless Tobacco (Ebbert, Patten, & Schroeder, 2006). Subjects also completed the Smokeless Tobacco Evaluation Questionnaire (STEQ) based upon the modified Cigarette Evaluation Questionnaire, a 12-item scale assessing the degree to which subjects experience the reinforcing effects of smoking (Cappelleri et al., 2007). The scale has five domains: smoking satisfaction, psychological reward, enjoyment of respiratory tract sensations, craving reduction, and aversion. We modified the scale for ST users, and only subjects reporting ST since the last visit completed the STEQ.
Assignment of Subjects to Condition
A computer-generated randomization sequence assigned subjects in a 1:1 ratio to treatment conditions. Using this randomization schedule, study personnel who did not have any subject contact dispensed the study medication into containers labeled according to study identification number. Consented subjects were assigned the next sequential subject identification number. Study subjects, investigators, and all other study staff were blinded to treatment assignment.
Treatment and Control Conditions
At the baseline visit (randomization), enrolled subjects were assigned to varenicline or matching placebo. Subjects received varenicline at a dose of 0.5 mg once daily for 3 days, which was increased to 0.5 mg twice daily for Days 4–7 and then to a target dose of 1 mg twice daily for 12 weeks of treatment. Subjects were instructed to continue using ST at their usual daily level during the first 7 days of varenicline therapy. Subjects were instructed to set a target quit date (TQD) for the eighth day of therapy.
All subjects received an individualized program containing four sessions of brief behavioral counseling approximately 10 min in duration to assist with tobacco abstinence. Interactions between subjects and project staff during scheduled visits adhered to a standardized set of activities. Behavior change strategies incorporated cognitive behavioral self-management strategies, including making a personal quitting contract, getting support, identifying and building coping strategies for high-risk situations, dealing with withdrawal, understanding and managing negative cognitions, and what to do in the event that lapses occur. Participants received a copy of an intervention manual (“Skip the Dip, Lose the Chew”) developed by our clinical and research team specifically for ST users and used in our previous ST studies. Study assistants used the intervention manual during the individual meetings as a reference source during the study.
Study Endpoints
The primary endpoint was the biochemically confirmed 7-day point prevalence all-tobacco abstinence rate at end of treatment (Week 12) defined as self-reported all-tobacco abstinence in the last seven days confirmed by a urine cotinine <50 ng/ml (Benowitz et al., 2002). ST point prevalence and prolonged abstinence rates were secondary endpoints. Subjects biochemically confirmed abstinent from all tobacco were considered abstinent from ST as were those who self-reported using a tobacco product other than ST but not using ST. Prolonged abstinence from ST was also assessed, and subjects were classified as failing criteria for prolonged ST abstinence if they reported using ST on 7 consecutive days or at least once per week for 2 consecutive weeks following a 2-week grace period after the TQD (Hughes et al., 2003). Point prevalence and prolonged abstinence rates were analyzed at end of treatment (Week 12) and 6 months postrandomization.
Withdrawal and Craving
To record tobacco withdrawal symptoms, subjects were asked to keep a diary for 6 weeks starting at the information session. The daily diary included the Minnesota Nicotine Withdrawal Scale (Hughes, 2007; Hughes & Hatsukami, 1998) modified for ST users, a 9-item measure consisting of the following symptoms rated on a 5-point Likert scale ranging from 0 (not present) to 4 (severe): desire to use tobacco (i.e., craving); anger, irritability, or frustration; anxiety or nervousness; difficulty concentrating; impatience; restlessness; hunger; awakening at night; and depression.
Adverse Events
All observed and self-reported adverse events were documented on case report forms and followed up according to a safety management protocol until the adverse events were resolved or the subject finished the study.
Statistical Analyses
The purpose of a Phase II trial is to decide whether additional studies of the experimental regimen are warranted and to provide preliminary data for designing a larger Phase III clinical trial to confirm efficacy. Although debate exists regarding the value of formal statistical comparisons in Phase II trials, we agree with those who propose that formal comparisons are appropriate under the caveat that Phase II studies are not expected to provide reliable definitive comparisons using a traditional two-sided Type I error rate of 0.05 (Ratain & Sargent, 2009; Rubinstein et al., 2005). For a randomized Phase II trial, a one-sided test with a false-positive (Type I error) rate of 0.20 is considered appropriate for the primary comparison to assess whether additional studies of the given regimen are warranted. In order to be consistent, we report one-tailed p values for treatment comparisons of both primary and secondary tobacco abstinence outcomes. For all other analyses, two-tailed p values are reported.
Tobacco abstinence outcomes were compared between groups using the chi-square test. For these analyses, subjects with missing information were classified as using tobacco. Daily diaries were used to assess nicotine withdrawal symptoms and craving. A composite nicotine withdrawal score was calculated as the mean of the individual withdrawal symptoms. Desire to use tobacco (i.e., craving) was analyzed separately. Baseline scores were calculated using diary data from the 7 days prior to starting medication. Data from the first 4 weeks following the start of medication were analyzed as change from baseline using generalized estimating equations with a lag-1 autoregressive covariance structure used to take into account multiple observations for each subject. Separate analyses were performed for the first week of study medication prior to TQD and the 3 weeks following TQD. The models included main effects for treatment group (varenicline vs. placebo) and time (in days treated as a continuous variable) as well as the time-by-treatment interaction effect. The frequency of adverse events considered to be possibly, probably, or definitely related to study drug were compared between treatment groups using Fisher's exact test. Weight change from baseline to the end of the medication phase was compared between groups using the two-sample t test. Medication adherence was quantified for each subject as the total amount of medication taken divided by the total prescribed dose according to the study protocol and analyzed using a dichotomous endpoint whereby subjects were considered adherent with medication if they took ≥80% of the prescribed dose. The percentage of subjects categorized as being adherent with medication was compared between groups using a chi-square test.
Results
Subjects
Of the 172 individuals calling into our research center for study participation, 139 (81%) passed a telephone screening and were invited to attend the information session. Of the 139 potential subjects invited, 85 (61%) attended and consented to study. Of the 85 who consented to study, 84 (99%) passed the initial study screen of whom 76 (90%) were randomized. Enrolled subjects were all male, and other baseline subject characteristics were similar between treatment groups (Table 1). Of the 76 subjects enrolled, 12 (16%; 6 varenicline and 6 placebo) discontinued study participation prior to the end-of-medication phase. The reasons for discontinuation included adverse events (zero varenicline and one placebo), consent withdrawn (two varenicline and three placebo), loss to follow-up (three varenicline and one placebo), and scheduling difficulties (one varenicline and one placebo).
Table 1.
Baseline Demographicsa
| Characteristic | Varenicline, N = 38 | Placebo, N = 38 |
| Age, years | 40.7 ± 10.1 | 41.0 ± 12.4 |
| Male, n (%) | 38 (100) | 38 (100) |
| Caucasian, n (%) | 38 (100) | 36 (95) |
| Marital status, n (%) | ||
| Married/living as married | 28 (74) | 28 (74) |
| Never married | 6 (16) | 6 (16) |
| Separated/divorced | 4 (11) | 3 (8) |
| Other | 0 (0) | 1 (3) |
| Highest level of education, n (%) | ||
| ≤High-school graduate | 7 (18) | 10 (26) |
| Some college | 21 (55) | 17 (45) |
| College graduate | 10 (26) | 11 (29) |
| FTND-ST | 5.2 ± 2.3 | 5.0 ± 2.2 |
| ST used per week, cans/pouches | 4.0 ± 3.5 | 3.2 ± 2.0 |
| Years of regular ST use, years | 19.1 ± 12.2 | 18.5 ± 10.4 |
| Current use of other tobacco productsb, n (%) | 4 (11) | 1 (3) |
| Close friends who use ST, n (%) | 29 (76) | 25 (66) |
| Number of serious stop attempts, n (%) | ||
| 0 | 9 (24) | 3 (8) |
| 1–2 | 9 (24) | 12 (32) |
| 3–4 | 7 (18) | 10 (26) |
| 5+ | 13 (34) | 13 (34) |
| Confidence in not using ST one year from now, n (%) | ||
| Not at all confident | 0 (0) | 0 (0) |
| Not very confident | 2 (5) | 0 (0) |
| Somewhat confident | 17 (45) | 13 (34) |
| Very confident | 17 (45) | 19 (50) |
| Completely confident | 2 (5) | 6 (16) |
| Contemplation ladder | 8.5 ± 1.6 | 8.4 ± 1.4 |
Note. FTND-ST = Fagerström Test for Nicotine Dependence-Smokeless Tobacco; ST = smokeless tobacco.
Data are presented as M ± SD or n (%) as indicated.
One subject in the placebo group reported smoking cigarettes (3 cigarettes\day [cpd]), two subjects in the varenicline group reported smoking cigarettes (<1 cpd and 2 cpd), one subject in the varenicline group reported smoking cigars (<1 per day), and one subject in the varenicline group reported smoking both pipe and cigar (both <1 per day).
Abstinence
Varenicline was associated with higher all tobacco and ST abstinence rates at end of treatment and 6 months (Table 2).
Table 2.
Abstinence Outcomesa
| n (%) Varenicline, N = 38 | n (%) Placebo, N = 38 | p Valueb | |
| End of treatment | |||
| Point prevalence ST abstinence | 22 (57.9) | 16 (42.1) | .084 |
| Point prevalence all-tobacco abstinence | 21 (55.3) | 16 (42.1) | .126 |
| Prolonged ST abstinence | 20 (52.6) | 15 (39.5) | .125 |
| 6 months | |||
| Point prevalence ST abstinence | 22 (57.9) | 12 (31.6) | .011 |
| Point prevalence all-tobacco abstinence | 18 (47.4) | 12 (31.6) | .080 |
| Prolonged ST abstinence | 17 (44.7) | 12 (31.6) | .119 |
Note. ST = smokeless tobacco.
Subjects met criteria for point prevalence all-tobacco abstinence if they reported not using any tobacco in the last seven days and had a urine cotinine <50 ng/ml. For point prevalence ST abstinence, subjects were considered abstinent if they were biochemically confirmed abstinent from all tobacco or if they reported using only non-ST tobacco products. To meet criteria for prolonged ST abstinence, subjects had to meet criteria for 7-day point prevalence ST abstinence and also report not using ST for 7 consecutive days or at least once each week on 2 consecutive weeks, since 2 weeks following their TQD. In all cases, subjects with missing information were assumed to be using ST.
One-tailed chi-square test.
Withdrawal Symptoms and Craving
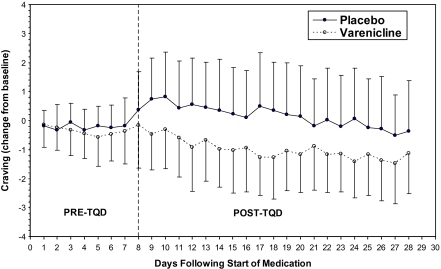
For the first week after starting medication (prior to TQD), changes in craving were small and similar between groups (Figure 1). After TQD, craving decreased with time (time effect = −0.06, SE = 0.02, p = .001) and was significantly less in those assigned varenicline versus placebo (treatment effect = −1.27, SE = 0.47, p = .006) with no evidence of a time-by-treatment interaction (time-by-treatment interaction = 0.01, SE = 0.02, p = .786). No significant effects were detected in the analysis of composite nicotine withdrawal scores.
Figure 1.
Craving on the Minnesota Nicotine Withdrawal Scale modified for smokeless tobacco users
Adverse Events
Nausea and sleep disturbance were the most commonly reported adverse effects associated with varenicline (Table 3).
Table 3.
Adverse Eventsa
| Event | n (%) Varenicline, N = 38 | n (%) Placebo, N = 38 |
| Nausea | 9 (23.7)b | 0 (0.0)b |
| Sleep disturbance | 7 (18.4) | 3 (7.9) |
| Vivid dreams | 3 (7.9) | 1 (2.6) |
| Headache | 1 (2.6) | 1 (2.6) |
| Confusion | 0 (0.0) | 1 (2.6) |
| Constipation | 1 (2.6) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 1 (2.6) |
| Dizziness | 1 (2.6) | 0 (0.0) |
| Drug reaction | 1 (2.6) | 0 (0.0) |
| Irritability | 1 (2.6) | 0 (0.0) |
| Mood disturbance | 0 (0.0) | 1 (2.6) |
| Restlessness | 0 (0.0) | 1 (2.6) |
Note. aAdverse events considered to be possibly, probably, or definitely related to study drug are summarized according to treatment group. Overall, there were 15 subjects in the varenicline group and 5 subjects in the placebo group who reported one or more adverse events, which were considered to be possibly, probably, or definitely related to study drug.
Fisher's exact test p = .002 comparing varenicline versus placebo.
Weight Gain
Among subjects who met criteria for prolonged abstinence at end of medication, the mean ± SD weight change from baseline to Week 12 did not differ significantly between groups (3.0 ± 2.9 kg for varenicline vs. 2.9 ± 3.0 kg for placebo; p = .924). Weight change from baseline to 6 months for subjects who met the criteria for prolonged abstinence at 6 months was also similar between treatment groups (3.9 ± 3.0 kg for varenicline vs. 4.1 ± 2.8 kg for placebo; p = .847).
Medication Adherence
Medication use was quantified for each subject as the total amount of medication taken expressed as a percentage of the total prescribed dose over the 12-week medication phase. In an analysis that included all subjects, the median percentage of medication taken was 94.2% for varenicline and 97.8% for placebo. The percentage of subjects who took >80% of the prescribed dose of medication was 76.3% (29/38) for varenicline and 71.1% (27/38) for placebo (p = .602). Among subjects who did not discontinue study participation prior to the end-of-medication phase, the percentage who took >80% of the prescribed dose of medication was 90.6% (29/32) for varenicline and 84.4% (27/32) for placebo (p = .450).
Discussion
We observed that varenicline was associated with higher point prevalence and prolonged tobacco and ST abstinence rates at end of treatment and 6 months among a sample of ST users in Midwestern United States. Side effects of nausea and sleep disturbance were consistent with the known common adverse effects reported with varenicline (Gonzales et al., 2006; Jorenby et al., 2006).
Similar to previous studies with varenicline for smoking cessation, we observed that varenicline decreased tobacco craving (Gonzales et al., 2006; Jorenby et al., 2006). However, we did not observe that varenicline had an impact on other withdrawal symptoms. Indeed, the effect of varenicline on other withdrawal symptoms (i.e., restlessness, negative affect) among cigarette smokers was small (Gonzales et al., 2006; Jorenby et al., 2006). Also similar to these previous trials, we did not observe an effect of varenicline on postcessation weight gain.
Varenicline is known to be efficacious among cigarette smokers, so why would it not be efficacious among ST users? We have observed that pharmacotherapies such as bupropion and nicotine replacement therapy (NRT; i.e., gum and patch) with known efficacy for increasing long-term (≥6 months) tobacco abstinence rates among cigarette smokers have not been shown to be efficacious for ST users (Ebbert et al., 2007). A number of hypotheses have been proposed for the lack of pharmacotherapeutic efficacy in ST users (Ebbert, Severson, Croghan, Danaher, & Schroeder, 2009) including (a) a “ceiling effect” for pharmacotherapy occurs when provided along with behavioral counseling, the efficacy of which produces high control group abstinence rates; (b) NRT may play a different or less important role in reducing the reinforcement of ST use; (c) underreplacement of serum nicotine concentrations with NRT; (d) a “priming” effect may occur with NRT, which may increase the risk for relapse; and (e) the nicotine gum similarity to ST (i.e., oral consumption) may increase the risk for relapse (Hatsukami, Jensen, Allen, Grillo, & Bliss, 1996; Hatsukami et al., 2000). In this regard, varenicline provides a novel mechanism for the treatment of tobacco dependence in ST users. The mechanism of action and preparation (i.e., pill form) of varenicline may circumvent the concerns of underreplacement, priming, and similarity in nicotine delivery with forms of NRT. Indeed, varenicline was observed to increase tobacco abstinence rates among Scandinavian snus users (Fagerstrom et al., 2010), but high placebo abstinence rates were observed (44% overall).
Since varenicline was observed to be efficacious among Scandinavian snus users (Fagerstrom et al., 2010), why would it not be effective among ST users in the United States? The results from the Scandinavian suggest that varenicline may be effective for the treatment of all ST users. However, important differences in health risks may exist between ST made in Sweden and in the United States. Cancer-related adverse health consequences of ST use are related to the more than 30 carcinogens present in ST, and strong evidence exists for the role of tobacco-specific nitrosamines (TSNAs) in the causation of cancer (Boffetta, Hecht, Gray, Gupta, & Straif, 2008). TSNAs have become a reference group of carcinogens in these products, and TSNA levels have defined the degree of risk (Stepanov, Jensen, Hatsukami, & Hecht, 2008). Analyses have demonstrated significantly higher levels of TSNAs in U.S. snuff compared with Swedish snus (Stepanov et al., 2008). Differences in TSNA concentrations between United States and Swedish ST probably account for the epidemiological data demonstrating increased risk of oral cancer (relative risk [RR]: 2.6; 95% CI: 1.3–5.2) in the United States and Asia, which has not been observed in northern European studies (RR: 1.0; 95% CI: 0.7–1.3; Boffetta et al., 2008). Because of these differences in health risks, we submit that it is important to validate the Scandinavian findings among U.S. ST users. Furthermore, the population of ST users seeking treatment in our study (i.e., all male, average age 41 years) is modestly different than those enrolled in the recent Scandinavian trial (i.e., 10% female, average age 44 years), suggesting that demographic differences may exist between the two populations.
The major strengths of our study are the randomized placebo-controlled design and the biochemical verification of tobacco abstinence. Our major limitation was the sample size. However, we designed this study as a Phase II clinical study to provide preliminary data for designing a larger Phase III trial to confirm efficacy and to decide whether additional studies of the experimental regimen are warranted. Additional studies of varenicline for the treatment of ST users in the United States may be warranted.
Funding
This work was supported by the National Cancer Institute Grant # CA132621 (Principal Investigator: JOE). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Declaration of Interests
None declared.
Acknowledgments
We would like to thank the subjects who participated in this research and the staff of the Mayo Clinic Nicotine Research Program who made this project possible. Research performance sites: Mayo Clinic College of Medicine, 200 1st Street SW, Rochester, MN 55905, USA.
References
- Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychology. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. doi:10.1037/0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncology. 2008;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. doi:10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addictive Behaviors. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. doi:10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Critchley JA, Unal B. Health effects associated with smokeless tobacco: A systematic review. Thorax. 2003;58:435–443. doi: 10.1136/thorax.58.5.435. doi:10.1136/thorax.58.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Montori V, Vickers KS, Erwin PC, Dale LC, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews. 2007;(4):CD004306. doi: 10.1002/14651858.CD004306.pub3. doi:10.1002/14651858.CD004306.pub3. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Patten CA, Schroeder DR. The Fagerstrom Test for Nicotine Dependence-smokeless tobacco (FTND-ST) Addictive Behaviors. 2006;31:1716–1721. doi: 10.1016/j.addbeh.2005.12.015. doi:10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A randomized clinical trial of nicotine lozenge for smokeless tobacco use. Nicotine & Tobacco Research. 2009;11:1415–1423. doi: 10.1093/ntr/ntp154. doi:10.1093/ntr/ntp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: Randomised double blind placebo controlled trial. British Medical Journal. 2010;341:c6549. doi: 10.1136/bmj.c6549. doi:10.1136/bmj.c6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi:10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Goodman MT, Morgenstern H, Wynder EL. A case-control study of factors affecting the development of renal cell cancer. American Journal of Epidemiology. 1986;124:926–941. doi: 10.1093/oxfordjournals.aje.a114482. Retrieved from http://aje.oxfordjournals.org/content/124/6/926.short. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Grillo M, Boyle R, Allen S, Jensen J, Bliss R, et al. Treatment of spit tobacco users with transdermal nicotine system and mint snuff. Journal of Consulting and Clinical Psychology. 2000;68:241–249. doi:10.1037/0022-006X.68.2.241. [PubMed] [Google Scholar]
- Hatsukami DK, Jensen J, Allen S, Grillo M, Bliss R. Effects of behavioral and pharmacological treatment on smokeless tobacco users. Journal of Consulting and Clinical Psychology. 1996;64:153–161. doi: 10.1037//0022-006x.64.1.153. doi:10.1037/0022-006X.64.1.153. [DOI] [PubMed] [Google Scholar]
- Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes and Control. 2005;16:347–358. doi: 10.1007/s10552-004-5519-6. doi:10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Minnesota Nicotine Withdrawal Scale—Revised. 2007. Retrieved from http://www.uvm.edu/∼hbpl/?Page=minnesota/default.html. [Google Scholar]
- Hughes JR, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. doi:10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. doi:10.1080/1462220031000070552. [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- McNeill A. Harm reduction. British Medical Journal. 2004;328:885–887. doi: 10.1136/bmj.328.7444.885. doi:10.1136/bmj.328.7444.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Hoffmann D, Wynder EL. The epidemiology of renal cell carcinoma. A second look. Cancer. 1995;75:2552–2557. doi: 10.1002/1097-0142(19950515)75:10<2552::aid-cncr2820751023>3.0.co;2-1. doi:10.1002/1097-0142(19950515) [DOI] [PubMed] [Google Scholar]
- Muscat JE, Stellman SD, Hoffmann D, Wynder EL. Smoking and pancreatic cancer in men and women. Cancer Epidemiology, Biomarkers and Prevention. 1997;6:15–19. doi:10.1158/1055-9965.EPI-03-0033. [PubMed] [Google Scholar]
- National Institutes of Health. National Institutes of Health state-of-the-science conference statement: Tobacco use: Prevention, cessation, and control. Annals of Internal Medicine. 2006;145:839–844. doi: 10.7326/0003-4819-145-11-200612050-00141. Retrieved from http://www.annals.org/content/145/11/839.long. [DOI] [PubMed] [Google Scholar]
- Posner K. Suicidality issues in clinical trials: Columbia suicide adverse event identification in FDA safety analyses. 2007. Retrieved from http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4306s1-01-CU-Posner.ppt. [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. doi:10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratain MJ, Sargent DJ. Optimising the design of phase II oncology trials: The importance of randomisation. European Journal of Cancer. 2009;45:275–280. doi: 10.1016/j.ejca.2008.10.029. doi:10.1200/JCO.2010.29.3787. [DOI] [PubMed] [Google Scholar]
- Rogers JD, Biener L, Clark PI. Test marketing of new smokeless tobacco products in four U.S. cities. Nicotine & Tobacco Research. 2010;12:69–72. doi: 10.1093/ntr/ntp166. doi:10.1093/ntr/ntp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. Journal of Clinical Oncology. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. doi:10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- Severson HH. Enough snuff: ST cessation from the behavioral, clinical, and public health perspectives. 1992. Monograph 2: Smokeless Tobacco or Health: An International Perspective (NIH Publication No. 93-3461): National Institutes of Health. Retrieved from http://cancercontrol.cancer.gov/tcrb/monographs/2/index.html. [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research. 2008;10:1773–1782. doi: 10.1080/14622200802443544. doi:10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell HG, Lyman GH. Impact of smoking and smokeless tobacco on the risk of cancer of the head and neck. Head & Neck Surgery. 1986;9:104–110. doi: 10.1002/hed.2890090206. doi:10.1002/hed.2890090206. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: National findings. 2009. (NSDUH Series H-36). Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration (SAMHSA) [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:64–71. doi: 10.1001/jama.296.1.64. doi:10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]