Abstract
It is difficult for rosacea patients to discern which products and ingredients will be beneficial to their skin and which products will lead to an exacerbation of the signs and symptoms of rosacea. In this paper, the authors provide a brief overview of rosacea, its pathogenesis, signs and symptoms, and the management of the two major rosacea subtypes—erythematotelangiectatic rosacea and papular pustular rosacea. Reviewed in greater detail are the common ingredients used in over-the-counter cleansers and moisturizers with discussion of how these ingredients potentially benefit or harm the skin of patients with rosacea. Clinical studies investigating the benefits of using certain over-the-counter cleansers and moisturizers in patients with erythematotelangiectatic rosacea and papular pustular rosacea with or without topical prescription therapy are also reviewed. The specific formulas used in the clinical studies include a sensitive skin synthetic detergent bar, a nonalkaline cleanser and moisturizer, polyhydroxy acid containing cleanser and moisturizer, and a ceramide-based cleanser and moisturizer formulated in a multivesicular emulsion. Based on review of available data, the authors conclude that the use of mild over-the-counter cleansers and moisturizers is beneficial for patients with erythematotelangiectatic rosacea and papular pustular rosacea. The properties of over-the-counter cleansers and moisturizers that contribute to their mildness include an acidic-neutral pH to minimize the flux in skin pH; surfactants or emulsifiers that will not strip the skin of its moisture or strip the lipids and proteins of the stratum corneum; moisturizing ingredients such as emollients, humectants, and occlusives; and formulas without potential irritants and allergens. The most consistent clinical benefits demonstrated in the reviewed studies were a subjectively perceived improvement in subjective symptoms of dryness and irritation as well as an objective improvement in dryness.
A common myth among the general public is that all over-the-counter (OTC) cleansers and moisturizers are created equal. This, however, is not the case. Many healthcare providers overlook the importance of OTC cleansers and moisturizers in the management of skin disease and may not consider their components to be actively involved in producing therapeutic benefit. Yet, use of OTC cleansers and moisturizers can potentially have multiple beneficial or deleterious effects on the skin and can induce changes in the superficial and deep layers of the epidermis.1 It is the specific ingredients in the formula and the properties of the formulation as a whole that determine whether the cleanser or moisturizer is truly a mild product and if the product will impact the skin, especially the integrity and function of the stratum corneum (SC), in a positive or negative manner.2
Because all cleansers and moisturizers comprise many different ingredients with diverse properties, it is difficult for rosacea patients to discern which products and ingredients will be beneficial to their skin and which products will lead to an exacerbation of the signs and symptoms of rosacea. It is our responsibility as dermatologists to guide our patients, especially those with rosacea who are innately prone to irritation and inflammation, as to which OTC products will benefit and not irritate their skin.3,4 In addition, it is also important to educate patients with rosacea that the daily use of a mild OTC cleanser and moisturizer, as specified by the dermatologist or a designated assistant, is an integral part of managing rosacea. Proper skin care in rosacea has been shown to contribute to improving signs and symptoms and can reduce potential skin irritation that is sometimes caused by topical medications.4
This article provides a brief overview of rosacea and the major subtypes—erythematotelangiectatic rosacea (ETR) and papular pustular rosacea (PPR). Also reviewed are the basic ingredients used in OTC cleansers and moisturizers with discussion of how specific ingredients can potentially benefit or harm the skin of rosacea patients. Scientific summaries of clinical trials that investigate the impact of using OTC cleansers and moisturizers in the treatment of ETR and PPR, with or without concurrent prescription medical therapy, are reviewed. Ultimately, the goal is to assist the clinician in discerning what benefits can be expected from routinely integrating specific OTC cleansers and moisturizers in the management of patients with ETR or PPR.
CLINICAL SUBTYPES OF ROSACEA DEFINED
Rosacea is actually an umbrella term for multiple clinical subtypes of rosacea that at times have overlapping features. In general, rosacea consists of the following five major subtypes: erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea, ocular rosacea, and granulomatous rosacea.
The two major subtypes are ETR and PPR, with the former being the most commonly encountered rosacea subtype (ETR:PPR ratio approximately 7:3).3 Similar to the way asteatotic dermatitis differs in its presenting symptoms from contact dermatitis, PPR differs in its signs and symptoms from ETR. The common thread between classic forms of cutaneous rosacea is a persistent erythema of the central portion of the face, while the other co-occurring signs, such as flushing, papules or pustules, ocular findings, phymatous changes, and/or telangiectasias, and symptoms of burning, stinging, and/or dryness dictate the subtype of the disease.3 This article focuses on the ETR and PPR subtypes of rosacea.
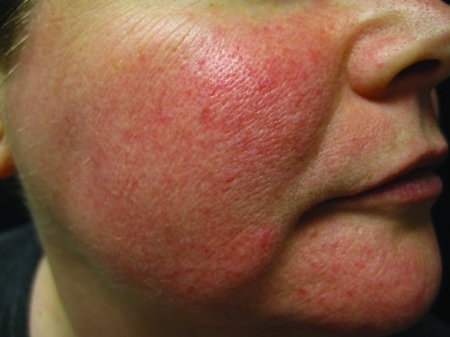
ETR presents as diffuse and confluent macular to slightly edematous central facial erythema with some telangiectasias present, but with absence of inflammatory papules, pustules, or nodules (Figure 1). The intensity of erythema in ETR may fluctuate; however, some persistence of fixed background macular erythema is always present (“fixed erythema” or “rouge erythema”). In ETR, patients typically describe a flushing of the central face that lasts longer than 10 minutes. The flushing characteristically spares the periocular skin but may involve the peripheral face. The flushing episodes can be caused by emotional stress, hot drinks, alcohol, spicy foods, exercise, cold or hot weather, hot baths or showers or without known stimuli. Other signs that accompany this subtype include telangiectasias and skin roughness with associated fine scaling in some cases. Patients often describe themselves as having dry and sensitive skin, and often relate skin tightness, dryness, itching, and burning and/or stinging without treatment or from topically applied substances including those meant to relieve redness and irritation.3–5
Figure 1.
Erythematotelangiectatic rosacea
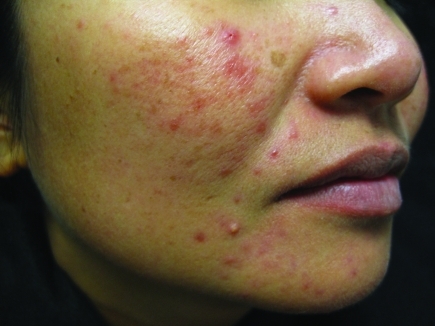
PPR is characterized by multiple inflammatory papules, pustules, and occasionally a few nodules involving predominantly the central face, perilesional erythema, absence of comedones, absence of associated scarring, and usually diffuse and confluent central facial erythema that may be macular or edematous (Figure 2). A history of flushing and irritation from external stimuli can be present in PPR patients, although these symptoms are usually milder than in ETR patients and present less commonly.5–7
Figure 2.
Papulopustular rosacea
The visible signs and symptoms of untreated PPR were collated in an experiment after a mandated washout period where the PPR patients refrained from using any topical or systemic therapies that may treat or impact the appearance or symptoms of PPR. The data from this experiment is summarized in Table 1. The findings depicted in Table 1 represent the signs and symptoms that are innately associated with untreated PPR graded in most cases as moderate in severity.
TABLE 1.
Baseline facial skin findings in patients with papulopustulatr rosacea. Signs and symptoms at baseline prior to treatment with topical therapies*
| CLINICAL CHARACTERISTICS | GROUP A, % (n=457) | GROUP B, % (n=127) | GROUP C, % (n=331) |
|---|---|---|---|
| Skin dryness | 65 | 66 | 69 |
| Scaling+ | 51 | 58 | 57 |
| Itching | 49 | 51 | 52 |
| Edema | 36 | 32 | 38 |
| Burning | 34 | 33 | 36 |
| Stinging | 29 | 34 | 29 |
| Pain (skin discomfort) | 17 | 14 | 21 |
Subjects washed out from all previous systemic and topical therapies and on no therapies felt to potentially impact the appearance or symptoms of PPR
Scaling reflective of baseline skin status in PPR and not sebhorreic dermatitis which was a study exclusion
Data adapted from references 80 and 81
The pathogenesis of rosacea is complex and multifactorial. Genetic disposition, dysfunction of the innate immune system, vascular changes and hyper-reactivity, skin barrier dysfunction, and microbial imbalance and growth may all play a role in the development of various subtypes of rosacea.3–5 Excessive ultraviolet (UV) exposure and photodamage may also play a role in the pathogenesis of ETR; however, the role of UV light in the pathogenesis of PPR is less certain.8–10
Disruption of the SC permeability barrier and its function may play an integral role in the symptoms of dryness and skin sensitivity experienced by rosacea patients. Dirschka et al11 found diminished SC barrier function evidenced by increased transepidermal water loss (TEWL) values in PPR patients (n=75) in areas of clinically involved skin, such as the perinasal cheek (p=0.001) and side of the nose (p=0.006), while finding a comparable decrease in TEWL values in areas of clinically healthy skin, such as the lateral cheek (p=0.61).11 The impaired permeability barrier function in PPR and ETR patients has also been demonstrated with the skin irritation test. Lonne-Rahm et al12 conducted a study involving 7 ETR, 25 PPR, and 32 healthy skin controls.12 In this study, a positive sting test reaction with 5% lactic acid was found in 100 percent of ETR patients, 68 percent of PPR patients, and 19 percent of control patients. The results from these two studies suggest decreased SC permeability barrier function in ETR and PPR compared to healthy skin, and also imply that decreased barrier function and skin sensitivity are more common in ETR patients compared to PPR patients. However, these conclusions would be stronger if the study by Dirschka et al11 had compared TEWL values of ETR patients and PPR patients to healthy central facial skin of normal control patients.
CONVENTIONAL MEDICAL THERAPIES FOR ROSACEA
General skin care and therapeutic strategies for patients with rosacea designed to restore the functional integrity of the SC, reduce facial redness, diminish inflammation, and suppress formation or activity of reactive oxygen species (ROS) can generally be applied to all cutaneous rosacea subtypes, although medical therapies have been most effective in PPR and the inflammatory phase of the phymatous subtype.13
The standard prescription topical therapies for PPR primarily include metronidazole, azelaic acid, and sulfacetamide-sulfur formulations. Oral therapy for PPR has been addressed for more than four decades by certain oral antibiotics, primarily tetracyclines (e.g., tetracycline, doxycycline, minocycline), and to a lesser extent macrolide derivatives (e.g., erythromycin, azithromycin). At present, doxycycline is commonly used for oral treatment of PPR, with a notable increase in the prescribing of subantimicrobial dose doxycycline (20mg twice daily; 40mg once daily using modified-release capsule) by dermatologists due to studies demonstrating therapeutic benefit for PPR without the need for an antibiotic effect. It is currently common belief that the above medical therapies are efficacious primarily due to their anti-inflammatory properties, although some authors purport that antimicrobial properties of some agents may play a role despite the lack of definitive evidence that bacteria are a component of rosacea pathogenesis.13
There is a considerable body of evidence to support that improper skin care can incite or worsen visible signs and symptoms of PPR and ETR, and that properly selected skin care contributes to the improvement of both disorders.2–4,11–16,19–22 Both female and male patients with rosacea commonly report that their skin is often very sensitive to many skin care and personal use products (Tables 2–4). It is thought that mild OTC cleansers and moisturizers may compliment prescription therapy in both ETR and PPR subtypes by promoting health and repair of the epidermis via gentle cleansing, hydration, and the use of gentle ingredients that will not aggravate their already inflamed and sensitive skin.
TABLE 2.
Skin care products and skin sensitivity in rosacea: Summary of overall findings
| National Rosacea Society Survey Completed in 1997 |
|---|
1,023 patients with rosacea
|
Data reported from: Torok HM. Rosacea skin care. Cutis. 2000;66(Suppl 4):14-16.
TABLE 4.
Skin care products and skin sensitivity in rosacea: Male respondents from National Rosacea Society 1997 Survey
| PRODUCT | % (N=1,023) |
|---|---|
| Soap | 24% |
| Cologne | 19% |
| Shaving lotion | 24% |
| Sunscreen | 13% |
| Shampoo | 12% |
| IRRITATING INGREDIENTS (SUSPECTED) | |
| Alcohol | 66% |
| Witch hazel | 30% |
| Fragrances | 29.5% |
| Menthol | 21% |
| Peppermint oil | 14% |
| Eucalyptos oil | 13% |
Data reported from: Torok HM. Rosacea skin care. Cutis. 2000;66(Suppl 4):14–16.
TABLE 3.
Skin care products and skin sensitivity in rosacea: Female respondents from National Rosacea Society 1997 Survey
| PRODUCT | % (N=1,023) |
|---|---|
| Astringents and toners | 49.5% |
| Soap | 40% |
| Exfoliating agents | 34% |
| Makeup | 29% |
| Perfume | 27% |
| Moisturizers | 25.5% |
| Hairspray | 20% |
| Shampoo | 12% |
Data reported from: Torok HM. Rosacea skin care. Cutis. 2000;66(Suppl 4):14–16.
SKIN CLEANSERS
Importance of a mild OTC cleanser for rosacea patients. The ideal cleanser would function by removing dirt, oil, environmental pollutants, and harmful bacteria from skin without disrupting or removing the beneficial lipids, proteins, and normal flora that contribute to the integrity, function, and health of normal skin. However, this is often not the case. Strong OTC cleansers that are efficient at removing dirt, oil, and bacteria cannot distinguish between good and bad lipids, proteins, or bacteria. Therefore, strong cleansers that provide an efficient skin cleaning are also, in essence, damaging the SC by damaging or stripping some of its essential components, such as lipids, proteins, and natural moisturizing factor (NMF).14 Because ETR and PPR patients already have a compromised SC permeability barrier and sensitive skin, it is recommended that rosacea patients use mild OTC cleansers that do not exacerbate their skin disease. Recognition of the importance of mild cleansers in the management of rosacea and other sensitive skin diseases has spurred the development of mild therapeutic cleansers with the sole purpose of gently cleansing the skin without stripping the skin of its functional components.
OTC cleansers, their ingredients, and what they do to the stratum corneum. There are four general categories of skin cleansing agents including soaps, synthetic detergent (syndet) bars and liquid cleansers, combination bar (combar) antimicrobials, and lipid-free liquid cleansers.
True soap. True soap (soap) is created through a process called saponification, which is the chemical reaction that occurs when a fat, such as tallow (beef fat), and an alkali, such as lye, are combined to create long chain fatty acid alkali salts. The typical pH of a true soap is 9 to 10.15 Soaps are strong OTC cleansers that do an excellent job of removing skin sebum and debris. Yet, in the process, soaps can remove beneficial intercellular lipids and damage SC proteins. Removing these beneficial lipids and proteins impairs the SC as evidenced by an increase in TEWL, dehydration, altered desquamation, and increased penetration of topically applied substances, therefore increasing skin sensitivity and irritation in rosacea patients.15
Synthetic detergent (syndet) cleansers. Syndet bars are synthetic detergent-based cleansers that contain less than 10 percent of soap and typically have a more neutral/acidic pH (5.5–7) similar to the pH of normal skin.15,16 Syndet bars in general are designed to provide an effective skin cleaning with minimal stripping of essential SC lipids and proteins, ultimately making these types of cleansing bars less irritating and drying than the traditional soap bars. Syndet liquid cleansers are also available.
In an experiment done on ex-vivo arm skin, the ultrastructural skin changes were monitored after washing with a soap and mild syndet bar using a combination of measurements including TEWL, environmental scanning electron microscopy, and transmission electron microscopy.17,18 The results of this study clearly demonstrated changes to the skin's ultrastructure after multiple washes with the soap bar. Environmental electron microscopy revealed changes in the skin surface morphology including a significant uplifting of cells and increase in surface roughness after washing with the soap. In addition, transmission electron microscopy revealed significant damage to both lipid and protein regions after washing with the soap bar. In contrast, under the same conditions, the syndet-washed skin showed well-preserved surface morphology and well-preserved lipid and protein regions. The study also demonstrated a correlation between high TEWL and damage to SC ultrastructure after use of the soap bar, clearly illustrating the potential for soap to damage the SC and the mildness of the syndet bar in comparison.17,18
Combination bars (combars). Combars are antibacterial soap bars that contain a combination of true soap surfactants and syndet bar surfactants with an added antibacterial agent. Although the antibacterial agents in combars are beneficial for reducing harmful bacteria, they may also eradicate the normal flora of the skin and can cause an increase in skin dryness and irritation.19 Since patients with rosacea may already have an imbalance of skin flora and problems with skin dryness and sensitivity,8 in general, combars are not ideal for patients with rosacea.
Lipid-free liquid cleansers. Lipid-free liquid cleansers are very mild as they clean without soap formation and are designed to leave behind a thin moisturizing film on the skin.19 Evidence that supports the use of several lipid-free cleansers in rosacea patients will be presented in the next sections of this paper.
Given the sensitive nature of ETR and PPR skin, lipid-free cleansers and syndet cleansers are well suited for rosacea patients. However, within the lipid-free cleanser and syndet bar subtypes there are several other properties of these cleansers that determine their mildness. These properties include the type of surfactant used in the cleanser, the extent of surfactant interaction with skin proteins and lipids, the pH of the cleanser, and the extent of skin hydration or dehydration caused by cleansing.20–22
Surfactants. Surfactants are the principle ingredients in cleansers responsible for removing oil and debris from the skin surface. The extent to which a surfactant is able to “clean” the skin is relative to its critical micelle concentration (CMC). The CMC is a measure of a surfactant's efficacy in solubilizing dirt and oil on the skin and dispersing them into solution. The lower the CMC, the higher the efficacy of the surfactant and the lower the amount needed in the cleanser formula. However, a high cleansing efficacy (or a low CMC) usually correlates with an increased number of deleterious effects on the SC integrity and function.15
Surfactants are subdivided into the following four main groups based on their molecular charge or lack of molecular charge: anionic, cationic, amphoteric, and nonionic.21–24 According to Ananthapadmanabhan et al15 and Effendy et al,24 the order of surfactant potential for SC alteration and skin irritation is cationic=anionic>amphoteric>nonionic. However, it cannot be generalized that all cationic and anionic surfactants are the most irritating group of surfactants to the skin because the specific surfactant used, in addition to its molecular charge, has a major effect on irritant potential. For example, when comparing the irritation potential and penetration of two anionic surfactants, sodium lauryl sulfate and sodium cocoyl isethionate, sodium lauryl sulfate can cause significant skin irritation and penetration while sodium cocoyl isethionate has shown excellent skin compatibility.21,25 Despite the potential for some anionic surfactants to irritate the skin and diminish skin health, they are still the primary surfactants used today, even in mild cleanser formulations, due to their excellent foaming and lathering characteristics. Cationic surfactants, such as benzalkonium chloride, can have high irritant and cytotoxic effects on the skin.24 Therefore cationic surfactants are generally used in antimicrobial washes due to their excellent antimicrobial properties.24
To minimize the irritation potential of anionic and cationic surfactants in a cleansing formula, these surfactants can be used in small amounts and can be combined with other amphoteric and nonionic surfactants to minimize their irritation potential and negative effects on the stratum corneum.15,24,26 For example, sodium laureth sulfate, an anionic surfactant that is a close relative to sodium lauryl sulfate, is often used in combination with the amphoteric surfactant cocamidopropylbetaine. It is assumed that the use of the anionic and amphoteric surfactants results in a milder cleansing formula with a decreased anionic surfactant concentration, decreased skin irritation, and decreased interaction with skin proteins and lipids.15,24
Surfactants and skin proteins. Surfactants that interact with SC proteins are deleterious to overall skin health because of their ability to disrupt and damage the proteins of the SC. This insult to the SC proteins can result in changes in SC integrity leading to cutaneous irritation, inflammation, and dessication, all of which can ultimately lead to visible changes associated with dryness and irritation. It is thought that interaction of the charged (polar) heads of the surfactants with the charged proteins of the SC facilitates the penetration of surfactants as well as other cleanser ingredients into the deeper skin layers. The deeper penetration of these ingredients can cause chemical irritation or an inflammatory biochemical response in healthy skin, or in the case of rosacea patients, exacerbate inflammation and irritation that may already be present depending on the current magnitude of underlying rosacea at that time.27–29 However, for a surfactant with a given chain length, the larger the head group size, the lower the tendency to cause protein swelling and subsequent damage. This may be one of the reasons why sodium cocoyl isethionate, with its larger head group, is milder than sodium lauryl sulfate as discussed above.15,30 In addition, the interaction of the charged head of the surfactant with the skin proteins also reduces the ability of these proteins to bind and hold water, allowing increased evaporation and resultant SC dehydration.27–29,31
In a study by Ananthapadmanabhan,15 the interaction of skin proteins with a soap, syndet bar, and lipid-free cleanser were compared using infrared spectroscopy. In this study, the true soap caused the most interaction and change in the SC protein structure, therefore supporting the idea that soaps are capable of producing the greatest magnitude of SC damage. The clinical relevance that soaps can produce greater cutaneous desiccation, lessened innate ability for SC repair, and increased skin drying may all easily progress to augmented potential to produce signs and symptoms of cutaneous irritation. In patients with ETR and PPR, a group already affected inherently with impairment of the SC permeability barrier, these negative effects produced by soaps and poorly formulated skin cleansers are further magnified.
Surfactants and skin lipids. The interaction of surfactants with skin lipids have also been studied extensively31–34 However, the mechanism by which surfactants interact with lipids and cause SC permeability barrier disruption remains somewhat elusive. It has been suggested that surfactants either solubilize SC lipids into micelles and thereby cause subsequent SC delipidation, or that the incorporation of surfactants, especially charged surfactants, into SC lipid bilayer results in bilayer destabilization and increased SC permeability33,35–37 Although it has been hypothesized that charged anionic surfactants have a greater effect on the lipid bilayer than nonionic surfactants, a greater skin defatting effect may actually occur with the use of nonionic surfactants compared to anionic surfactants. This consideration stems from the fact that nonionic surfactants have a greater tendency to dissolve stearic acid than do anionic surfactants.15 Also, transmission electron microscopic studies have shown that nonionic-surfactant-based cleansers alter the lipid region to a greater extent than do mild cleansing bars with sodium cocoyl isethionate (an anionic surfactant).17,18
Mendelsohn and Moore used infrared spectroscopy to compare the perturbation of the lipid layers in the SC after the use of a true soap, syndet bar, or water alone (as the control).38,39 The results showed significant disruption of the lipid layer after the use of a soap as compared to the syndet bar and water alone. In comparing the effects of the syndet bar versus the control (water alone), analysis showed no discernable difference in lipid chain fluidity or rotational freedom. However, further analysis of the syndet bar did show a significant decrease in the cooperativity of the lipid bilayer after syndet bar use indicating a significant alteration in lipid organization and a resultant decrease in SC cohesion. Such changes can be a result of alterations in SC lipid composition either through the removal of endogenous lipid fractions or subfractions or the incorporation of surfactant molecules into the SC lipid layer. Overall, these results further support the common belief that true soaps impart the greatest magnitude of deleterious effects on SC integrity, followed by syndet bars and water, respectively.
Stratum corneum pH. Maintaining SC pH in an acid range of 4 to 6 is important for the overall health, integrity, and function of the SC, as discussed in a previous paper by Levin et al.40 Due to their inherent alkaline pH, soaps have the potential to change the pH of skin and therefore are not ideal for support of the permeability barrier of the SC. It is important to emphasize that a single or occasional use of an alkaline soap is not likely to significantly affect skin pH given the innate buffering capacity of skin.40 However, Fluhr et al41 have demonstrated that small and sustained pH increases, such as those caused by the daily use of soaps, induces changes in skin pH and adversely influences the SC barrier repair mechanism.41 Syndet bars and lipid-free cleansers are generally formulated to have a neutral or slightly acidic pH to ensure skin compatibility, decrease the alkaline flux of skin pH, and decrease the compromise of SC barrier function caused by alkaline pH alterations.
Deposition characteristics of some cleanser formulations. An additional benefit of some syndet bars, syndet liquid cleansers, and lipid-free cleansers is that they can be designed to deposit beneficial ingredients onto the skin even with short contact during cleansing and after rinsing. However, compared to bar technology, advances in liquid cleansing technology allow more efficient cutaneous deposition of beneficial agents, such as lipids.15 These cleansers, in essence, are using the same technology that is used in shampoos to deposit conditioning agents onto the hair. Some of the liquid cleansers currently available contain vegetable oils such as sunflower or soybean oil, occlusives such as petrolatum, humectants such as glycerol, and other ingredients such as ceramides and cholesterol that have beneficial effects on the skin. Cleanser formulations that allow for true deposition of specific major ingredients produce greater benefit and are less likely to damage the SC. Deposition technology with proper cleanser formulation, including with liquid cleansers, has the potential to minimize surfactant-mediated depletion of skin lipids, reduce the visible signs of dryness after cleansing, and assist in mitigating increases in TEWL. Today, in the OTC cleanser market, we now see some specific wash-off systems offering novel combinations of ingredients that can be deposited on the skin by cleansers, leading to a range of skin care claims that are supported by cogent scientific evidence.15
Proper cleanser use. In addition to using a mild OTC cleanser, it is also important for patients with rosacea to use the cleanser properly. Physicians should advise their rosacea patients to wash the face using gentle motion with the fingertips. Washing the face with hot or cold water may trigger a flush and washing the face with a rough surface or vigorous scrubbing may further irritate the already sensitive skin of a patient with ETR or PPR.22,42,43
Putting it all together, it becomes clear that certain cleansers can disrupt the health of the SC more than others. Using a cleanser that may truly be more effective at removing surface oils and debris, such as a true soap or a cleanser with low CMC surfactants, on the sensitive skin of a patient with ETR or PPR, will likely cause exacerbation of their underlying SC impairment and can trigger signs and symptoms. Patients with ETR or PPR who use well-formulated, mild cleansing agents such as syndet cleansers and lipid-free cleansers with a neutral to slightly acidic pH may not only avoid exacerbation of their skin disorder, but also may note adjunctive benefit in combination with therapies being used to treat their rosacea.
Clinical data concerning OTC cleansers and rosacea. There are clinical trials that have investigated the benefits of mild syndet cleansers and lipid-free cleansers in patients with ETR or PPR. Table 5 provides a summary of these clinical trials.
TABLE 5.
Summary of studies investigating over-the-counter cleansers in patients with rosacea including the papulopustular subtype
| STUDY | ROSACEA PATIENT SUBTYPE IN STUDY SELECTION | PATIENT # (N) | STUDY DESIGN | EXPERIMENTAL GROUP REGIMEN | CONTROL GROUP REGIMEN | WASHOUT PERIOD | STUDY PERIOD LENGTH |
|---|---|---|---|---|---|---|---|
| Subramanyan et al14 | Undisclosed | N=70 | Randomized, double-blind, inter-individual comparison study | Sensitive skin syndet bar plus metronidazole | True soap plus topical metronidazole | None | 4 weeks |
| Draelos et al44 | PPR | N=30 | Intraindividual comparison study with two weeks using the sensitive skin syndet+ bar versus two weeks of using the nonalkaline cleanser | Nonalkaline lipid-free liquid cleanser | Sensitive skin syndet bar | two weeks of using only the sensitive skin syndet bar* | 2 weeks |
| STUDY | SUBJECTIVE CLINICAL IMPROVEMENTS IN THE EXPERIMENTAL GROUP | OBJECTIVE CLINICAL IMPROVEMENTS IN THE EXPERIMENTAL GROUP | OBJECTIVE MEASUREMENT IMPROVEMENTS IN THE EXPERIMENTAL GROUP |
|---|---|---|---|
| Subramanyan et al14 |
|
Objective improvement in erythema, dryness, burning, stinging, itching, and tightness (n.s.) | None** |
| Draelos et al44 | No subjective reports of erythema, scaling, dryness, stinging, or skin roughness | Clinical Rosacea Severity Score demonstrated improvement (n.s.) |
|
- PPR
papulupustular rosacea
- Syndet
synthetic detergent
The two-week washout period is also the two-week control period in this study
- n.s.
Nonsignificant conclusion stastistically (not statistically significant)
Objective measurements were not taken in this study
Subramanyan et al14 evaluated the benefit of mild cleansing with a sensitive skin syndet bar (Dove sensitive skin bar, Unilever) versus a true soap bar (type undisclosed) in the inherently sensitive skin of atopic dermatitis, acne, vulgaris, and rosacea patients, as well as the induced sensitive skin of patients on topical tretinoin therapy and those after a single chemical peel. The results of these five individual substudies in all cases of inherent and induced sensitive skin showed a self-assessed and a dermatologist-assessed improvement in the patients using the syndet bar as compared to the soap bar.14
The above study by Subramanyan et al14 involving rosacea patients was a randomized, double-blind clinical trial that compared the effect of using the sensitive skin syndet bar versus a soap bar in addition to topical metronidazole on 70 patients with moderate rosacea (type unspecified).14 Thirty-five patients used the syndet bar plus topical metronidazole and 35 patients used the soap bar plus topical metronidazole over the four-week study period. The dermatologists assessed the patients for erythema, peeling, and dryness and inquired about burning, stinging, tingling, itching, and tightness over the four-week study period. The patients favored the use of syndet bar and reported a statistically significant improvement in itching, irritation, and tingling compared to those using the soap bar (P<0.005) and a statistically significant improvement from baseline in dryness, itching, irritation, and tingling with use of the syndet bar (P<0.05). The skin assessment made by the dermatologist showed improvement in all signs and symptoms evaluated when using the syndet bar compared to the soap bar except for peeling; however, none of these results were statistically significant. Looking at all the results of this study, there was definitive patient-perceived benefit to using the syndet bar that is supported by the improvements seen by the objective investigator assessments. This study would have been stronger with the following modifications: the inclusion of a washout period, objective measurements of hydration status and SC barrier status using TEWL and electrical capacitance, disclosure of the subtype(s) of rosacea, disclosure of the type of soap bar, and disclosure of the type of erythema being monitored (i.e., perilesional versus background erythema). Even with all the limitations of the study as listed above, in the authors' opinion, one of the most important aspects of any treatment regimen is patient-perceived improvement in their skin disease and patient-perceived improvement was demonstrated with the use of the syndet bar versus the soap bar in these rosacea patients.
Draelos44 reported a clinical study that subjectively and objectively evaluated the benefit of using a lipid-free nonalkaline cleanser (Cetaphil Gentle skin Cleanser, Galderma Laboratories, L.P., Fort Worth, Texas) in 30 patients with mild-to-moderate PPR whose disease had been clinically stable over a six-month time period. The study included a two-week washout period before the two-week active study period where the patients only used a sensitive skin syndet bar (Dove sensitive skin bar). No additional moisturizers or medications were allowed during the washout period or the study itself. The use of a two-week washout period where the subjects only used the designated sensitive skin syndet bar and a two-week study period where the subjects only used the nonalkaline cleanser allowed an intra-individual comparison between the syndet bar and nonalkaline cleanser. Although an intra-individual comparison is beneficial, an improved study design may have been a split-face comparison of products to minimize other confounding variables. However, split-face studies are fraught with their own limitations, especially with cleansers.
The following three methods were used to objectively assess the PPR patients during both the washout and study period: TEWL, corneometry, and a clinical rosacea severity score. Assessments for TEWL and corneometry were performed before beginning the washout period (at the screening visit), at the end of the two-week washout period (at baseline), and at Weeks 1 and 2 during the active study period. From screening to baseline (during the washout period), use of the syndet bar resulted in an increase in TEWL and decrease in corneometry values with significance (p≤=0.001) implying a decrease in SC barrier function and hydration with the syndet bar. Over the study period while using the nonalkaline cleanser, the TEWL values decreased with significance (p<0.05) and then returned to the baseline value at the end of the two-week study period. The corneometric readings showed no changes in hydration over the two-week study period. This study concluded that this decrease in TEWL at Week 1 of the study period demonstrates an improvement of SC barrier function with the nonalkaline gentle skin cleanser. However, this conclusion does not address the fact that the TEWL values return to baseline at two weeks. It is the authors' opinion that a sustained decrease in TEWL at Week 2 would be needed to conclude that the nonalkaline gentle skin cleanser produced a sustained improvement in SC skin barrier function. The study also concludes that the syndet bar demonstrates a decrease in skin hydration compared to the nonalkaline cleanser. However, looking closely at the data there is a clear decrease in hydration in the washout period, yet this decrease in hydration was not improved or worsened during the two-week active study period. This data is difficult to interpret. The lack of change in hydration status during the study period could be looked at in two different ways. First, the nonalkaline cleanser is as dehydrating as the syndet bar or second, the nonalkaline cleanser causes no change in the hydration status of skin. It is the authors' opinion that this study design did not allow an accurate comparison between the two products and their hydration level.
The clinical rosacea severity score was assessed by the investigator and was recorded at Weeks 1 and 2 of the washout period and Weeks 1 and 2 of the study period. A screening rosacea severity score before the washout period was not reported. The investigator rated the PPR patients on a scale of 0 (no signs of rosacea) to 3 (severe signs of rosacea). The results of the severity score showed a significant drop during the study period compared to the washout period suggesting an improvement in the signs of rosacea with the use of the nonalkaline cleanser. However, without reporting a clinical severity score during the screening visit (i.e., before the wash out period), it is not clearly possible to determine whether there was improvement or not of the patient's skin disease during the washout period. It is entirely possible that the severity score during the washout period was an improvement of the patient's skin disease or that the washout period represented a worsening of skin disease. Either scenario is impossible to determine without knowing the patient's rosacea severity scores prior to the start of the washout period.
In addition to the objective assessments described above, subjective patient tolerability, patient adherence, and a subjective overall rating of the nonalkaline cleanser was reported. The tolerability assessment included a subjective rating of erythema, scaling, dryness, stinging, burning, and lack of smoothness in the skin. While the author reported no tolerability issues during the study, the details of these results were not given. The method for adherence determination was also not reported; however, the author reported a compliance rate of 99.5 percent. The subjective rating of the nonalkaline cleanser involved subjects rating the nonalkaline gentle skin cleanser on a scale of 1 (worst) to 10 (best). It was reported that 80 percent of patients (N=30) in the study ranked the nonalkaline cleanser with a score of 10 (best).
In summary, this study shows greater improvement in study parameters with use of the nonalkaline lipid-free cleanser versus the designated syndet bar including objective clinical severity score, subjective tolerability, and subjective overall rating. Perhaps further experimentation involving a more direct comparison of the two products with better baseline capture of certain parameters would allow for more concrete results and conclusions.
Looking at both clinical studies by Subramanyan et al14 and Draelos,44 there is a definite patient satisfaction and patient-perceived clinical benefit in rosacea signs and symptoms with the use of mild cleansers, such as the lipid-free nonalkaline liquid cleanser and the sensitive skin syndet bar. In addition, there is also objective dermatologist-assessed clinical improvement with the use of both mild cleanser formulations. The study by Draelos44 does suggest superiority of the nonalkaline lipid-free liquid cleanser over the designated syndet bar; however, the study was small and the authors have suggested certain study limitations that obviate definitive conclusions without additional studies.
Gentle cleansers and rosacea. Looking at both clinical studies by Subramanyan et al14 and Draelos et al,44 there is a definite patient satisfaction and patient-perceived clinical benefit to their rosacea symptoms with the use of mild cleansers such as the nonalkaline cleanser and the sensitive skin syndet bar. In addition, there is also objective dermatologist-assessed clinical improvement with the use of mild cleansers. Given that both studies discussed above used the same syndet bar in their experimentation, it seems reasonable to loosely conclude that the nonalkaline cleanser may deliver more benefits to ETR and PPR patients than the sensitive skin syndet bar, which will deliver more benefits than the true soap bar.
However, overall, there is a conspicuous absence of data and large-scale clinical trials comparing specific gentle cleanser products in rosacea patients. Therefore, clinicians must draw their own conclusions based on clinical experience and available data as to product preference.
MOISTURIZERS
Importance of a properly selected moisturizer for rosacea patients. A combination of the right ingredients in a moisturizing formula has the potential to mitigate the innate SC permeability barrier dysfunction characteristic of ETR and PPR and has the potential to alleviate many of the signs and symptoms associated with cutaneous rosacea.4,11,13,19–21 During periods of both quiescence or during active flares, patients with ETR or PPR can both commonly experience symptoms of sensitive skin or, with greater progression of SC dysfunction, experience dryness and scaling of facial skin. This dryness and fine scaling affecting primarily the central forehead, cheeks, and chin is essentially a primary “rosacea dermatitis” related to the innate disturbance of the SC permeability barrier that has been correlated with increased centrofacial TEWL and which appears to account for skin sensitivity common to many patients with ETR or PPR.3,4,11 Therefore, patients with ETR or PPR stand to benefit immensely from proper product selection of skin care products that increase skin hydration, mitigate damage to SC proteins, limit damage and stripping of SC lipids, do not contain “special additives” that augment cutaneous irritation or SC integrity, and optimally deposit lipids, humectants, and cosmetically acceptable occlusive agents that expedite SC repair.2–4,11,13–16,19–22,42–47
In the next section, the authors dissect and analyze the function of the key ingredients used in most OTC moisturizing formulas today explaining how these ingredients have the potential to benefit and/or harm SC integrity and function. After this general review of moisturizer ingredients, summaries of clinical studies that investigate the benefits of using certain mild OTC moisturizers in the management of ETR and PPR are presented. The authors then discern the specific benefits ETR and PPR patients can expect by adding mild OTC cleansers and moisturizers to their skin care regimen.
OTC moisturizers, their ingredients, and what they do to the stratum corneum. The standard components in a conventional oil-in-water OTC facial moisturizer include approximately 80% water, 5% humectants, 4% emollients/occlusives, 6% emulsifiers, 2% silicate, 0.3% thickener, 0.4% preservative, and 0.2% fragrance.45 Several exceptions exist in the marketplace as far as ingredients and their relative ratio. Variations in formulations are based on advances in vehicle and ingredient technologies; however, the fundamental characteristics remain.45 These advances in technology include 1) the ability to deposit lipids and other permeability barrier-enhancing ingredients within the SC to expedite repair; 2) the ability to deposit specific ingredients that serve to enhance skin hydration through humectancy (e.g., glycerin, hyaluronic acid, etc; 3) the ability to provide occlusivity, which causes a more immediate reduction in TEWL; 4) incorporation of specific ingredients into the formulation with appropriate concentrations and ratios; 5) a high degree of patient satisfaction and cosmetic acceptability in terms of overall product texture and “feel”, spreadability, perception of elegance, and lack of odor or tackiness; and 6) avoidance as much as possible of ingredients with a high propensity for allergic or irritant cutaneous tolerability reactions.
The basic function of a moisturizer is to provide SC hydration through direct and or indirect mechanisms. A direct mechanism is via pure occlusion (e.g., petrolatum), as dehydration is retarded quickly via inhibition of evaporative TEWL, but the total improvement in skin hydration is less than with use of other ingredients that may provide humectancy and/or get incorporated into the intercellular lipid bilayer of the SC.2,4,45,46 Indirect mechanisms include use of humectants that function to retain water within the SC and/or physiologic lipids that are actively packaged into lamellar bodies at the level of the stratum granulosum and deposited into the upper SC within intercellular lipid bilayer. The latter indirect mechanism exhibits a slower onset of SC barrier repair as it takes time for physiologic incorporation of lipids to occur; however, the reparative effects on the SC permeability barrier are more prolonged.
Skin hydration is an integral component of homeostatic SC function in all individuals, regardless of the absence or presence of underlying skin disorders such as rosacea. Skin desiccation and dryness that is not corrected and allowed to progress leads to morphologic epidermal changes that incite cutaneous inflammation and visible progression from noninflamed xerotic-appearing skin to secondary asteatotic eczematous dermatitis.46
Importantly, the level of hydration from an OTC moisturizer or barrier repair cream is not directly correlated with the amount of water within the formulation. Exogenous water exposure is only capable of delivering a transient moisturization to the skin if occluded and may be counterproductive in patients with existing SC impairment if not used in combination with appropriate skin care formulations. What most contributes to the therapeutic benefits associated with use of a designated moisturizer is the proper combination of ingredients, including occlusives, emollients, humectants, and other potential additives (e.g., natural lipids, pseudoceramides, and niacinamide), combined with patient and clinical satisfaction.45
Occlusives. Occlusive agents form a layer on the skin surface that is poorly permeated by water and moisturize by retarding the evaporation of water. Examples of occlusive agents used in moisturizers include petrolatum, mineral oil, caprylic/capric triglyceride, silicone derivatives (dimethicone and cyclomethicone), lanolin, ceatyl alcohol, and stearyl alcohol.47 Naturally anhydrous occlusive petrolatum jelly can reduce SC water loss by more than 98 percent, whereas other occlusive oils can create a 20 to 30-percent reduction of SC water loss. The excellent occlusive properties of petrolatum may be due to its potential to diffuse into the intercellular lipid domain of the skin.48 However, this same ability to diffuse into the skin may also interfere with barrier recovery and therefore may not be the best choice for those with an impaired SC permeability barrier associated skin disorder such as ETR and PPR.45
Humectants. Humectants increase SC hydration by attracting and holding water in the SC. The degree of skin hydration offered by each humectant is dependent upon its water-binding capacity and its ability to penetrate the skin. Examples of humectants include glycerin (glycerol), propylene glycol, sodium lactate, sodium pyrrolidonic carboxylic acid (PCA), hyaluronic acid, ammonium lactate, potassium lactate, sorbitol, urea, polyglycerylmethacrylate, and the alpha hydroxyl acids (AHAs)—lactic acid, glycolic acid, and tartaric acid. Glycerin (glycerol), a polyol, is one of the most widely used and effective humectants, although high concentrations may produce tackiness after application.49
Some of the humectants listed above are naturally occurring skin components, many of which mirror physiologically derived products of filaggrin degradation in the SC and are referred to collectively as natural moisturizing factor (NMF). NMF consists primarily of amino acids and their derivatives, such as PCA, urocanic acid, lactic acid, citrate and urea, and other osmolytes that collectively are highly hygroscopic.50 As such, NMF functions as “nature's humectant” within the corneocytes of the epidermis.51 The importance of proper corneocyte water balance is that proteolytic enzymes involved in the breakdown of corneodesmosomes and subsequent “single corneocyte” desquamation are hydrolytic and require adequate water content to function optimally. In the absence of adequate SC water content, these enzymes function suboptimally, leading to incomplete cleavage of corneodesmosomes, which results in shedding of clumped corneocytes that are visible clinically as scales.50,51
Corneocytes that possess the highest amount of NMF retain more water and appear swollen under electron microscopy. The swelling of hydrated corneocytes is advantageous to the skin because it creates a more tortuous path for molecules to penetrate SC, thereby improving permeability barrier function and decreasing TEWL.52,53
Emollients. Emollients are materials designed to make the skin feel and appear smooth. In addition to improving the organoleptic qualities of the skin, some emollients exhibit occlusive properties, thereby contributing to reduction in TEWL. Examples of emollients include glycerol stearate, lanolin, soy sterol, and sunflower seed oil.54
Potential negative effects of occlusives, humectants, and emollients. In contrast to their moisturizing properties, certain occlusives, humectants, and emollients can sometimes incite adverse cutaneous effects, including significant irritant potential, that may outweigh their moisturizing benefits in patients with ETR or PPR. Examples of such ingredients include petrolatum, which may sometimes paradoxically delay SC barrier recovery; propylene glycol, which is unlikely to cause skin irritation in low concentrations, but may rarely produce dermatitic changes in exquisitely sensitive individuals in concentrations as low as 2%; AHAs such as lactic acid, which can induce stinging and irritation; and lanolin, which can induce allergic or irritant reactions.2,45,55,56 However, not all formulas that contain potential irritants elicit clinical irritant reactions. This is because ingredients can be included in small enough concentrations that they do not cause an irritant effect or they can be combined with other ingredients that negate their irritating properties. Either way, ingredients that have potential to cause irritation should generally be avoided in ETR and PPR patients who are likely to have lower threshold for irritancy. A more comprehensive list of ingredients to avoid in patients with rosacea due to irritation are listed later in this section.
Emulsifiers. Agents added to produce emulsification reduce surface tension between and combine the hydrophilic and lipophilic components in a moisturizing formula.2 Emulsifiers are able to create a multiphase mixed product that otherwise would separate into two distinct phases, akin to oil and water. This unique ability is due to their structure, which has one nonpolar hydrocarbon end and one polar end. However, because of this structure, they tend not to be wholly soluble in either oil or water. Instead they collect at the interface of the two phases and create a multiphase formula in which one phase contains droplets of the other. Most simple emulsions are oil-in-water (O/W), which means that the oil droplets are suspended in the continuous water phase, whereas others are water-in-oil (W/O). There are also more complicated emulsions (for example oil-in water-in oil) that are used to enhance the delivery and stability of certain active ingredients. The choice of emulsification system is highly dependent on the ingredients in the formula as well as the consumer-perceived benefits that are being targeted.2
Emulsifiers are essentially surfactants. Therefore, as discussed previously, they can be classified as anionic (negatively charged), nonionic (no charge), or cationic (positively charged). Anionic surfactants have the most potential to interact with skin lipids and proteins and therefore are viewed as having the greatest potential to produce negative effects on the SC. Sodium lauryl sulfate is an example of an anionic surfactant that is well known for its irritation potential and is used less commonly, except sometimes in low concentrations. Stearic acid and palmitic acid are examples of long chain fatty acids that are commonly used anionic emulsifiers. Despite the potential for anionic surfactants to induce SC permeability barrier disruption, desiccation, and cutaneous irritation, they are often used in mild OTC moisturizers as emulsifiers because of their excellent organoleptic qualities as discussed previously. However, the irritation potential of some anionic emulsifiers can be diminished by partially neutralizing them with other cationic ingredients or adding other nonionic emulsifiers to keep their concentration low in the formula.2
Nonionic emulsifiers depend chiefly upon hydroxyl groups and ether linkages (from polyhydric alcohol anhydrides and polyoxyethylene chains) for their hydrophobic effects. As discussed previously, nonionic emulsifiers are usually less irritating than their ionic counterparts.57 However, TEWL measurements indicate that some nonionic emulsifiers may produce microscopic but not visibly perceptible SC damage in normal skin.57 Perhaps this is due to their proven interaction with skin lipids that comprise the lipid bilayer of the SC.15 Examples of nonionic emulsifiers include cholesterol, a natural component of the lipid bilayer, polyethylene glycol, cetearyl alcohol, ceteareth-20, and stearyl alcohol. Polyethelyene glycol is susceptible to oxidation and the formation of free radical species. At least theoretically, as rosacea patients exhibit increased levels of ROS as evidenced by an inverse correlation between superoxide dismutase levels and rosacea severity, the use of polyetheylene glycol as an emulsifier in a moisturizer formulation may not be ideal for rosacea patients.8,58
Cationic emulsifiers have the potential to cause both positive and negative effects on the skin. Cationic emulsifiers may actually benefit patients who are prone to skin dryness such as rosacea patients. This is because the cationic emulsifier can bind to the negative charge of the keratin proteins in the skin and persist after washing, providing more prolonged moisturization.45,59 However, certain cationic surfactants can harm the SC even at low concentrations due to their cytotoxic effects.24 Examples of cationic surfactants include behentrimonium methosulfate, benzalkonium chloride, quaternium-15, quaternium-19, stearalkonium chloride, quaternium-23, and stearalkonium hectorite.
Despite the potential disadvantages associated with emulsifiers used in moisturizers, they are a “necessary evil” in product formulation just as surfactants are in cleansers. Without emulsifiers we would not be able to combine the hydrophilic and hydrophobic components of a moisturizer into one cosmetically elegant formulation.
Lipids. In addition to the standard hydrating components of moisturizers (i.e., occlusives, emollients, and humectants), many moisturizers on the market today boast the additional benefit of accelerated SC permeability barrier repair via the addition of lipids to the formula.45,52 Examples of such lipids include fatty acids, cholesterol, ceramides, and some pseudoceramides. Permeability barrier dysfunction of the SC, characterized by an increase in TEWL, is an integral feature of rosacea pathogenesis.60 The impaired SC barrier function in ETR and PPR likely explains the sensitive skin and its associated signs and symptoms that are commonly experienced by rosacea patients.11 However, most of the studies investigating the improvement of SC barrier repair with the use of these lipid ingredients have not been conducted on rosacea patients. Rather, clinical studies have focused on the use of lipid-enriched moisturizers, especially ceramide-based formulations, primarily in atopic dermatitis, where certain ceramides have been shown to be innately deficient in both disease and normal-appearing skin (“atopic skin”). It has been shown in atopic dermatitis that formulas containing specific ceramides, cholesterols, and fatty acids, along with appropriate humectants and occlusives, diminish TEWL, produce visible clinical benefit, and decrease pruritus and other signs and symptoms of cutaneous irritation.61,62
Theoretically, moisturizer formulations designed to replace deficient SC components associated with specific disease states, such as certain lipid subfractions in atopic dermatitis, would be expected to provide optimal clinical benefit as the included ingredients are targeted to meet disease-specific needs. Although many such formulations provide effective moisturization and clinical benefit, large-scale, well-controlled studies comparing various moisturizer formulations in the management of specific disease states are conspicuously absent overall and are also fraught with complexities related to proper study design. Nevertheless, advances in moisturizer formulation technology have provided clinicians and patients with several favorable choices that achieve clinical benefit and provide cosmetic elegance.
With regard to patients with ETR and PPR, although studies are lacking in this patient subgroup with many individual moisturizer formulations, it may be reasonable to anticipate that lipid-enriched (e.g., ceramide-based) moisturizers designed specifically to promote SC corneum repair may produce clinical benefit similar to what is seen in atopic dermatitis.63 Restoration of SC function with decrease in TEWL serves to decrease skin sensitivity and to partially reduce background erythema.64
Impact of relative ratios of lipid components in moisturizers. It is important to recognize that some study results suggest that the optimal relative lipid ratios for SC repair may vary for different subtypes of sensitive skin and certain associated disease states.65–69 For example, in acetone-disrupted mice skin, unequal mixtures of cholesterol, fatty-acids, and ceramides delayed SC barrier recovery instead of accelerating SC barrier repair as was expected. On the other hand, an equimolar mixture of ceramides, fatty acid, and cholesterol or pure cholesterol alone facilitated normal SC permeability barrier recovery.67
In sodium lauryl sulfate-induced SC damage, a moisturizer containing ceramide-3, cholesterol, and fatty acids, or ceramide 3B alone in a petrolatum-rich emulsion delayed SC barrier repair as compared to pure petrolatum alone.70,71 Similarly, in tape-stripped skin, a moisturizer containing ceramide-3, cholesterol, and fatty acids in a petrolatum-rich emulsion failed to accelerate SC barrier repair compared to petrolatum alone or cholesterol alone.69,71
These results suggest that simply incorporating specific ingredients into moisturizer formulations does not in and of itself produce an optimized final product. The interaction of specific ingredients when blended together as a formulation, and relative ratios of some ingredients such as lipids, ultimately determine the therapeutic and aesthetic qualities of the final product. Additionally, as the permeability barrier defects of the SC may vary inherently among patients with different skin types and genetic backgrounds, and in different disease states, it is unreasonable to believe that a single moisturizer formulation is superior to others in all situations. At present, it is not known if there are specific ingredients or ratios that are clearly optimal for use in patients with ETR and PPR as compared to others.
However, it can be assumed that use of well-formulated moisturizers that are devoid of common irritants are beneficial overall as an integral component of rosacea management.2,4,13,16,20,21,42–44
Gentle OTC moisturizers and rosacea. In summary, patients with ETR or PPR can benefit from moisturizers that contain adequate hydrating ingredients, promote SC barrier repair, and incorporate emulsifiers that are least damaging to SC integrity. However, it is also important that rosacea patients use moisturizers that do not contain potential irritants that could exacerbate their symptoms.47,72 In general, these patients should avoid skin care regimens that contain toners, astringents, abrasives, and sensory stimulants.73,74 A list of common ingredients that can potentially incite cutaneous irritation for rosacea patients is listed in Table 6. It is the lack of potentially irritating ingredients or the presence of these ingredients at low concentrations that contributes to the mildness of a formulation.
TABLE 6.
| EXAMPLES OF SKIN IRRITANTS USED IN OTC PRODUCTS |
|---|
| Acetone |
| Alcohol |
| Propylene glycol |
| Alpha-hydroxy acids |
| Sodium lauryl sulfate |
| Benzalkonium chloride |
| Formaldehyde releasers |
| Menthol |
| Benzyl alcohol |
| Camphor |
| Urea |
| Pyrriolidonic carboxylic acid |
| Lanolin |
| Fragrances |
“Priming the skin” in rosacea. It has been emphasized in the literature that mild OTC cleansers and moisturizers can have a clinically relevant positive impact on decreasing skin sensitivity and at least partially reducing the signs and symptoms of ETR and PPR.72,75 Del Rosso (personal communication)75 has discovered in his clinical experience for patients with a flare of ETR or PPR, especially when associated with intensification of erythema and increased severity of symptoms, that first priming the skin with proper skin care before initiating topical therapy is very beneficial therapeutically. Skin priming essentially “resets the SC permeability barrier to a more neutral playing field,” and involves initiating topical management over the first 3 to 5 days with only a specifically designated mild OTC cleanser and moisturizer (with or without oral therapy based on the clinical situation). This approach corrects SC impairment that is related innately to rosacea, especially during a flare, and also counters any previous patient-induced SC damage related to poor skin care practices. Additionally, once the cycle of facial cutaneous irritation and inflammation is set in motion, an acutely flared patient with ETR or PPR rosacea may further progress to a clinical state of status cosmeticus. Status cosmeticus in a patient with rosacea is comparable to angry back syndrome in a patient with contact dermatitis. That is, just as a patient with acute contact dermatitis with angry back syndrome will manifest a brisk reaction to almost any potential allergen or irritant placed on his or her skin, a rosacea patient with status cosmeticus presents with brisk facial erythema and symptomatic inflammation that is further incited by almost any potential irritant or allergen placed on the skin. Priming the skin as defined above allows for mitigation of SC permeability barrier dysfunction and reduces the potential for cutaneous irritation that may sometimes be associated with topical medication when started immediately without skin priming. Priming the skin is akin to preparing a wall that is covered with old flaking paint and the effects of environmental wear and tear before repainting that wall with a new fresh coat. The skin of patients with ETR and PPR, especially when inherently flared and/or irritated by improper skin care practices and procedures, appreciates the therapeutic benefit of returning the SC permeability barrier to baseline.
Clinical data and OTC moisturizers in rosacea. The clinical benefits of a mild skin care regimen comprising many Dove brand mild cleansers and moisturizers were evaluated by Hawkins et al76 in three separate clinical studies on normal skin, self-perceived sensitive skin, and dermatologist-assessed sensitive skin. In the first study, subjects with healthy skin were photographed with a calibrated high-resolution digital camera over a two-week study period, and their average improvement from using the mild cleanser and moisturizer was calculated using image morphing and facial averaging techniques. In a second study, subjects with self-perceived sensitive facial skin used the mild skin care regimen for a three-week study period and changes in skin hydration, skin dryness, and skin sensitivity were determined by means of a lactic acid sting test and subject self-assessment. The third study involved subjects with dermatologist-assessed highly sensitive skin. These subjects, who mostly were rosacea patients with or without an atopic background, used the mild OTC skin care regimen over a four-week study period and were assessed by both subjective and objective measurements of skin improvement.
The results of all three studies by Hawkins et al, conducted on three different skin types, demonstrated significant improvements in skin health and skin quality by means of expert assessments, instrumental evaluations, and subjective self-assessment. Although these studies are only briefly presented here, the reason for their inclusion in this review is to demonstrate the benefits of using mild cleansers and moisturizers in all skin types whether it is healthy skin, self-perceived sensitive skin, dermatologist-assessed sensitive skin, and skin of rosacea patients.
Draelos et al77 conducted a 12-week, single-site, investigator-blinded, randomized study of 66 patients with mild-to-moderate PPR. All of the patients in the study used azelaic acid 15% gel (AzA) in addition to either a self-selected skin care regimen or a dermatologist-selected skin care regimen. Group 1 utilized AzA plus their own self-selected cleanser and moisturizer and Group 2 used AzA plus the dermatologist-selected polyhydroxy acid (PHA)-containing cleanser and moisturizer (NeoStrata Facial Cleanser and Ultra Moisturizing Face Cream, NeoStrata, Princeton, New Jersey). Subjects were instructed not to use any systemic medications or new products four weeks prior to the study and to discontinue all topical skin medications two weeks before the study. During the study period, clinical evaluations were performed at Weeks 2, 4, 8, and 12. The investigator assessed the erythema, dryness, and telangiectasias on a 3-point scale and the subjects completed self-assessments of stinging, burning, itching, tightness, and tingling on a 5-point scale. Digital photographs were also taken at each visit. Improvements were seen in skin sensitivity, dryness, texture, smoothness, and overall skin condition with statistical significance (p<0.05) in the patients using the dermatologist-recommended regimen (Group 2) compared to those using their own self-selected regimen (Group 1). However, it should be noted that neither regimen altered the magnitude of inflammatory lesion reduction produced by AzA in treating the papules and pustules of PPR. Draelos also noted an appreciable clinical improvement in background erythema in patients using the dermatologist-selected regimen and postulates that this improvement in background erythema may be a result of improved SC function from the gluconolactone in the formula.77 Gluconolactone is a PHA that exhibits humectant properties, which can improve SC barrier function temporarily by inducing a swelling of corneocytes as discussed previously.52,53,78
There are two possible conclusions that we can draw from this study. First, signs and symptoms of PPR improve with use of a PHA-containing cleanser and moisturizer regimen used adjunctively with AzA. Second, PPR patients with sensitive skin will have improvements in their signs and symptoms of rosacea by using a dermatologist-recommended skin care regimen over a self-selected regimen in addition to topical therapy.42 However, the second conclusion is more difficult to substantiate given that the study evaluated only one dermatologist-recommended regimen. In an ideal world, the conclusion supporting the importance of a dermatologist-selected mild cleansing and moisturizing regimen would have been stronger if the study design had included comparisons among several dermatologist-recommended skin care regimens in addition to the PHA-containing regimen. However, this study was company-sponsored as the vast majority are, and as expected, comparisons are typically limited to just a few study arms. This is not meant to imply that company-sponsored studies do not provide valuable and relevant information, as many obviously do.
In another study, Laquieze et al79 evaluated the benefit of a nonalkaline moisturizer cream (Cetaphil Moisturizing Cream, Galderma Laboratories, L.P.) in combination with metronidazole 0.75% gel (Metro, Rozex, Galderma Laboratories) in a randomized, intra-individual comparison study with 20 ETR patients. This study involved a two-week washout period where patients used metronidazole 0.75% gel twice daily (BID) with their own self-selected moisturizers (if any were used) and a two-week study period where the ETR patients started using the nonalkaline moisturizer cream to one half of the face BID in addition to using metronidazole 0.75% gel BID on both sides of the face. Therefore, during the two-week study period an intra-individual comparison could be made evaluating the nonalkaline moisturizer cream plus metronidazole 0.75% gel therapy versus metronidazole 0.75% gel therapy alone (control). The skin was assessed at baseline (after the two-week washout period) and after the 15-day study period using a 5-point severity scale for erythema, dryness, desquamation, and roughness by subjective self-assessment and objective investigator assessment. Objective measurements of skin hydration, SC barrier function, and skin sensitivity were also assessed by electrical capacitance, TEWL measurements, and the lactic acid 10% stinging test, respectively. Patients also completed a questionnaire rating their overall impression of the products and evaluating their intent to purchase or not purchase the nonalkaline moisturizer cream.
The results of both the objective investigator assessments and subjective self-assessments demonstrated a statistically significant improvement only for skin dryness (p<0.05) over the two-week study period with use of the nonalkaline moisturizer cream. However, there were noted improvements in skin roughness, desquamation, and skin discomfort with use of the nonalkaline moisturizer cream that were not statistically significant. No appreciable improvements in erythema were noted in either the nonalkaline moisturizer cream group or the control group. Given these results, it was no surprise that skin hydration evaluated by electrical capacitance (corneometry) and skin sensitivity evaluated by the lactic acid stinging test both demonstrated marked improvement with the nonalkaline moisturizer cream over the control. SC barrier function as evaluated by TEWL measurements demonstrated improvement with the nonalkaline moisturizer cream; however, this difference was not statistically significant. In the results of the survey, all of the ETR patients expressed a desire to buy the nonalkaline moisturizer cream for continued use suggesting a noticeable self-assessed improvement in the signs and symptoms of ETR and overall skin appearance.
Unfortunately, this well-designed study tested only 20 patients with ETR and was of short duration. Nevertheless, this study demonstrated both patient-perceived improvement in ETR signs and symptoms and objective improvements in skin hydration and skin sensitivity with the use of this nonalkaline moisturizer cream concurrently with topical metronidazole.79 As data is limited on therapy for ETR, this information is valuable and will hopefully lead to additional studies.
Given the list of ingredients in the nonalkaline moisturizer cream used in this study, it is not surprising that the TEWL only showed slight improvement over the two-week study period. We hypothesize that this slight improvement is mostly because of the moisturizing attributes of the formula and that a statistically significant improvement was not achieved because the formula contains no physiologic additives such as specified lipids. The addition of ingredients specifically designed to both directly and indirectly improve the SC permeability barrier would be more likely to result in a greater reduction in TEWL and in the signs and symptoms of ETR to an even greater extent than what was demonstrated in this study.
In a split-face, open-label, multicenter study, 102 patients with mild-to-moderate PPR applied AzA 15% gel to both sides of the face while only applying a nonalkaline moisturizer cream (Cetaphil Moisturizing Cream) or a ceramide-based moisturizer cream (CeraVe Moisturizing Cream, Coria Laboratories, Ltd., Fort Worth, Texas) to one side of the face BID for seven days.4 The intent of the study design was to evaluate a mild OTC moisturizer in treating PPR. The results demonstrated a statistically significant decrease (p=0.008) in stinging, burning, tingling, and itching with use of nonalkaline or ceramide-based creams in conjunction with AzA versus using AzA alone.4 Interestingly, these results were reported by comparing the cumulative symptom score (CSS) at baseline to the CSS score during the seven-day trial for the treatment group and control group separately. These results showed significant improvement for the treatment side (p=0.008), as reported above, and nonsignificant improvement for the control side (p=0.015). However, it was never reported whether there was a significant difference in CSS scores between the moisturizer-treated side and the control side. We can only assume that this was not the case, as otherwise it would have been reported.4 In conclusion, this large but short study showed a reduction in the propensity for cutaneous irritation with the use of a nonalkaline or ceramide-based moisturizer along with AzA. The results of this study would have been even stronger with slightly different data analysis and if the study design included objective measurements such as electrical conductance, TEWL, and/or the lactic acid stinging test.
Table 7 summarizes the clinical studies discussed above. Looking at all of these studies collectively, we can conclude that the use of mild cleansers and moisturizers therapeutically benefit the sensitive skin of ETR and PPR patients through SC repair and reduce the potential for cutaneous irritation sometimes associated with topical therapy. In addition, although the benefits of using a mild cleanser and moisturizer alone for the first 3 to 5 days of an acute rosacea flare is logical, further studies are needed to substantiate this anecdotal evidence.
TABLE 7.
Summary of studies investigating over-the-counter moisturizers in patients with erythematotelangiectatic rosacea and papulopustular rosacea
| STUDY | ROSACEA PATIENT SUBTYPE IN STUDY SELECTION | PATIENT # (N) | STUDY DESIGN | EXPERIMENTAL GROUP REGIMEN | CONTROL GROUP REGIMEN | WASHOUT PERIOD | STUDY PERIOD LENGTH |
|---|---|---|---|---|---|---|---|
| Draelos et al77 | PPR | N=66 | Single site, investigator blinded, randomized, inter-individual comparison study | PHA cleanser and moisturizer plus AzA 15% gel BID (Group 2) | Self-selected cleanser and moisturizer plus AzA 15% gel BID (Group 1) | two weeks with no topical products or systemic therapeutics | 12 weeks |
| Laquieze et al79 | ETR | N=20 | Randomized, intra-individual comparison study | Nonalkaline moisturizer cream plus metronidazole 0.75% gel BID | Metronidazole 0.75% gel BID | two weeks using any moisturizer plus metronida zole 0.75% gel BID | 2 weeks |
| Del Rosso4 | PPR | N=102 | Multicenter, open label, intra- individual compariosn study | Nonalkaline or ceramide-based cleanser and ccream plus AzA 15% gel BID (51) | AzA 15% gel BID (51) | None | 1 week |
| STUDY | SUBJECTIVE CLINICAL IMPROVEMENTS IN THE EXPERIMENTAL GROUP | OBJECTIVE CLINICAL IMPROVEMENTS IN THE EXPERIMENTAL GROUP | OBJECTIVE MEASUREMENT IMPROVEMENTS IN THE EXPERIMENTAL GROUP |
|---|---|---|---|
| Draelos et al77 | None* |
|
None** |
| Laquieze et al79 |
|
|
|
| Del Rosso4 | Improvement in stinging, burning, itching, and tingling (cumulative symptom score) from baseline in the experimental group (p=0.008) | None† | None** |
- PPR
Papulopustular rosacea
- ETR
Erythematotelangiectatic rosacea
- n.s.
Nonsignificant conclusion stastistically (not statistically significant)
Subjective clinical improvements were not evaluated in this study
Objective measurements were not evaluated in this study
Objective clinical Improvements were not evaluated in this study
Further studies are needed to subjectively and objectively assess the effects of OTC moisturizer formulations that incorporate specific SC repair components (e.g., physiologic lipids) in patients with rosacea. It is the authors' belief that moisturizers with ingredients intended to repair the SC permeability barrier, such as niacinamide or ceramides, will result in a significant decrease in TEWL detectable during active use and during the postapplication regression phase. In addition, SC barrier repair reduces cutaneous irritation and contributes to reduction of signs and symptoms of ETR or PPR.
Proper moisturizer use. Rosacea patients need to be educated about what cleansers and moisturizers to use as well as how to use these OTC products, including with regard to coordination with topical medications. During an acute flare of ETR or PPR, facial skin sensitivity is intensified leading to decreased skin tolerability with symptoms of stinging and burning occurring after application of many common contactants, including many skin care products. In such cases, patients need to be instructed to allow for gentle drying after cleansing their face, sometimes with a delay of several minutes, before applying a moisturizer. In some cases, a delay of up to 30 minutes may be necessary. The alleged reasoning behind the delay in moisturizer application after facial cleansing is the observation that many skin care products tend to be most irritating when the skin is wet. Subsequently, over a period of time as tolerability improves, patients may decrease the delay time by five minutes per week until they are able to moisturize right after cleansing.54 In reality, the lifestyle of many patients may not allow for such complexity with routine skin care. Fortunately, with the current availability of gentle cleansers and moisturizers that are less prone to inducing skin irritation due to their formulation characteristics, the time involved with gentle cleansing followed by moisturizer application may be markedly compressed.
Order of moisturizer and topical medication application. Dermatologists commonly recommend use of designated skin care products to be used along with topical medications for certain disease states. For example, use of a gentle cleanser and moisturizer is commonly recommended in patients with atopic dermatitis, asteatotic eczema, and in patients using a topical retinoid to circumvent retinoid-induced signs and symptoms of skin irritation. However, there is very little scientific data on whether or not the temporal order of application of a moisturizer and the topical medication affects the efficacy or activity of the products that are applied. Does it matter if the moisturizer is applied before or after the topical medication? Is the answer to this question dependent on the nature of the formulations involved or the characteristics of the active medication ingredient(s)?
Does the order of application affect the percutaneous penetration of the active ingredient in a prescription topical medication? The answer to this question was addressed in a human skin in-vitro study incorporating a modified Franz cell assay system.63 In this study, a fixed amount of AzA (15% gel) was applied to human skin alone, before, and after the application of three different commercially available moisturizer lotions. The AzA molecules were radiolabeled (AzA–14C) to allow for quantification. The percutaneous penetration profile after a single application of AzA was determined, along with profiles of AzA percutaneous penetration from applications both before and after each of the three tested moisturizer lotions. The tested lotions included a nonalkaline lotion (Cetaphil Moisturizing Lotion), a sensitive skin lotion (Dove lotion), and a ceramide-based lotion (CeraVe Moisturizing Lotion). Fifteen minutes separated the first and second dosing application to each skin section. Calculated parameters included the amount of AzA absorbed (total penetration into the reservoir solution over 48 hours), rate of AzA penetration (flux), and mass distribution of AzA (epidermal and dermal AzA content). Interestingly, the results of the penetration profile of AzA did not change with significance (p>0.05) when it was dosed before or after the three moisturizer lotions for all of the parameters tested. In fact, applying the sensitive skin lotion or the ceramide-based lotion before the application of AzA resulted in a greater magnitude of penetration of the AzA as compared to application of either lotion after AzA, although the differences were not statistically significant. This trend was not appreciated for the nonalkaline lotion, perhaps due to a partial occlusive effect from the macadamia nut oil and other emollients in this formulation. In conclusion, applying any of the three OTC moisturizer lotions before AzA does not decrease the percutaneous absorption of AzA and may enhance its delivery into skin in some cases. Although this study sheds new light on the question of when moisturizer can or should be applied, the results of this study cannot be applied definitively to use of other topical medications or moisturizer formulations. Additional studies of this type, which assess results with other topical medications and moisturizers, are welcome.
SUMMARY
Do no harm. This is the first rule of medicine and may be the most important aspect of any cleanser and moisturizer for ETR and PPR patients. However, as demonstrated in this paper, certain OTC products and ingredients can be beneficial or damaging to the skin both clinically and microscopically and can potentially improve or aggravate the sensitive skin of a rosacea patient.
Based on the above thorough review, the authors conclude overall that use of a mild OTC cleanser and moisturizer is therapeutically beneficial in patients with ETR and PPR based on several observations that are outlined in Table 8. However, as discussed above, the ingredients used in the formula and the formula as a whole determine the effect the product will have on the skin. The properties of OTC cleansers and moisturizers that contribute to their mildness include an acidic-neutral pH to minimize the flux in skin pH; surfactants or emulsifiers that will not strip the skin of its moisture or strip the lipids and proteins of the skin barrier; moisturizing ingredients such as emollients, humectants, and occlusives; and formulas without potential irritants and allergens. Other technologies that may benefit ETR and PPR patients include cleaning without soap formation and ingredients designed for barrier repair.
TABLE 8.
Gentle over-the-counter cleanser and moisturizer use as an integral component of rosacea management: Summary of findings based on literature review and clinical experience3,4,8,11,13–16,20,21,42–44,46,47,60,64,72,76,77,80–82
Stratum corneum permeability barrier impairment is an inherent component of the pathophysiology of rosacea in patients with ETR* and PPR+
|
Rosacea associated with self-reported tendency for sensitive skin
|
| Dermatologist-selected skin care recommended to reduce the risk of poor product selection and untoward outcomes |
| Dermatologist-directed designated skin care regimen inclusive of a gentle cleanser and moisturizer shown to reduce facial dryness, burning, stinging, itching, and skin tightness |
| Proper skin care reduces potential risk of skin tolerability reactions to topical medications |
In this paper, the authors presented studies that demonstrated the clinical and microscopic benefit of using certain mild OTC cleansers and moisturizers on the market today in ETR and PPR patients. The benefits reported in these studies included subjective and objective improvement in rosacea signs and symptoms as well as objectively measured improvement in skin health parameters. Tables 5 and 6 summarize the clinical studies investigating the benefits of using mild OTC cleansers and moisturizers in ETR and PPR patients. Looking closely at the results, the most consistent finding in all studies presented was a subjective clinical improvement in symptoms, such as dryness, burning, stinging, itching, and tightness with the use of OTC cleansers and moisturizers.
Given the differing levels of soap formation in the true soap bar, sensitive skin syndet bar, and nonalkaline cleanser, and the two results of the two studies presented here, the nonalkaline cleanser may provide the most objective and subjective benefits to ETR and PPR patients while the sensitive skin syndet bar may provide more benefits than the true soap bar. However, larger direct comparison studies are needed.
In the studies involving the mild OTC PHA, nonalkaline, and ceramide-based moisturizers, the results showed a fairly consistent improvement in objectively assessed skin dryness. Therefore, it seems that ETR and PPR patients can generally expect improvements in their subjective symptoms of dryness and irritation and signs of dryness when adding mild OTC cleansers and moisturizers to their treatment regimen.
Although the most consistent finding in all the clinical studies presented in this review was a subjective improvement, the authors feel that the most important aspect of any treatment regimen may be a patient-perceived improvement to their skin and easing the discomfort associated with their skin condition. This self-perceived improvement will likely lead to patient satisfaction, increased patient compliance, and improved tolerability of prescription products.
From the studies presented here, it seems that we cannot expect a consistent improvement in barrier function as evidence by a decrease in TEWL or improvement in the papules of PPR, or improvement in background erythema in ETR and PPR patients with the addition of a mild OTC cleanser and moisturizer to their treatment regimen. Further research is needed in these areas in large-scale, randomized, double-blind clinical trials involving formulas with targeted ingredients to improve these specific skin problems.
ACKNOWLEDGMENT
James Q. Del Rosso, DO, served as consultant to the lead author during the planning and writing of this article.
REFERENCES
- 1.Levin J, Del Rosso JQ, Momin S. How much do we really know about our favorite cosmeceuticals? J Clin Aesthet Dermatol. 2010;3(2):22–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4(11):771–778. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 3.Crawford GH, Pelle MH, and James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004:327–341. doi: 10.1016/j.jaad.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Del Rosso JQ. The use of moisturizers as an integral component of topical therapy for rosacea: clinical results based on assessment of skin characteristic study. Cutis. 2009;84:72–76. [PubMed] [Google Scholar]
- 5.Lonne-Rahm SB, Fischer T, Berg M. Stinging and rosacea. Acta Derm Venerol. 1999;79:460–461. doi: 10.1080/000155599750009915. [DOI] [PubMed] [Google Scholar]
- 6.Chen DM, Crosby DL. Periorbital edema as an initial presentation of rosacea. J Am Acad Dermatol. 1997;37:346–348. [PubMed] [Google Scholar]
- 7.Harvey DT, Fenske NA, Glass LF. Rosaceous lymphedema: a rare variant of a common disorder. Cutis. 1998;61:321–324. [PubMed] [Google Scholar]
- 8.Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buechner SA. Rosacea: an update. Dermatology. 2005;210:100–108. doi: 10.1159/000082564. [DOI] [PubMed] [Google Scholar]
- 10.McAleer M, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33–39. doi: 10.1016/j.jaad.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Dirschka T, Tronnier H, Folster-Holst R. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br J Dermatol. 2004;150:1136–1141. doi: 10.1111/j.1365-2133.2004.05985.x. [DOI] [PubMed] [Google Scholar]
- 12.Lonne-Rahm SB, Fischer T, Berg M. Stinging and Rosacea. Acta Derm Venereol (Stockh) 1999;79:460–461. doi: 10.1080/000155599750009915. [DOI] [PubMed] [Google Scholar]
- 13.Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51(4):499–512. doi: 10.1016/j.jaad.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17:26–34. doi: 10.1111/j.1396-0296.2004.04s1003.x. [DOI] [PubMed] [Google Scholar]
- 15.Ananthapadmanabhan KP, Moore DJ, Subramanyan K, Misra M, Meyer F. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleanser. Dermatol Ther. 2004;17:16–25. doi: 10.1111/j.1396-0296.2004.04s1002.x. [DOI] [PubMed] [Google Scholar]
- 16.Draelos ZD. Skin care for the sensitive skin and rosacea patient: the biofilm and new cleansing technology. Cosmet Dermat. 2006;19(8):520–522. [Google Scholar]
- 17.Misra M, Ananthapadmanabhan KP, Hoyberg K, et al. Correlation between surfactant-induced ultrastructural changes in epidermis and transepidermal water loss. J Soc Cosmet Chem. 1997;48:219–234. [Google Scholar]
- 18.Misra M, Ananthapadmanabhan KP. Quantitative analysis of surfactant induced ultrastructural chenages in lipids. Supramolecular and Colloidal Structures in Biomaterials and Biosubstrates. In: Lal M, Lillford PJ, Naik VM, Prakash V, editors. Proceedings of the 5th Royal Society Unilever-Indo-UK Forum in Materials Science and Engineering, January 10–14, 1999. London: Imperial College Press; 2000. pp. 183–180. [Google Scholar]
- 19.Draelos ZD. Concepts in skin care maintenance. Cutis. 2005;76(6) Suppl:19–25. [PubMed] [Google Scholar]
- 20.Draelos ZD. Facial hygiene and comprehensive management of rosacea. Cutis. 2004;73:183–187. [PubMed] [Google Scholar]
- 21.Bikowski JB. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(5):12–19. [PubMed] [Google Scholar]
- 22.Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209–214. doi: 10.1053/sder.2001.27556. [DOI] [PubMed] [Google Scholar]
- 23.Kirsner RS, Froelich CW. Soaps and detergents: understanding their composition and effect. Ostomy Wound Management. 1998;44(3A):62S–70S. [PubMed] [Google Scholar]
- 24.Effendy I, Maibach HI. Detergent and skin irritation. Clin Dermatol. 1996;14:15–21. doi: 10.1016/0738-081x(95)00103-m. [DOI] [PubMed] [Google Scholar]
- 25.Ertel K. Modern skin cleansers. Dermatol Clin. 2000;18:561–575. doi: 10.1016/s0733-8635(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 26.Dominquez JG, Balaguer F, Parra JL, Pelejero CM. The inhibitory effect of some amphoteric surfactants on the irritation potential of alkyl sulfayes. Int J Cosmet Soc. 1981;3(2):57–68. doi: 10.1111/j.1467-2494.1981.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 27.Rhein LD, Robbins CR, Kernee K, Cantore R. Surfactant structure effects on swelling of isolated human stratum corneum. J Soc Cosmet Chem. 1986;37:125–139. [Google Scholar]
- 28.Wilhelm KP, Cua AB, Wolff HH, Maibach HI. Predicting surfactant induced stratum corneum hydration in vivo: prediction of the irritation potential of anionic surfactants. J Invest Dermatol. 1994;101:310–315. doi: 10.1111/1523-1747.ep12365467. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm KP, Wolff HH, Maibach HI. Effects on surfactants on skin hydration. In: Elsner P, Berardesca E, Maibach HI, editors. Bioengineering of the Skin: Water and the Stratum Corneum. Boca Raton, FL: CRC Press; 1994. pp. 254–290. [Google Scholar]
- 30.Ananthapadmanabhan KP, Yu KK, Meyers CL, Aronson MP. Binding of surfactants to stratum corneum. J Soc Cosmet Chem. 1996;47:185–200. [Google Scholar]
- 31.Imokawa G, Sumra K, Katsumi M. Study on skin roughness caused by surfactants. II. Correlation between protein denaturation and skin roughness. J Am Oil Chem Soc. 1975;52:484–489. doi: 10.1007/BF02640737. [DOI] [PubMed] [Google Scholar]
- 32.Rhein LD. In vitro interactions: biochemical and biophysical effects of surfactants on skin. Surfactants in Cosmetics. In: Rieger MM, Rhein LD, editors. Surfactant Science Series. New York: Marcel Dekker; 1997. pp. 397–320. [Google Scholar]
- 33.Imokawa G. Surfactant mildness. Surfactants in Cosmetics. In: Riegerc MM, Rhein LD, editors. Surfactant Science Series. New York: Marcel Dekker; 1997. pp. 427–330. [Google Scholar]
- 34.Rawlings AW, Watkinson A, Rogers J, Mayo HJ, Scott IR. Abnormalities in stratum corneum structure, lipid composition, and desmosome degradation in soap-induced winter xerosis. J Soc Cosmet Chem. 1994;45:203–220. [Google Scholar]
- 35.de la Maza A, Coderch L, Lopez O, Baucells J, Parra JL. Permeability changes caused by surfactants in liposomes that model the stratum corneum lipid composition. J Am Oil Chem Soc. 1997;74(1):1–8. [Google Scholar]
- 36.Frebe CL, Simion FA, Rhein LD, Cagan RH, Kligman A. Stratum corneum lipid removal by surfactants: relation to in vivo irritation. Dermatologica. 1990;181:277–283. doi: 10.1159/000247822. [DOI] [PubMed] [Google Scholar]
- 37.Downing DT, Abraham W, Wegner KK, William KW, Marshal JL. Partition of sodium dodecyl sulfate into stratum corneum lipid liposomes. Arch Dermatol Res. 1993;285(3):151–157. doi: 10.1007/BF01112918. [DOI] [PubMed] [Google Scholar]
- 38.Mendelsohn R, Moore DJ. Infrared determination of conformational order and phase behavior in ceramides and stratum corneum models. Meth Enzymol. 2000;312:228–247. doi: 10.1016/s0076-6879(00)12913-1. [DOI] [PubMed] [Google Scholar]
- 39.Moore DJ. Stratum corneum lipids and barrier function: biophysical studies of molecular organization in sphingomyelin and ceramide [NS] bilayers. Recent Res Dev Lipids. 2002;6:55–63. [Google Scholar]
- 40.Levin J, Maibach HI. Human skin buffering capacity: overview. In: Zhai H, Wilhelm KP, Maibach HI, editors. Marzulli and Maibach's Dermatotoxicology. Seventh Edition. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2008. pp. 971–400. [Google Scholar]
- 41.Fluhr JW, Kao J, Jain M, et al. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117:52–58. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 42.Del Rosso JQ. The role of maintaining proper barrier function in the management of rosacea. Cosmet Dermatol. 2007;20(8):485–490. [Google Scholar]
- 43.Bikowski JB. Rosacea: a tiered approach to therapy. Cutis. 2000;66(4):3–6. [PubMed] [Google Scholar]
- 44.Draelos ZD. The effect of Cetaphil Gentle Skin Cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77(4):27–33. [PubMed] [Google Scholar]
- 45.Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatol Ther. 2004;17:49–56. doi: 10.1111/j.1396-0296.2004.04s1006.x. [DOI] [PubMed] [Google Scholar]
- 46.Loden M. The clinical benefit of moisturizers 2005. J Euro Acad Dermatol Venereol. 2005;19:672–688. doi: 10.1111/j.1468-3083.2005.01326.x. [DOI] [PubMed] [Google Scholar]
- 47.Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75:17–21. [PubMed] [Google Scholar]
- 48.Ghadially R, Halkiersorenson L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387–396. doi: 10.1016/0190-9622(92)70060-s. [DOI] [PubMed] [Google Scholar]
- 49.Johnson AW. The moisturizer marketplace. In: Leyden J, Rawlings A, editors. Skin Moisturization. New York: Marcel Dekker, Inc.; 2002. pp. 1–490. [Google Scholar]
- 50.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17:43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 51.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103(5):731–740. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 52.Menon GK, Norlen L. Stratum corneum ceramides and their role in skin barrier function. In: Leyden J, Rawlings A, editors. Skin Moisturization. New York: Marcel Dekker, Inc.; 2002. pp. 31–520. [Google Scholar]
- 53.Bouwstra JA, de Graaff A, Gooris GS, et al. Water distribution and related morphology in human stratum corneum at different hydration levels. J Invest Dermatol. 2003;120:750–758. doi: 10.1046/j.1523-1747.2003.12128.x. [DOI] [PubMed] [Google Scholar]
- 54.Odom R, Dahl M, Dover J, et al. Standard management options for rosacea, part 1: overview and broad spectrum of care. Cutis. 2009;84:43–47. [PubMed] [Google Scholar]
- 55.Fan W, Kinnunen T, Niinimake A, et al. Skin reactions to glycols used in dermatological and cosmetic vehicles. Am J Contact Dermat. 1991;2:181–183. [Google Scholar]
- 56.Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem. 1977;28:197–209. [Google Scholar]
- 57.Barany E, Lindberg M, Loden M. Unexpected skin barrier influence from nonionic emulsifiers. Int J Pharm. 2000;195:189–195. doi: 10.1016/s0378-5173(99)00388-9. [DOI] [PubMed] [Google Scholar]
- 58.Donbrow M, Azaz E, Pillersdorf A. Autoxidation of polysorbates. J Pharm Sci. 1978;67:1676–1681. doi: 10.1002/jps.2600671211. [DOI] [PubMed] [Google Scholar]
- 59.Goddard ED. Substantivity through cationic substitution. Cosmetics & Toiletries. 1987;102:71–80. [Google Scholar]
- 60.Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135–141. [PubMed] [Google Scholar]
- 61.Potts RO. Stratum corneum hydration: experimental techniques and interpretations of results. J Soc Cosmet Chem. 1986;37:9–33. [Google Scholar]
- 62.Draelos ZD. The effect of ceramide-containing skin care products on eczema resolution duration. Cutis. 2008;81:87–91. [PubMed] [Google Scholar]
- 63.Del Rosso JQ, Lehman PA, Raney SG. Impact of order of application of moisturizers on percutanous absorption kinetics: evaluation of sequential application of moisturizer lotions and azelaic acid gel 15% using a human skin model. Cutis. 2009;83:119–124. [PubMed] [Google Scholar]
- 64.Del Rosso JQ. Understanding skin cleansers and moisturizers: the correlation of formulation with the art of clinical use. Cosmet Dermatol. 2003;16:19–31. [Google Scholar]
- 65.Mao-Qiang M, Brown BE, Wu-Pong S, et al. Exogenous nonphysiologic vs. physiologic lipids: divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 66.Thornfeldt C. Critical and optimal molar ratios of key lipids. In: Loden M, Maibach HI, editors. Dry Skin and Moisturizers: Chemistry and Function. Boca Raton, FL: CRC Press; 2000. pp. 337–660. [Google Scholar]
- 67.Man MQ, Feingold KR, Elias PM. Exogenous lipids influence permeability barrier recovery in acetone-treated marine skin. Arch Dermatol. 1993;129:728–738. [PubMed] [Google Scholar]
- 68.Held E, Lund H, Agner T. Effect of different moisturizers on SLS-irritated human skin. Contact Dermatits. 2001;44:229–234. doi: 10.1034/j.1600-0536.2001.044004229.x. [DOI] [PubMed] [Google Scholar]
- 69.Zettersten EM, Ghadially R, Feingold KR, et al. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J Am Acad Dermatol. 1997;37:403–408. doi: 10.1016/s0190-9622(97)70140-3. [DOI] [PubMed] [Google Scholar]
- 70.Loden M, Barany E. Skin—identical lipids versus petrolatum in the treatment of tape-stripped and detergent-perturbed human skin. Acta Derm Venereol. 2000;80:412–415. doi: 10.1080/000155500300012774. [DOI] [PubMed] [Google Scholar]
- 71.De Paepae K, Derde M-P, Reseeuw D, et al. Incorporation of ceramide 3B in dermatocosmetic emulsions: effect of the trans epidermal water loss of sodium lauryl sulphate-damaged skin. J Eur Acad Dermatol Venerol. 2000;14:272–279. doi: 10.1046/j.1468-3083.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- 72.Bikowski J, Torok H, Torok H. Rosacea management: a three-pronged approach. Practical Dermatology. 2007:65–68. [Google Scholar]
- 73.Bikowski J. The use of cleansers as therapeutic in various dermatologic disorders. Cutis. 2001;68(5):12–19. [PubMed] [Google Scholar]
- 74.Draelos ZD. Clinical situations conductive to proactive barrier enhancement. Cutis. 2002;70(6):17–23. [PubMed] [Google Scholar]
- 75.Personal communication: James Q. Del Rosso, DO. October 2010; Priming the skin concept first presented by Dr. Del Rosso at Fall Clinical Dermatology; Las Vegas, Nevada. Nov 13, 2010. [Google Scholar]
- 76.Hawkins SS, Subramanyan K, Liu D, et al. Cleansing, moisturizing, and sun-protection regimens for normal skin, self-percieved sensitive skin, and dermatologist-assessed sensitive skin. Dermatol Ther. 2004;17:63–68. doi: 10.1111/j.1396-0296.2004.04s1008.x. [DOI] [PubMed] [Google Scholar]
- 77.Draelos ZD, Green BA, Edison BL. An evaluation of a polyhydroxy skin care regimen in combination with azelaic acid 15% gel in rosacea patients. J Cosmet Dermatol. 2006;5:23–29. doi: 10.1111/j.1473-2165.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 78.Berardesca E, Distante F, Vignoli GP, Oresajo C, Green B. Alpha hydroxyacids modulate stratum corneum barrier function. Br J Dermatol. 1997;137:934–938. [PubMed] [Google Scholar]
- 79.Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil Moisturizing Cream as a part of a daily skin care regimen for individuals with rosacea. J Dermatol Treat. 2007;18:158–162. doi: 10.1080/09546630601121078. [DOI] [PubMed] [Google Scholar]
- 80.Elewski BE, Fleischer AB, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea. Arch Dermatol. 2003;139:1444–1450. doi: 10.1001/archderm.139.11.1444. [DOI] [PubMed] [Google Scholar]
- 81.Del Rosso JQ, Baum EW. Comprehensive medical management of rosacea: an interim study report and literature review. J Clin Aesthet Dermatol. 2008;1:20–25. [PMC free article] [PubMed] [Google Scholar]
- 82.Torok HM. Rosacea skin care. Cutis. 2000;66(Suppl 4):14–16. [PubMed] [Google Scholar]