Abstract
Understanding the mechanisms of augmented bacterial pathogenicity in post-viral infections is the first step in the development of an effective therapy. This study assessed the effect of human coronavirus NL63 (HCoV-NL63) on the adherence of bacterial pathogens associated with respiratory tract illnesses. It was shown that HCoV-NL63 infection resulted in an increased adherence of Streptococcus pneumoniae to virus-infected cell lines and fully differentiated primary human airway epithelium cultures. The enhanced binding of bacteria correlated with an increased expression level of the platelet-activating factor receptor (PAF-R), but detailed evaluation of the bacterium–PAF-R interaction revealed a limited relevance of this process.
Introduction
The concept of excessive morbidity and mortality of bacterial infection occurring during or shortly after viral infection was first formulated for influenza virus in the early 19th century. Analysis of influenza pandemics showed that the incidence of bacterial pneumonia was increased and contributed substantially to mortality rates (Abrahams et al., 1919; Muir & Wilson, 1919; Stone & Swift, 1919; Wilson & Steer, 1919). Comparison of bacteriological and virological data from children hospitalized for respiratory disease shows a high degree of occurrence of viral and bacterial infections positively correlating with the severity of illness (Duttweiler et al., 2004; Kneyber et al., 2005; Randolph et al., 2004; Thorburn et al., 2006). Although the role of a preceding viral infection in development and severity of bacterial respiratory diseases is a clinically well-documented phenomenon, the exact mechanism has not been elucidated fully.
Initially, it was proposed that respiratory viruses facilitate bacterial colonization through physical damage of the respiratory tract epithelium, with exposed basement membrane components being responsible for increased bacterial adherence (Louria et al., 1959; Muir & Wilson, 1919; Wilson & Steer, 1919; Wolbach, 1919). Such a mechanism undoubtedly occurs for highly pathogenic viral species, but it does not explain the occurrence of increased severity of bacterial infection during and shortly after relatively mild viral infections. Analysis of published data suggests that interplay between viruses and bacteria is a complex process, where the final outcome depends heavily on multiple factors, including modulation of innate immune responses resulting in delayed clearance of bacteria, hypersensitization of infected cells leading to enhanced immune-mediated lung damage and modulation of bacterial adherence (Okamoto et al., 2004). The increase in bacterial adherence occurs due to exposure of novel binding sites for bacteria on the epithelial surface, either by expression of highly glycosylated viral proteins (McCullers & Bartmess, 2003; Peltola & McCullers, 2004) or by the alteration of a bacterial receptor expression pattern (McCullers & Rehg, 2002; Patel et al., 1995; Terajima et al., 1997).
Bacterial pathogens predominantly involved in secondary infection of the respiratory tract are recruited from respiratory tract pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa and Staphylococcus aureus. In addition, they may include pathogens involved in the development of aspiration pneumonia. Aspiration pneumonia is commonly caused by anaerobic bacteria derived from the supra- or subgingival dental plaque (Brook & Frazier, 1993), with Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia and Fusobacterium nucleatum as the most prevalent pathogens (Brook, 2002; Carlisle et al., 2009; Finegold, 1991; Potempa & Pike, 2009; Scannapieco, 1999; Schreiner, 1979).
Mixed viral/bacterial infections have been studied in vitro and in vivo. Influenza virus infections exacerbate the severity of secondary streptococcal pneumonia, and increased pneumococcal adherence is proposed to be mediated at least partially by expression of the influenza virus neuraminidase protein. This protein promotes adherence and invasion of Streptococcus pneumoniae by cleavage of sialic acid from the surface of host cells, resulting in exposure of cryptic receptors for pneumococci (McCullers & Bartmess, 2003; Peltola et al., 2005; Tong et al., 2001). Other studies show a positive interaction between respiratory syncytial virus (RSV) infection and Streptococcus pneumoniae, H. influenzae and Pseudomonas aeruginosa. RSV is known to upregulate the surface expression of cellular receptors for these bacteria, namely intercellular adhesion molecule 1 (ICAM-1), carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) and platelet-activating factor receptor (PAF-R), and it was reported previously that the virus is able to act as a coupling agent between bacteria (Streptococcus pneumoniae and Pseudomonas aeruginosa) and epithelial cells (Avadhanula et al., 2006, 2007; Hament et al., 2004, 2005; Van Ewijk et al., 2007). Furthermore, a pathogenic synergism between rhinovirus and Staphylococcus aureus and Streptococcus pneumoniae has been identified. It was demonstrated that human rhinovirus infection promotes internalization of Staphylococcus aureus into epithelial cells by the secretion of inflammatory cytokines [interleukin (IL)-6 and IL-8] and overexpression of ICAM-1 on infected cells. Infection with rhinovirus stimulates adherence of Streptococcus pneumoniae to human tracheal epithelial cells via binding to overexpressed PAF-R molecules (Ishizuka et al., 2003; Passariello et al., 2006). In contrast, it was shown that increased adhesion of pneumococcus to influenza virus-infected cells is not PAF-R dependent (McCullers & Rehg, 2002; McCullers et al., 2008). It was also confirmed that adenovirus infections facilitate Streptococcus pneumoniae colonization by increasing bacterial binding to epithelial cells (Håkansson et al., 1994).
Although many studies on viral and bacterial co-infections have been performed to date, no data are available on human coronaviruses. Human coronavirus NL63 (HCoV-NL63) is a relatively recently discovered human respiratory pathogen with a high worldwide prevalence (Fouchier et al., 2004; Golda & Pyrc, 2008; Hofmann et al., 2005; Pyrc et al., 2004, 2006, 2007a, b, 2008; Schildgen et al., 2006; van der Hoek et al., 2004, 2006). Arguably, HCoV-NL63 is among the most clinically important human coronaviruses and is associated with upper and lower respiratory tract infections, occurring most frequently in the winter season and presenting more severe symptoms in children, the elderly and immunocompromised patients (Bastien et al., 2005; Chiu et al., 2005; Dijkman et al., 2008; Kaiser et al., 2005; Suzuki et al., 2005; Vabret et al., 2005; Wu et al., 2008). HCoV-NL63 is currently considered to be the major pathogen involved in the development of croup in young children (van der Hoek et al., 2005).
In this study, we evaluated the effect of HCoV-NL63 on adherence of a number of bacterial species to epithelial cells and showed that coronovirus infection results in increased adherence of Streptococcus pneumoniae to epithelial cells.
Results
Effect of HCoV-NL63 infection on adherence of bacteria to LLC-MK2 cells
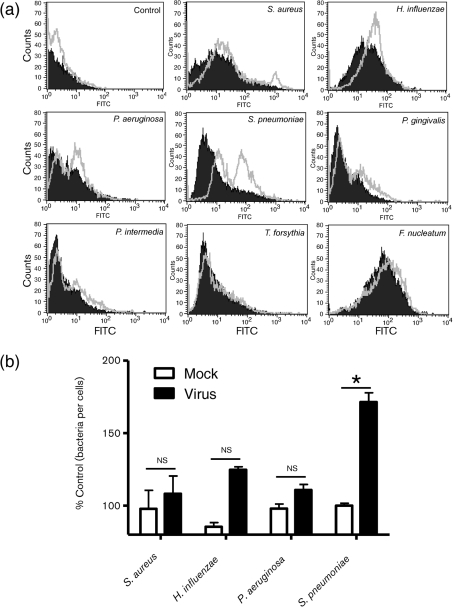
The in vitro adherence of Staphylococcus aureus, Pseudomonas aeruginosa, H. influenza, Streptococcus pneumoniae, Porphyromonas gingivalis, T. forsythia, F. nucleatum and Prevotella intermedia to LLC-MK2 cells (a monkey kidney cell line) infected with HCoV-NL63 or mock-infected cells was evaluated. For the primary screening assay, flow cytometry analysis was used, as it allows high-throughput testing of a large number of samples. Briefly, cells were incubated with Streptococcus pneumoniae at an m.o.i. of 500. Such a high dose of bacteria was required to visualize the binding of bacteria to mock-treated cells. Analysis revealed that at day 6 post-inoculation (p.i.), HCoV-NL63 infection significantly enhanced Streptococcus pneumoniae adherence to LLC-MK2 cell monolayers (Fig. 1a). In contrast, adhesion of the other bacteria tested to virus-infected cells was not markedly altered compared with mock-treated cells (Fig. 1a). To ensure that the observed effect was not related to binding of dead bacteria lacking membrane integrity, we evaluated bacteria viability with propidium iodide staining and subsequent fluorescence-activated cell sorting (FACS) analysis in the experimental setting described above. A significant change in bacterial binding was observed for the population of living cells, and the residual population of damaged cells did not influence the result (data not shown).
Fig. 1.
HCoV-NL63-mediated modulation of bacterial adherence to LLC-MK2 cells. (a) Bacterial adhesion was quantified by FACS. The histograms illustrate the fluorescence intensities and corresponding numbers of FITC-labelled bacteria attached to mock-treated or virus-infected cells (filled areas and grey lines, respectively). Data are representative of three independent experiments. (b) The number of adherent bacteria was quantified by plating and c.f.u. counting. Bars represent the number of bacteria that attached to mock- or HCoV-NL63-infected LLC-MK2 cells as a percentage of the control. Adhesion is expressed as a percentage of the control. The results are shown as means±sd of three independent experiments. The statistical significance of observed differences was estimated using Student’s t-test; *, P<0.01; ns, not significant.
To confirm this phenomenon and to rule out the possibility that FITC staining modulates the binding capacities of certain bacteria, a c.f.u.-based adherence assay was carried out for selected pathogens. We evaluated the adherence of Staphylococcus aureus, H. influenza, Pseudomonas aeruginosa and Streptococcus pneumoniae to LLC-MK2 cells infected with HCoV-NL63 or mock-treated cells at day 6 p.i. LLC-MK2 cells were incubated with bacteria under the conditions described above and, after an extensive washing step, the bacteria were harvested by trypsinization and plated on agar plates (as described in Methods). Analysis of c.f.u. numbers revealed a significant increase in the number of Streptococcus pneumoniae cells adhering to HCoV-NL63-infected cells (Fig. 1b). Adhesion of Staphylococcus aureus, H. influenza and Pseudomonas aeruginosa to virus-infected cells did not increase significantly compared with mock-treated cells (Fig. 1b).
Adherence of Streptococcus pneumoniae to HCoV-NL63-infected epithelium
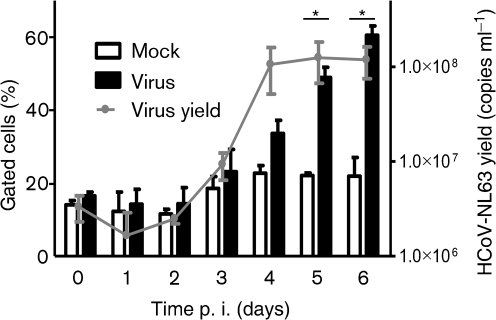
To determine whether there is a link between HCoV-NL63 replication and pneumococcal adhesion to LLC-MK2 cells, modulation of bacterial adhesion was studied on consecutive days following virus inoculation. Concurrently, the HCoV-NL63 yield was assessed by employing real-time PCR. Flow cytometric analysis detected no increase in the number of adhered Streptococcus pneumoniae in control and infected cells up to day 3 p.i. Compared with mock-treated LLC-MK2 cells, a significant enhancement in Streptococcus pneumoniae adhesion to virus-infected LLC-MK2 cells was observed at day 4 p.i. The number of cells binding Streptococcus pneumoniae progressively increased over the 2 days following inoculation, coinciding with virus replication (Fig. 2). After an initial lag period, HCoV-NL63 replicated efficiently in LLC-MK2 cells, with a steep rise in virus yield at day 3 p.i. Importantly, Streptococcus pneumoniae did not adhere to cells inoculated with UV-inactivated virus, suggesting that increased adherence is dependent on virus replication (data not shown).
Fig. 2.
Adhesion of Streptococcus pneumoniae to HCoV-NL63-infected LLC-MK2 cells increases in a time-dependent manner that correlates with virus yield. Pneumococcal adhesion was quantified by FACS and is expressed as the percentage of cells showing increased fluorescence. The results are shown as means±sd of three independent experiments. The statistical significance of observed differences was estimated using Student’s t-test; *, P<0.05.
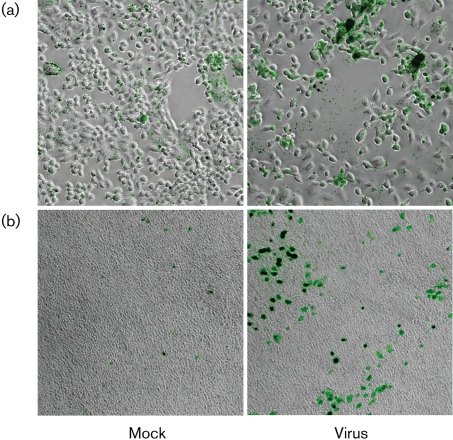
Fluorescent microscopy was also used to visualize differences in the adherence of Streptococcus pneumoniae to mock- and HCoV-NL63-infected cells and to further substantiate and confirm this phenomenon. The analysis confirmed the significant increase in Streptococcus pneumoniae adherence to virus-infected LLC-MK2 cells. The bacteria attached to HCoV-NL63-infected cells in a non-random pattern and were found in clusters associated with a subset of cells (Fig. 3a).
Fig. 3.
Adhesion of Streptococcus pneumoniae to LLC-MK2 cells (a) and HAE cultures (b) is increased following infection with HCoV-NL63. Fluorescence images from FITC-labelled bacteria and bright-field images were obtained using a fluorescence microscope. Magnification: ×200 (a); ×100 (b). Data are representative of two independent experiments.
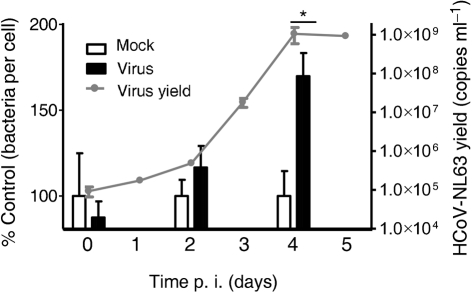
To add physiological meaning to the finding, binding of Streptococcus pneumoniae to fully differentiated human airway epithelium (HAE) cells during consecutive days following HCoV-NL63 infection was examined. Virus replication in HAE cultures was assessed in the same manner as described above. A c.f.u.-based adherence assay revealed a major increase in the number of Streptococcus pneumoniae bacteria adhered to virus-infected cells at day 4 p.i., although no significant difference was observed on days 0 and 2 p.i. (Fig. 4). The observed increase in bacterial adherence coincided with virus replication. Furthermore, to visualize the increased adherence of Streptococcus pneumoniae to HCoV-NL63-infected HAE cultures, we also assessed the increase in bacterial adherence to infected epithelium by fluorescent microscopy. The results clearly showed a marked increase in Streptococcus pneumoniae adherence to HCoV-NL63-infected HAE cultures (Fig. 3b).
Fig. 4.
HCoV-NL63 affects pneumococcal adherence to the apical surface of HAE cultures. Adherence of Streptococcus pneumoniae to virus-infected HAE cultures increased in a time-dependent manner that correlated with virus yield. The number of adherent bacteria was quantified by plating and c.f.u. counting. Bars represent the number of Streptococcus pneumoniae that attached to mock- or HCoV-NL63-infected HAE cultures as a percentage of the control. The results are shown as means±sd of two independent experiments. Statistical significance of the observed differences was estimated using Student’s t-test; *, P<0.05.
Effect of HCoV-NL63 infection on eukaryotic receptors for Streptococcus pneumoniae expression
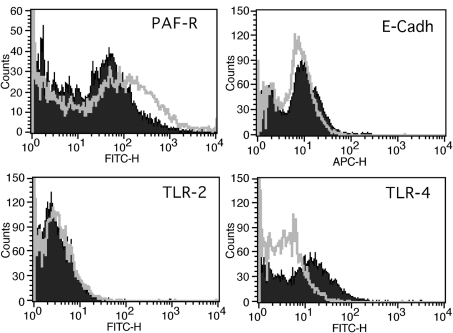
In order to reveal the mechanism of the enhanced bacterial binding to coronavirus-infected cells, the level of surface expression of known epithelial receptors for Streptococcus pneumoniae, including PAF-R, epithelial cadherin (E-cadherin), Toll-like receptor (TLR)-2 and TLR-4, was determined on HCoV-NL63-infected and mock-infected cells. The surface expression of receptors was assayed by flow cytometry. At day 6 p.i., PAF-R surface levels increased significantly compared with mock-treated cells, whilst E-cadherin levels did not change (Fig. 5). TLR-2 and TLR-4 expression on both virus-infected and mock-infected cells was marginal.
Fig. 5.
Infection with HCoV-NL63 affects receptor expression on the cell surface. Levels of bacterial receptors on the cell surface were determined by FACS on HCoV-NL63- or mock-infected LLC-MK2 cells (grey lines and filled areas, respectively). Analysis was performed for PAF-R, E-cadherin (E-Cadh), TLR-2 and TLR4. The data are representative of three independent experiments.
Inhibition of PAF-R does not alter pneumococcal adherence to LLC-MK2 cells
It has been reported previously that PAF-R is a common receptor for Streptococcus pneumoniae that may be essential for the observed increase in bacterial adherence during viral infection (Ishizuka et al., 2003). Indeed, we observed a marked increase in PAF-R surface levels during HCoV-NL63 infection. To test whether this phenomenon might serve as a mechanistic explanation for the observed increase in Streptococcus pneumoniae adherence, we evaluated platelet-activating factor (PAF), a PAF-R antagonist (ABT-491) and PAF-R-specific polyclonal antibody, previously reported to hamper the bacterium–PAF-R interaction (Radin et al., 2005). Pre-incubation of HCoV-NL63-infected cells with PAF, PAF-R antagonist or PAF-R-specific antibody prior to the flow cytometry-based adherence assay did not affect the adhesion of the pneumococci to LLC-MK2 cells (Fig. 6). We were also not able to detect any decrease in Streptococcus pneumoniae adherence to human respiratory epithelial cultures following incubation with PAF.
Fig. 6.
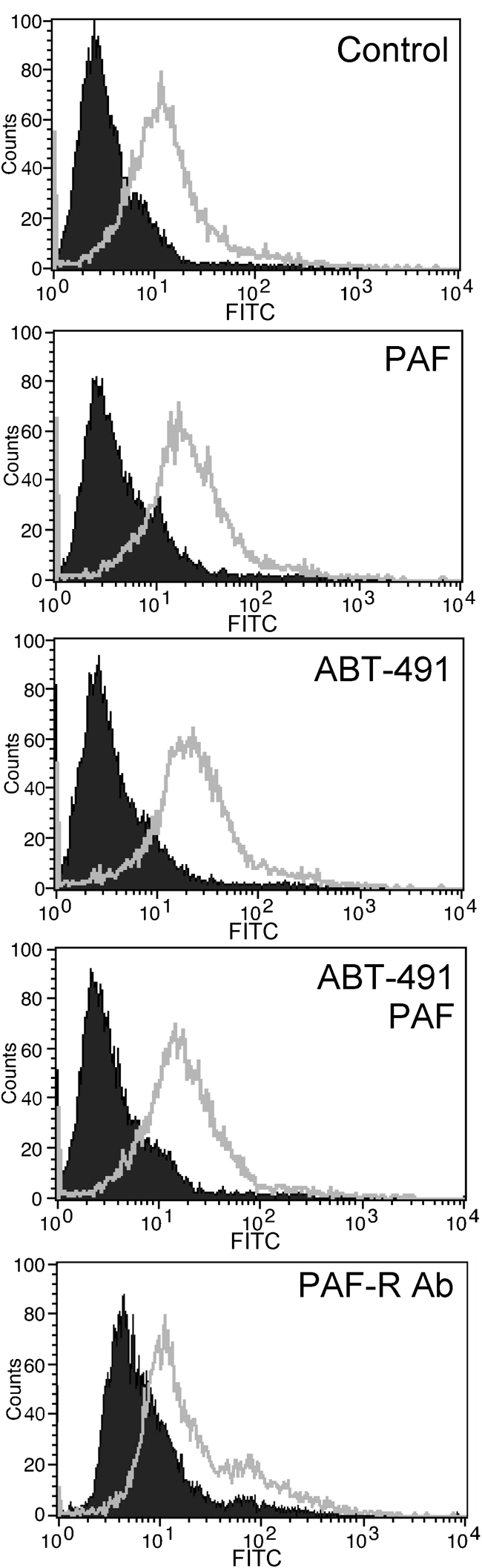
Inhibition of PAF-R with PAF, ABT-491 and PAF-R-specific polyclonal antibodies does not decrease the adherence of pneumococci to LLC-MK2 cells. Adherence of Streptococcus pneumoniae to the cell surface was determined by FACS on HCoV-NL63- or mock-infected cells (grey lines and filled areas, respectively). Data are representative of three independent experiments.
Discussion
Previous reports have clearly shown that pneumococcal infections occurring during or shortly after respiratory viral illness are characterized by increased severity and more frequent hospitalization (Stille et al., 1961). To illustrate the magnitude of the problem, it is worth mentioning that preceding viral infection could be detected in up to 90 % of children with acute otitis media, which is the most common bacterial infectious sequela of initial viral infection. Moreover, clinical and epidemiological data showed that Streptococcus pneumoniae is a leading pathogen, involved in primary or secondary community-acquired pneumonia in hospitalized children, and in more than half of all cases a secondary viral agent could be detected (McCullers, 2006).
This study was carried out to evaluate whether infection with HCoV-NL63, a recently discovered viral respiratory pathogen, resulted in increased vulnerability to secondary bacterial infection. The bacterial pathogens included in the study were chosen because of their prevalence and their association with respiratory tract infections. Within the studied group, Staphylococcus aureus, H. influenzae, Pseudomonas aeruginosa and Streptococcus pneumoniae were taken as representatives of bacteria frequently associated with respiratory illness. Porphyromonas gingivalis, T. forsythia, F. nucleatum and Prevotella intermedia were chosen because they are aetiological agents of aspiration pneumonia, most prevalent in young children and elderly patients (Brook, 2002; Okuda et al., 2005; Scannapieco, 1999).
Detailed analysis showed that adhesion of Streptococcus pneumoniae to epithelial cells was vastly enhanced by a preceding HCoV-NL63 infection. This finding is in accordance with the concept that viral infections augment pneumococcal adherence to the local epithelium, thereby facilitating bacterial infection (Avadhanula et al., 2006, 2007; Hament et al., 2004, 2005; McCullers & Bartmess, 2003; Peltola et al., 2005; Tong et al., 2001). As the viral infection had no effect on adherence of the other bacteria tested, we assumed that there is a specific synergistic interaction between Streptococcus pneumoniae and HCoV-NL63.
To evaluate our findings in a more natural environment for human respiratory pathogens, we employed the fully differentiated HAE cell line generated by primary human airway epithelial cells cultivated in an air–liquid interface. This system results in tissue culture that morphologically, structurally and functionally mimicks human cartilaginous airway epithelium. Mature, fully differentiated HAE cells contain multiple cell species forming multi-layered, ciliated, three-dimensional tissue culture that has been shown previously to support a wide range of respiratory viruses, including human coronavirus HKU1 (Pyrc et al., 2010), severe acute respiratory syndrome coronavirus (Sims et al., 2006), HCoV-NL63 (Orenstein et al., 2008), influenza virus (Thompson et al., 2006), parainfluenza virus (Zhang et al., 2005) and respiratory syncytial virus (Zhang et al., 2002). HCoV-NL63 infection of HAE culture resulted in a significant increase in the number of adherent Streptococcus pneumoniae cells in comparison with control, mock-infected culture. As it has been reported previously that virus-mediated modulation of bacterial adherence is highly cell-line specific (Avadhanula et al., 2006), these data validated the results obtained with LLC-MK2 cells as clinically relevant.
The mechanistic explanations for increased bacterial binding to cells infected with other viral pathogens are ambiguous and several factors have been considered (Hament et al., 1999). One hypothesis suggests that an increase in the density of cellular receptors facilitates bacterial adherence. Here, we studied the levels of specific cell-surface proteins identified previously as receptors for Streptococcus pneumoniae. Although we observed no effect of viral infection on TLR-2, TLR-4 and E-cadherin expression, there was a marked increase in the surface level of the PAF-R protein. PAF-R is a cell-surface G protein-coupled receptor, facilitating adherence of Streptococcus pneumoniae to epithelial cells by binding to phosphorylcholine, a component of the bacterial cell wall (Barletta et al., 2002; Cundell et al., 1995). It has been suggested previously that PAF-R is an essential factor for the development of secondary bacterial pneumonia after viral infection (McCullers & Rehg, 2002). During rhinovirus and RSV infection, upregulation of PAF-R surface levels has been documented (Avadhanula et al., 2006; Ishizuka et al., 2003). In an in vivo experiment, it was also shown that influenza virus infection in mice caused enhanced PAF-R expression in the lungs, further supporting the contention of PAF-R importance for Streptococcus pneumoniae adhesion. Moreover, it was demonstrated that Streptococcus pneumoniae employs PAF-R to invade the host’s epithelium previously infected with influenza virus (van der Sluijs et al., 2006).
In this study, we found that HCoV-NL63 infection was associated with a marked increase in PAF-R expression on infected cells, suggesting that enhanced adherence of Streptococcus pneumoniae through binding to PAF-R may be one of the mechanisms involved in the development of secondary bacterial infections after HCoV-NL63-associated respiratory illness. However, the presence of PAF, PAF-R antagonist (ABT-491) or PAF-R-specific antibody did not affect the adherence of Streptococcus pneumoniae to cells pre-infected with HCoV-NL63. This strongly suggests that increased adherence of pneumococci following HCoV-NL63 infection is not solely mediated by PAF-R, and that some other cellular receptors are involved in this interaction. It is also possible that increased bacterial adherence is caused by virus-mediated modification of the cellular glycan layer.
Taken together, we have provided a possible reason for the severity of Streptococcus pneumoniae-related illness during or shortly after HCoV-NL63 infection. As this virus is a relatively recently described human pathogen, available clinical data are relatively limited and further epidemiological studies on the interactions between Streptococcus pneumoniae and HCoV-NL63 are required to elucidate fully the clinical relevance of the described process.
Methods
Cell culture.
LLC-MK2 cells were maintained in minimal essential medium (MEM), containing two parts Hanks’ MEM and one part Earle’s MEM (PAA Laboratories) supplemented with 3 % heat-inactivated FBS (PAA Laboratories), penicillin (100 U ml−1) and streptomycin (100 µg ml−1). Cells were cultured on six-well plates (Sarstedt) at 37 °C with 5 % CO2.
Human tracheobronchial epithelial cells were obtained from airway specimens resected from patients undergoing surgery under protocols approved by the bioethical committee of the Silesian Center for Heart Diseases, Poland (approval no: KNW/0022/KB1/17/10 dated 16 February 2010). Primary cells were expanded on plastic to generate passage 1 cells and plated at a density of 3×105 cells per well on permeable Transwell inserts (6.5 mm diameter) supports. HAE cultures were generated by provision of an air–liquid interface for 6–8 weeks to form well-differentiated, polarized cultures that resemble in vivo pseudo-stratified mucociliary epithelium.
Virus preparation, titration and infection.
HCoV-NL63 stocks were generated by infecting LLC-MK2 cells. Cells were lysed at 6 days p.i. by two freeze–thaw cycles. The virus-containing fluid was aliquotted and stored at −80 °C. A control LLC-MK2 cell lysate from mock-infected cells was prepared in the same manner as the virus stocks.
Virus yield was assessed by virus titration on fully confluent LLC-MK2 cells in 96-well plates, according to the method of Reed & Muench (1938). Plates were incubated at 32 °C for 6 days and the occurrence of a cytopathic effect was scored using an inverted microscope.
In subsequent experiments, fully confluent cells were exposed to HCoV-NL63 at a concentration of 2000 TCID50 ml−1. Cell-culture supernatant samples used for real-time PCR were collected every 24 h for 7 days.
HAE cultures were infected with HCoV-NL63, which was inoculated at the apical surface. Following 2 h incubation at 32 °C, unbound virus was removed by extensive washing with PBS and the HAE cultures were maintained at an air–liquid interface for the rest of the experiment. To generate replication curves at specific times, 100 µl PBS was applied to the apical surface of the HAE cells and collected after 10 min incubation at 32 °C. All samples were stored at −80 °C.
Bacterial strains, storage and growth conditions.
Pseudomonas aeruginosa, H. influenzae and Streptococcus pneumoniae were kindly provided by J. Fiett (National Medicines Institute, Warsaw, Poland) and Staphylococcus aureus strain Newman by T. J. Foster (Moyne Institute of Preventive Medicine, Trinity College, Dublin, Ireland). Porphyromonas gingivalis, Prevotella intermedia, T. forsythia and F. nucleatum were obtained from S. Eick (University Hospital of Jena, Jena, Germany).
Staphylococcus aureus, Pseudomonas aeruginosa and Streptococcus pneumoniae were cultured in tryptic soy broth (TSB). H. influenzae was grown in brain–heart infusion broth (BHI) with haemin (10 µg l−1) and NAD (2 µg ml−1). Porphyromonas gingivalis, Prevotella intermedia, T. forsythia and F. nucleatum were grown anaerobically in Schaedler broth supplemented with l-cysteine (0.05 g ml−1), 1 % DTT, menadione (0.5 mg ml−1) and haemin (1 mg ml−1). Bacteria were stored in TSB or BHI medium supplemented with glycerol (50 %, v/v) at −80 °C. During experiments, 10 ml cultures were inoculated with bacterial stock. Staphylococcus aureus and Pseudomonas aeruginosa were grown overnight under constant rotation (180 r.p.m.) to stationary growth phase at 37 °C. H. influenzae and Streptococcus pneumoniae were grown for 6 h to stationary phase at 37 °C (anaerobic conditions).
Prior to each inoculation, bacteria were washed twice in PBS. The concentration of bacteria was assessed based on optical density at 600 nm. Absolute numbers were determined based on a standard growth curve generated previously by counting c.f.u. on agar plates. Each time, in order to verify the absolute numbers of cells, bacteria were plated and colonies were counted after 16 h of incubation at 37 °C.
Labelling of bacteria.
In order to label bacterial cells with a fluorescent dye for subsequent analysis, bacteria were collected by centrifugation at 4000 r.p.m. in a microfuge for 6 min and resuspended in 900 µl PBS. Samples were mixed with 100 µl FITC (1 mg ml−1) and incubated for 20 min at 37 °C in the dark. Subsequently, bacteria were washed three times with sterile PBS and resuspended in PBS to a final concentration of 1×109 c.f.u. ml−1.
Adherence of bacteria to LLC-MK2 cells (flow cytometry-based adherence assay).
To examine the effect of HCoV-NL63 infection on the adherence of bacteria to epithelial cells, LLC-MK2 monolayers were inoculated with the virus and mock-infected as described above. To assess the ability of bacteria to adhere to the cell surface, cells were incubated with FITC-labelled bacteria (1×109 c.f.u. ml−1, m.o.i. 500) for 1 h at 4 °C and subsequently rinsed three times with ice-cold PBS to remove non-adherent bacteria. The cells were then detached from the plate surface by scraping. Collected cells were resuspended in 1 ml PBS, spun at 260 g for 6 min and fixed in 3 % paraformaldehyde (PFA). Samples were analysed on a FACSCalibur instrument (Becton Dickinson) using CellQuest software. A total of 10 000 events was acquired. During the procedure, the cell culture medium was not supplemented with antibiotics.
Adherence of bacteria to LLC-MK2 cells (c.f.u.-based adherence assay).
Cell monolayers were incubated with bacteria (1×109 c.f.u. ml−1, m.o.i. 500) for 1 h at 4 °C and subsequently rinsed three times with ice-cold PBS to remove non-adherent bacteria. Cells were detached from the plate with 1× trypsin/EDTA solution (PAA Laboratories) and immediately placed on ice. Serial dilutions were prepared in ice-cold PBS and plated onto agar plates. Cell number was verified using a haemocytometer. After overnight incubation at 4 °C, colonies were counted.
To test whether the increase in PAF-R surface protein levels might alter Streptococcus pneumoniae adherence, virus-infected cells were incubated with 10 µM platelet activating factor-16 (PAF; Calbiochem), 10 µM PAF-R antagonist (ABT-491; Calbiochem) or PAF-R-specific polyclonal antibody (20 µg ml−1; Cayman Chemical) for 30 min. Subsequently, the cells were incubated with Streptococcus pneumoniae and tested in a flow cytometry-based adherence assay as described above.
Adherence of Streptococcus pneumoniae to HAE cultures (c.f.u.-based adherence assay).
HAE cultures were incubated with bacteria overlaid on the apical surface of the culture (1×108 c.f.u. ml−1) for 1 h at 4 °C and subsequently rinsed three times with ice-cold PBS to remove non-adherent bacteria. Cells were detached from the plate with 1× trypsin/EDTA solution and instantly placed on ice. Serial dilutions were prepared in ice-cold PBS and plated onto agar plates. After overnight incubation at 4 °C, colonies were counted.
Virus detection by reverse transcription and quantitative PCR.
HCoV-NL63 total nucleic acids were isolated from cell-culture supernatant or apical washes from HAE cultures using a Total RNA Mini kit (A&A Biotechnology), according to the manufacturer’s instructions. Reverse transcription was carried out with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems), according to the manufacturer’s instructions. HCoV-NL63 virus yield was determined using real-time PCR. Five microlitres of cDNA was amplified in a 20 µl reaction mixture containing 1× TaqMan Universal PCR Master Mix, No AmpEraseUNG (Applied Biosystems), 200 nM specific probe labelled with 6-carboxyfluorescein (FAM) and 6-carboxytetramethylrhodamine (TAMRA) and 900 nM each primer. Rox was used as a reference dye. The following primers were used for HCoV-NL63 amplification: sense, 5′-AAACCTCGTTGGAAGCGTGT-3′; antisense, 5′-CTGTGGAAAACCTTTGGCATC-3′; and probe, 5′-FAM-ATGTTATTCAGTGCTTTGGTCCTCGTGAT-TAMRA-3′. The reaction was monitored on a 7500 Fast Real-Time PCR machine (Applied Biosystems) with the following settings: 2 min at 50 °C and 10 min at 92 °C, followed by 45 cycles of 15 s at 92 °C and 1 min at 60 °C.
Surface expression of molecules involved in bacterial adherence.
LLC-MK2 cells were scraped from plates, washed with PBS, incubated with 1 % BSA (Sigma-Aldrich) in PBS and fixed with 3 % PFA. To assess the surface expression of PAF-R, cells were incubated for 2 h at room temperature with a 1 : 50 dilution of rabbit anti-human PAF-R polyclonal antibody (Cayman Chemicals), followed by incubation with a 1 : 200 dilution of Alexa Fluor 488-labelled goat anti-rabbit antibody (Molecular Probes) for 1 h prior to analysis. To analyse surface expression of E-cadherin, cells were incubated with a 1 : 50 dilution of Alexa Fluor 647-conjugated anti-E-cadherin (CD324; eBioscience) antibody. To analyse surface expression of TLR-2 and TLR-4, cells were incubated with mouse anti-TLR-2 mAb (Abcam) and mouse anti-TLR-4 mAb (Abcam). For TLR staining, 1 : 200-diluted goat Alexa Fluor 488-conjugated anti-mouse secondary antibody (Molecular Probes) was used. In each case, after incubation with antibodies, cells were washed, suspended in PBS and analysed with Becton Dickinson FACSCalibur using CellQuest software.
Fluorescence microscopy.
LLC-MK2 monolayers were inoculated with virus and mock infected as described above. FITC-labelled bacteria (1×109 c.f.u. ml−1) were added to cell cultures and incubated for 1 h at 4 °C. Cell monolayers were washed three times with ice-cold PBS and subsequently fixed with 3 % PFA (Sigma-Aldrich). Samples were examined under a fluorescent microscope (Nikon Eclipse Ti).
HCoV-NL63-infected or mock-infected HAE cultures were cooled, inoculated at the apical surface with bacteria (1×108 c.f.u. ml−1) and subsequently incubated for 1 h at 4 °C. The apical side of the cultures was then rinsed three times with ice-cold PBS to remove non-adherent bacteria. Samples were examined under a fluorescent microscope.
Statistical analysis.
All experiments were performed in triplicate and results are expressed as means±sd. To determine the significance of the obtained results, comparisons between groups were made using Student’s t-test. P values <0.05 were considered significant.
Acknowledgements
We gratefully thank Lia van der Hoek, Janusz Fiett and Sigrun Eick for supplying cell lines, viral stocks and bacterial cultures. This work was supported in part by the Foundation for Polish Science within the HOMING Programme (K. P.), a grant from the Ministry of Scientific Research, Poland (0095/B/P01/2009/37) (K. P.), the Jagiellonian University statutory funds DS/9/WBBiB (J. P. and K. P.) and grants from the National Institutes of Health, USA (DE 09761) (J. P.), the Department of Scientific Research, Polish Ministry of Science and Education (1642/B/P01/2008/35) (J. P.) and the Foundation for Polish Science (TEAM project DPS/424-329/10) (J. P.). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union (grant no. POIG.02.01.00-12-064/08 – ‘Molecular biotechnology for health’). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Abrahams A., Hallows N., French H. (1919). A further investigation into influenzo-pneumococcal and influenzo-streptococcal septicaemia: epidemic influenzal pneumonia of highly fatal type and its relation to puruleng bronchitis. Lancet 193, 1–11 10.1016/S0140-6736(01)22115-1 [DOI] [Google Scholar]
- Avadhanula V., Rodriguez C. A., Devincenzo J. P., Wang Y., Webby R. J., Ulett G. C., Adderson E. E. (2006). Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 80, 1629–1636 10.1128/JVI.80.4.1629-1636.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhanula V., Wang Y., Portner A., Adderson E. (2007). Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J Med Microbiol 56, 1133–1137 10.1099/jmm.0.47086-0 [DOI] [PubMed] [Google Scholar]
- Barletta E., Mugnai G., Ruggieri S. (2002). Platelet activating factor inhibits the expression of matrix metalloproteinases and affects invasiveness and differentiation in a system of human neuroblastoma clones. Biol Chem 383, 189–197 10.1515/BC.2002.019 [DOI] [PubMed] [Google Scholar]
- Bastien N., Robinson J. L., Tse A., Lee B. E., Hart L., Li Y. (2005). Human coronavirus NL-63 infections in children: a 1-year study. J Clin Microbiol 43, 4567–4573 10.1128/JCM.43.9.4567-4573.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. (2002). Anaerobic infections in children. Microbes Infect 4, 1271–1280 10.1016/S1286-4579(02)01656-8 [DOI] [PubMed] [Google Scholar]
- Brook I., Frazier E. H. (1993). Aerobic and anaerobic microbiology of empyema. A retrospective review in two military hospitals. Chest 103, 1502–1507 10.1378/chest.103.5.1502 [DOI] [PubMed] [Google Scholar]
- Carlisle M. D., Srikantha R. N., Brogden K. A. (2009). Degradation of human α- and β-defensins by culture supernatants of Porphyromonas gingivalis strain 381. J Innate Immun 1, 118–122 10.1159/000181015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. S., Chan K. H., Chu K. W., Kwan S. W., Guan Y., Poon L. L., Peiris J. S. (2005). Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis 40, 1721–1729 10.1086/430301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell D. R., Gerard N. P., Gerard C., Idanpaan-Heikkila I., Tuomanen E. I. (1995). Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377, 435–438 10.1038/377435a0 [DOI] [PubMed] [Google Scholar]
- Dijkman R., Jebbink M. F., El Idrissi N. B., Pyrc K., Müller M. A., Kuijpers T. W., Zaaijer H. L., van der Hoek L. (2008). Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol 46, 2368–2373 10.1128/JCM.00533-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttweiler L., Nadal D., Frey B. (2004). Pulmonary and systemic bacterial co-infections in severe RSV bronchiolitis. Arch Dis Child 89, 1155–1157 10.1136/adc.2004.049551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M. (1991). Aspiration pneumonia. Rev Infect Dis 13 Suppl. 9S737–S742 [DOI] [PubMed] [Google Scholar]
- Fouchier R. A., Hartwig N. G., Bestebroer T. M., Niemeyer B., de Jong J. C., Simon J. H., Osterhaus A. D. (2004). A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A 101, 6212–6216 10.1073/pnas.0400762101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golda A., Pyrc K. (2008). Recent antiviral strategies against human coronavirus-related respiratory illnesses. Curr Opin Pulm Med 14, 248–253 10.1097/MCP.0b013e3282f7646f [DOI] [PubMed] [Google Scholar]
- Håkansson A., Kidd A., Wadell G., Sabharwal H., Svanborg C. (1994). Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect Immun 62, 2707–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hament J. M., Kimpen J. L., Fleer A., Wolfs T. F. (1999). Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol 26, 189–195 10.1111/j.1574-695X.1999.tb01389.x [DOI] [PubMed] [Google Scholar]
- Hament J. M., Aerts P. C., Fleer A., Van Dijk H., Harmsen T., Kimpen J. L., Wolfs T. F. (2004). Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory syncytial virus. Pediatr Res 55, 972–978 10.1203/01.PDR.0000127431.11750.D9 [DOI] [PubMed] [Google Scholar]
- Hament J. M., Aerts P. C., Fleer A., van Dijk H., Harmsen T., Kimpen J. L., Wolfs T. F. (2005). Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr Res 58, 1198–1203 10.1203/01.pdr.0000188699.55279.1b [DOI] [PubMed] [Google Scholar]
- Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pöhlmann S. (2005). Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A 102, 7988–7993 10.1073/pnas.0409465102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka S., Yamaya M., Suzuki T., Takahashi H., Ida S., Sasaki T., Inoue D., Sekizawa K., Nishimura H., Sasaki H. (2003). Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 188, 1928–1939 10.1086/379833 [DOI] [PubMed] [Google Scholar]
- Kaiser L., Regamey N., Roiha H., Deffernez C., Frey U. (2005). Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J 24, 1015–1017 10.1097/01.inf.0000183773.80217.12 [DOI] [PubMed] [Google Scholar]
- Kneyber M. C. J., Blussé van Oud-Alblas H., van Vliet M., Uiterwaal C. S. P. M., Kimpen J. L. L., van Vught A. J. (2005). Concurrent bacterial infection and prolonged mechanical ventilation in infants with respiratory syncytial virus lower respiratory tract disease. Intensive Care Med 31, 680–685 10.1007/s00134-005-2614-4 [DOI] [PubMed] [Google Scholar]
- Louria D. B., Blumenfeld H. L., Ellis J. T., Kilbourne E. D., Rogers D. E. (1959). Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest 38, 213–265 10.1172/JCI103791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers J. A. (2006). Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 19, 571–582 10.1128/CMR.00058-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers J. A., Bartmess K. C. (2003). Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 187, 1000–1009 10.1086/368163 [DOI] [PubMed] [Google Scholar]
- McCullers J. A., Rehg J. E. (2002). Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 186, 341–350 10.1086/341462 [DOI] [PubMed] [Google Scholar]
- McCullers J. A., Iverson A. R., McKeon R., Murray P. J. (2008). The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand J Infect Dis 40, 11–17 10.1080/00365540701477568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir R., Wilson G. H. (1919). Influenza and its complications. BMJ 1, 3–5 10.1136/bmj.1.3027.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Akuta T., Tamura F., van Der Vliet A., Akaike T. (2004). Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol Chem 385, 997–1006 10.1515/BC.2004.130 [DOI] [PubMed] [Google Scholar]
- Okuda K., Kimizuka R., Abe S., Kato T., Ishihara K. (2005). Involvement of periodontopathic anaerobes in aspiration pneumonia. J Periodontol 76 Suppl.2154–2160 10.1902/jop.2005.76.11-S.2154 [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Banach B., Baker S. C. (2008). Morphogenesis of coronavirus HCoV-NL63 in cell culture: a transmission electron microscopic study. Open Infect Dis J 2, 52–58 10.2174/1874279300802010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passariello C., Schippa S., Conti C., Russo P., Poggiali F., Garaci E., Palamara A. T. (2006). Rhinoviruses promote internalisation of Staphylococcus aureus into non-fully permissive cultured pneumocytes. Microbes Infect 8, 758–766 10.1016/j.micinf.2005.09.013 [DOI] [PubMed] [Google Scholar]
- Patel J. A., Kunimoto M., Sim T. C., Garofalo R., Eliott T., Baron S., Ruuskanen O., Chonmaitree T., Ogra P. L., Schmalstieg F. (1995). Interleukin-1α mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol 13, 602–609 [DOI] [PubMed] [Google Scholar]
- Peltola V. T., McCullers J. A. (2004). Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J 23 Suppl.S87–S97 10.1097/01.inf.0000108197.81270.35 [DOI] [PubMed] [Google Scholar]
- Peltola V. T., Murti K. G., McCullers J. A. (2005). Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis 192, 249–257 10.1086/430954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J., Pike R. N. (2009). Corruption of innate immunity by bacterial proteases. J Innate Immun 1, 70–87 10.1159/000181144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Jebbink M. F., Berkhout B., van der Hoek L. (2004). Genome structure and transcriptional regulation of human coronavirus NL63. Virol J 1, 7 10.1186/1743-422X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Dijkman R., Deng L., Jebbink M. F., Ross H. A., Berkhout B., van der Hoek L. (2006). Mosaic structure of human coronavirus NL63, one thousand years of evolution. J Mol Biol 364, 964–973 10.1016/j.jmb.2006.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Berkhout B., van der Hoek L. (2007a). Identification of new human coronaviruses. Expert Rev Anti Infect Ther 5, 245–253 10.1586/14787210.5.2.245 [DOI] [PubMed] [Google Scholar]
- Pyrc K., Berkhout B., van der Hoek L. (2007b). The novel human coronaviruses NL63 and HKU1. J Virol 81, 3051–3057 10.1128/JVI.01466-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Jebbink M. F., Berkhout B., van der Hoek L. (2008). Detection of new viruses by VIDISCA. Virus discovery based on cDNA-amplified fragment length polymorphism. Methods Mol Biol 454, 73–89 10.1007/978-1-59745-181-9_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Sims A. C., Dijkman R., Jebbink M., Long C., Deming D., Donaldson E., Vabret A., Baric R., et al. (2010). Culturing the unculturable: human coronavirus HKU1 infects, replicates, and produces progeny virions in human ciliated airway epithelial cell cultures. J Virol 84, 11255–11263 10.1128/JVI.00947-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. N., Orihuela C. J., Murti G., Guglielmo C., Murray P. J., Tuomanen E. I. (2005). β-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun 73, 7827–7835 10.1128/IAI.73.12.7827-7835.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph A. G., Reder L., Englund J. A. (2004). Risk of bacterial infection in previously healthy respiratory syncytial virus-infected young children admitted to the intensive care unit. Pediatr Infect Dis J 23, 990–994 10.1097/01.inf.0000143647.88873.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. J., Muench H. (1938). A simple method of estimating fifty percent endpoints. Am J Hyg 27, 493–497 [Google Scholar]
- Scannapieco F. A. (1999). Role of oral bacteria in respiratory infection. J Periodontol 70, 793–802 10.1902/jop.1999.70.7.793 [DOI] [PubMed] [Google Scholar]
- Schildgen O., Jebbink M. F., de Vries M., Pyrc K., Dijkman R., Simon A., Müller A., Kupfer B., van der Hoek L. (2006). Identification of cell lines permissive for human coronavirus NL63. J Virol Methods 138, 207–210 10.1016/j.jviromet.2006.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A. (1979). Anaerobic pulmonary infections. Scand J Infect Dis Suppl 19, 77–79 [PubMed] [Google Scholar]
- Sims A. C., Yount B., Burkett S. E., Baric R. S., Pickles R. J. (2006). SARS CoV replication and pathogenesis in human airway epithelial cultures. Adv Exp Med Biol 581, 535–538 10.1007/978-0-387-33012-9_97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stille W. T., Pierce W., Crawford Y. E. (1961). Multiple infections in acute respiratory illness. I. Severity of illness of naval recruits and independence of infectious agents. J Infect Dis 109, 158–165 [DOI] [PubMed] [Google Scholar]
- Stone W. J., Swift G. W. (1919). Influenza and influenzal pneumonia at Fort Riley, Kansas. JAMA 72, 487–493 [Google Scholar]
- Suzuki A., Okamoto M., Ohmi A., Watanabe O., Miyabayashi S., Nishimura H. (2005). Detection of human coronavirus-NL63 in children in Japan. Pediatr Infect Dis J 24, 645–646 10.1097/01.inf.0000168846.71517.ee [DOI] [PubMed] [Google Scholar]
- Terajima M., Yamaya M., Sekizawa K., Okinaga S., Suzuki T., Yamada N., Nakayama K., Ohrui T., Oshima T., et al. (1997). Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1β. Am J Physiol 273, L749–L759 [DOI] [PubMed] [Google Scholar]
- Thompson C. I., Barclay W. S., Zambon M. C., Pickles R. J. (2006). Infection of human airway epithelium by human and avian strains of influenza a virus. J Virol 80, 8060–8068 10.1128/JVI.00384-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn K., Harigopal S., Reddy V., Taylor N., van Saene H. K. (2006). High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 61, 611–615 10.1136/thx.2005.048397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H. H., Weiser J. N., James M. A., DeMaria T. F. (2001). Effect of influenza A virus infection on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. Infect Immun 69, 602–606 10.1128/IAI.69.1.602-606.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A., Mourez T., Dina J., van der Hoek L., Gouarin S., Petitjean J., Brouard J., Freymuth F. (2005). Human coronavirus NL63, France. Emerg Infect Dis 11, 1225–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M. F., Vermeulen-Oost W., Berkhout R. J., Wolthers K. C., Wertheim-van Dillen P. M., Kaandorp J., Spaargaren J., Berkhout B. (2004). Identification of a new human coronavirus. Nat Med 10, 368–373 10.1038/nm1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M. F., Petersen G., Forster J., Berkhout B., Uberla K. (2005). Croup is associated with the novel coronavirus NL63. PLoS Med 2, e240 10.1371/journal.pmed.0020240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M. F., Petersen G., Forster J., Berkhout B., Uberla K. (2006). Human coronavirus NL63 infection is associated with croup. Adv Exp Med Biol 581, 485–491 10.1007/978-0-387-33012-9_86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs K. F., van Elden L. J., Nijhuis M., Schuurman R., Florquin S., Shimizu T., Ishii S., Jansen H. M., Lutter R., van der Poll T. (2006). Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am J Physiol Lung Cell Mol Physiol 290, L194–L199 10.1152/ajplung.00050.2005 [DOI] [PubMed] [Google Scholar]
- Van Ewijk B. E., Wolfs T. F., Aerts P. C., Van Kessel K. P., Fleer A., Kimpen J. L., Van der Ent C. K. (2007). RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res 61, 398–403 10.1203/pdr.0b013e3180332d1c [DOI] [PubMed] [Google Scholar]
- Wilson C. B. P. S., Steer P. (1919). Bacteriological and pathological observations on influenza as seen in France during 1918. Br Med J 1, 634–635 10.1136/bmj.1.3047.634 [DOI] [Google Scholar]
- Wolbach S. B. (1919). Comments on the pathology and bacteriology of fatal influenza cases, as observed at Camp Devens, Mass. John Hopkins Hosp Bull 30, 104–109 [Google Scholar]
- Wu P.-S., Chang L.-Y., Berkhout B., Hoek L., Lu C.-Y., Kao C.-L., Lee P.-I., Shao P.-L., Lee C.-Y., et al. (2008). Clinical manifestations of human coronavirus NL63 infection in children in Taiwan. Eur J Pediatr 167, 75–80 10.1007/s00431-007-0429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Peeples M. E., Boucher R. C., Collins P. L., Pickles R. J. (2002). Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76, 5654–5666 10.1128/JVI.76.11.5654-5666.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Bukreyev A., Thompson C. I., Watson B., Peeples M. E., Collins P. L., Pickles R. J. (2005). Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 79, 1113–1124 10.1128/JVI.79.2.1113-1124.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]