Abstract
In genome-wide association studies (GWAS) of common genetic variants associated with circulating alpha- and gamma-tocopherol concentrations in two adult cohorts comprising 5006 men of European descent, we observed three loci associated with alpha-tocopherol levels, two novel single-nucleotide polymorphisms (SNPs), rs2108622 on 19pter-p13.11 (P= 1.7 × 10−8) and rs11057830 on 12q24.31 (P= 2.0 × 10−8) and confirmed a previously reported locus marked by rs964184 on 11q23.3 (P= 2.7 × 10−10). The three SNPs have been reported to be associated with lipid metabolism and/or regulation. We replicated these findings in a combined meta-analysis with two independent samples, P= 7.8 × 10−12 (rs964184 on 11q23.3 near BUD13, ZNF259 and APOA1/C3/A4/A5), P= 1.4 × 10−10 (rs2108622 on 19pter-p13.11 near CYP4F2) and P= 8.2 × 10−9 (rs11057830 on 12q24.31 near SCARB1). Combined, these SNPs explain 1.7% of the residual variance in log alpha-tocopherol levels. In one of the two male GWAS cohorts (n= 992), no SNPs were significantly associated with gamma-tocopherol concentrations after including data from the replication sample for 71 independent SNPs with P< 1 × 10−4 identified.
INTRODUCTION
Vitamin E has been examined for its possible role in preventing chronic diseases including heart disease, diabetes and cancer through its anti-oxidant and other properties (1–7). It is an essential fat-soluble micronutrient encompassing four tocopherols and four tocotrienols that protect the cellular membranes, low-density lipoprotein (LDL) cholesterol and other molecules from oxidative damage. Alpha-tocopherol, the primary vitamin E compound in humans, also inhibits cell proliferation and angiogenesis (8), induces apoptosis (9) and enhances immune function (10).
A growing literature of novel genetic determinants of circulating nutrients identified from genome-wide association studies (GWAS) [e.g. vitamin B12 (11) and vitamin D (12)] is enhancing our ability to investigate their associations with human disease. With respect to vitamin E compounds, only one GWAS of the circulating alpha-tocopherol phenotype has been conducted, which found a single-nucleotide polymorphism (SNP) close to the apolipoprotein A5 (APOA5) gene (rs12272004) to be associated with higher plasma alpha-tocopherol levels (13). The association between the autosomal recessive neurodegenerative disease, AVED (ataxia with isolated vitamin E deficiency) and mutations in the gene, TTPA, on chromosome 8q13 encoding the alpha-tocopherol transfer protein (14), has been established. We report here findings from a GWAS meta-analysis of circulating alpha-tocopherol and gamma-tocopherol.
RESULTS
Alpha-tocopherol
In 4014 participants in the ATBC Study (Table 1), we found evidence of novel quantitative trait loci for log-transformed circulating alpha-tocopherol concentration. Figure 1 depicts the Manhattan plot for genetic variants in relation to alpha-tocopherol levels achieving genome-wide significance on 11q23.3, 19pter-p13.11 and 12q24.31. In each region, there are plausible candidate SNPs for follow-up: rs964184 near BUD13 [(yeast) budding site selection protein 13], ZNF259 (zinc finger protein 259) and APOA1/C3/A4/A5 (apolipoproteins A1, C3, A4 and A5) on 11q23.3, rs2108622 in CYP4F2 (cytochrome p450, family 4, subfamily F, polypeptide 2) on 19pter-p13.11 and rs11057830 in SCARB1 (scavenger receptor class B member 1) 12p24.31 (Table 2). Our most significant findings were replicated in silico using two independent data sets, with the combined meta-analysis revealing markers below the threshold for genome-wide significance, namely, P= 7.8 × 10−12 for rs964184, P= 1.4 × 10−10 for rs2108622 and P= 8.2 × 10−9 for rs11057830.
Table 1.
Baseline characteristics of GWAS study cohorts
| Characteristic | ATBC (n= 4014) | PLCO (n= 992) | NHS (n= 2775) |
|---|---|---|---|
| Cases/controls, n | 2402/1612 | 475/517 | 1416/1359 |
| Age at randomization (years) | |||
| Mean ± SD | 58.1 ± 5.0 | 64.6 ± 4.9 | 59.2 ± 6.3 |
| Median (range) | 58 (49–70) | 65 (55–74) | 60 (43–70) |
| BMI (kg/m2) | |||
| Mean ± SD | 26.2 ± 3.7 | 27.4 ± 3.7 | 25.8 ± 4.9 |
| Median (range) | 25.9 (16.1–49.4) | 27.0 (19.7–43.7) | 24.7 (15.6–52.2) |
| Serum total cholesterol (mg/dL) | |||
| Mean ± SD | 241.4 ± 44.8 | 236.2 ± 74.6 | 224.7 ± 32.9 |
| Median (range) | 239.0 (110.2–460.2) | 222.0 (77.0–579.3) | 224.0 (116.0–414.6) |
| Serum HDL cholesterol (mg/dL) | |||
| Mean ± SD | 46.0 ± 11.6 | — | — |
| Median (range) | 11.1 (11.3–139.2) | — | — |
| Alpha-tocopherol (mg/L) | |||
| Mean ± SD | 11.9 ± 3.4 | 19.1 ± 9.7 | 13.3 ± 5.8 |
| Median (range) | 11.5 (1.9–52.9) | 16.8 (5.8–102.5) | 12.0 (0.5–61.6) |
| Gamma-tocopherol (mg/L) | |||
| Mean ± SD | — | 3.3 ± 1.9 | 2.1 ± 1.2 |
| Median (range) | — | 2.9 (2.2–13.2) | 1.9 (0.2–11.6) |
SD, standard deviation.
Figure 1.
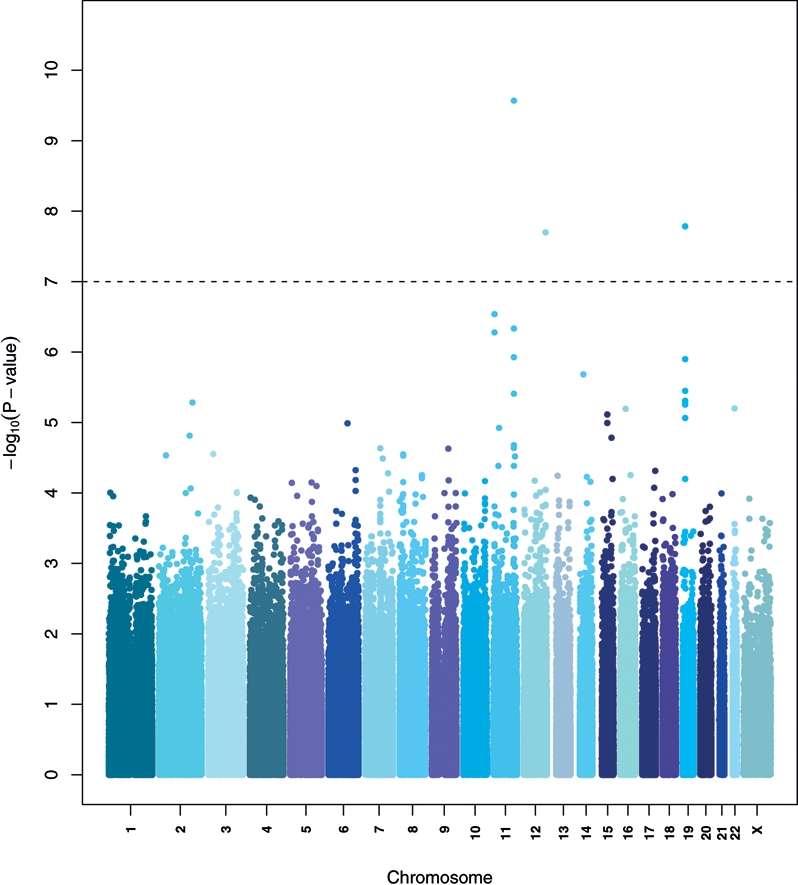
Manhattan plot of the P-values in the serum alpha-tocopherol GWAS (ATBC). The x-axis represents chromosomal locations and the y-axis shows P-values on a logarithmic scale.
Table 2.
Genetic variants associated with circulating alpha-tocopherol concentrations in the GWAS
| SNP | Chr | Location | Gene | MAF (allele) | Study | P-value | Beta | SE | Mean alpha-tocopherol (mg/L) by genotype (number of minor alleles) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | |||||||||
| rs964184 | 11 | 116154127 | BUD13/ZNF259/APOA5 | 0.15 (G) | |||||||
| GWAS | ATBC | 2.7 × 10−10 | 0.04 | 0.01 | 11.6 | 12.5 | 13.6 | ||||
| Replication | PLCO | 5.9 × 10−1 | −0.01 | 0.02 | 19.3 | 18.6 | 19.2 | ||||
| Total NHS | 7.2 × 10−4 | 0.05 | 0.01 | — | — | — | |||||
| NHS-CGEMS | 5.6 × 10−2 | 0.03 | 0.02 | 13.2 | 13.9 | 13.3 | |||||
| NHS-CHD | 1.9 × 10−2 | 0.08 | 0.03 | 13.0 | 13.8 | 16.3 | |||||
| NHS-T2D | 2.5 × 10−2 | 0.09 | 0.04 | 13.1 | 14.9 | 17.4 | |||||
| Combined meta-analysis | 7.8 × 10−12 | 0.04 | 0.01 | — | — | — | |||||
| rs2108622 | 19 | 15851431 | CYP4F2 | 0.21 (T) | |||||||
| GWAS | ATBC | 1.7 × 10−8 | 0.04 | 0.01 | 11.8 | 12.1 | 12.7 | ||||
| Replication | PLCO | 1.8 × 10−2 | 0.05 | 0.02 | 18.6 | 19.6 | 20.3 | ||||
| Total NHS | 2.7 × 10−2 | 0.02 | 0.01 | — | — | — | |||||
| NHS-CGEMS | 1.7 × 10−2 | 0.03 | 0.01 | 13.2 | 13.5 | 14.3 | |||||
| NHS-CHD | 1.0 × 10−1 | 0.04 | 0.03 | 12.7 | 13.6 | 14.7 | |||||
| NHS-T2D | 1.5 × 10−1 | −0.05 | 0.03 | 13.9 | 13.5 | 12.7 | |||||
| Combined meta-analysis | 1.4 × 10−10 | 0.03 | 0.01 | — | — | — | |||||
| rs11057830 | 12 | 123873006 | SCARB1 | 0.15 (A) | |||||||
| GWAS | ATBC | 2.0 × 10−8 | 0.04 | 0.01 | 11.8 | 12.2 | 12.7 | ||||
| Replication | PLCO | 7.3 × 10−1 | −0.01 | 0.03 | 19.1 | 18.5 | 18.0 | ||||
| Total NHS | 3.9 × 10−2 | 0.03 | 0.01 | — | — | — | |||||
| NHS-CGEMS | 3.3 × 10−1 | 0.02 | 0.02 | 13.4 | 13.5 | 13.9 | |||||
| NHS-CHD | 4.4 × 10−2 | 0.06 | 0.03 | 12.9 | 14.3 | 13.1 | |||||
| NHS-T2D | 3.8 × 10−1 | 0.03 | 0.04 | 13.6 | 13.5 | 15.1 | |||||
| Combined meta-analysis | 8.2 × 10−9 | 0.03 | 0.01 | — | — | — | |||||
MAF, minor allele frequency; SE, standard error. The regression beta and standard error were based on logarithmic scale.
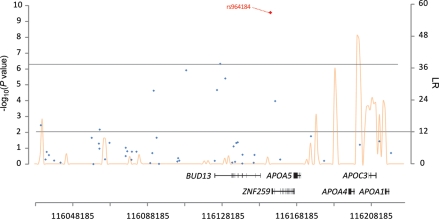
Our GWAS identified an association between serum alpha-tocopherol and rs964184 on chromosome 11q23.3. Four other correlated SNPs in the BUD13/ZNF259 region reached genome-wide significance and were in moderate linkage disequilibrium (LD) with rs964184 (range of r2= 0.2–0.6): rs180326 (r2= 0.27), rs7350481 (r2= 0.56), rs12292921 (r2= 0.26) and rs12272004 (r2= 0.20) (Fig. 2). We fit sequential general linear regression models to test whether the association of each of these four SNPs was independent of that of rs964184 using a likelihood ratio test. Results showed none of the SNPs to be independently associated with circulating alpha-tocopherol after controlling for rs964184 and the covariates used in the ATBC GWAS analysis (likelihood ratio test P-values for the four SNPs are 0.13, 0.89, 0.11 and 0.18, respectively), suggesting that the five variants represent an association signal derived from a common source and rs964184 is the marker with the strongest association with alpha-tocopherol concentrations. One of the statistically significant (although, not independent) signals, rs12272004 (P= 5.1 × 10−8), was previously reported to be associated with serum alpha-tocopherol (P= 3.9 × 10−7) in a previous GWAS (13).
Figure 2.
Association and recombination hotspot for rs964184 SNP on chromosome 11. LR = the recombination rate on a logarithmic scale with 12 being ‘notable’ for a hotspot.
This chromosomal region contains several genes involved in cholesterol and lipid metabolism including APOA4, APOA5 and APOC3 (15–17). The rs12272004 SNP has been reported to be correlated with the S19W APOA5 SNP (13). The rs964184 variant allele, associated with increased alpha-tocopherol, has also been associated with decreased circulating HDL cholesterol and increased triglyceride concentrations (18–20). Although not reaching genome-wide significance, an association has been suggested between rs12292921 and triglyceride concentrations (P= 1.3 × 10−3) (15). Of the five variants, rs12272004 and rs12292921 were not highly correlated with our primary SNP related to alpha-tocopherol concentrations (rs964184; LD r2's= 0.20 and 0.26, respectively), but were highly correlated with each other (rs12292921 and rs12272004; LD r2= 0.88). Many of the other SNPs (n= 41) previously identified in meta-analyses as being related to circulating lipids (21,22) were not, however, significantly associated with alpha-tocopherol levels in our study, although most (75%) were consistent with respect to effect directionality (Supplementary Material, Table S2).
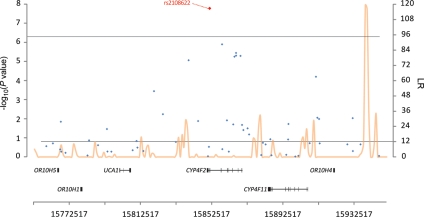
We also found a novel association between increased circulating alpha-tocopherol and a variant in CYP4F2 (rs2108622; P= 1.7 × 10−8) on chromosome 19pter-p13.1. One other nearby SNP, rs2074901 approached genome-wide significance (P= 1.3 × 10−6; LD r2= 0.50, Fig. 3). After conditioning on rs2108622, however, rs2074901 was no longer significantly associated with alpha-tocopherol (P= 0.31), indicating the association was not independent of rs2108622. CYP4F2 contributes to vitamin E metabolism by catalyzing tocopherol phytyl side-chain oxidation (23–25). The rs2108622 variant has also been associated with elevated hepatic vitamin K1 concentrations (26) and altered fatty acid metabolism (23). Carriers of one or two copies of the variant allele were recently shown to require higher warfarin dosing for adequate coagulation compared with those homozygous for the common allele in a GWAS study of Japanese patients (27). According to results of recent meta-analyses, rs2108622 is not associated with triglycerides (P-values ranging from 0.39 to 0.97) (19,20), supporting a non-lipid transport function for CYP4F2 in vitamin E metabolism.
Figure 3.
Association and recombination hotspot for rs2108622 SNP on chromosome 19. LR = the recombination rate on a logarithmic scale with 12 being ‘notable’ for a hotspot.
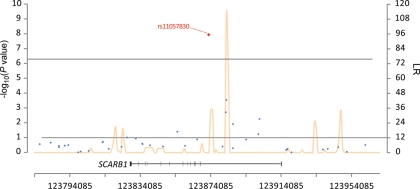
A significant association between a common variant in SCARB1 (rs11057830; P= 2.0 × 10−8) and alpha-tocopherol levels was also observed; no other SNPs approached genome-wide significance (Fig. 4). A candidate-gene study showed two other SNPs in SCARB1 (i.e. in exons 1 and 8) to be significantly related to plasma concentrations of gamma-tocopherol (P= 0.027) and alpha-tocopherol (P= 0.037) in men (28). The SCARB1 protein, which is encoded in the region of chromosome 12q24.31, has been implicated in the transfer of alpha-tocopherol from HDL to tissues (29). Other SCARB1 SNPs (e.g. rs9919713, three variants at exons 1 and 8 and intron 5, and rs838880) have been associated with insulin-resistance, increased HDL, lower LDL and/or higher body mass index (21,30,31). Our finding is of interest in that promising signals from SNP markers in this region also appear associated with risk for renal cell carcinoma (32), a cancer for which body mass index (BMI) and obesity are established risk factors. The LD, however, was low (r2= 0.01) between our SCARB1 signal and rs838880; the other SCARB1 SNPs (including surrogates) were not available in our scan data.
Figure 4.
Association and recombination hotspot for rs11057830 SNP on chromosome 12. LR = the recombination rate on a logarithmic scale with 12 being ‘notable’ for a hotspot.
The three SNP markers (rs964184, rs2108622, rs11057830) together explain 1.7% of the variance in log-transformed alpha-tocopherol concentration in the ATBC samples after adjusting for age, BMI, cholesterol and cancer status.
Gamma-tocopherol
A GWAS analysis of gamma-tocopherol levels was conducted in 992 participants in the PLCO study and identified associations between log-transformed circulating levels and variants in the following four regions (Supplementary Material, Table S1): rs847915 [TAS2R2 (taste receptor, type 2, member 2), P= 2.7 × 10−7] on chromosome 7; rs6865300 (NA, P= 4.7 × 10−7) and rs442392 (NA, P= 6.0 × 10−7; LD r2= 0.54) on chromosome 5; and rs3815951 in ARHGAP17 (Rho GTPase activating protein 17), P= 9.1 × 10−7 on chromosome 16. The loci associated with the alpha-tocopherol levels were not related to gamma-tocopherol (rs964184, P= 0.81; rs2108622, P= 0.09; rs11057830, P= 0.58). ARHGAP17 plays a central role in epithelial apical polarity through regulation of CDC42, a small G protein. For the two SNPs on chromosome 5, results of sequential general linear models showed rs6865300 to be independently associated with gamma-tocopherol levels.
DISCUSSION
Three independent genetic loci were identified in association with circulating alpha-tocopherol concentrations. Furthermore, the SNP markers localize to candidate genes that have a strong biological basis in the transport and metabolism of vitamin E, including association with circulating lipoproteins. The magnitudes of the estimated betas reported in this discovery study were small, ranging from 0.03 to 0.04, and in combination explained only 1.7% of the variance in concentrations among individuals. Additional studies are needed to refine the estimates of the associations and to accurately confirm the effect sizes.
To our knowledge, only one previous GWAS examined the circulating alpha-tocopherol phenotype (13). Consistent with that investigation, we observed a marker with genome-wide significance, rs12272004, but this common variant was in moderate LD and not independent of our most significant SNP, rs964184.
Because our analysis for discovering common variants associated with gamma-tocopherol levels was not well-powered and could represent a false positive finding, additional studies are needed to re-examine those associations. It is, however, notable that our findings for gamma-tocopherol, a compound differing from alpha-tocopherol only by the absence of one methyl group at the C-5 position of the chroman ring identified different candidate regions. This underscores the complexity of the pathways that contribute to the metabolism of circulating vitamin E congeners.
Among the inherent strengths of our study is the large sample size and confirmation of findings in independent populations. Because baseline information on factors known (or suspected) to modify levels of circulating vitamin E existed in the parent study, we were able to elucidate the independent effects of SNPs by multivariable adjustment. Our study is limited in that triglycerides were not measured and therefore we were unable to directly account for them in the GWAS; however, we did adjust for total cholesterol and non-HDL levels which did not alter the study findings. Because triglyceride data were unavailable in the present study, we are unable to rule out the possibility that the observed signal may be secondary to, or influenced by, the associations previously reported for triglycerides (21). It is possible that the association we observed for rs964184 is reflecting the strong association previously reported for triglycerides (P= 7 × 10−240). For rs11057830 (SCARB1), the relation with triglycerides is not well-established (P-values= 0.001, 0.30 and 0.80) according to previous meta-analyses (19–21). We do believe that our finding for rs2108622 (CYP4F2) is novel and does not reflect an influence of triglycerides or transport in circulation. Previous meta-analyses have not reported a significant association for this CYP4F2 SNP and triglyceride levels (P-values range from 0.39 to 0.93) (19–21). Another potential limitation is that all participants in our primary scan were male smokers; therefore, possible modifying effects of smoking status on the observed associations cannot be excluded. Previous studies have demonstrated that cigarette smoking increases the concentration of triglycerides and decreases HDL cholesterol (33–35). In addition, smoking-associated free radicals deplete vitamin E and other anti-oxidants (36). Our main findings for the three loci did, however, replicate in an independent population of women comprised of primarily never smokers, suggesting that heterogeneity in the circulating vitamin E-genetic associations based on smoking status is unlikely.
In conclusion, we found strong evidence that common genetic variants on 11q23.3, within or near BUD13, ZNF259 and the APOA1/C3/A4/A5 gene cluster are associated with circulating alpha-tocopherol levels. Our study identified two additional SNP markers, one that maps to 19pter-p13.11, which includes the biologically plausible candidate gene CYP4F2 and another on 12q24.31, which harbors SCARB1. Further work is required to fine map the regions discovered in our GWAS in order to nominate the optimal variants for functional studies designed to understand the biological basis of the reported observations. Our findings have identified regions of the genome that influence vitamin E biochemical status and provide a framework for investigation of this membrane-integrated micronutrient that is important in complex chronic diseases, such as cardiovascular disease, diabetes, and cancer.
MATERIALS AND METHODS
We conducted a GWAS of serum alpha-tocopherol concentrations within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study cohort and replicated the findings in a combined meta-analysis with the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) Study and the Nurses’ Health Study (NHS) (Table 1). Similarly, a separate GWAS was performed for gamma-tocopherol concentrations using available data from the PLCO Study.
Briefly, the ATBC Study was a randomized, double-blind, placebo-controlled intervention trial conducted to determine whether supplementation with alpha-tocopherol, beta-carotene or both could prevent cancer (37). The participants were all male smokers at study entry, aged 50–69 years, and residents of southwestern Finland. Men were not eligible for study inclusion if they reported a history of cancer, had severe diseases limiting long-term participation or took supplements of vitamins E (>20 mg/day) or A (>20 000 IU/day) or beta-carotene (>6 mg/day). Fasting blood samples were collected at baseline and stored at −70°C until analyzed. Serum alpha-tocopherol levels were measured by high-performance liquid chromatography (38), with a coefficient of variation (CV) of 2.2%. Gamma-tocopherol was not measured at study baseline. Serum cholesterol levels were measured with an enzymatic assay by the CHOD-PAP method (Boehringer Mannhein) (39). GWAS data from the Illumina 550K platform were available for 4014 men that were also previously analyzed with respect to circulating vitamin D levels (12).
The PLCO Study was a multi-center trial conducted in the US to evaluate the effectiveness of cancer screening and examine early markers of cancer (40). PLCO male participants of Caucasian descent, aged 55–74 years, were included in the present GWAS (n= 992). Plasma concentrations of alpha- and gamma-tocopherol were measured by CLIA. The CVs for alpha- and gamma-tocopherol concentrations were 5.8 and 8.9%, respectively. Cholesterol was measured enzymatically by a standard procedure at 37°C on a Hitachi 912 autoanalyzer. GWAS genotyping used both the Illumina 317K and 240K platforms, and as a result, SNP coverage (relative to the Illumina 550K used for ATBC) for two of the loci associated with circulating alpha-tocopherol, was incomplete. Genotype imputation was therefore performed for rs964184 and rs11057830 using IMPUTE2 to identify SNPs with the 1000 genomes project June 2010 release and HapMap 3 release 2 as the reference set. The imputed SNPs had a high imputation quality score.
Data from the NHS, a cohort of US women, was also used to replicate the most significant findings (approximately 100 SNPs with P < 1 × 10−5 or higher) obtained in the original GWAS. For the NHS samples, plasma tocopherol levels were measured using reversed-phase, high-performance liquid chromatography. The CVs for each batch were ≤13% with the exception of one batch which had a CV of 22%. Total cholesterol was assayed from plasma using the enzymatic methods described by Allain et al. (41). The Affymetrix 6.0 platform was used for nested case–control studies of coronary heart disease (CHD; n= 425) and type 2 diabetes (T2D; n= 394), and Illumina 550K for breast cancer [Cancer Genetic Markers of Susceptibility (CGEMS); n= 1929]. Each study sample used the MACH to impute up to approximately 2.5 million autosomal SNPs with NCBI build 36 of Phase II HapMap CEU data (release 22) as the reference panel. All of the imputed SNPs had a high imputation quality score.
Prior to analysis, tocopherol levels were log-transformed to normalize the distributions. A linear model adjusted for age, BMI and cancer status was used to relate the log-transformed outcomes to a SNP by assuming an additive mode of inheritance. Furthermore, because it is well established that vitamin E levels are affected by circulating lipids, we further adjusted the analyses for total cholesterol. Because HDL cholesterol was available in the ATBC Study, we performed a sensitivity GWAS analysis that adjusted for ‘non-HDL’ cholesterol levels (total minus HDL, or essentially LDL + VLDL, which we would expect to more closely reflect triglycerides) and yielded SNP findings identical to those adjusted for total cholesterol. Additional models that included both total cholesterol and HDL also provided similar results. The likelihood ratio test was used to detect the association between the tocopherol levels and the SNPs, adjusting for the above covariates. To identify the independent effect of other SNPs in the region of the most significant SNP, the likelihood ratio test was again used in the initial GWAS sample with the most significant SNP and those covariates involved in the basic model. We used a fixed effects meta-analysis on the GWAS and replication studies. The meta-analysis was conducted by combining the study-specific beta-estimates weighted by the inverse of the corresponding variances. We performed a sensitivity analysis on the alpha-tocopherol GWAS, excluding subjects who reported any use of vitamin E supplements (including those from multivitamins); the identified SNPs remained significant and accentuated among non-users. Additionally adjusting the GWAS for HDL levels did not change study findings. Study protocols for ATBC, PLCO and NHS were approved by their respective institutional review boards and eligible participants provided written consent.
SUPPLEMENTARY MATERIAL
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the Intramural Program of the US National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute. Additionally, the research was supported by Public Health Service contracts (N01-CN-45165, N01-RC-45035, N01-RC-37004 and HHSN261201000006C) from the National Cancer Institute, Department of Health and Human Services. S.H. is supported in part by training grant NIH 5 T32 CA09001-35. H.E. is supported in part by grant NIH R01 CA131218.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J.M., Ross C., Arnold A., Sleight P., Probstfield J., et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 2.Marchioli R. Antioxidant vitamins and prevention of cardiovascular disease: laboratory, epidemiological and clinical trial data. Pharmacol Res. 1999;40:227–238. doi: 10.1006/phrs.1999.0480. [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R., Schweiger C., Levantesi G., Tavazzi L., Valagussa F. Antioxidant vitamins and prevention of cardiovascular disease: epidemiological and clinical trial data. Lipids. 2001;36(suppl.):S53–63. doi: 10.1007/s11745-001-0683-y. [DOI] [PubMed] [Google Scholar]
- 4.Salonen J.T., Nyyssonen K., Tuomainen T.P., Maenpaa P.H., Korpela H., Kaplan G.A., Lynch J., Helmrich S.P., Salonen R. Increased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in men. BMJ. 1995;311:1124–1127. doi: 10.1136/bmj.311.7013.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Davis E.J., Costacou T., King I., Zaccaro D.J., Bell R.A. Plasma and dietary vitamin E in relation to incidence of type 2 diabetes: The Insulin Resistance and Atherosclerosis Study (IRAS) Diabetes Care. 2002;25:2172–2177. doi: 10.2337/diacare.25.12.2172. [DOI] [PubMed] [Google Scholar]
- 6.Lippman S.M., Klein E.A., Goodman P.J., Lucia M.S., Thompson I.M., Ford L.G., Parnes H.L., Minasian L.M., Gaziano J.M., Hartline J.A., et al. JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal B.B., Sundaram C., Prasad S., Kannappan R. Tocotrienols, the vitamin E of the 21(st) century: it's potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010;80:1613–1631. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shklar G., Schwartz J.L. Vitamin E inhibits experimental carcinogenesis and tumour angiogenesis. Eur. J. Cancer B. Oral Oncol. 1996;32B:114–119. doi: 10.1016/0964-1955(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 9.Sigounas G., Anagnostou A., Steiner M. dl-Alpha-tocopherol induces apoptosis in erythroleukemia, prostate, and breast cancer cells. Nutr. Cancer. 1997;28:30–35. doi: 10.1080/01635589709514549. [DOI] [PubMed] [Google Scholar]
- 10.Meydani S.N., Beharka A.A. Recent developments in vitamin E and immune response. Nutr. Rev. 1998;56:S49–58. doi: 10.1111/j.1753-4887.1998.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 11.Hazra A., Kraft P., Selhub J., Giovannucci E.L., Thomas G., Hoover R.N., Chanock S.J., Hunter D.J. Nat. Genet. 2008;40:1160–1162. doi: 10.1038/ng.210. Common variants of FUT2 are associated with plasma vitamin B12 levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn J., Yu K., Stolzenberg-Solomon R., Simon K.C., McCullough M.L., Gallicchio L., Jacobs E.J., Ascherio A., Helzlsouer K., Jacobs K.B., et al. Hum. Mol. Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. Genome-wide association study of circulating vitamin D levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrucci L., Perry J.R., Matteini A., Perola M., Tanaka T., Silander K., Rice N., Melzer D., Murray A., Cluett C., et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am. J. Hum. Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouahchi K., Arita M., Kayden H., Hentati F., Ben Hamida M., Sokol R., Arai H., Inoue K., Mandel J.L., Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat. Genet. 1995;9:141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 15.Sabatti C., Service S.K., Hartikainen A.L., Pouta A., Ripatti S., Brodsky J., Jones C.G., Zaitlen N.A., Varilo T., Kaakinen M., et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N.P., Rieder M.J., Cooper G.M., Roos C., Voight B.F., Havulinna A.S., et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooner J.S., Chambers J.C., Aguilar-Salinas C.A., Hinds D.A., Hyde C.L., Warnes G.R., Gomez Perez F.J., Frazer K.A., Elliott P., Scott J., et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat. Genet. 2008;40:149–151. doi: 10.1038/ng.2007.61. [DOI] [PubMed] [Google Scholar]
- 18.Weissglas-Volkov D., Aguilar-Salinas C.A., Sinsheimer J.S., Riba L., Huertas-Vazquez A., Ordonez-Sanchez M.L., Rodriguez-Guillen R., Cantor R.M., Tusie-Luna T., Pajukanta P. Investigation of variants identified in caucasian genome-wide association studies for plasma high-density lipoprotein cholesterol and triglycerides levels in Mexican dyslipidemic study samples. Circ. Cardiovasc. Genet. 2010;3:31–38. doi: 10.1161/CIRCGENETICS.109.908004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kathiresan S., Willer C.J., Peloso G.M., Demissie S., Musunuru K., Schadt E.E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willer C.J., Sanna S., Jackson A.U., Scuteri A., Bonnycastle L.L., Clarke R., Heath S.C., Timpson N.J., Najjar S.S., Stringham H.M., et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M., Pirruccello J.P., Ripatti S., Chasman D.I., Willer C.J., et al. Nature. 2010;466:707–713. doi: 10.1038/nature09270. Biological, clinical and population relevance of 95 loci for blood lipids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen C.T., Kathiresan S., Hegele R.A. J. Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. Genetic determinants of plasma triglycerides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardwick J.P. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem. Pharmacol. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Sontag T.J., Parker R.S. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 25.Bardowell S.A., Stec D.E., Parker R.S. J. Nutr. 2010;140:1901–1906. doi: 10.3945/jn.110.128579. Common variants of cytochrome P450 4F2 exhibit altered vitamin E-{omega}-hydroxylase specific activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald M.G., Rieder M.J., Nakano M., Hsia C.K., Rettie A.E. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 2009;75:1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha P.C., Mushiroda T., Takahashi A., Kubo M., Minami S., Kamatani N., Nakamura Y. Hum. Mol. Genet. 2010;19:4735–4744. doi: 10.1093/hmg/ddq389. Genome-wide association study identifies genetic determinants of warfarin responsiveness for Japanese. [DOI] [PubMed] [Google Scholar]
- 28.Borel P., Moussa M., Reboul E., Lyan B., Defoort C., Vincent-Baudry S., Maillot M., Gastaldi M., Darmon M., Portugal H., et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J. Nutr. 2007;137:2653–2659. doi: 10.1093/jn/137.12.2653. [DOI] [PubMed] [Google Scholar]
- 29.Goti D., Reicher H., Malle E., Kostner G.M., Panzenboeck U., Sattler W. High-density lipoprotein (HDL3)-associated alpha-tocopherol is taken up by HepG2 cells via the selective uptake pathway and resecreted with endogenously synthesized apo-lipoprotein B-rich lipoprotein particles. Biochem. J. 1998;332:57–65. doi: 10.1042/bj3320057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy J.J., Somji A., Weiss L.A., Steffy B., Vega R., Barrett-Connor E., Talavera G., Glynne R. Polymorphisms of the scavenger receptor class B member 1 are associated with insulin resistance with evidence of gene by sex interaction. J. Clin. Endocrinol. Metab. 2009;94:1789–1796. doi: 10.1210/jc.2008-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acton S., Osgood D., Donoghue M., Corella D., Pocovi M., Cenarro A., Mozas P., Keilty J., Squazzo S., Woolf E.A., et al. Association of polymorphisms at the SR-BI gene locus with plasma lipid levels and body mass index in a white population. Arterioscler Thromb. Vasc. Biol. 1999;19:1734–1743. doi: 10.1161/01.atv.19.7.1734. [DOI] [PubMed] [Google Scholar]
- 32.Purdue M.P., Johansson M., Zelenika D., Toro J.R., Scelo G., Moore L.E., Prokhortchouk E., Wu X., Kiemeney L.A., Gaborieau V., et al. Nat. Genet. 2011;43:60–65. doi: 10.1038/ng.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig W.Y., Palomaki G.E., Haddow J.E. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298:784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meenakshisundaram R., Rajendiran C., Thirumalaikolundusubramanian P. Tob. Induc. Dis. 2010;8:11. doi: 10.1186/1617-9625-8-11. Lipid and lipoprotein profiles among middle aged male smokers: a study from southern India. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatesan A., Hemalatha A., Bobby Z., Selvaraj N., Sathiyapriya V. Effect of smoking on lipid profile and lipid peroxidation in normal subjects. Indian J. Physiol. Pharmacol. 2006;50:273–278. [PubMed] [Google Scholar]
- 36.Kharb S., Singh G.P. Clin. Chim. Acta. 2000;302:213–219. doi: 10.1016/s0009-8981(00)00343-0. [DOI] [PubMed] [Google Scholar]
- 37.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann. Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 38.Milne D.B., Botnen J. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin. Chem. 1986;32:874–876. [PubMed] [Google Scholar]
- 39.Kattermann R., Jaworek D., Moller G., Assmann G., Bjorkhem I., Svensson L., Borner K., Boerma G., Leijnse B., Desager J.P., et al. Multicentre study of a new enzymatic method of cholesterol determination. J. Clin. Chem. Clin. Biochem. 1984;22:245–251. doi: 10.1515/cclm.1984.22.3.245. [DOI] [PubMed] [Google Scholar]
- 40.Hayes R.B., Sigurdson A., Moore L., Peters U., Huang W.Y., Pinsky P., Reding D., Gelmann E.P., Rothman N., Pfeiffer R.M., et al. Methods for etiologic and early marker investigations in the PLCO trial. Mutat. Res. 2005;592:147–154. doi: 10.1016/j.mrfmmm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.