Abstract
The medicinal mushroom Agaricus blazei Murill from the Brazilian rain forest has been used in traditional medicine and as health food for the prevention of a range of diseases, including infection, allergy, and cancer. Other scientists and we have examined whether there is scientific evidence behind such postulations. Agaricus blazei M is rich in the immunomodulating polysaccharides, β-glucans, and has been shown to have antitumor, anti-infection, and antiallergic/-asthmatic properties in mouse models, in addition to anti-inflammatory effects in inflammatory bowel disease patients. These effects are mediated through the mushroom's stimulation of innate immune cells, such as monocytes, NK cells, and dendritic cells, and the amelioration of a skewed Th1/Th2 balance and inflammation.
1. Introduction
The edible Basidiomycetes mushroom Agaricus blazei Murill (AbM), a.k.a. Agaricus subrufescens Peck (already described in 1893), and Agaricus brasiliensis Wasser [1] (Figure 1), of Brazilian rain forest origin is used in traditional medicine against cancer and various diseases [2, 3]. It is related to the champignon (Agaricus bisporus) and is shown to be rich in immunomodulating substances such as highly branched β-1,3-/1,6-glucans [4] and proteoglycans [5]. These are known ligands for CD11b/18 (complement receptor 3, CR3) [6], dectin-1 [7], and toll-like receptor 2 (TLR2) [8] on monocytes, dendritic cells (DC), granulocytes, and NK cells [9] of the innate immune system. AbM is also shown to contain agaritine and ergosterol (provitamin D2) that is found to induce apoptosis in leukemic cells [10] and inhibit tumor-induced angiogenesis [11], respectively, as well as isoflavonoids with potent hypoglycemic action that could be useful against diabetes mellitus [12]. AbM is reported to have antitumor properties in mouse models of fibrosarcoma, myeloma, ovarian, lung, and prostate cancer, and in human studies against gynecological cancer (increased NK cell activity and quality of life) and leukemia [13].
Figure 1.

Agaricus blazei Murill. Photo NutriCon. The mushroom is cultivated commercially for the health food market in Japan, China, and Brazil. The AbM-based AndoSan extract is produced in Japan and developed and distributed by ImmunoPharma AS, Oslo, Norway.
2. Effects on Infection and Allergy
We found that an AbM-based extract (AndoSan, http://www.immunopharma.net/), also containing the medicinal Basidiomycetes mushrooms Hericium erinaceum (15%) and Grifola frondosa (3%), given orally increased survival from bacterial sepsis in mice inoculated i.p. a day afterward with pneumococci (Figure 2) [14] or fecal bacteria [15]. The mixed mushroom extract also protected against IgE-mediated allergy in a mouse model when given p.o. either before or after ovalbumin s.c. sensitization of the animals (Figure 3) [16]. In supernatants of cultured spleen cells from the sacrificed AbM-treated mice, there was an increased T-helper cell 1 response relative to the allergy-inducing Th2 response. The observation fits with the reduced specific serum IgE levels in these animals and shows that also adaptive immunity is engaged by the mushroom. Since the original Th1/Th2 dichotomy [17] says that the antitumor and anti-infection Th1 response is inversely related to the Th2 response, the spleen cell finding above also helps explain the concomitant antiallergic, antitumor, and antiinfection effects of AbM. Moreover, this agrees with the very interesting report finding that AbM extract ameliorated a skewed Th1/Th2 balance both in asthma-induced and in tumor-bearing mice [18]. It is previously known that patients with advanced cancer have malfunctional Th1 cells and a Th2-skewed immune system [19]. However, it is not known whether AbM contributed to rectify a possibly induced Th1/Th2 imbalance in the above-mentioned sepsis models in mice [14, 15].
Figure 2.
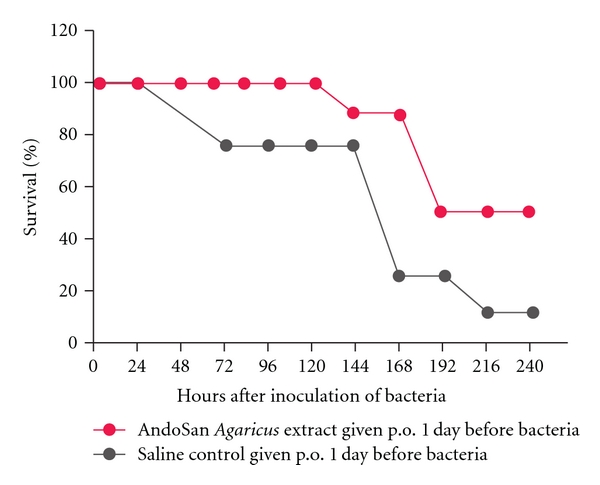
5-6-week-old female inbred NIH/Ola mice were given either 200 μL of AndoSan AbM extract or phosphate-buffered saline (PBS) intragastrically a day before i.p. injection of 1 million colony-forming units of Streptococcus pneumoniae serotype 6B. There was a significant difference (P < 0.05) between survival after treatment with AndoSan (red line) and PBS (black line). From [14], permission granted for republication by Scand J Immunol, where the figure was originally published.
Figure 3.
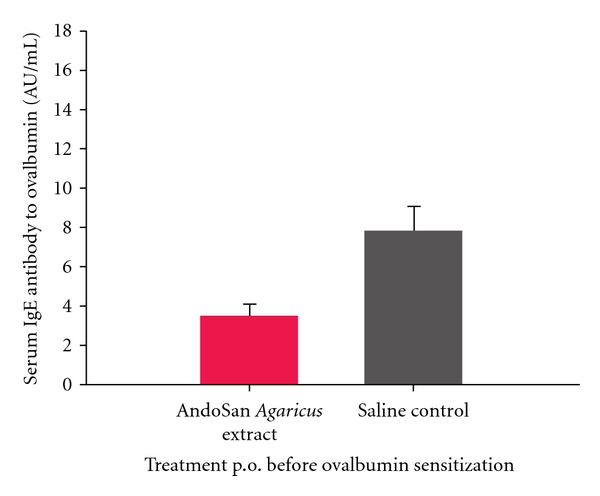
Female NIH/Ola mice were given either 200 μl of the AndoSan AbM-based extract or PBS intragastrically on day 1 and 10 μg of ovalbumin s.c. on day 0 and again on day 20, before exsanguination for serum on day 26. IgE antiovalbumin levels were lower in the AbM- than in the PBS-treated groups (P = 0.002). Similar results were found if AbM extract or PBS was given 3 weeks after the allergen immunization (not shown, please see [16]). IgG2a antiovalbumin levels (Th1 response) tended to show the opposite result (not shown). From [16], previously published by a BMC journal, which allows reuse.
We have previously compared the biological potency of 5 different AbM products orally in a blinded experiment in the pneumococcal sepsis model and found that only AndoSan, given orally 24 h prior to bacterial challenge, induced statistically significant lower bacteremia and higher survival rate than did saline given prechallenge in control mice [13]. The outcome of this experiment, actually done in 2003 but not published until 2008, was the basis for choosing AndoSan (then called AbM extract A) in our further studies. Synergies between components from the three mushrooms in the said extract may explain its enhanced efficacy against sepsis. Tuberculosis is another serious infection although it actually only develops into active disease in 10% of those infected with M. tuberculosis bacilli. Hence, in contrast to the exposed but healthy individuals, the tuberculosis patients represent a selected group, which is not prone to the tubercle bacilli's strong ability to elicit Th1-type cellular immune responses, for example, the normal reaction to the BCG vaccine. In fact, the Th1/Th2 imbalance in these patients is shown by their higher frequency of allergy when compared with healthy controls [20, 21]. Although a β-glucan from yeast had a protective effect against M. bovis, BCG infection in a mouse model [22], the effect of AbM has not to our knowledge been examined in tuberculosis.
3. Anti-Inflammatory Effect
In a human phase I study in 15 healthy volunteers, we recently found no side effects after intake of the AbM-based AndoSan extract [23, 24]. In particular, there were no significant changes in general blood parameters and no negative effects on kidney, liver, or pancreas function. This agrees with a toxicity study over two years in rats [25], which rather found that animals ingesting the highest AbM concentration lived the longest, presumably due to reduced cancer development. In the mentioned phase I study, AbM extract had an anti-inflammatory effect as shown by significantly reduced levels of proinflammatory cytokines. Although this is contrary to our in vitro findings of increased production of proinflammatory cytokines by monocytes, monocyte-derived DC (MDDC), and human umbilical vein endothelial cells (HUVEC), we did, as a consequence of the phase I trial, conduct a clinical pilot study at our hospital on patients with the inflammatory bowel diseases, ulcerative colitis and Crohn's disease. The result was a significant decrease in plasma levels of proinflammatory cytokines after 12 days of AndoSan intake orally and also decreased levels of the inflammatory marker calprotectin in feces of ulcerative colitis patients [24]. Figure 4 shows that MIP-1β, IL-6, IL-8, MCP-1, IL-1β, G-CSF, and GM-CSF levels were reduced in the blood of ulcerative colitis patients and MIP-1β, MCP-1, IL-8, IL-1β, G-CSF, IL-17, GM-CSF, and IL-2 levels in blood of Crohn's patients, respectively. This anti-inflammatory property of AbM may also be of importance for the mushroom's therapeutic effect on allergy and asthma in mouse models [18, 21], both of which are inflammatory conditions, and it may bear promise for use against autoimmune diseases. In addition, it may explain some of AbM's antitumor effects discussed below.
Figure 4.
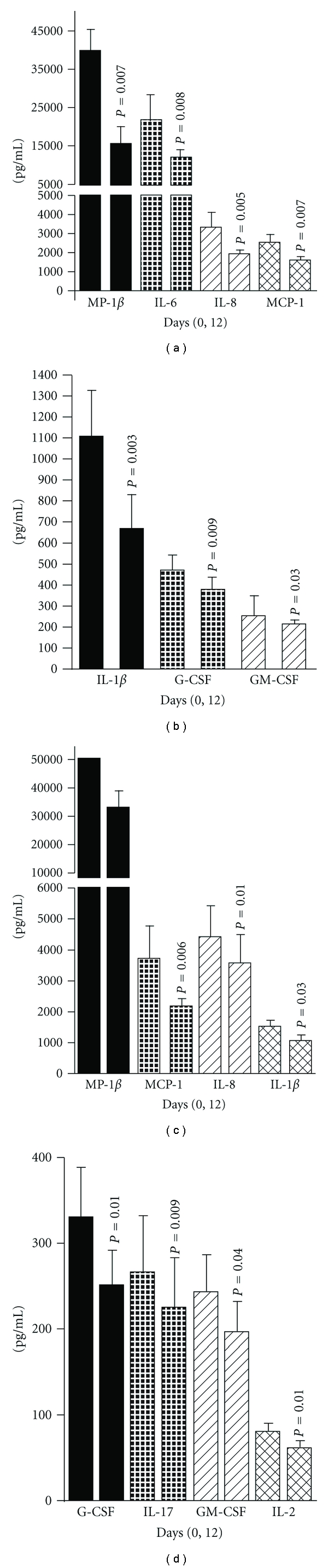
Bar graphs (mean and standard error the mean (SEM)) with levels of cytokines (pg/mL) MIP-1β, IL-6, IL-8, and MCP-1 (a) and IL-1β, G-CSF, and GM-CSF (b) in stimulated (LPS 1 ng/mL) whole blood ex vivo from eleven patients (unless otherwise stated) with ulcerative colitis prior to (day 0) and after AndoSan consumption for 12 days. Days 0 and 12 after stimulation are depicted by the first and second bars from the left, respectively. For MIP-1β and IL-8, measurements in nine out of ten patients were available. Corresponding measurements from eleven patients with Crohn's disease (unless otherwise stated) were significantly reduced for cytokines MIP-1β, MCP-1, IL-8, and IL-1β (c) and G-CSF, IL-17, GM-CSF, and IL-2 (d). For MIP-1β and IL-8, measurements in ten out of eleven patients were available. Despite remeasurement of MIP-1β after dilution of plasma 1/10, high out of range values still occurred. Accordingly, the true concentrations of MIP-1β were even higher. The P values between the bar graphs compare with the cytokine levels at day 0 prior to the intake of AndoSan. From [24] where the data was part of scatter plots in Scand J Immunol.
4. Mechanism: Stimulation of Immune Cells
The reason for the forceful and swift engagement of innate immunity when encountering an edible and harmless mushroom, such as AbM, is its sharing of pathogen-associated molecular patterns (PAMP) with other highly poisonous species. Such mushrooms and fungi are usually a health threat due to the action of their toxins, for example, muscimol from Amanita muscaria and the vasoconstrictor ergotamine from Claviceps purpurea, or invasion in immunodeficient patients (e.g., Aspergillus fumigatus) or normal individuals (e.g., Stachybotrys chartarum).
PAMP, such as β-glucans, which form the main cell wall skeleton in mushrooms and fungi and are their signature molecule, are recognized immediately by so-called pattern-recognition receptors (PRR) [26], such as TLR2, dectin-1, and CR3. One can exploit this immune reaction by using an innocent mushroom such as Agaricus blazei M to enhance the body's protection against serious diseases. Although AbM induced NF-κB activation via stimulation of TLR2 on cells in vitro [27], the AbM-based mushroom extract AndoSan had anti-inflammatory effect in inflammatory bowel disease patients in vivo [24]. In addition to monocytes, granulocytes, and DC, also NK cells bear such PRR [9] and are stimulated by AbM in vitro, for example, to induce increase in cytokine production, expression of the adhesion molecule CD11b on monocytes and granulocytes, and ROS and NO− production in the latter cells [28]. An AbM extract also gave a dose-dependent increase in release of proinflammatory cytokines from HUVEC in culture [29]. Since human skin endothelial cells can express all 10 TLR genes [30], TLR-binding of AbM was probably one mechanism behind the above finding in HUVEC. This demonstrates that AbM also affects EC, which are important parttakers in the innate immune response. In a human study, AbM has been shown to increase NK cell activity in cancer patients [31]. Another important group of receptors on macrophages and other innate immune cells are cytosolic NOD (nucleotide-binding and oligomerization domain-) like receptors (NLR). These receptors detect conserved bacterial molecular signatures within the host cells, similar to recognition of β-glucans via surface receptors or so-called “danger signals,” alerting the immune system of hazardous environments [32], and “cross-talk” with TLR on DC [33].
It is also known that AbM can activate the alternative pathway of complement [34], giving binding of the CR3 ligand, iC3b, to particulate AbM and thus contribute to the AbM engagement of the phagocytic CR3. Moreover, the formation of complement activation split products and chemotaxins—C3a and C5a—when also the terminal complement pathway is activated will lead to their binding to C3a and C5a (CD88) receptors, respectively, and chemotaxis of the immune cells towards a C3a and C5a gradient and hence towards the AbM source.
Animal studies have shown that although β-glucans can enter the proximal small intestine, the specific β-1,3-glucan backbone is indigestible [35]. In vivo, AbM most probably engage Peyer's patches in the intestines both via their abundant β-glucans and other immunomodulating substances such as proteoglycans, which may be transported to macrophages and DC after being taken up from the gastrointestinal lumen by M cells. Another possibility is direct uptake of or stimulation by such substances of DC via their processes into the gastrointestinal lumen or by intestinal macrophages that may fragment the glucans for transport to the bone marrow and reticuloendothelial system for further release and stimulation of other immune cells [35]. Previously, AbM has in fact been shown to stimulate endothelial cells in vitro [29]. Sorimachi et al. [36] demonstrated that ethanol (50%) precipitation of water extracts of the AbM fruiting body or its supernatant, rather than of the mycelium, induced TNFα, IL-8, and NO− secretion in bone-marrow-derived rat macrophages. Since the complex β-1,3-/1,6-glucansfrom AbM induced different cellular activities than the mainly glucose-containing β-1,4-glucan from its nonmedicinal cousin A. bisporus (the champignon) [37], both differences in content, structure, and branching of their β-glucans must be highly important for the mushrooms' biological properties. Especially, β-1,3-glucans seem to be essential for immunological activity [37]. Interestingly, also a low-molecular-weight polysaccharide isolated from AbM has been reported to suppress tumor growth and angiogenesis in vivo [38]. Pyroglutamate is found to be another such small substance [39]. Since also the anti-allergic effect of AbM extract seems to be owing to low-molecular-weight substances (Hetland G, unpublished results) in a mouse model, other smaller and simpler substances than β-glucans in AbM could very well be as important for the mushroom's biological effects.
Most probably the mushroom extract also affects the intestinal flora, which comprises 10 times more bacteria than cells in our entire body. Bacteria in the gut are known to produce essential vitamins and so forth, for example, bacteria that can ferment soy beans and produce K2 vitamin that is important for calcium metabolism [49]. It is probable that also intestinal bacteria either produce metabolites during digestion of AbM extract or produce analytes after themselves being stimulated by AbM. Such molecules may be biologically active after uptake from the gut and may affect the host.
The PAMP or NOD signature-bearing pathogens are annihilated immediately by the patrolling innate immune cells. Some of these like monocytes and DC—directors of the immune system orchestrating the linkage of innate and adaptive immunity—process antigens from the mushrooms/fungi and present them together with self HLA II molecules for CD4 T helper cells, thus engaging adaptive immunity against the intruders.
Recently, we examined AbM-stimulated MDCC and found a significantly increased production of various cytokines [50]. This agrees with microarray studies in vitro of promonocytic THP-1 cells incubated with the AbM extract, which showed upregulation for most of genes associated with immune function, including the gene for IL-23α subunit—a Th1 cytokine in the IL-12 family [10]. In fact, the AbM extract induced even higher production of cytokines TNFα, IL-1β and MCP-1, and G-CSF than did cells stimulated with an appropriate concentration of 0.5 μg/mL of LPS [50]. Hence, MDDC may in some respect be even more primed to defense against mushrooms/fungi than against Gram-negative bacteria. At our Department of Cellular Therapy, we construct autologous DC vaccines for clinical trials against various human cancers, by electroporating isolated cancer mRNA into dendritic cells harvested from the same patients. Benko et al. [33] have proposed the emerging of ligands of the innate recognition systems as new adjuvant candidates for vaccine design. In line with this school of thought, we are currently examining whether the above AbM extract may be used as immune adjuvant in such anti-cancer DC vaccines, similar to studies on DNA vaccines in the mouse against hepatitis B virus and mouth-and-foot diseases [51, 52]. In these vaccines, AbM was found to increase levels of antigen-specific antibodies as well as to promote proliferation of T cells, illustrating engagement of adaptive immunity. Figure 5 shows a cartoon of the proposed role of AbM in immune system modulation and the resulting disease control.
Figure 5.
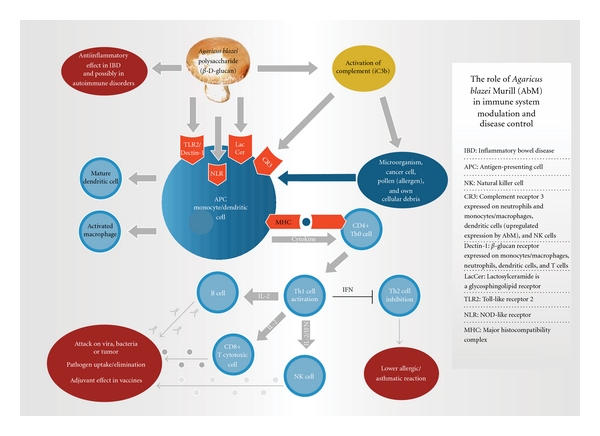
Role of the mushroom Agaricus blazei Murill in immune system modulation and disease control. It is assumed that besides β-glucans also other, yet unknown substances in the mushroom, probably of low molecular weight, do parttake in the action. IL-12 is the cytokine from the monocyte/dendritic cell that stimulates Th0.
In Figure 6, the balance between the different Th responses is depicted as the Ying and Yang of adaptive immunity. The committed cells are characterized by expression of the specific transcription factors; T-bet for Th1, GATA-3 for Th2, FoxP3 for Tregs, and RORγt for Th17 cells [53]. The cartoon shows that whereas both Th1 and Th2, cells inhibit Th17 and Treg cells, Th1 inhibits Th2, and Th17 inhibits Treg cells. It is noteworthy that in Crohn's disease, which is proposed to be a Th1/Th17-type autoimmune disease, we found significant reduction in plasma levels of IL-2 (Th1 cytokine) and IL-17 after intake of the AbM-based AndoSan extract by the patients [24]. In the other patients with ulcerative colitis, which is proposed to have a Th2-type autoimmune pathogenesis, we found that the IL-4 and IL-13 levels tended to decrease, although not statistically significantly, after AndoSan's intake [24].
Figure 6.
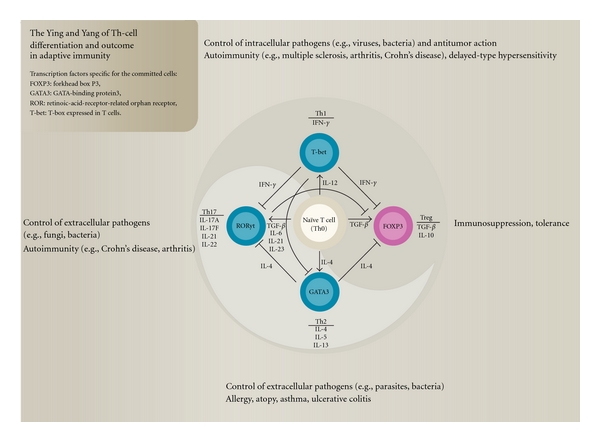
The Ying and Yang of adaptive immunity. There is a balance between the Th responses in such a way that Th1 inhibits Th2, which inhibits Th17 and which again inhibits the Treg response. “Over-shoot” of Th1 and Th17 responses in susceptible individuals can result in autoimmune disorders. The regulation by T reg cells results in immunosuppression and tolerance. The Agaricus mushroom shifts the Th1/Th2 balance towards increased Th1 response, which in addition to intracellular pathogens also fights cancer.
5. Antitumor Effects and Proposed Mechanism behind
As mentioned, the Agaricus mushroom is reported to inhibit various tumors [10], including hematological cancers such as myeloma in a recent mouse model [43] and leukemia in a human study [42]. One proposed mechanism behind the antitumor effects of AbM is the induction of apoptosis in tumor cells, which is demonstrated in vitro [40]. This is confirmed by the microarray finding of increased expression of genes inducing apoptosis as well as inhibition of cell division [46] in PBMC from patients with hepatitis C virus infection who drank AbM extract for 1 week. Other contributing mechanisms are (i) the known antitumor action of ergosterol [11, 54] contained in AbM extract, (ii) the anti-inflammatory effect of AbM [23, 24], which may reduce levels of the “tumor-friendly” neoangiogenic and granulocyte-chemoattractant factor IL-8 and thus may also decrease intratumor formation of reactive nitrogen and oxygen species that may hamper the infiltration of cytotoxic T cells [55], and (iv) the amelioration of the proposed skewed Th1/Th2 balance in advanced cancer [18]. There is a well-established connection between inflammation and tumorigenesis [56], and it is known that organs with chronic inflammation are prone to cancer, for example, the colon in inflammatory bowel diseases, the pancreas after chronic pancreatitis, and the liver secondary to chronic viral hepatitis. Since up to 1/4 of all cancers are estimated to be caused by underlying infections and inflammation [57], there has lately been a novel interest for the use of anti-inflammatory treatment in cancer therapy [45]. Hence, both the anti-inflammatory [23, 24] and anti-infection [14, 15] properties of AbM that we have disclosed may contribute to the mushroom's antitumor activity. Interestingly, whereas there was a little, nonsignificant reduction in HCV load in serum in the mentioned clinical pilot trial in 4 patients with chronic IFNα-resistant HCV infection, the gene for IFNαβ receptor was significantly upregulated by the 7 days AbM treatment [46].
Currently at our hematological department, we are conducting a placebo-controlled, double-blinded phase II trial in patients with multiple myeloma, who have been drinking the AbM-based AndoSan extract as a supplementary treatment. These patients are subjected to standard high-dose chemotherapy and autologous hematopoietic stem cell transplantation after the harvesting of the cells at our Department of Cellular Therapy. Patients with multiple myeloma were chosen for such a clinical trial because there is no curative therapy for this cancer today-only life-extending treatment. In the other known clinical studies with AbM, the mushroom was reported to have positive effect against nonlymphocytic leukemia [42] and to increase quality of life and NK-cell activity in blood of gynaecological cancer patients on high-dose chemotherapy [31]. In a small AbM study on prostate cancer in patients enrolled after radical prostatectomy, there was no reduction in their prostate-specific antigen (PSA) levels [58]. However, there were no placebo controls, and the study included many with PSA values below the guideline of 0.2 ng/mL of PSA. That study is in contrast with the findings of AbM-induced apoptosis for prostate cancer cells and inhibited tumor growth and antiangiogenic effect in a mouse model [44]. Interestingly, a recent paper reported evidence for suppressed growth and invasiveness of human breast cancer cells in vitro after treatment with a blend of AbM and other medicinal mushrooms [59]. Table 1 gives an overview over the reported in vivo antitumor effects of the Agaricus bM mushroom, and Table 2 shows clinical studies with this mushroom.
Table 1.
Reported in vivo antitumor effect of the mushroom Agaricus blazei Murill.
| Tumor/related activity | In vivo effects of Agaricus bM | Refs. (no., year, country) |
|---|---|---|
| Fibrosarcoma | Inhibition of tumor size and neoangiogenesis | [4] 1994, [40] 1998, [3] 2001, [39] 2004, Japan |
| Gynecological cancer* | Increased NK-cell activity and increased quality of life (QOL) | [31] 2004, Republic of Korea |
| Ovarian cancer | Inhibition of metastasis | [41], 2005 Japan |
| Lung cancer | Inhibition of metastasis | [39] 2004, [41] 2005, Japan |
| Leukemia* | Inhibition | [42] 1994, Japan |
| Myeloma | Inhibition of tumor size | [43] 2007, Japan |
| Prostate cancer | Reduced tumor growth and neoangiogenesis No significant effect on PSA values* |
[44] 2009, Taiwan [45] 2010, Japan |
| Carcinogenicity | No | [25] 2008, Republic of Korea |
*Human studies, otherwise studies in mice.
Table 2.
Clinical studies with Agaricus blazei Murill (AbM).
| Patients disease | No. of subjects | Treatment | Clinical effect | Refs. (no., year, country) |
|---|---|---|---|---|
| Acute nonlymphocytic leukemia | 10 | Chemo + AbM | Inhibition of leukemic cells | [42], 1994, Japan |
| Gynecological cancer | 100 | Chemo + AbM Kyowa or placebo, 9 wk | ↑ NK cell activity, ↑ QOL | [31], 2004, Republic of Korea |
| IFNα-resistant chron. HCV infection | 4 | AndoSan extract 60 mL/d for 7 d | Insignificant ↓ HCV load, but ↑ IFNαβ receptor and “antitumor gene” expression | [46], 2006, Norway |
| Chron. HBV infection | 4 | AbM extract 1.5 g/d, 12 mo | Normalized liver function; ↓ASAT ↓ ALAT levels | [47], 2007, Taiwan |
| Diabetes type 2 | 72 | AbM 1.5 g/d or placebo 12 wk | Improved insulin resistance | [48], 2008, Taiwan |
| Healthy volunteers | 15 | AndoSan 60 mL/d for 12 d | No toxicity, Anti-inflammatory | [23], 2009, Norway |
| Inflammatory bowel diseases (*Ulcerative colitis and Mb Crohn) | 21 | AndoSan 60 mL/d for 12 d | Anti-inflammatory; ↓ proinflammatory cytokines in blood and ↓*calprotectin in feces | [24], 2011, Norway |
Abbreviations: ASAT aspartate aminotransferase, ALAT alanine aminotransferase, QOL quality of life. AndoSan is an AbM-based extract also containing 15% H. erinaceum and 3% G. frondosa.
6. Conclusions
The medicinal mushroom Agaricus bM has been shown to have beneficial effects on a range of diseases including cancer, infections, allergy/asthma, and inflammatory disorders. The explanation is the mushroom's engagement of innate immunity, which is “broad-spectered.” When adaptive immunity then is engaged through the stimulation of DC, it results in an enhanced Th1 antitumor and anti-infection response relative to the proallergic Th2 response. Thus, AbM ameliorates the Th2-skewed balance found in advanced cancer, mycobacterial infections and allergy and asthma. In addition, there may be a general anti-inflammatory effect of AbM, which may be therapeutical for inflammatory bowel diseases and augment the mushroom's antitumor and antiallergy/antiasthma properties. Hence, AbM extract may show promise as a prophylacticum and as an additive treatment for quite different and some serious diseases.
Disclosure
T. Lyberg and G. Kvalheim have no relationships to declare. G. Hetland and E. Johnson are option and stock holders in ImmunoPharma AS.
Acknowledgments
Research funding was from The Norwegian Institute of Public Health, Oslo and the Oslo University Hospital, Norway. The authors thank Rafal Biedron (http://www.ishf.org/) for illustrative help.
References
- 1.Kerrigan RW. Agaricus subrufescens, a cultivated edible and medicinal mushroom, and its synonyms. Mycologia. 2005;97(1):12–24. doi: 10.3852/mycologia.97.1.12. [DOI] [PubMed] [Google Scholar]
- 2.Huang N. Brazilian mushroom (Gee Song Rong) In: Huang N, editor. Cultivation of Eigth Rare and Precious Gourmet Mushrooms. Chinese Agriculture University Press; 1997. pp. 95–101. [Google Scholar]
- 3.Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: a modern perspective. Critical Reviews in Immunology. 1999;19(1):65–96. [PubMed] [Google Scholar]
- 4.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T. Antitumor β-glucan from the cultured fruit body of Agaricus blazei . Biological and Pharmaceutical Bulletin. 2001;24(7):820–828. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 5.Itoh H, Ito H, Amano H, Noda H. Inhibitory action of a (1→6)-β-D-glucan-protein complex (F III-2-b) isolated from Agaricus blazei Murill (’Himematsutake’) on Meth A fibrosarcoma-bearing mice and its antitumor mechanism. Japanese Journal of Pharmacology. 1994;66(2):265–271. doi: 10.1254/jjp.66.265. [DOI] [PubMed] [Google Scholar]
- 6.Czop JK, Valiante NM, Janusz MJ. Phagocytosis of particulate activators of the human alternative complement pathway through monocyte beta-glucan receptors. Progress in Clinical and Biological Research. 1989;297:287–296. [PubMed] [Google Scholar]
- 7.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, Korting HC. Toll-like receptors as key mediators in innate antifungal immunity. Medical Mycology. 2004;42(6):485–498. doi: 10.1080/13693780400011112. [DOI] [PubMed] [Google Scholar]
- 8.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. Journal of Experimental Medicine. 2003;197(9):1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodridge HS, Wolf AJ, Underhill DM. β-glucan recognition by the innate immune system. Immunological Reviews. 2009;230(1):38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo M, Beppu H, Akiyama H, et al. Agaritine purified from Agaricus blazei Murrill exerts anti-tumor activity against leukemic cells. Biochimica et Biophysica Acta. 2010;1800(7):669–673. doi: 10.1016/j.bbagen.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Takaku T, Kimura Y, Okuda H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. Journal of Nutrition. 2001;131(5):1409–1413. doi: 10.1093/jn/131.5.1409. [DOI] [PubMed] [Google Scholar]
- 12.Oh TW, Kim YA, Jang WJ, et al. Semipurified fractions from the submerged-culture broth of Agaricus blazei Murill reduce blood glucose levels in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry. 2010;58(7):4113–4119. doi: 10.1021/jf9036672. [DOI] [PubMed] [Google Scholar]
- 13.Hetland G, Johnson E, Lyberg T, Bernardshaw S, Tryggestad AMA, Grinde B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer. Scandinavian Journal of Immunology. 2008;68(4):363–370. doi: 10.1111/j.1365-3083.2008.02156.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernardshaw S, Johnson E, Hetland G. An extract of the mushroom Agaricus blazei Murill administered orally protects against systemic Streptococcus pneumoniae infection in mice. Scandinavian Journal of Immunology. 2005;62(4):393–398. doi: 10.1111/j.1365-3083.2005.01667.x. [DOI] [PubMed] [Google Scholar]
- 15.Bernardshaw S, Hetland G, Grinde B, Johnson E. An extract of the mushroom Agaricus blazei Murill protects against lethal septicemia in a mouse model of fecal peritonitis. Shock. 2006;25(4):420–425. doi: 10.1097/01.shk.0000209526.58614.92. [DOI] [PubMed] [Google Scholar]
- 16.Ellertsen LK, Hetland G. An extract of the medicinal mushroom Agaricus blazei Murill can protect against allergy. Clinical and Molecular Allergy. 2009;7, article no. 6 doi: 10.1186/1476-7961-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romagnani S. The Th1/Th2 paradigm. Immunology Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 18.Takimoto H, Kato H, Kaneko M, Kumazawa Y. Amelioration of skewed Th1/Th2 balance in tumor-bearing and asthma-induced mice by oral administration of Agaricus blazei extracts. Immunopharmacology and Immunotoxicology. 2008;30(4):747–760. doi: 10.1080/08923970802279092. [DOI] [PubMed] [Google Scholar]
- 19.Pelligrini P, Berghella AM, Del Beato T, et al. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progession. Cancer Research. 1998;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lienhardt C, Azzurri A, Amedei A, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. European Journal of Immunology. 2002;32(6):1605–1613. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Ellertsen LK, Wiker HG, Egeberg NT, Hetland G. Allergic sensitisation in tuberculosis and leprosy patients. International Archives of Allergy and Immunology. 2005;138(3):217–224. doi: 10.1159/000088722. [DOI] [PubMed] [Google Scholar]
- 22.Hetland G, Løvik M, Wiker HG. Protective effect of β-glucan against Mycobacterium bovis, BCG infection in BALB/c mice. Scandinavian Journal of Immunology. 1998;47(6):548–553. doi: 10.1046/j.1365-3083.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson E, Førland DT, Sætre L, Bernardshaw SV, Lyberg T, Hetland G. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scandinavian Journal of Immunology. 2009;69(3):242–250. doi: 10.1111/j.1365-3083.2008.02218.x. [DOI] [PubMed] [Google Scholar]
- 24.Førland DT, Johnson E, Sætre L, Lyberg T, Lygren I, Hetland G. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on expression of cytokines and calprotectin in patients with ulcerative colitis and Crohn’s disease. Scandinavian Journal of Immunology. 2011;73(1):66–75. doi: 10.1111/j.1365-3083.2010.02477.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee IP, Kang BH, Roh JK, Kim JR. Lack of carcinogenicity of lyophilized Agaricus blazei Murill in a F344 rat two year bioassay. Food and Chemical Toxicology. 2008;46(1):87–95. doi: 10.1016/j.fct.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Kumagai Y, Akira S. Identification and functions of pattern-recognition receptors. Journal of Allergy and Clinical Immunology. 2010;125(5):985–992. doi: 10.1016/j.jaci.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 27.Tryggestad AMA, Espevik T, Førland DT, et al. The medical mushroom Agaricus blazei Murill activates NF-κB via TLR2. In: Proceedings of the 13th International Congress of Immunology; August 2007; Rio de Janeiro, Brazil. p. 1193. P2.23 INI-02 Signalling pathways of innate immune receptors. [Google Scholar]
- 28.Bernardshaw S, Lyberg T, Hetland G, Johnson E. Effect of an extract of the mushroom Agaricus blazei Murill on expression of adhesion molecules and production of reactive oxygen species in monocytes and granulocytes in human whole blood ex vivo . Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2007;115(6):719–725. doi: 10.1111/j.1600-0463.2007.apm_619.x. [DOI] [PubMed] [Google Scholar]
- 29.Bernardshaw S, Hetland G, Ellertsen LK, Tryggestad AMA, Johnson E. An extract of the medicinal mushroom Agaricus blazei Murill differentially stimulates production of pro-inflammatory cytokines in human monocytes and human vein endothelial cells in vitro. Inflammation. 2005;29(4–6):147–153. doi: 10.1007/s10753-006-9010-2. [DOI] [PubMed] [Google Scholar]
- 30.Fitzner N, Clauberg S, Essmann F, Liebmann J, Kolb-Bachofen V. Human skin endothelial cells can express all 10 TLR genes and respond to respective ligands. Clinical and Vaccine Immunology. 2008;15(1):138–146. doi: 10.1128/CVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn WS, Kim DJ, Chae GT, et al. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. International Journal of Gynecological Cancer. 2004;14(4):589–594. doi: 10.1111/j.1048-891X.2004.14403.x. [DOI] [PubMed] [Google Scholar]
- 32.Geddes K, Magalhães JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nature Reviews Drug Discovery. 2009;8(6):465–479. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 33.Benko S, Magyarics Z, Szabó A, Rajnavölgyi E. Dendritic cell subtypes as primary targets of vaccines: the emerging role and cross-talk of pattern recognition receptors. Biological Chemistry. 2008;389(5):469–485. doi: 10.1515/bc.2008.054. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Ohno N, Saito K, Yadomae T. Activation of the complement system by (1→3)-β-D-glucans having different degrees of branching and different ultrastructures. Journal of Pharmacobio-Dynamics. 1992;15(6):277–285. doi: 10.1248/bpb1978.15.277. [DOI] [PubMed] [Google Scholar]
- 35.Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. Journal of Hematology & Oncology. 2009;2:p. 25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorimachi K, Akimoto K, Ikehara Y, Inafuku K, Okubo A, Yamazaki S. Secretion of TNF-α, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell Structure and Function. 2001;26(2):103–108. doi: 10.1247/csf.26.103. [DOI] [PubMed] [Google Scholar]
- 37.Volman JJ, Helsper JPFG, Wei S, et al. Effects of mushroom-derived β-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-κb transactivation in Caco-2 reporter cells: can effects be explained by structure? Molecular Nutrition and Food Research. 2010;54(2):268–276. doi: 10.1002/mnfr.200900009. [DOI] [PubMed] [Google Scholar]
- 38.Niu YC, Liu JC, Zhao XM, Wu XX. A low molecular weight polysaccharide isolated from Agaricus blazei suppresses tumor growth and angiogenesis in vivo. Oncology Reports. 2009;21(1):145–152. [PubMed] [Google Scholar]
- 39.Kimura Y, Kido T, Takaku T, Sumiyoshi M, Baba K. Isolation of an anti-angiogenic substance from Agaricus blazei Murill: its antitumor and antimetastatic actions. Cancer Science. 2004;95(9):758–764. doi: 10.1111/j.1349-7006.2004.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimiya Y, Suzuki Y, Oshiman KI, et al. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunology Immunotherapy. 1998;46(3):147–159. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi H, Yoshida R, Kanada Y, et al. Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. Journal of Cancer Research and Clinical Oncology. 2005;131(8):527–538. doi: 10.1007/s00432-005-0672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiaohui T, Lun Z, Wang J, et al. Clinical observation on treatment of acute nonlymphocytic leukemia with Agaricus blazei Murill. Journal of Lanzhou Medical College. 1994;20:169–171. [Google Scholar]
- 43.Murakawa K, Fukunaga K, Tanouchi M, Hosokawa M, Hossain Z, Takahashi K. Therapy of myeloma in vivo using marine phospholipid in combination with Agaricus blazei Murill as an immune respond activator. Journal of Oleo Science. 2007;56(4):179–188. doi: 10.5650/jos.56.179. [DOI] [PubMed] [Google Scholar]
- 44.Yu CH, Kan SF, Shu CH, Lu TJ, Sun-Hwang L, Wang PS. Inhibitory mechanisms of Agaricus blazei Murill on the growth of prostate cancer in vitro and in vivo. Journal of Nutritional Biochemistry. 2009;20(10):753–764. doi: 10.1016/j.jnutbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clinical Pharmacology and Therapeutics. 2010;87(4):401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 46.Grinde B, Hetland G, Johnson E. Effects on gene expression and viral load of a medicinal extract from Agaricus blazei in patients with chronic hepatitis C infection. International Immunopharmacology. 2006;6(8):1311–1314. doi: 10.1016/j.intimp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Hsu CH, Hwang KC, Chiang YH, Chou P. The mushroom Agaricus blazei Murill extract normalizes liver function in patients with chronic hepatitis B. Journal of Alternative and Complementary Medicine. 2008;14(3):299–301. doi: 10.1089/acm.2006.6344. [DOI] [PubMed] [Google Scholar]
- 48.Hsu CH, Liao YL, Lin SC, Hwang KC, Chou P. The mushroom Agaricus blazei Murill in combination with metformin and gliclazide improves insulin resistance in type 2 diabetes: a randomized, double-blinded, and placebo-controlled clinical trial. Journal of Alternative and Complementary Medicine. 2007;13(1):97–102. doi: 10.1089/acm.2006.6054. [DOI] [PubMed] [Google Scholar]
- 49.Zittermann A. Effects of vitamin K on calcium and bone metabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 2001;4(6):483–487. doi: 10.1097/00075197-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Førland DT, Johnson E, Tryggestad AMA, Lyberg T, Hetland G. An extract based on the medicinal mushroom Agaricus blazei Murill stimulates monocyte-derived dendritic cells to cytokine and chemokine production in vitro. Cytokine. 2010;49(3):245–250. doi: 10.1016/j.cyto.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Chen L, Shao HJ, Su YB. Coimmunization of Agaricus blazei Murill extract with hepatitis B virus core protein through DNA vaccine enhances cellular and humoral immune responses. International Immunopharmacology. 2004;4(3):403–409. doi: 10.1016/j.intimp.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Shao H. Extract from Agaricus blazei Murill can enhance immune responses elicited by DNA vaccine against foot-and-mouth disease. Veterinary Immunology and Immunopathology. 2006;109(1-2):177–182. doi: 10.1016/j.vetimm.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 53.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clinical and Experimental Immunology. 2007;148(1):32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yazawa Y, Yokota M, Sugiyama K. Antitumor promoting effect of an active component of polyporus, ergosterol and related compounds on rat urinary bladder carcinogenesis in a short-term test with concanavalin A. Biological and Pharmaceutical Bulletin. 2000;23(11):1298–1302. doi: 10.1248/bpb.23.1298. [DOI] [PubMed] [Google Scholar]
- 55.Viola A. Improving cancer immunotherapy by preventing chemokine nitration. European Journal of Cancer Supplment. 2010;8:p. 89. [Google Scholar]
- 56.Schetter AJ, Heegaard NHH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2009;31(1):37–49. doi: 10.1093/carcin/bgp272. Article ID bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. International Journal of Cancer. 2007;121(11):2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura K, Kamoto T, Ogawa O, et al. Medical mushrooms used for biochemical failure after radical treatment for prostate cancer: an open-label study. International Journal of Urology. 2010;17(6):548–554. doi: 10.1111/j.1442-2042.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 59.Jiang J, Sliva D. Novel medicinal mushroom blend suppresses growth and invasiveness of human breast cancer cells. International Journal of Oncology. 2010;37(6):1529–1536. doi: 10.3892/ijo_00000806. [DOI] [PubMed] [Google Scholar]


