Abstract
We developed an accurate and valid medication order algorithm to identify from electronic health records the definitive medication order intended for dispensing and applied this process to identify a cohort of patients and to stratify them into one of three medication adherence groups: early non-persistence, primary non-adherence, or ongoing adherence. We identified medication order data from electronic health record tables, obtained the orders, and linked the orders to dispensings. These steps were then used to identify patients newly prescribed antihypertensive, antidiabetic, or antihyperlipidemic medications and to determine the adherence group of each patient. Record review validated each process step, thus increasing the accuracy of group assignment as well as the criteria used to select patients. This work is an important first step to accurately identify study-specific patient adherence cohorts and allow more comprehensive estimates of population medication adherence.
Background
Poor medication adherence contributes to increased morbidity and mortality in many chronic diseases.1–9 Therefore, accurate assessments of medication adherence are important when interpreting clinical outcomes. Commonly used adherence measurements, such as the proportion of days covered, estimate adherence from pharmacy databases using a surrogate marker of medication possession, but require at least two medication dispensings.2 10–12 Therefore, such estimators systematically and routinely exclude patients who do not fill the medication order (primary non-adherence) and those who only fill the medication order once and do not refill it (early non-persistence).
Electronic health records (EHRs) support clinician order entry and enhance our ability to estimate medication adherence.13 14 Integrated healthcare delivery systems that utilize EHRs have a particular advantage in that EHR medication order information can be readily linked to pharmacy dispensing data, as opposed to systems where order and dispensing data are generally separate and without established electronic interfaces. Even within integrated systems, an initial order may not be the dispensed order (ie, the ‘definitive order’) because the original order may be amended (based on formulary, dosage adjustments due to renal dysfunction, or other factors). If order revisions are not considered when identifying patients with primary non-adherence or early non-persistence, adherence estimates can be incorrect.
As part of a medication adherence project, the objectives of this exploratory work were (a) to determine the programming process necessary to accurately identify from the EHR the definitive medication order and (b) to apply this process to identify a study-specific patient cohort.
Methods
This study was conducted at Kaiser Permanente Colorado (KPCO), a group model, integrated healthcare delivery system providing care to over 470 000 people in the Denver/Boulder, Colorado, USA metropolitan area. It was approved by the KPCO Institutional Review Board with a waiver of informed consent.
KPCO utilizes an ambulatory, fully integrated automated medical record system known as HealthConnect. HealthConnect is the Spring 07 Hyperspace version of Epic Systems Corporation's Electronic Medical Record software (proprietary to Epic, Verona, Wisconsin). HealthConnect includes scheduling, patient registration, and billing modules, and an inpatient/outpatient automated clinical record. The system is deployed on workstations in all medical facility areas and allows real time data capture and simultaneous multiple user access 7 days per week, 24 h per day. Clinicians transmit medication orders to KPCO pharmacies via an HL7 interface between HealthConnect and the pharmacy information management system (PIMS). Medication information is transferred in real-time from HealthConnect to PIMS and from PIMS to HealthConnect.
The study patient cohort included all KPCO members with a newly initiated order for an oral antihypertensive, antidiabetic, or antihyperlipidemic medication between January 1, 2007 and June 30, 2008. Patients were required to have at least 365 days of membership with pharmacy benefits before and at least 180 days after the initial order.
We prepared an inclusive list of oral antidiabetic, antihyperlipidemic, and antihypertensive medications from the First Data Bank data categorization scheme included in the HealthConnect medication tables. This was verified and cross-referenced by drug name to the PIMS product table and by the National Drug Code system codes (NDC) to the Medi-Span Generic Product Identifier (GPI) (proprietary to Medi-Span; licensed through McKesson, San Francisco, California) to ensure all drugs in their respective classes were captured.
Medication orders for newly initiated therapies were identified from the HealthConnect ORDER_MED table. The medication order data for each patient were sorted by order date, order medication number, and dispense date, and the medication order occurring on the earliest date was considered the index order. If the index order was revised within 30 days without being dispensed in the intervening time, the last revision was chosen as the definitive index order.
Dispensings associated with the index order were determined from PIMS. The medication orders were linked to PIMS by a patient identifier, the dispense date, and the GPI Drug Name level. Any dispensings of drug(s) within the GPI Drug Name level within 180 days after the index order were pulled. If no matching record was found in PIMS, the medication order was considered not dispensed.
Approximately 5% of the medication orders were designated for dispensing at a pharmacy external to the KPCO system. External orders are identified in HealthConnect through two different variables: (a) the RSN_FOR_DISCON_C field (Stop Reason) with an indication of ‘Transferred to an outside pharmacy’, or (b) the PHARM_NAME field (Dispense Pharmacy) with an indication of ‘External’. An accurate proportion of days covered cannot be calculated for patients with external medication orders. Therefore, if HealthConnect indicated the prescription was externally dispensed at any time during the prescription refill history, that order was not included.
After linking medication orders with dispensings, the proportion of days covered was calculated for each patient and the study cohort was stratified into drug adherence groups for analyses as follows:
Early non-persistence: newly initiated chronic medication orders dispensed within 30 days of the initial order with no refills within 180 days
Primary non-adherence: newly initiated chronic medication orders not dispensed within 30 days of the initial order
Ongoing: newly initiated chronic medication orders dispensed within 30 days of the initial order with at least one refill within 180 days.
Results
Multiple iterations of programming and chart review were required to accurately identify the definitive medication order. Table 1 summarizes the impacts these challenges had on identifying the study cohort.
Table 1.
Programming challenges encountered in identifying final medication orders intended for dispensing
| Programming challenge/iteration | Impact on study cohort identification |
| Unfamiliar drug categorization scheme | Time to familiarize team to new scheme and verify correct drug inclusion |
| Complex electronic health record (clarity) schema | |
| Table/variable identification | Time to verify information via chart review |
| Overwritten dates | Change medication order date to combination of order date, start date, and dispense date |
| Missing days supply field | Merged to dispensing records, loss of 0.2% of study cohort |
| Clinician processes | |
| Identifying external prescriptions | Correctly identified all external prescriptions and prescription refills transferred to external pharmacies and removed them from the study cohort |
| Diagnosis confirmation | Add diagnoses from ambulatory visits to confirm diagnosis associated with prescription |
| Determining final order | Checked for any revised or amended prescriptions within 30 days of index order and determined final index order as last revision |
The drug categorization schemes differed between HealthConnect (First Data Bank and KP-specific) and PIMS (Medi-Span). We therefore matched the generic drug name to the categorization scheme used in PIMS and then matched it to the Medi-Span GPI by NDC to ensure complete drug capture. We then extracted medication order data from HealthConnect by individual medication identification number.
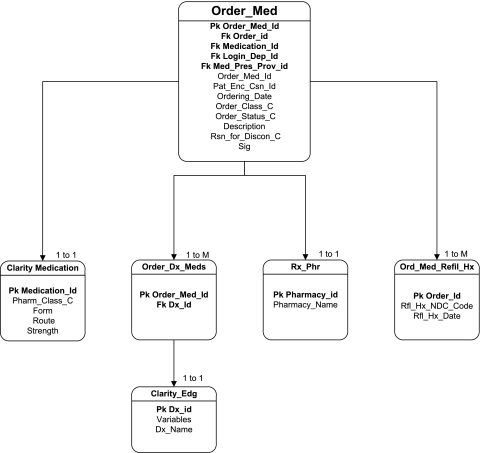
We initially identified 16 medication-related tables in HealthConnect; all were explored to identify useful data fields, verify field meaning, and verify data in that field. We determined several fields that were either not used at all or not used as defined. For example, the field for medication discontinuation (Stop Reason) was not helpful in accurately identifying canceled orders. The final programming code to obtain complete medication order data needed for this study queried six tables to obtain 16 different variables (figure 1).
Figure 1.
Electronic health record schema to identify medication order data.
Determining the order date was challenging in that, when prescriptions were renewed, the original order date was overwritten with the new refill date—for the entire refill history. The prescription start, order, and dispense dates were therefore compiled to determine the definitive order date.
The days supply variable required to calculate the proportion of days covered was not available in HealthConnect tables; we merged orders from HealthConnect to dispensings in PIMS to obtain days supply. We were unable to find a matching dispensing record in PIMS for 29 (0.2% of 15 417) index orders. We used the PIMS data as the source of refills for the proportion of days covered calculations and re-grouped the 18 (0.1%) patients whose adherence changed from ongoing to early non-persistent as a result of this decision.
The two fields in HealthConnect that identified prescriptions dispensed externally were inconsistently informative. Therefore, we used both the Stop Reason field with an indication of ‘Transferred to an outside pharmacy’ and the Dispense Pharmacy field with an indication of ‘External’ to identify external prescriptions.
To confirm the indication for use, we used the diagnosis field associated with the medication order (the clinician chooses an ICD-9 code from a drop-down menu to associate with the order). However, because the clinician can modify the text string (resulting in a change in field description), we also confirmed indication for use based on diagnoses from outpatient ambulatory visits. The number of patients eligible for inclusion in the study cohort dropped by 24% in the hypertension group when the diagnosis associated with the prescription was used alone compared to also using ambulatory visits diagnoses. Reductions of 7% and 5%, respectively, occurred in the diabetes and lipid cohorts when only the diagnosis associated with the medication order was used.
Human error also complicated determination of the final order. Examples include multiple orders sent to an internal pharmacy that were intended for an external pharmacy and orders sent to incorrect internal pharmacy dispensing locations.
Finally, it was difficult to identify the definitive order if the order had been revised. In HealthConnect, all revisions of the medication orders were shown; that is, canceled orders still appeared in the medication tables without clear association or sequencing. We initially allowed up to 72 h for revisions (if not dispensed in the interim), but chart review revealed that revisions were often made past 72 h. We therefore modified the allowable interval for revisions to up to 30 days (if no interim dispensings) and chose the last revised order as the final index order.
Programming revisions based on overcoming each challenge affected the number of members included in the study cohort and in each adherence group. For example, in the hypertension cohort, our initial programming attempt identified 927 patients in the primary non-adherence and early non-persistent adherence groups. After refining the programming codes, the number of patients more than doubled to 2003.
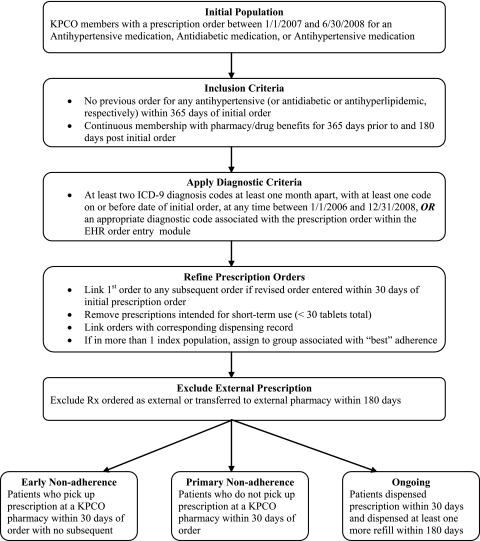
Figure 2 illustrates the final selection criteria used to identify the study cohort for each drug class. Prior to applying any selection criteria, we identified 136 805 orders across the three drug classes. After applying the final selection criteria, the study dataset included 15 417 unique orders.
Figure 2.
Selection of patients for adherence cohorts. EHR, electronic health record; KPCO, Kaiser Permanente Colorado; Rx, prescription.
Discussion
Much adherence research has been conducted using information available in pharmacy claims databases and includes only patients with at least two dispensings of the medication. Comprehensive adherence measurement also requires the inclusion of patients who are primarily non-adherent and those who are early non-persistent. Linking medication orders to dispensings is an important preliminary step to capture these two groups of patients, but little research has been done on this linkage. In this paper we document that, even in a system where medication orders and dispensings can be linked, assumptions about the inherent completeness, appropriateness, and accuracy of the information available can be misleading, and can result in classification of patients into incorrect adherence groups. If attention is not given to iterative evaluation and refinement of the programming code required to identify primary non-adherence and early non-persistence, erroneous results and interpretations will occur. The above narrative provides valuable guidance about both the challenges encountered in striving to accurately identify adherence as well as the approaches taken to overcome those challenges in a database where medication orders and dispensings can be linked.
Others have attempted to assess medication adherence solely from EHRs. Some have assessed adherence information extracted from the clinical narrative.15 16 However, these studies were only able to secure simple elements of medication orders by natural language processing and were unable to include elements such as days supply. One study was successful in linking orders with dispensed medications17; however, the medication orders were not verified via chart review.
There were multiple strengths to our study that allowed us to link medication orders and dispensings, such as the robust nature of the data within our integrated system, universal clinician medication order entry into HealthConnect, accessible electronic pharmacy dispensing data, and the ability to identify prescriptions intended for external dispensing. Another strength was that we used medical chart review to assess the impact of each programming refinement on adherence category assignment and made necessary programming adjustments in the next iteration.
One limitation of our work is that we were not able to differentiate suboptimal medication adherence from medication intolerance or adverse events in the early non-persistence group. For example, we could not identify the subset of patients who stopped taking medication on the advice of the clinician due to an adverse reaction to the new medication.
In conclusion, identifying definitive medication orders in the EHR was challenging. In particular, determining the correct order date, identifying external prescriptions, and identifying amended orders was difficult. When scientific investigations include assessment of primary non-adherence and early non-persistence, it is particularly important to accurately identify these factors and to internally validate data extraction methods. Particular attention to iterative evaluations and refinement of the programming code may help develop an accurate medication order algorithm.
Acknowledgments
We acknowledge Elizabeth Bayliss, MD, Brandy McGinnis, PharmD, Susan M Shetterly, MS, and Stan Xu, PhD for their contributions to the building of the algorithm.
Footnotes
Funding: The work presented in this manuscript was supported by an internal grant from the Kaiser Permanente Institute for Health Research.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Balkrishnan R. The importance of medication adherence in improving chronic-disease related outcomes: what we know and what we need to further know. Med Care 2005;43:517–20 [DOI] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschkte T. Adherence to medication. N Engl J Med 2005;353:497. [DOI] [PubMed] [Google Scholar]
- 3.Walker EA, Molitch M, Kramer MK, et al. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care 2006;29:1997–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JR, Moser DK, DeJong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am Heart J 2009;157:285–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521–30 [DOI] [PubMed] [Google Scholar]
- 6.Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med 2008;168:271–6 [DOI] [PubMed] [Google Scholar]
- 7.McGinnis BD, Olson KL, Delate TM, et al. Statin adherence and mortality in patients enrolled in a secondary prevention program. Am J Manag Care 2009;15:689–95 [PubMed] [Google Scholar]
- 8.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–41 [DOI] [PubMed] [Google Scholar]
- 9.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7 [DOI] [PubMed] [Google Scholar]
- 10.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565–74 [DOI] [PubMed] [Google Scholar]
- 11.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40:1280–8 [DOI] [PubMed] [Google Scholar]
- 12.Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–61 [DOI] [PubMed] [Google Scholar]
- 13.Shah NR, Hirsch AG, Zacker C, et al. Factors associated with first-fill adherence rates for diabetic medications: a cohort study. J Gen Intern Med 2008;24:233–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens 2009;22:392–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mork JG, Bodenreider O, Demner-Fushman D, et al. Extracting Rx information from clinical narrative. J Am Med Inform Assoc 2010;17:536–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turchin A, Wheeler HI, labreche M, et al. Indentification of documented medication non-ahderence in physician notes. Proceedings of the American Medical Informatics Association Symposium. Washington, DC, 2008:732–6 [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med 2010;25:284–90 [DOI] [PMC free article] [PubMed] [Google Scholar]