Abstract
The cyclin proteolysis that accompanies the exit from mitosis in diverse systems appears to be essential for restoration of interphase. The early syncytial divisions of Drosophila embryos, however, occur without detectable oscillations in the total cyclin level or Cdk1 activity. Nonetheless, we found that injection of an established inhibitor of cyclin proteolysis, a cyclin B amino-terminal peptide, prevents exit from mitosis in syncytial embryos. Similarly, injection of a version of Drosophila cyclin B that is refractory to proteolysis results in mitotic arrest. We infer that proteolysis of cyclins is required for exit from syncytial mitoses. This inference can be reconciled with the failure to observe oscillations in total cyclin levels if only a small pool of cyclins is destroyed in each cycle. We find that antibody detection of histone H3 phosphorylation (PH3) acts as a reporter for Cdk1 activity. A gradient of PH3 along anaphase chromosomes suggests local Cdk1 inactivation near the spindle poles in syncytial embryos. This pattern of Cdk1 inactivation would be consistent with local cyclin destruction at centrosomes or kinetochores. The local loss of PH3 during anaphase is specific to the syncytial divisions and is not observed after cellularization. We suggest that exit from mitosis in syncytial cycles is modified to allow nuclear autonomy within a common cytoplasm.
Keywords: Drosophila, mitosis, proteolysis, cyclin, histone H3
Although the past decade of cell cycle research has revealed a bewildering complexity of cell cycle control, one simple generalization has been supported by observations in a variety of experimental settings and in numerous species. The kinase activity of a cyclin:Cdk1 complex promotes mitosis, whereas the destruction of the cyclin partner and loss of kinase activity is associated with and is required for exit from mitosis. The early evidence for the requirement for cyclin destruction came from the production of deleted versions of cyclins that resisted mitotic destruction and arrested cells in mitosis (Murray et al. 1989; Glotzer et al. 1991; Gallant and Nigg 1992; Surana et al. 1993; Sigrist et al. 1995a). However, some complexities have been introduced even into this most global generalization about the cell cycle. We now recognize that exit from mitosis is complex and that sequential destruction of many different regulators is likely to regulate distinct steps in the process, as detailed below.
Mitosis may be considered a progression of discrete steps, with the ultimate goal being the equal division of the genomic contents. Among all mitotic phases, metaphase appears to represent the pinnacle of the mitotic state, with the spindle and the condensed chromosomes poised to segregate daughter genomes to the opposite poles of the cell. Release from this state requires a ubiquitin-conjugating enzyme complex, APC (anaphase promoting complex; King et al. 1995), or cyclosome (Sudakin et al. 1995) that targets cyclins and several other proteins for rapid proteolytic destruction (for review, see King et al. 1996a; Nasmyth 1996). The original demonstration that the transition from metaphase requires proteolysis was shown in Xenopus egg extract experiments (Holloway et al. 1993). In these extracts, introduction of high levels of a target peptide for ubiquitin-mediated proteolysis (containing the “destruction box”) led to mitotic arrest, apparently by competing with endogenous substrates and interfering with their destruction (Holloway et al. 1993; van der Velden and Lohka 1993). Genetic analyses in yeast and biochemical experiments in mammalian systems established the universal requirement for ubiquitin-mediated proteolysis in exiting mitosis (for review, see King et al. 1996a; Nasmyth 1996). Although recent work focusing on the mechanisms that govern the exit from mitosis has identified new and important targets of APC-mediated proteolysis (e.g., see Cohen-Fix et al. 1996; Funabiki et al. 1996; Juang et al. 1997), the destruction of mitotic cyclins is still thought to represent a key and essential step in the return to an interphase state.
Removal of the destruction box sequences from the amino terminus of mitotic cyclins protects these proteins from mitotic degradation (Murray et al. 1989; Glotzer et al. 1991; Gallant and Nigg 1992; Surana et al. 1993; Sigrist et al. 1995a). Stable cyclins block exit from mitosis, but stabilization of different cyclins blocks exit from mitosis at different steps (Sigrist et al. 1995a). In cellularized embryos of Drosophila melanogaster, stabilization of cyclin A (which has a mitotic function in Drosophila; Lehner and O’Farrell 1989, 1990; Knoblich and Lehner 1993) results in a metaphase-like arrest, with unsegregated sister chromosomes aligned at the metaphase plate. In contrast, mitotic arrest that results from stabilization of another mitotic cyclin, cyclin B, shows partially segregated condensed chromosomes and therefore, resembles early anaphase. Finally, stabilization of the third Drosophila mitotic cyclin, cyclin B3, results in a mitotic arrest with fully segregated chromosomes, thus resembling late anaphase/telophase. Similar to the situation with stabilized cyclin B3 of Drosophila, stable B-type cyclins can lead to postmetaphase arrest in budding and fission yeasts, Xenopus extracts, and mammalian cells (Holloway et al. 1993; Sigrist et al. 1995a; Surana et al. 1993; Yamano et al. 1996). Interestingly, mitotic arrest that results from stabilization of each mitotic cyclin, A, B, and B3, in Drosophila embryos resembles the mitotic stage at which degradation of each cyclin is completed (Sigrist et al. 1995a). This observation has led to the proposal that sequential degradation of mitotic cyclins dictates the sequential order of some of the events at exit from mitosis (Sigrist et al. 1995a; for review, see Follette and O’Farrell 1997).
The precellular mitotic divisions of the Drosophila embryo appear to provide a striking counterpoint to the demonstrated importance of cyclin degradation and Cdk1 inactivation to cell cycle progression. Drosophila embryogenesis begins with 13 metasynchronous mitotic cycles within a syncytial cytoplasm (Foe et al. 1993). These cycles consist only of S and M phases, rely on maternally supplied activities, and do not require zygotic gene expression. The first 10 syncytial cycles last ∼9 min each; subsequently, the cycles slow gradually, leading to a transition from maternal to zygotic control of the cell cycle in cycle 14. Previous studies indicate that cyclin levels and Cdk1 activity remain high during the first eight cycles (Edgar et al. 1994). Thereafter, oscillations of these key cell cycle regulators set in gradually, with the amplitude of the oscillation increasing in successive mitoses. Given that mitotic cyclin degradation and Cdk1 inactivation appear essential for exiting mitosis in all systems tested, we asked how syncytial cycles occur in the continuous presence of mitotic regulators.
Two cases in which cells exit mitosis without cyclin degradation are worth noting. In budding yeast, hct1/cdh1 mutants are defective in the proteolysis of a mitotic cyclin, Clb2, and yet these cells are able to exit mitosis (Schwab et al. 1997). Second, in budding yeast cells that are arrested in mitosis because of spindle defects, a mutation in the phosphatase regulatory subunit CDC55 allowed the exit from mitosis (Minshull et al. 1996). In this case, exit from mitosis occurred in the presence of high cyclin levels, apparently by inhibitory phosphorylation of Cdk1. The absence of an inhibitory phosphate on Cdk1 in Drosophila syncytial cycles, however, argues against Cdk1 phosphorylation being responsible for exit from syncytial mitoses (Edgar et al. 1994). Instead, the fact that mitotic arrest follows cyclin stabilization in diverse experimental systems argues that Cdk1 inactivation by cyclin degradation may be the universal way of exiting mitosis in unperturbed cells.
In this report we demonstrate that despite the constant presence of cyclins and Cdk1 activity, proteolysis and cyclin degradation appear to be essential for syncytial nuclei to exit mitosis. Using phosphorylation on the first serine of histone H3 (PH3) as a reporter for Cdk1 activity, we present evidence for local changes in Cdk1 activity as syncytial nuclei exit mitosis. Such local changes are absent in the mitoses of the cellular blastoderm embryo in which mitotic cyclins are degraded completely at exit from mitosis. We propose that syncytial embryos experience local changes in Cdk1 activity through degradation of small, local pools of cyclins. We infer that in cellularized embryos global degradation of cyclins throughout the cell results in global changes such as the near-uniform loss of PH3 from chromosomes.
Results and Discussion
Injection of a destruction box peptide results in mitotic arrest in syncytial embryos
To assess the role of ubiquitin-mediated proteolysis in the syncytial cycles of Drosophila, we injected syncytial stage embryos with a known inhibitor of the process, an amino-terminal peptide of sea urchin cyclin B. This peptide contains the signal sequence for the ubiquitin-mediated degradation by the APC pathway, preceded by a T7 epitope tag. It is identical to the peptide used in an earlier demonstration of the importance of proteolysis for mitotic progression in Xenopus extracts (referred to as 13-110 in Holloway et al. 1993). Injection of the 13-110 peptide into syncytial stage embryos and staining for the epitope tag revealed a gradient of peptide with the high point at the site of injection (not shown). Such injections blocked the nuclear cycles in mitotic-like states near the point of injection, whereas nuclei distant from the site of injection progressed to the expected nuclear density (Fig. 1A). Nearly all injected embryos showed condensed chromosomes within a mitotic spindle near the point of injection, although not all arrested mitoses had the appearance of a normal metaphase. Such arrests were seen in syncytial embryos of all ages, including precycle 8 embryos, which ordinarily exhibit no detectable oscillations in cyclin levels or Cdk1 activity (Edgar et al. 1994). As a control, we injected a peptide in which the amino acids RAAL of the destruction box consensus sequence have been mutated to AARL to render it unrecognizable by the ubiquitin pathway (called 13-110* in Holloway et al. 1993). We found that the control peptide did not cause a mitotic arrest under similar conditions (Fig. 1B). We conclude that the presence of 13-110 interfered with mitotic progression, whereas 13-110* did not. The specificity implicates the destruction box in the inhibition of the nuclear cycles. The destruction box sequences target cyclin B for ubiquitination and proteolysis and the peptide containing this sequence interferes with these processes (van der Velden and Lohka 1993; King et al. 1996b). Therefore, our results suggest that APC-mediated ubiquitination, and presumably proteolysis, is required for exit from mitosis in the early syncytial cycles of Drosophila, although we cannot eliminate the possibility that ubiquitination might drive exit from mitosis without associated destruction of proteins.
Figure 1.
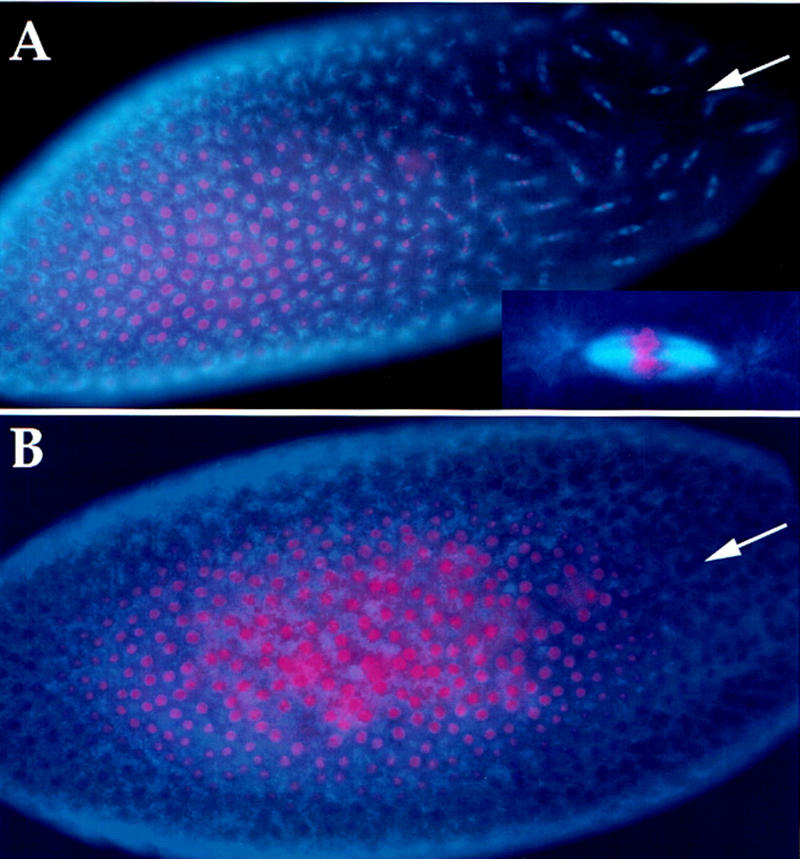
Amino-terminal peptide of cyclin B causes mitotic arrest. (A) Syncytial embryos (0- to 1-hr-old, before cycle 7) were injected with 13-110 peptide, fixed 30–45 min later, and stained for DNA (red) and β-tubulin (blue) to visualize microtubules. Nuclei near the site of injection (arrow) are arrested in mitosis and show condensed chromosomes and mitotic spindles. In contrast, in the remainder of the embryo syncytial divisions have progressed such that nuclear density is higher and nuclei are not arrested in mitosis. An arrested mitotic figure is magnified fivefold and shown in the inset. (B) An embryo injected with the control peptide bearing two point mutations in the destruction box consensus sequence, 13-110*, shows no mitotic arrest. The site of injection is indicated with an arrow.
Injection of a stable cyclin results in mitotic arrest in syncytial embryos
Studies in diverse systems identified mitotic cyclins as proteolytic substrates that must be removed to allow exit from mitosis. Recent work in yeast and Aspergillus, however, has identified additional substrates that must be degraded to progress through events of mitosis (Pu and Osmani 1995; Cohen-Fix et al. 1996; Funabiki et al. 1996). These include proteins that regulate sister chromosome segregation and proteins associated with the mitotic spindle. Although the actual restoration of an interphase state is thought to usually require destruction of cyclins and inactivation of Cdk1, neither cyclin levels nor Cdk1 activity oscillate in the early syncytial cycles. This raises the possibility that it is the degradation not of cyclins but of other proteins that drives the exit from these mitoses. Thus, we assessed the importance of cyclin degradation to the syncytial cycles.
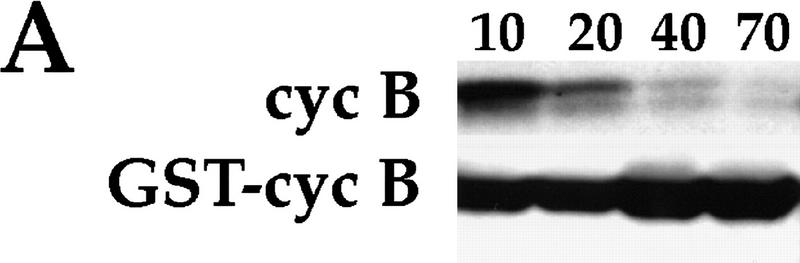
The importance of cyclin destruction to exiting mitosis has been demonstrated by the production of a mitotic arrest by stabilized cyclins when these proteins are introduced into cells or cycling Xenopus extracts (Murray et al. 1989; Gallant and Nigg 1992; Holloway et al. 1993; Surana et al. 1993; Sigrist et al. 1995a). To test the importance of cyclin degradation to the early mitotic cycles, we prepared a stable derivative of Drosophila cyclin B protein for injection into syncytial embryos. In addition to truncations that removed sequences inferred to represent the destruction box (Sigrist et al. 1995a; Sprenger et al. 1997), a fusion of glutathione S-transferase (GST) to the amino terminus of full-length Drosophila cyclin B proved to be stable. GST–cyclin B reconstituted kinase activity upon addition of in vitro-translated Drosophila Cdk1 (Campbell et al. 1995), and incubation in Xenopus mitotic extracts demonstrates its stability (Fig. 2A). The GST tag simplified purification of stable cyclin B from bacterial extracts (see Materials and Methods).
Figure 2.
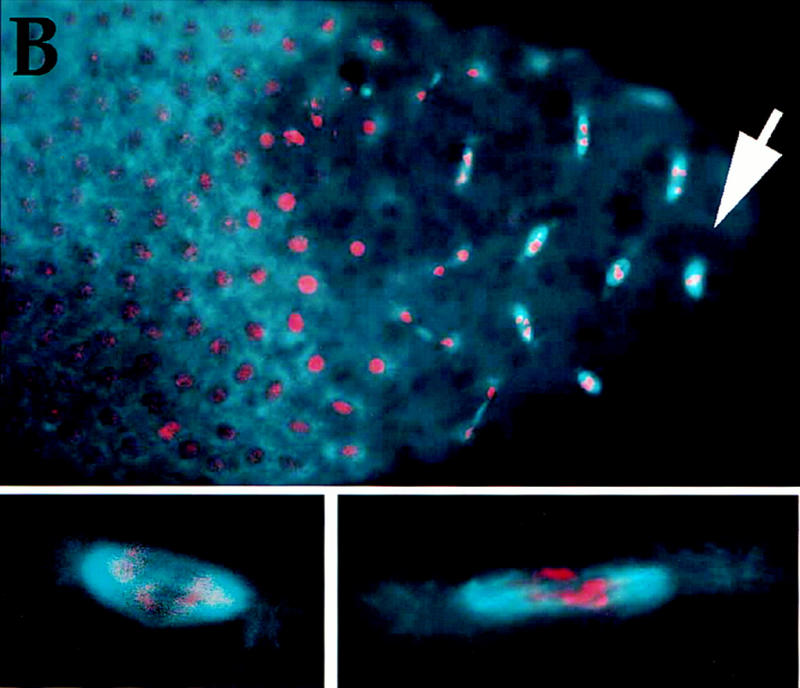
GST–cyclin B causes mitotic arrest. (A) GST–cyclin B is stable in mitotic extracts. In vitro translated 35S-labeled Drosophila cyclin B (cyc B) or a bacterially produced GST–cyclin B fusion (GST–cyc B) was incubated in Xenopus mitotic extracts for various times (minutes) as indicated. Proteins were separated on denaturing gels and cyclin B was detected by autoradiography (cyc B) or by immunoblotting with polyclonal antibodies to GST (GST–cyc B). Although cyc B was degraded in mitotic extracts, GST–cyc B remained stable. (B) Syncytial embryos (0- to 1-hr-old, before cycle 7) were injected with GST–cyclin B, fixed after 30 min, and stained for DNA (red) and β-tubulin (blue) to visualize microtubules. Nuclei near the site of injection (arrow) are arrested in mitosis and show condensed chromosomes and mitotic spindles. In contrast, in the remainder of the embryo syncytial divisions have progressed and the nuclear density is higher, and nuclei are not arrested in mitosis. Two representative arrested mitotic figures are magnified and shown below.
Injection of GST–cyclin B protein into syncytial embryos (cycles 1–7) resulted in arrest of the mitotic cycle surrounding the point of injection (Fig. 2B). Arrested mitoses were not seen with control injections of GST. Many of the arrested mitoses show condensed chromosomes that are segregated partially (Fig. 2 insets). Similarly, the arrest induced by expression of stable cyclin B in later cycles also shows an arrest with the chromosomes neither paired at the metaphase plate nor at the spindle poles (Sigrist et al. 1995a; see also Fig. 3). The amount of injected GST–cyclin B approximated that of endogenous cyclin B as determined by immunoblotting (not shown), thus doubling the amount of total cyclin B. Increasing endogenous cyclin B by eightfold does not result in mitotic arrest (G. Schubiger, pers. comm.). This is consistent with observations after induced expression of normal and stable cyclin constructs during the later cycles; these observations indicate that only the stabilized versions of the cyclins have the capacity to arrest the cycle (Sigrist et al. 1995a; Fig. 3G–L). On the basis of the GST–cyclin B-induced block to mitotic progression, we infer that destruction of cyclin B, and presumably other mitotic cyclins as well, is required for exit from syncytial mitoses.
Figure 3.
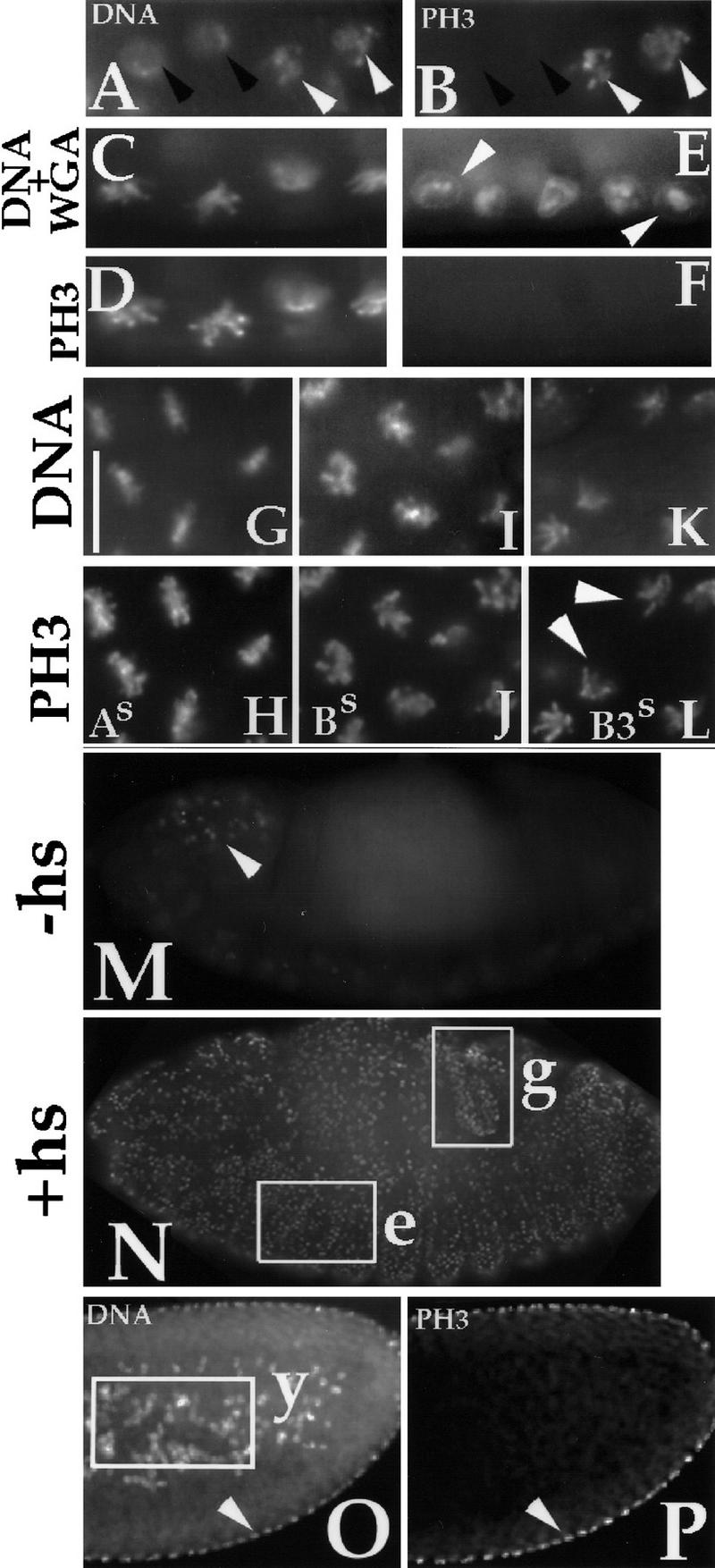
The dependence of PH3 on Cdk1 activity in Drosophila embryos. (A,B) PH3 staining at entry into mitosis. Drosophila embryos in division cycle 14–15 were fixed and stained for DNA (A) and PH3 (B). PH3 stain is absent in interphase nuclei (black arrowheads) but observed on condensing chromosomes as cells enter prophase (white arrowheads). (C–F) Inactivation of Cdk1 during a mitotic arrest led to the loss of PH3 signal. Syncytial stage Cdk1ts embryos were arrested in mitosis for 20 min at the permissive temperature of 18°C using colchicine. During this arrest, chromosomes are condensed (C) and stained for PH3 (D). After inactivation of Cdk1ts at 30°C for 15 min, chromosomes decondense and become surrounded by the nuclear envelope (E, arrowheads point to wheat germ agglutinin (WGA) staining of nuclear pores) and PH3 signal is lost (F). (G–L) PH3 during mitotic arrests with nondegradable mitotic cyclins A (As in G,H), B (Bs in I,J) or B3 (B3s in K,L). After heat-induction of each stable cyclin, embryos were fixed and stained for DNA and PH3. PH3 stain is present at all three arrests. (M,N) Ectopic expression of Cdk1AF and cyclin A leads to induction of PH3. Embryos carrying a heat shock inducible Cdk1AF and cyclin A transgenes were fixed in stages 13–15 (Campos-Ortega and Hartenstein 1985) either directly (M) or 2 hr after heat induction (N) and were stained for PH3. In M, the normally mitotic cells of the CNS show PH3 staining (arrowhead), but this stain is absent in most other cells, including the epidermis and the gut. In N, PH3 staining is widespread and includes nonmitotic epidermal cells (e) and hindgut cells (g) that are arrested in a postmitosis 16 quiescent phase and are destined to undergo endocycles. The hindgut was identified according to position and morphology viewed by differential interference contrast (DIC) (not shown). (O,P) PH3 staining of a syncytial embryo demonstrates that cortical nuclei are in a mitotic state and exhibit the PH3 stain (O, arrowhead), whereas the yolk nuclei (y, boxed in N) that endoreplicate lack the PH3 stain. Note that the PH3 signal in internal tissues is detectable by our methods (Su et al. 1988); therefore, the failure of yolk nuclei to stain for PH3 is unlikely to be attributable to technical reasons. N shows the DNA stain. (A–L) Bar, 10 μm; (M–O) bar, 40 μm.
Mitotic and nonmitotic nuclei coexist within the syncytial cytoplasm
To reconcile the above observations with the lack of oscillation in total cyclin levels in the early syncytial cycles of the Drosophila embryo and their incomplete destruction during the later syncytial mitoses (Edgar et al. 1994), we suggest that a small pool of cyclins is destroyed during the exit from mitosis. If destruction of cyclins is confined to the locale of each mitotic spindle, local variations in Cdk1 activity may be created that allow syncytial nuclei to exit mitosis in the presence of high levels of Cdk1 activity throughout the rest of the embryo. This proposal predicts that the nuclei of the syncytium would exit mitosis independently of one another. Although the synchrony of the early syncytial cycles is well known, this synchrony is broken in a number of instances that argue for a substantial degree of autonomy among syncytial nuclei.
For example, the products of female meiosis that do not contribute to the zygotic nucleus are not partitioned off as separate cells but remain as nuclei within the syncytium; these “polar bodies” progress through a first mitotic cycle, then arrest in a mitotic-like state with condensed chromosomes, but without a mitotic spindle (Foe et al. 1993; the absence of an associated spindle is presumed to be attributable to the absence of centrosome, which is provided to the zygotic nucleus by the incoming sperm). Importantly, these polar bodies remain in a condensed mitotic state, whereas the nuclei that surround them progress through rapid mitotic cycles. If we inactivate mitotic kinase Cdk1 by raising the temperature in a Cdk1ts mutant, these polar bodies decondense, indicating that the persistent mitotic state requires Cdk1 activity (T.T. Su and P.H. O’Farrell, unpubl.; see below for Cdk1ts experiments). Thus, the mitotic configuration of the polar bodies is consistent with the high levels of Cdk1 kinase. Presumably, when adjacent zygotic mitotic nuclei return to interphase they inactivate Cdk1 locally without eliminating Cdk1 activity in the environment of the polar bodies.
Three other examples suggest that the polar bodies are not unique in the autonomy of their cell cycle. Yolk nuclei forgo mitotic events and endoreplicate, whereas the cortical nuclei in the same cytoplasm undergo mitotic divisions (for example, Fig. 3O,P). In addition, posterior nuclei that will later become progenitors of germ cells have a division program that first deviates from somatic nuclei when both types of nuclei still share a common cytoplasm (Su et al. 1998). Finally, in Drosophila mutants bearing an abnormally long chromosome, syncytial nuclei are observed to initiate anaphase autonomously (Sullivan et al. 1993). These observations lead us to suggest that exit from mitosis in the syncytial embryo may be under local control.
PH3 signal reflects Cdk1 activity
We reasoned that if local variations in Cdk1 activity are involved in the exit from mitosis in the early syncytial cell cycles, these variations might be detected as local variations in the action of Cdk1. We found that PH3, which can be detected using a specific antibody (Shibata et al. 1990; Bradbury 1992; Ajiro et al. 1996a,b), correlates with Cdk1 activity in Drosophila embryos. H3 phosphorylation is thought to contribute to chromatin condensation during mitosis (Hanks et al. 1983; Th’ng et al. 1994; Ajiro et al. 1996a,b). In both syncytial and cellularized embryos, phosphorylation of histone H3 (PH3) signal is absent in interphase and appears on chromosomes as cells enter mitosis (for example, Fig. 3A,B). We carried out three experimental manipulations to test whether the levels of PH3 might be coupled to the activity of Cdk1: (1) inactivation of Cdk1 during mitosis, (2) prolongation of Cdk1 activity during mitosis, and (3) ectopic induction of Cdk1 activity in interphase.
Inactivation of Cdk1 during mitosis led to a rapid loss of PH3 (Fig. 3C–F). Syncytial embryos bearing a temperature-sensitive version of Cdk1, Cdk1ts (Sigrist et al. 1995b), were arrested in mitosis using a microtubule depolymerization agent (Fig. 3C,D). Subsequent inactivation of Cdk1ts in these embryos by shifting to the restrictive temperature led to loss of PH3 within 15 min (Fig. 3E,F). Similarly treated wild-type embryos retained PH3 staining as did the control Cdk1ts embryos that remained at the permissive temperature (not shown). These data indicate that Cdk1 activity is required to maintain PH3 in mitosis.
Conversely, experimental maintenance of Cdk1 activity in mitosis led to maintenance of PH3 (Fig. 3G–L). We induced expression of stable versions of cyclins from transgenes under the control of the hsp70 promoter in cellularized embryos (note that heat induction of these transgenes is only possible after cellularization; heat shock before cellularization results in lethality). As shown previously, expression of stable versions of each of the three mitotic cyclins, stable cyclin A (As), stable cyclin B (Bs), and stable cyclin B3 (B3s), lead to mitotic arrest (Sigrist et al. 1995a). The arrests, however, resemble different stages between metaphase and telophase; As arrests mitosis with chromosomes at the metaphase plate; Bs arrests mitosis with sister chromosomes separated to varying degrees (early to mid-anaphase-like configuration); and B3s arrests mitosis with fully separated but fully condensed chromosomes (see arrowheads in Fig. 3L). Importantly, at each arrest point only the stabilized cyclin persists (Sigrist et al. 1995a). PH3 signal was maintained at all three arrests, suggesting that Cdk1 in complex with any of these mitotic cyclins can maintain PH3.
Finally, we found that ectopic induction of Cdk1 activity led to induction of PH3. To induce Cdk1 activity ectopically, we used heat-inducible promoters to express Cdk1AF in conjunction with either cyclin A or B. Cdk1AF contains mutations in inhibitory phosphorylation sites (T14A and Y15F) and cannot be inhibited by Wee1 kinase (Sprenger et al. 1997). Consistent with the demonstrated role of inhibitory phosphorylation on Cdk1 in controlling the progress into mitosis, induction of Cdk1 with a cyclin triggered G2-arrested cells to enter mitosis and to accumulate PH3 (Su et al. 1998; N. Yakubovich and P.H. O’Farrell, unpubl.). Endocycling cells and G1-arrested cells also responded to induction of Cdk1 and cyclin A by accumulating PH3 (Fig. 3M,N) although these cells did not enter mitosis. Thus, accumulation of PH3 in response to Cdk1 activity does not require mitosis.
Data presented above suggest that PH3 levels are governed by Cdk1 activity such that Cdk1 can induce PH3 and Cdk1 is required to maintain PH3 in syncytial mitoses. At present we do not know whether Cdk1 acts directly on histone H3 in Drosophila.
Localized loss of PH3 signal at exit from syncytial mitoses
A more detailed analysis of PH3 staining upon exit from mitosis reveals an unexpected feature of PH3 loss during the syncytial cycles. Immunostaining of syncytial embryos for PH3 demonstrates that chromosomes in metaphase, early anaphase, and mid-anaphase show PH3 stain along their length (Fig. 4A, top two mitotic figures; data not shown). As anaphase progresses, loss of PH3 begins at the kinetochore regions of chromosomes (Fig. 4A, the third mitotic figure) and continues as chromosomes decondense in telophase (Fig. 4A, bottom mitotic figure). Such local gradients of PH3 are seen in syncytial mitoses from at least cycle 4 (M4; the earliest we have analyzed; M6 is shown in Fig. 4B) up to and including the last syncytial mitosis, M13 (Fig. 4C), although the PH3 gradient appears increasingly shallower as nuclear cycles progress. The localized loss of H3 phosphorylation during anaphase demonstrates that nonuniform conditions occur during exit from syncytial mitosis. A local gradient of kinase activity or a local gradient of phosphatase activity, or a combination of both, could result in the gradient of PH3 staining we observed. Given the strict correlation between PH3 and Cdk1 activity in Drosophila embryos described above, we suggest that a likely basis for the localized loss of PH3 is a localized decline in Cdk1 activity. The localized loss of PH3 is blocked by injection of the 13-110 peptide (not shown), suggesting that proteolysis contributes to local loss of Cdk1 activity and PH3.
Figure 4.
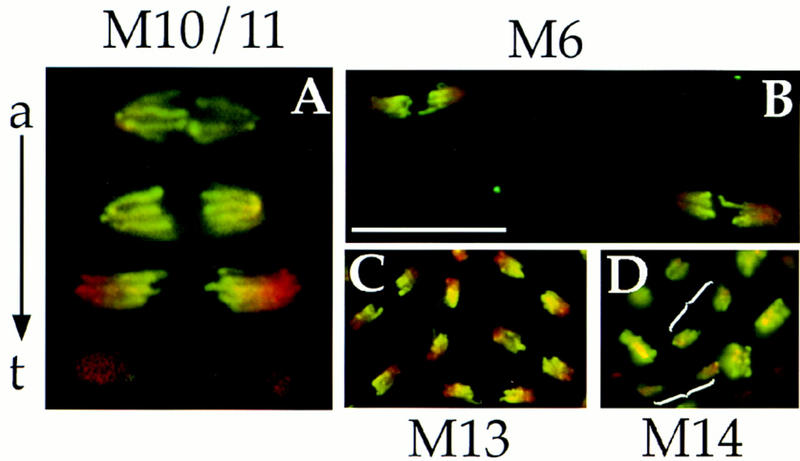
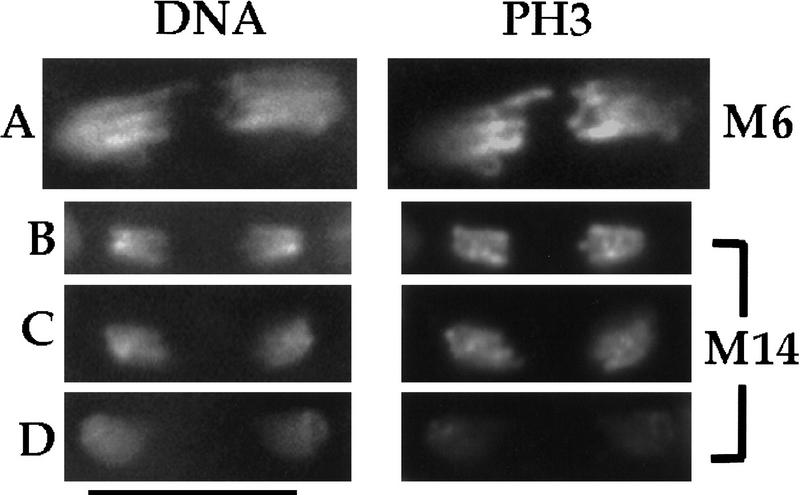
PH3 staining at exit from mitoses. Wild-type Drosophila embryos were fixed and stained for DNA (red) and PH3 (green). Colocalization of red and green signals result in yellow. PH3 disappears in a gradient on chromosomes at exit from syncytial mitoses (A–C), but this gradient is not readily apparent in cellular blastoderm mitoses (D). (A) A composite of mitotic figures from syncytial mitoses 10/11 is shown to depict the progression from anaphase (a) to telophase (t). Anaphase–telophase progression is characterized by a greater separation between kinetochore (poleward) regions of sister chromosomes, lengthening of chromosome arms, and increasing degree of chromosome decondensation. Loss of PH3 begins at the kinetochore regions of chromosomes in late anaphase/telophase. PH3 gradients are seen in M6 (B) and in M13 (C). Only slight color gradients are seen in late anaphase figures of M14 (D, brackets show two pairs of late anaphase/telophase chromosomes) and these gradations appear to be caused by nonhomogenous Hoechst 33258 staining of DNA rather than by nonhomogenous PH3 stain (see Fig. 5). Bar (A) 10 μm; (B–D) 20 μm.
Localized Cdk1 inactivation would explain how syncytial nuclei are able to exit mitosis despite the presence of cyclins and Cdk1 activity in the rest of the embryo. Our data are consistent with the degradation of a small and local pool of cyclins, generating a local decline in Cdk1 activity. This would explain the ability of an inhibitor of the APC and a stabilized cyclin to block exit from mitosis during these syncytial cycles.
A priori we might have expected that exit from mitosis requires the complete elimination of cyclins in the environment of the mitotic figures. However, there are indications that any loss of cyclin that might occur is more limited. Staining for cyclins A and B during the syncytial cycles revealed strikingly nonuniform distributions, but did not detect a general disappearance of cyclins in the region of anaphase spindles (Maldonado-Codina and Glover 1992). The gradient of PH3 on anaphase chromosomes in syncytial mitoses is consistent with dephosphorylation events originating at the kinetochore region or at the spindle pole. We suggest that changes in protein degradation and phosphorylation status of specific components within the mitotic spindle can guide exit from mitosis.
Localized changes in the phosphorylation state of kinetochore proteins have been implicated previously in mitotic spindle function in insect cells (Nicklas et al. 1995, Nicklas 1997). In addition, certain components of the ubiquitin-mediated degradation pathways are localized to the kinetochore region and at the centrosomes (Tugendreich et al. 1995; Bai et al. 1996; Connelly and Heiter 1996; Jorgensen et al. 1998). These and the observations presented here lead us to suggest that mitotic proteolytic activities may also be localized at the kinetochore region or the centrosomes and contribute to mitotic progression. Such local proteolysis of cyclins and inactivation of Cdk1 could explain the local gradient in PH3 loss we observed in syncytial embryos.
PH3 loss is near uniform in mitoses of cellularized embryos
At the conclusion of the last syncytial division (M13), the embryo undergoes many changes associated with the maternal to zygotic transition [(MZT; similar to mid-blastula transition (MBT) of vertebrates], including cellularization and the transition from the maternal to zygotic control of the cell cycle (Foe et al. 1993). Of these changes, the most pertinent to this work are the changes in cyclin degradation; whereas no mitotic cyclin destruction is detected before the ninth mitosis, at each subsequent mitosis a larger and larger fraction of cyclins A and B are degraded until, subsequent to the MZT, cyclin destruction at mitosis 14 appears to be complete (Lehner and O’Farrell 1989, 1990; Edgar et al. 1994; Sigrist et al. 1995a). Paralleling these changes, there is a change in histone H3 dephosphorylation at exit from mitosis. The gradient of PH3 staining along the anaphase chromosomes becomes less marked in the later syncytial divisions, and then in M14, PH3 persists on chromosomes throughout anaphase and disappears approximately uniformly from chromosomes in telophase (Figs. 4D and 5). Although the two-color images in Figure 4D suggest that a slight gradient might persist into mitosis 14, the separate staining for DNA shown in Figure 5 suggests that uneven color ratios in M14 largely results from the nonuniformity of the Hoechst 33258 staining of the DNA. PH3 staining in the two remaining embryonic mitoses in the epidermis, M15 and M16, is similar to that of M14 (data not shown).
Figure 5.
PH3 staining disappears uniformly as cellular blastoderm nuclei exit mitosis. Wild-type Drosophila embryos were fixed and stained for DNA and PH3 as indicated. (B–D) A progression from late anaphase through telophase of M14. These mitotic chromosomes are from the same embryo and have been processed identically. A decline in PH3 signal from B to D is evident but does not occur in a gradient as in syncytial mitoses (to allow comparison, A shows M6 chromosomes from Fig. 4B). Bar, 10 μm.
What might underlie the gradual change in histone H3 dephosphorylation as divisions progress? We suggest that it reflects the change in cyclin destruction. As suggested above, the incomplete cyclin destruction and local loss of PH3 during the syncytial cycles might be attributable to localization of cyclin destruction machinery, in which case the delocalization of this machinery in cycle 14 might result in the complete cyclin destruction and near-uniform loss of PH3 seen during M14. Regardless of the mechanism, it is noteworthy that the mode of histone H3 dephosphorylation changes at the time of profound transitions that comprise the MZT. Hence, the manner in which exit from mitosis is executed may be developmentally regulated, just as the entry into mitosis is developmentally regulated (Edgar et al. 1994). We suggest that localized cyclin destruction is a specialization of early syncytial cycles that allows local control of the exit from mitosis despite the absence of cell membranes and that this specialization is lost in concert with cellularization.
Materials and methods
Fly stocks
The Cdk1ts(A171T) stock and stocks harboring heat shock-inducible stable cyclins Cdk1AF and cyclins A and B have been described before (Sigrist et al. 1995a; Sprenger et al. 1997).
Embryo-staining procedures
For antibody staining embryos were fixed for 20 min in PBS + 10% formaldehyde or for 5 min in 37% formaldehyde (injected embryos, see below), using standard procedures. DNA was stained with 10 μg/ml bizbenzamide (Hoechst 33258). FITC-labeled wheat germ agglutinin (WGA) was used at 500 ng/ml (Molecular Probes). PH3 was detected with a purified rabbit polyclonal antibody (1:1000 dilution) against the peptide ARKSTGGKAPRKQL (present in three Drosophila H3 variants) in which S was phosphorylated (Upstate Biotechnologies). β-Tubulin antibodies (Amersham) were used at 1:100 dilution. Primary antibodies were probed with rhodamine or FITC-conjugated secondary antibodies diluted 1:500 (Jackson).
Injection
Expression and purification of 13-110 (amino terminus of sea urchin cyclin B) and 13-110* (identical to 13-110 except for two-point mutations described in the text) peptides was performed as previously described (Holloway et al. 1993), except for a modification of the column chromatography. Supernatant after 85°C incubation was applied to a phosphocellulose column equilibrated in 20 mm PIPES (pH 6.5), 50 mm NaCl. The column was washed sequentially with three column volumes 100, 300, and 500 mm of NaCl in 20 mm PIPES (pH 6.5). The peptide eluted predominantly in the 300 mm NaCl step elution and was >90% pure by Coomassie blue staining on a SDS gel. Fractions from the 300 mm NaCl elution were pooled, concentrated using a Centriprep-3 (Amicon), dialyzed overnight at 4°C against 5 mm NaCl, 0.1 mm NaHPO4 (pH 7.7) injection buffer, and concentrated again to a final concentration of 50 mg/ml using a Microcon-3 (Amicon).
Sevelin embryos (0–1 hr) were prepared and microinjected, as described previously (Su et al. 1997), with 5 mg/ml peptide in 5 mm NaCl, 0.1 mm NaHPO4 (pH 7.7). Estimated injection volume was between 2% and 5% of the total volume of the embryo. Injected embryos were aged at 18°C for 30 min before fixing. The GST–cyclin B protein used in this study was a gift from Douglas Kellogg (University of California, Santa Cruz) and the GST was kindly provided by Danesh Moazed (University of California, San Fransisco). GST–cyclin B was injected at 6 mg/ml and GST at 12 mg/ml in 25 mm HEPES (pH 7.4), 125 mm KCl, 10% glycerol. Injected embryos were aged at 18°C for 30 min, fixed for 5 min in 37% formaldehyde, devitellinized manually as described previously (Su et al. 1997), and stained with anti-tubulin antibody (1:100, Amersham) and propidium iodide or Hoechst 33258 to visualize DNA.
Miscellaneous procedures
To arrest Cdk1ts embryos in mitosis, syncytial stage embryos were permeabilized according to standard procedures and incubated in Schneider’s medium containing 500 μg/ml colchicine (Sigma). To arrest with stable cyclins, 2- to 4-hr-old embryos were exposed to 37°C for 30 min, allowed to recover at room temperature for 2 hr (As and Bs) or 1 hr (B3s) to achieve maximal arrest per embryo, and fixed as above. To induce Cdk1AF + cyclin A or B, 10- to 12-hr-old embryos were heat-shocked as above and fixed after 2 hr rest at room temperature.
Incubation in Xenopus mitotic extracts were performed as described previously (King et al. 1996b).
Division cycle in syncytial embryos was determined from nuclei number (n = 2 for cycle 2; n = 4 for cycle 3, etc.) and nuclear location with respect to the embryo surface (in embryo interior through cycle 8, migrate during cycle 9, and reach the surface at the end of cycle 9; Foe et al. 1993).
Acknowledgments
We thank D. Kellogg for GST–cyclin B, D. Moazed for GST protein, the A.W. Murray laboratory for peptide expression plasmids, Alex Szidon for help with the peptide purification, and C. Detweiler and M. Maxon for critical reading of the manuscript. F.S. was supported by the Human Frontier Science Program (H.F.S.P.) and the Max-Planck Society, S.D.C. was supported by a Centennial fellowship from the Medical Research Council of Canada, and T.T.S. was supported by a Herbert Boyer postdoctoral fellowship. This work was supported by National Institutes of Health grant GM37193 to P.H.O.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL ofarrell@cgl.ucsf.edu; FAX (415) 502-5143/5145.
References
- Ajiro K, Yasuda H, Tsuji H. Vanadate triggers the transition from chromosome condensation to decondensation in a mitotic mutant (tsTM13) inactivation of p34cdc2/H1 kinase and dephosphorylation of mitosis-specific histone H3. Eur J Biochem. 1996a;241:923–930. doi: 10.1111/j.1432-1033.1996.00923.x. [DOI] [PubMed] [Google Scholar]
- Ajiro K, Yoda K, Utsumi K, Nishikawa Y. Alteration of cell cycle-dependent histone phosphorylations by okadaic acid. Induction of mitosis-specific H3 phosphorylation and chromatin condensation in mammalian interphase cells. J Biol Chem. 1996b;271:13197–13201. doi: 10.1074/jbc.271.22.13197. [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Bradbury EM. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992;14:9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- Campbell SD, Sprenger F, Edgar BA, O’Farrell PH. Drosophila Wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Cdc2 in vitro. Mol Biol Cell. 1995;6:1333–1347. doi: 10.1091/mbc.6.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin, Germany: Springer-Verlag; 1985. [Google Scholar]
- Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes & Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Connelly C, Heiter P. Budding yeast SKP1 encodes an evolutionarily conserved kinetochore protein required for cell cycle progression. Cell. 1996;86:275–285. doi: 10.1016/S0092-8674(00)80099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Sprenger F, Duronio RJ, Leopold P, O’Farrell PH. Distinct molecular mechanisms regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes & Dev. 1994;8:440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe VE, Odell GM, Edgar BA. Mitosis and morphogenesis in the Drosophila embryo. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 149–300. [Google Scholar]
- Follette PJ, O’Farrell PH. Cdks and the Drosophila cell cycle. Curr Opin Genet Dev. 1997;7:17–22. doi: 10.1016/s0959-437x(97)80104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Gallant P, Nigg EA. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J Cell Biol. 1992;117:213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Rodriguez LV, Rao PN. Relationship between histone phosphorylation and premature chromosome condensation. Exp Cell Res. 1983;148:293–302. doi: 10.1016/0014-4827(83)90153-2. [DOI] [PubMed] [Google Scholar]
- Holloway SL, Glotzer M, King RW, Murray AW. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Jorgensen PM, Brundell E, Starborg M, Hoog C. A subunit of the anaphase-promoting complex is a centromere-associated protein in mammalian cells. Mol Cell Biol. 1998;18:468–476. doi: 10.1128/mcb.18.1.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YL, Huang J, Peters JM, McLaughlin ME, Tai CY, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996a;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- King RW, Glotzer M, Kirschner MW. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996b;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Lehner CF. Synergistic action of Drosophila cyclins A and B during the G2–M transition. EMBO J. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, O’Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Codina G, Glover DM. Cyclins A and B associate with chromatin and the polar regions of spindles, respectively, and do not undergo complete degradation at anaphase in syncytial Drosophila embryos. J Cell Biol. 1992;116:967–976. doi: 10.1083/jcb.116.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. At the heart of the budding yeast cell cycle. Trends Genet. 1996;12:405–412. doi: 10.1016/0168-9525(96)10041-x. [DOI] [PubMed] [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Ward SC, Gorbsky GJ. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol. 1995;130:929–939. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu RT, Osmani SA. Mitotic destruction of the cell cycle regulated NIMA protein kinase of Aspergillus nidulans is required for mitotic exit. EMBO J. 1995;14:995–1003. doi: 10.1002/j.1460-2075.1995.tb07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Shibata K, Inagaki M, Ajiro K. Mitosis-specific histone H3 phosphorylation in vitro in nucleosome structures. Eur J Biochem. 1990;192:87–93. doi: 10.1111/j.1432-1033.1990.tb19199.x. [DOI] [PubMed] [Google Scholar]
- Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995a;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S, Ried G, Lehner CF. Dmcdc2 kinase is required for both meiotic divisions during Drosophila spermatogenesis and is activated by the Twine/cdc25 phosphatase. Mech Dev. 1995b;53:247–260. doi: 10.1016/0925-4773(95)00441-3. [DOI] [PubMed] [Google Scholar]
- Sprenger F, Yakubovich N, O’Farrell PH. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr Biol. 1997;7:488–499. doi: 10.1016/s0960-9822(06)00220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TT, Yakubovich N, O’Farrell PH. Cloning of Drosophila MCM homologs and analysis of their requirement during embryogenesis. Gene. 1997;192:283–289. doi: 10.1016/s0378-1119(97)00107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, T.T., S.D. Campbell, and P.H. O’Farrell. 1998. Cell cycle program in drosophila germ cells. Dev. Biol. (in press). [DOI] [PubMed]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W, Daily DR, Fogarty P, Yook KJ, Pimpinelli S. Delays in anaphase initiation occur in individual nuclei of the syncytial Drosophila embryo. Mol Biol Cell. 1993;4:885–896. doi: 10.1091/mbc.4.9.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Th’ng JP, Guo XW, Swank RA, Crissman HA, Bradbury EM. Inhibition of histone phosphorylation by staurosporine leads to chromosome decondensation. J Biol Chem. 1994;269:9568–9573. [PubMed] [Google Scholar]
- Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- van der Velden HM, Lohka MJ. Mitotic arrest caused by the amino terminus of Xenopus cyclin B2. Mol Cell Biol. 1993;13:1480–1488. doi: 10.1128/mcb.13.3.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]