Abstract
Aims:
To report the use of transesophageal Doppler (TED), a minimally invasive cardiac output (COP) monitor, before, during and after reperfusion and study its effect on anesthetic management during living donor liver transplantation (LDLT).
Setting and Design:
A prospective observational study.
Methods:
A total of 25 consecutive recipients with a MELD score between 15 and 20 were enrolled. Data were recorded at baseline (TB); anhepatic phase (TA); and post-reperfusion — 1, 5, 10 and 30 minutes. Fluid therapy was guided by corrected flow time (FTc) of the TED. Packed red blood cells (RBCs) were only given when hematocrit was less than 25%. Rotational thromboelastometry (ROTEM) and standard laboratory tests were used to guide component blood products requirements.
Results:
Post-reperfusion, the COP, Cardiac Index (CI) and stroke volume (SV) increased significantly at all points of measurements; this was associated with a significant decrease in systemic vascular resistance (SVR) (P ;< .05). Immediately post-reperfusion, for 5 minutes, mean arterial blood pressure (ABP) dropped significantly (P < .05), and 14 out of the 25 patients required boluses of epinephrine (10 μg) to restore the mean ABP; 3 of the 14 patients required norepinephrine infusion till the end of surgery. Central venous pressure (CVP) and urine output (UOP) at all measures were maintained adequately with FTc-guided fluid replacement. Eight out of the 25 patients required no blood transfusion, and 4 of the 8 patients required no catecholamine support.
Conclusion:
TED as a sole monitor for COP was able to present significant and reliable changes in the cardiovascular status of the recipients during reperfusion, which could help to guide fluid- and drug-supportive therapy in this population of patients. This preliminary study needs to be applied on a larger scale.
Keywords: Cardiac output, liver transplantation, transesophageal Doppler reperfusion
INTRODUCTION
Living donor liver transplantation (LDLT) was first done at the Liver Institute, Menoufiya University, Egypt, in 1991, with a total of 104 cases managed till November 2010. LDLT helps in organ availability, especially in the Middle East, where the concept of brain death is still not widely implemented. Preparations for LDLT program were started at the National Liver Institute (NLI), Menoufiya University, Egypt, in 1991 by a joint team headed by Prof. Nagi Habib from Hammer Smith Hospital, London, UK; they managed 3 pediatric cases.[1] These cases were the first in Egypt and Africa. Later in April 2003 and after most of the transplant team staff from different specialties returned from their overseas training scholarship programs, which involved doing transplant research as well as training, the LDLT program was again initiated. In April 2003, LDLT program was developed in two phases. In the first phase, the first 23 cases were managed (including 17 pediatric cases) with the cooperation of Prof. Tanaka and his team from Kyoto University. Japan; but in the second phase, after July 2007 till July 2010, the rest of the cases were managed entirely and independently by a Egyptian national team, with several visits from time to time by Prof. Tanaka and his team.
To manage the changes in hemodynamic variables during the different phases of liver transplantation operation that was sometimes complicated by bleeding in a likely critically ill patient has been a challenge.[2] So the primary goal of hemodynamic monitoring and therapy is the prevention of inadequate tissue perfusion and inadequate oxygenation. There are several invasive methods to measure cardiac output (COP) in such critically ill surgical patients.[3] Pulmonary artery catheters (PACs) have been used for many years to monitor COP changes.[4] Recently, several significant studies in the field of cardiac output (COP) measurement have presented different techniques of measurement to overcome the limitations of PACs due to their invasive nature and of the direct Fick method used for measuring the stroke volume. These are divided into invasive, minimally invasive and noninvasive techniques. Transesophageal Doppler (TED) is one of the minimally invasive monitors that allow continuous monitoring of the COP. This enables optimization of intravascular volume and tissue perfusion in major abdominal surgery and has been found to improve short-term outcome in patients following major surgery. The use of a minimally invasive approach (like the TED) to measure COP could minimize the risks with the associated coagulopathy in cirrhotic patients undergoing living donor liver transplantation.[5–8]
TED has been reported by several studies on cadaveric liver transplantation to be an applicable method and might be able to substitute the use of pulmonary artery catheters as it has a good correlation with the thermodilution technique; and also when fluid therapy was guided by corrected flow time (FTc) of the TED, better stroke volume and hemodynamic status were achieved.[9–10]
The aim of this prospective study was to report the use of TED intraoperatively as a sole monitor for COP and as a guide for fluid administration among recipients during living donor liver transplantation; and also to report the effect of TED on anesthetic management, as well as the lessons learned.
METHODS
After approval by the ethics committee of the Liver Institute, Menoufiya University, and after obtaining written consent, 25 consecutive adult patients scheduled for LDLT were enrolled in this prospective study between March 2010 and November 2010. Exclusion criteria included pediatric patients and recipients with known cardiac diseases or dysrrhythmia.
Esophageal Doppler ultrasonography was first introduced in 1971. Its principle is based on the following equation:
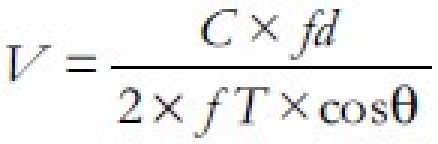
where V is the flow velocity; C is speed of sound (in body tissue, 1540 m/s); fd is frequency shift (in Hz); cosθ is the cosine angle between sound beam axis and velocity vector; and fT is frequency of transmitted ultrasound (in Hz). It was subsequently refined to provide a minimally invasive means of continuously monitoring cardiac function; with this technique, a pulsed, competitive-frequency continuous-wave Doppler signal is emitted from a probe placed in the distal esophagus and directed to the descending thoracic aorta.[11]
After standard monitoring was in place, anesthesia was induced using propofol 2 mg/kg and rocuronium 1.2 mg/ kg to facilitate endotracheal intubation. Anesthesia was maintained with sevoflorane in O2:air mixture (FiO2 = 0.4), fentanyl, and rocuronium, keeping spectral entropy (GE Healthcare, Helsinki, Finland) between 40% and 60%. Normothermia was achieved with a forced-air warming device. An arterial line was placed in the left radial artery, and central lines were inserted in the right internal jugular vein with triple-lumen catheter and large-bore single-lumen catheter 7.5 F. The esophageal Doppler probe (Cardio QTM; Deltex Medical, Chichester, UK) was inserted orally. The ideal probe tip location is at the level between the fifth and sixth thoracic vertebrae because at that level, the aorta is adjacent and parallel to the esophagus. This location is achieved by superficially “landmarking” the distance to the third sternocostal junction anteriorly and is approximately 30 to 40 cm in an adult of average build. After insertion, the TED probe is rotated on its axis to achieve an optimal signal prior to taking measurements.
Esophageal Doppler monitor parameters include measuring stroke volume (SV) (50-100 cc/beat); cardiac output (COP) (4-8 L/min); FTc flow time corrected, which represents the systolic flow time corrected for heart rate (330-360 ms); systemic vascular resistance (SVR) (900-1300 dynes.sec/cm5).
Our fluid regimen consisted of Ringer acetate solutions at 6 mL/kg/h. Albumin 5% only was given to treat hypoalbuminemia.
Hypovolemia was treated with bolus of colloid 5 mL/kg (HAES 130/0.4 — Voluven, Fresenius, Kabi) as needed (maximum dose, 30 mL/kg), in patients with corrected flow time (FTc) of less than 350 ms (which suggests hypovolemia). Thereafter, the following protocol was applied[12]:
If stroke volume (SV) remained the same or increased and FTc was <350 ms, then the fluid challenge was repeated.
If SV increased by 10% and FTc was >350 ms, then the fluid challenge was repeated till there was no increase in SV.
If FTc was >400ms, then no further fluids were required till FTc or SV decreased by 10%.
Arterial blood samples were collected hourly or when needed for laboratory analysis. Ionized hypocalcemia was treated appropriately. The threshold for blood transfusion aims to keep the hematocrit between 25% and 32%. Hemodynamic data were collected in six phases: TB, baseline (when the patient was hemodynamically stable after the start of surgery); TA, anhepatic phase (30 minutes after partial clamping of the vena cava); T1, T5, T10 and T30 were 1, 5, 10 and 30 minutes after reperfusion, respectively. If the patient developed post-reperfusion hypotension, a single bolus of epinephrine (10 μg) was given to restore hemodynamic stability.[13] A norepinephrine infusion was initiated in cases of persistent hypotension with decreased systemic vascular resistance. The surgical team was the same in all surgeries and they did not use venovenous bypass but performed the venous anastomosis with lateral clamping of the recipient vena cava (piggyback technique).[14] All patients were reperfused by portal vein first. All patients were admitted to the intensive care unit (ICU) at the end of the procedure.
Statistical analysis
Data were statistically analyzed using SPSS (statistical package for social sciences — Chicago, IL) program, version 16, for Windows. A P value < .05 was considered statistically significant. All data were tested with Kolmogorov-Smirnov Z test, and most of them were found normally distributed and so presented in terms of mean ± standard deviation (SD). Parametric tests were used for association or correlation. Repeated-measures ANOVA test was performed for the differentiated changes for follow-up results of normally distributed variables.
RESULTS
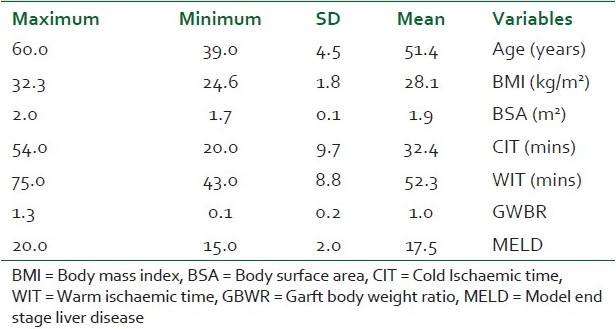
Patient characteristics included in the study are outlined in Table 1. Twenty-five (n = 25) recipients with end-stage liver disease scheduled for living donor liver transplantation fulfilled the inclusion criteria. Fifteen patients suffered from end-stage liver disease due to hepatitis C; 7, due to hepatitis C with hepatocellular carcinoma; and the remaining 3, due to hepatocellular carcinoma with negative viral markers.
Table 1.
Patients' demographic and operative data
In this study, 1 patient was excluded due to development of progressive metabolic acidosis and acute rise in the anion gap. These changes required NaHCO3 repeatedly and readjustment of the ventilation settings, aiming to keep a reasonable level of pH. A hepatic Doppler study revealed graft venous outflow obstruction with graft congestion, and this was corrected surgically.
In the present work, the mean blood pressure decreased significantly post–graft reperfusion. Fifteen (n =15; 60%) patients developed post-reperfusion syndrome but rapidly recovered their hemodynamic stability after a single bolus of epinephrine (10 μg). Three patients did not respond to repeated epinephrine boluses, and they required continuous infusion of norepinephrine, initiated between 30 and 45 minutes after reperfusion, till the end of the procedure. However, COP slightly decreased during the anhepatic stage (TA) when compared to the baseline (TB); but after reperfusion, it significantly increased to a value more than the TB value till the end of measurement at T30 [Figure 1]. This was accompanied with a significant increase at all points of measurements of COP, Cardiac Index (CI) and stroke volume (SV); a further decrease in SVR was also reported in comparison with preoperative values (P < .05) [Table 2, Figures 2 and 3].
Figure 1.
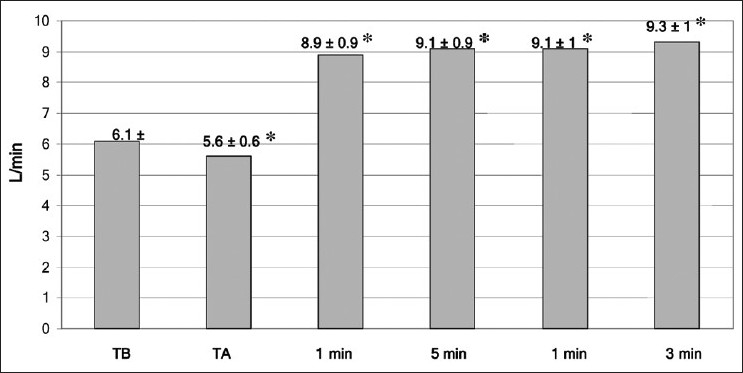
Mean (SD) of cardiac output at all times of measurement. TB = baseline time,TA = anhepatic stage — 1, 5, 10 min post-reperfusion. * = P < .05
Table 2.
Hemodynamic data at specific stages of liver transplantation
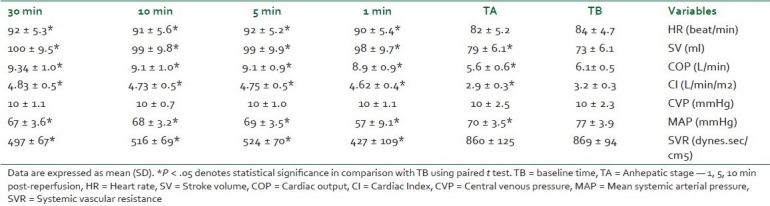
Figure 2.
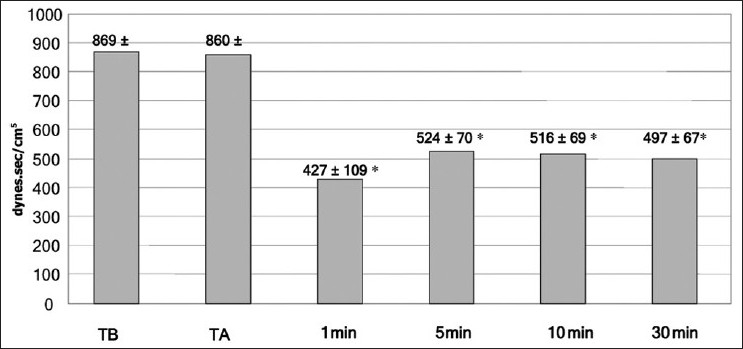
Mean (SD) of systemic vascular resistance at all times of measurement. TB = baseline time, TA = anhepatic stage — 1, 5, 10 min post-reperfusion. * = P < .05
Figure 3.
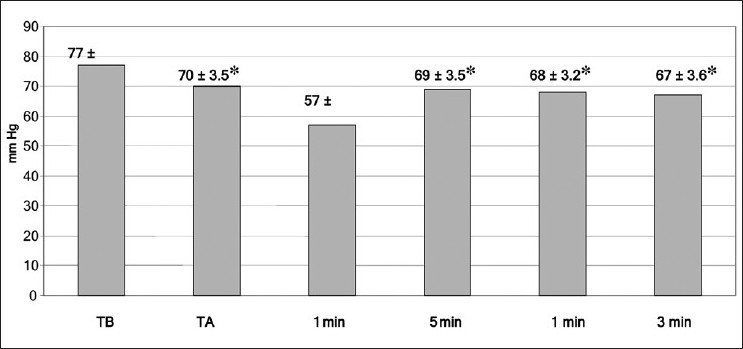
Mean (SD) of arterial blood pressure at all times of measurements. TB = baseline time, TA = anhepatic stage — 1, 5, 10 min post-reperfusion.* = P < .05
Eight patients required no blood transfusion, and 4 of these 8 patients required no catecholamine support. The median and interquartiles amounts of packed red blood cells (RBCs) and fresh frozen plasma (FFP) used were 4 [1-8] and 6 [4-9], respectively.
CVP and urine output (UOP) at all measures were maintained adequately with FTc-guided fluid replacement. CVP was found to be within normal range with no dramatic increase during the dissection phase of the diseased liver, that could lead to severe blood loss and hence affect the hemodynamic status in the anhepatic phase. An abnormally high CVP also could lead to graft congestion on reperfusion due to hepatic venous blood outflow resistance with a subsequent early graft congestion leading to early post-reperfusion graft dysfunction and persistent academia.
Twenty-three patients survived the procedure, 1 patient died on the 10th postoperative day from portal vein thrombosis and 1 patient died 3 months later from hepatic vein thrombosis and outflow obstruction.
DISCUSSION
In the present study, the mean ABP decreased in all recipients immediately after graft reperfusion due to the effects of post-reperfusion syndrome (PRS). PRS is a transient and occasionally profound cardiovascular collapse that occurs after the release of the portal clamp and was initially defined as a 30% decrease in mean arterial pressure (MAP) within the first 5 minutes after reperfusion.[15] The incidence of PRS in our study was 56%. This decrease in the MAP was found to be associated with a decrease in the SVR, not due to a decrease in myocardial contractility.[16,17] This responded to repeated boluses of epinephrine (10 μg) in 14 patients. Three patients required continuous intravenous norepinephrine till the end of the surgery, to support circulation.
De La Morena et al.[18] in a transesophageal echo (TEE) study of ventricular function during liver reperfusion, found no alteration in left ventricular function to justify PRS. Aggarwal et al.[19] studied more than 69 recipients and stated that acute hyperkalemia, hypothermia and acidosis did not appear to be major causes of reperfusion hypotension in their study. In a similar work, Jugan et al.[20] suggested that there was failure of venovenous bypass to prevent graft liver post-reperfusion syndrome. In another study done at the Australian Liver Transplantation Center, Nanashima and his colleagues[21] found that 29% of the patients exhibited PRS during orthotopic liver transplantation (OLT) (PRS group), and there was a higher incidence among older donors (age >50 years) in the PRS group (48% vs. 23%).
Daniela et al.[22] evaluated the influence of retrograde reperfusion in liver transplantation (LT) on the incidence of post-reperfusion syndrome. Liver transplantation in 53 patients was performed with piggyback technique with retrograde reperfusion via the caval vein and antegrade reperfusion via the portal vein. They suggested that PRS could be diminished with retrograde reperfusion.
In the present study, the fluid intake was manipulated cautiously according to FTc. CVP was found to be nearly stable during all the times of measurements, and this had a great benefit in maintaining a smooth hepatic venous blood outflow with subsequent prevention of early graft congestion, which may lead to early post-reperfusion graft dysfunction and persistent academia. There have been various other studies that have demonstrated a reduced postoperative morbidity and shorter length of hospital stay in patients managed with TED when compared with patients managed with conventional clinical techniques, suggesting that TEDmay be a valuable supplement.[23,24]
TED monitoring was not without limitations. One of the recognized limitations during the current study was the effect of diathermy on the performance of TED. Diathermy activity during surgery interferes with the readings and the graphs of stroke volume. Also the repeated need to rotate the probe in the distal esophagus and direct it at the descending thoracic aorta whenever a surgical maneuver affects its position is another limitation.
On placing the probe for the first time, caution is necessary and good lubrication is mandatory in order to avoid any trauma to the lower esophagus, which could host several esophageal varices. One patient in the current study had some sort of limited bleeding in the mouth, probably pharyngeal, during placement and due to associated coagulopathy, which was self-limiting and responded to a mouth pack.
A TED is a minimally invasive method of monitoring perioperative optimization of stroke volume, with a very low risk to the patient in comparison with other less invasive COP monitors available in the market, such as pulse indicator continuous cardiac output (PiCCO) monitor. TED requires lesser skills to insert than PiCCO. The operator can learn the use of TED in a short time due to the simplicity of the method of its insertion.
TED also can provide information about COP and SV with less risk of complications as no central venous access is required for its insertion, as needed when using the pulmonary artery catheter, and this makes it useful for monitoring donors for living-related liver resection during the procedure of liver resection, where inserting a pulmonary artery catheter is not at all required. Perioperative stroke volume optimization with TED has potential for cost saving, decreased complications, decreased nursing and physician attendance time, and improved patient outcomes, as reported by several studies.[25,26] Madan et al. in their comparative study concluded that FTc parameter in TED is a better indicator of preload than pulmonary catheter wedge pressure (PCWP).[27]
In conclusion, a possible role is emerging for TED during liver transplantation. The rapid learning curve and the ease of use encourage wider application of the device. TED findings helped to explain the transient hypotension that follows graft reperfusion as being due to the significant decrease in peripheral vascular resistance and not due to cardiac contractility depression. Also, TED was found to help maintain fluid during management, which kept an adequate CVP throughout the procedure. Further studies on a larger scale are still needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Abdeldayem HM, Allam NA, Salah E, Mostafa Aziz A, Kashkoush S, Adawy NM, et al. Moral and ethical issues in living-donor liver transplant in Egypt. Exp Clin Transplant. 2009;7:18–24. [PubMed] [Google Scholar]
- 2.Nissen P, Frederiksen HJ, Secher NH. Intraoperative hemodynamic monitoring during liver transplantation: Goals and devices. Minerva Gastroenterol Dietol. 2010;56:261–77. [PubMed] [Google Scholar]
- 3.Cholley B, Payen D. Noninvasive techniques for measurements of cardiac output. Curr Opin Crit Care. 2005;11:424–9. doi: 10.1097/01.ccx.0000176698.51456.5a. [DOI] [PubMed] [Google Scholar]
- 4.Siniscalchi A, Dante A, Spedicato S, Riganello L, Zanoni A, Cimatti M, et al. Hyperdynamic circulation in acute liver failure: Reperfusion syndrome and outcome following liver transplantation. Transplant Proc. 2010;42:1197–9. doi: 10.1016/j.transproceed.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 5.Dark PM, Singer M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med. 2004;30:2060–6. doi: 10.1007/s00134-004-2430-2. [DOI] [PubMed] [Google Scholar]
- 6.Bernardin G, Tiger F, Fouche R, Mattei M. Continuous noninvasive measurement of aortic blood flow in critically ill patients with a new esophageal echo-Doppler system. J Crit Care. 1998;13:177–83. doi: 10.1016/s0883-9441(98)90003-x. [DOI] [PubMed] [Google Scholar]
- 7.Valtier B, Cholley BP, Belot JP, de la Coussaye JE, Mateo J, Payen DM. Noninvasive monitoring of cardiac output in critically ill patients using transesophageal Doppler. Am J Respir Crit Care Med. 1998;158:77–83. doi: 10.1164/ajrccm.158.1.9707031. [DOI] [PubMed] [Google Scholar]
- 8.Sharma J, Bhise M, Singh A, Mehta Y, Trehan N. Hemodynamic Measurements After Cardiac Surgery: Transesophageal Doppler Versus Pulmonary Artery Catheter. J Cardiothorac Vasc Anesth. 2005;19:746–50. doi: 10.1053/j.jvca.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Parra V, Fita G, Rovira I, Matute P, Gomar C, Paré C. Transoesophageal echocardiography accurately detects cardiac output variation: A prospective comparison with thermodilution in cardiac surgery. Eur J Anaesthesiol. 2008;25:135–43. doi: 10.1017/S0265021507001354. [DOI] [PubMed] [Google Scholar]
- 10.Boucaud C, Bouffard Y, Dumortier J, Gaillac N, Sagnard P, Graber MC, et al. Transoesophageal echo-Doppler vs.thermodilution cardiac output measurement during hepatic vascular exclusion in liver transplantation. Eur J Anaesthesiol. 2008;25:485–9. doi: 10.1017/S0265021508003670. [DOI] [PubMed] [Google Scholar]
- 11.Singer M. Oesophageal Doppler. Curr Opin Crit Care. 2009;15:244–8. doi: 10.1097/MCC.0b013e32832b7083. [DOI] [PubMed] [Google Scholar]
- 12.Tote SP, Grounds RM. Performing perioperative optimization of the high risk patient. Br J Anaesth. 2006;97:4–11. doi: 10.1093/bja/ael102. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Postreperfusion syndrome: Cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19:54–5. [PubMed] [Google Scholar]
- 14.Meunier B, Bardaxoglou E, Chareton B, Landen S, Camus C, Roumeas J, et al. ‘Piggyback’ method in hepatic transplantation. Chirurgie. 1993;119:682–5. [PubMed] [Google Scholar]
- 15.Chui AK, Shi L, Tanaka K, Rao AR, Wang LS, Bookallil M, et al. Postreperfusion syndrome in orthotopic liver transplantation. Transplant Proc. 2000;32:2116–7. doi: 10.1016/s0041-1345(00)01595-5. [DOI] [PubMed] [Google Scholar]
- 16.Goode HF, Webster NR, Howdle PD, Leek JP, Lodge JP, Sadek SA, et al. Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology. 1994;19:354–9. [PubMed] [Google Scholar]
- 17.Emery RW, Estrin JA, Wahler GM, Booth AM, Swayze CR, Fox IJ. Reflex hypotension due to regional activation of left ventricular mechanoreceptors to explain hypotension noted in clinical myocardial ischemia or reperfusion. Cardiovasc Res. 1986;20:161–70. doi: 10.1093/cvr/20.3.161. [DOI] [PubMed] [Google Scholar]
- 18.De La Morena G, Acosta F, Villegas M, Bento M, Sansano T, Bueno FS, et al. Ventricular function during liver reperfusion in hepatic transplantation. Transplantation. 1994;58:306–10. [PubMed] [Google Scholar]
- 19.Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19:54–5. [PubMed] [Google Scholar]
- 20.Jugan E, Albaladejo P, Jayais P, Ecoffey C. The failure of venovenous bypass to prevent graft liver postreperfusion syndrome. Transplantation. 1992;54:81–4. doi: 10.1097/00007890-199207000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Nanashima A, Pillay P, Crawford M, Nakasuji M, Verran DJ, Painter D. Analysis of postrevascularization syndrome after orthotopic liver transplantation: The experience of an Australian liver transplantation center. J Hepatobiliary Pancreat Surg. 2001;8:557–63. doi: 10.1007/s005340100025. [DOI] [PubMed] [Google Scholar]
- 22.Daniela K, Michael Z, Florian I, Silvia S, Estrella J, Doris D, et al. Influence of retrograde flushing via the caval vein on the post-reperfusion syndrome in liver transplantation. Clin Transplant. 2004;18:638–41. doi: 10.1111/j.1399-0012.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 23.Perilli V, Avolio AW, Sacco T, Modesti C, Gaspari R, Caserta R, et al. Use of an Esophageal Echo-Doppler Device During Liver Transplantation: Preliminary Report. Transplant Proc. 2009;41:198–200. doi: 10.1016/j.transproceed.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Schober P, Loer S, Schwarte LA. Perioperative Hemodynamic Monitoring with Transesophageal Doppler Technology. Anesth Analg. 2009;109:340–53. doi: 10.1213/ane.0b013e3181aa0af3. [DOI] [PubMed] [Google Scholar]
- 25.Gan TJ, Horacek A, Maroof M. Intraoperative volume expansion guided by esophageal Doppler improved postoperative outcome and shorten hospital stay. Anesth Analg. 1999;88:S1-424–S179. [Google Scholar]
- 26.Gan TJ. The Esophageal Doppler as an alternative to the pulmonary artery catheter. Curr Opin Crit Care. 2000;6:214–21. [Google Scholar]
- 27.Madan AK, UyBarreta VV, Aliabadi-Wahle S, Jesperson R, Hartz RS, Flint LM, et al. Esophageal Doppler ultrasound monitor versus pulmonary artery catheter in the hemodynamic management of critically ill surgical patients. J Trauma. 1999;46:607–11. doi: 10.1097/00005373-199904000-00008. [DOI] [PubMed] [Google Scholar]


