Abstract
Arthropods and vertebrates are constructed of many serially homologous structures whose individual patterns are regulated by Hox genes. The Hox-regulated target genes and developmental pathways that determine the morphological differences between any homologous structures are not known. The differentiation of the Drosophila haltere from the wing through the action of the Ultrabithorax (Ubx) gene is a classic example of Hox regulation of serial homology, although no Ubx-regulated genes in the haltere have been identified previously. Here, we show that Ubx represses the expression of the Wingless (Wg) signaling protein and a subset of Wg- and Decapentaplegic-activated genes such as spalt-related, vestigial, Serum Response Factor, and achaete-scute, whose products regulate morphological features that differ between the wing and haltere. In addition, we found that some genes in the same developmental pathway are independently regulated by Ubx. Our results suggest that Ubx, and Hox genes in general, independently and selectively regulate genes that act at many levels of regulatory hierarchies to shape the differential development of serially homologous structures.
Keywords: Ultrabithorax, haltere, development, Drosophila, serial homology
Arthropods and chordates possess many serially iterated homologous structures (segments, vertebrae, limbs, etc.) that differ in number, morphology, and function between taxa. In both phyla, different Hox genes regulate the development of initially similar developmental fields into distinct structures, presumably by controlling different sets of target genes (Krumlauf 1994; Carroll 1995). Differences in gene expression between certain serial homologs such as the Drosophila leg and antenna (Wagner-Bernholz et al. 1991) and vertebrate fore- and hindlimb (Peterson et al. 1994; Gibson-Brown 1996) have been described. However, the identity of the Hox-regulated target genes and developmental pathways that determine the differences in morphology between any homologous structures are not known. It is therefore not known whether Hox genes act upon a few genes at the top of, or upon many genes throughout the gene hierarchies that govern the formation and patterning of homologous structures.
Here, we examine the Hox-regulated gene hierarchy governing the differential development of the serially homologous dipteran (two-winged insects) wing and haltere (Fig. 1A,B). Dipterans evolved from a four-winged ancestor, with the resulting posterior flight appendages, the halteres, being morphologically distinct and reduced in size compared to wings. In Drosophila, the Hox gene Ultrabithorax (Ubx) controls the differential development between wing and haltere. Ubx is expressed throughout haltere development but not in the developing wing (Struhl 1982; Beachy et al. 1985; White and Wilcox 1985a) (Fig. 1C,D). Reduced Ubx function in imaginal discs or in Ubx mutant clones results in transformation of haltere tissue into wing tissue (Lewis 1963; Morata and Garcia-Bellido 1976; Morata and Kerridge 1981; Kerridge and Morata 1982) (Fig. 1E). Total loss of Ubx function in the developing halteres results in the complete transformation of halteres to wings, giving rise to a four-winged fly (Lewis 1978) (Fig. 1F). Conversely, mutations that cause ectopic expression of Ubx in the developing wing disc [e.g., Contrabithorax (Cbx)] (Cabrera et al. 1985; White and Akam 1985; White and Wilcox 1985b; Castelli-Gair et al. 1990) transform wing into haltere tissue (Lewis 1955, 1978, 1982; Morata and Lawrence 1975; Casanova et al. 1985; Micol and García-Bellido 1988; González-Gaitán et al. 1990). Although these spectacular Ubx mutant phenotypes have been known for decades, no Ubx-regulated genes in the haltere have been identified.
Figure 1.
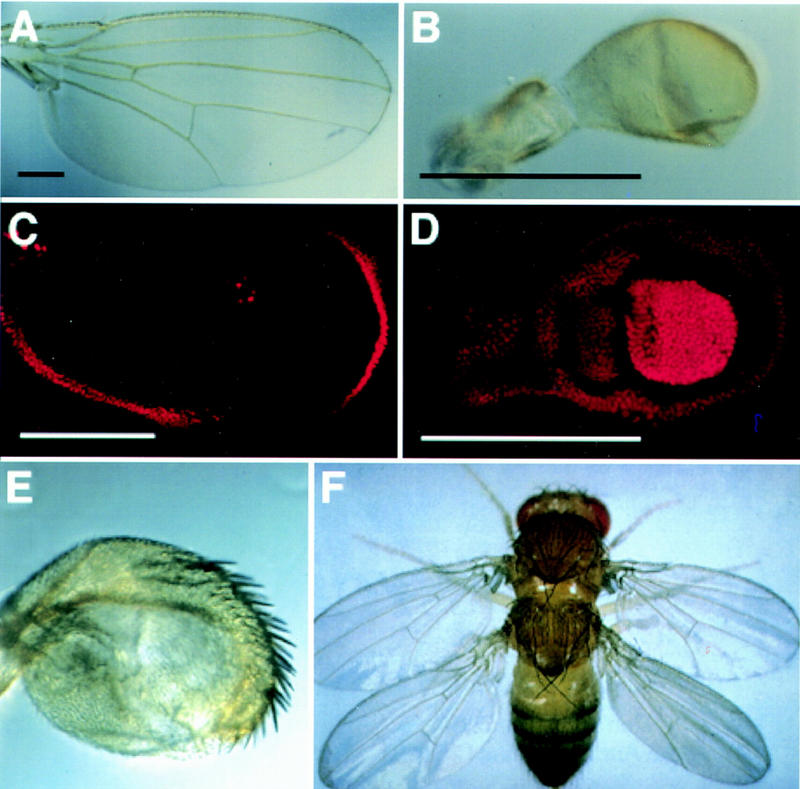
Ubx controls the differential development of the haltere. The wild-type wing (A) and haltere (B) differ in size, shape, and the presence of veins and margin bristles. (C,D) antibody staining of third instar wing and haltere discs. (C) Ubx expression (red) in the wing disc is limited to the peripodial membrane and is not necessary for proper wing development (Struhl 1982). (D) Ubx expression fills the haltere disc, with strongest expression in the “pouch”, which will give rise to capitellar tissue (Beachy et al. 1985). Reduction of Ubx activity in the halteres leads to transformations toward wing identity. (E) Haltere from a Ubx6.28/bx34E fly (Kerridge and Morata 1982), in which Ubx gene activity is <50% of wild-type (shown at the same magnification as B). A large number of ectopic margin bristles appear on the haltere, which is increased in size. (F) Total loss of Ubx activity in the developing haltere results in a complete transformation toward wing identity (Lewis 1978). Black scale bars, 0.25 mm; white scale bars, 0.2 mm.
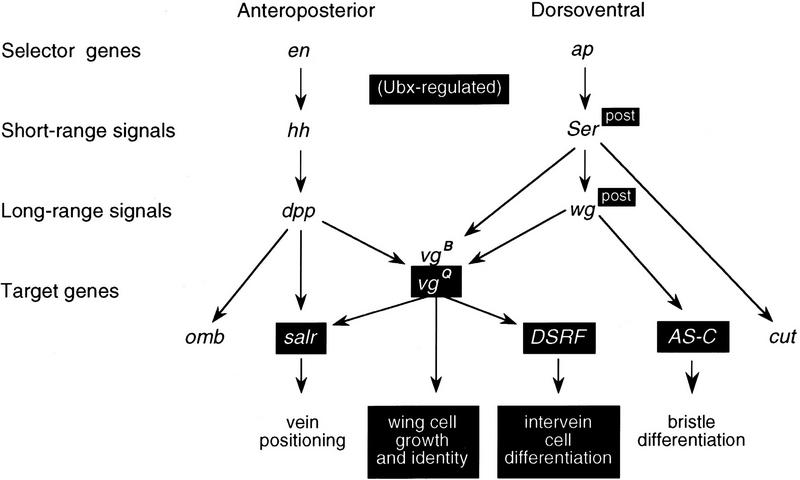
Recent progress in understanding the genetic mechanisms that govern the formation and patterning of the insect wing has created the opportunity to identify genes that are regulated differently between wings and halteres. In the Drosophila wing disc, growth and patterning are organized by the Decapentaplegic (Dpp) and Wingless (Wg) long-range signaling proteins (for review, see Serrano and O’Farrell 1997), which are produced by cells along the anteroposterior (AP) and dorsoventral (DV) compartment boundaries, respectively, and organize growth and patterning via the regulation of numerous downstream wing-patterning target genes. The expression of Dpp and Wg is regulated by the short-range signaling proteins Hedgehog (Hh) and Serrate (Ser), which are in turn regulated by the posterior engrailed (en) and dorsal apterous (ap) selector genes (for review, see Burke and Basler 1997; Irvine and Vogt 1997; Neumann and Cohen 1997a).
We have investigated how Ubx modifies a wing field into a haltere field by focusing on these global signaling systems and their target genes. We discovered that Ubx regulates the expression of the Wg signaling protein, selected Dpp- and Wg-activated target genes or cis-regulatory elements, and genes that are further downstream of Ubx-regulated genes. We also examined whether the ectopic expression of these genes was sufficient to induce wing-like characters on the haltere. Our findings reveal that Ubx represses haltere development by independently regulating selected genes that act at different levels of the wing patterning hierarchy.
Results
The anteroposterior axis: Ubx represses selected Dpp target genes
The expression pattern of en is essentially the same in the haltere disc as in the wing disc (Fig. 2A,B), indicating that Ubx is not regulating haltere identity by altering the expression of this compartmental selector gene. Similarly, the expression of dpp in the developing haltere on the anterior side of the AP compartment boundary resembles that in the wing disc (Fig. 2A,B). Because these discs give rise to very different appendages, there may be genes downstream of the Dpp signal that are regulated by Ubx. To identify these, we examined how a number of genes involved in the development of specific wing characters are expressed and regulated in the developing haltere.
Figure 2.
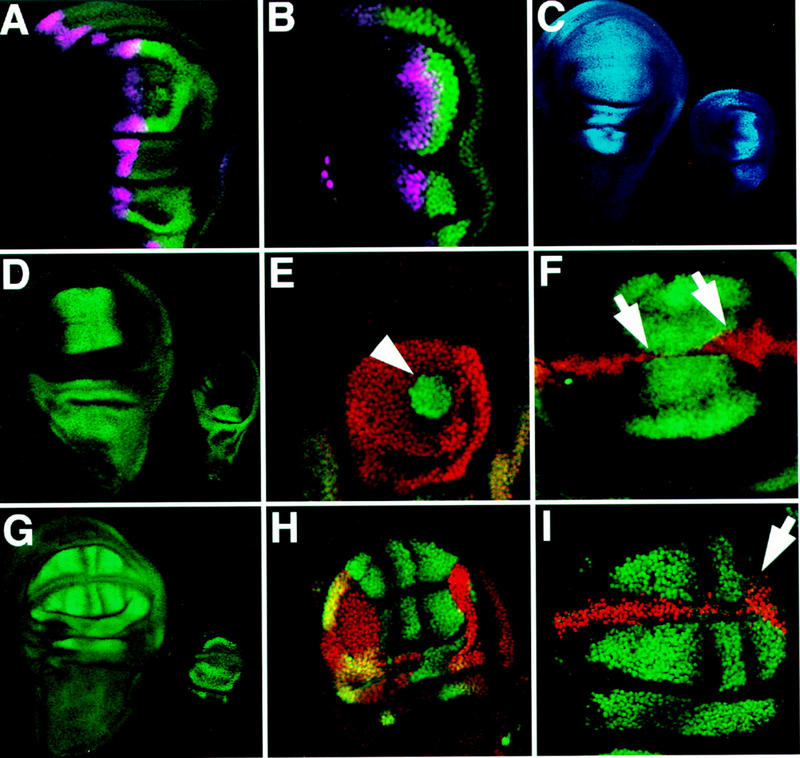
Ubx represses genes downstream of AP patterning signals in the haltere. (A,B) En (green) and dpp (purple, visualized by a lacZ reporter transgene) expression patterns are similar in the wing (A) and haltere (B). (C) omb expression (blue, visualized by a lacZ reporter transgene) is found in the haltere disc (right) in a pattern similar to that found in the wing (left), indicating that Dpp signaling is not repressed by Ubx. (D–F) Antibody staining detecting Salr is shown in green; Ubx is shown in red. (D) Salr expression in a wing disc (left) and in a haltere disc (right). Salr is not expressed in the haltere pouch, indicating that this Dpp target gene is repressed by Ubx. (E) Ubx− clone close to the AP boundary shows cell-autonomous derepression of Salr expression (arrowhead). Ubx− clones more than eight cells anterior to the AP boundary and posterior clones do not show Salr derepression (not shown). (F) Ectopic Ubx expression in a CbxM1 heterozygous wing pouch represses Salr expression in a cell autonomous fashion (arrows). (G–I) antibody staining detecting DSRF is shown in green; Ubx is shown in red. (G, left), DSRF is expressed in the future intervein cells of the Drosophila wing imaginal disc. (right) Expression of DSRF in the haltere is limited to extreme ventral and dorsal crescents in the pouch, and is also present in pedicellar and notal portions of the disc. (H) Ubx− clone in the haltere (lack of red staining) showing DSRF derepression in the haltere pouch in a cell-autonomous manner. A winglike pattern forms in the clone, whereas the haltere expression pattern is still visible where Ubx is expressed (yellow overlap). (I) Ectopic Ubx expression in a CbxM1/+ wing disc represses a portion (ventral intervein D) of the normal DSRF expression (arrw). Note that omb expression does not extend into the posterior of the haltere nearly as far as it does in the anterior (Fig. 2C), and Salr expression is not derepressed in posterior Ubx− clones close to the AP boundary (not shown) which suggests that Dpp signaling may somehow be reduced in the posterior haltere disc.
Dpp acts as a morphogen from its source to organize wing growth, AP pattern, and to activate target gene expression over a distance. The optomotor blind (omb), spalt (sal), and spalt related (salr) genes are expressed in nested patterns centered on the Dpp stripe and are necessary for proper development of the central wing region including veins II–IV (de Celis et al. 1996; Grimm and Pflugfelder 1996; Lecuit et al. 1996; Nellen et al. 1996; Sturtevant et al. 1997). We examined the expression of these Dpp target genes in the haltere disc and found that although omb is expressed in the developing haltere pouch straddling the Dpp stripe as it does in the wing disc (Fig. 2C), salr and sal are not expressed in the haltere pouch (Fig. 2D; data not shown). These results show that the Dpp signal transduction machinery operates in the haltere disc but that selected wing target genes are not activated by the Dpp signal.
To determine whether Ubx represses salr expression in the haltere disc, we generated homozygous Ubx− clones. Indeed, salr is derepressed in Ubx− clones in the anterior compartment of the haltere disc. As in the wing disc, salr expression in these clones depended on their distance from the Dpp source (Fig. 2E). To determine whether Ubx is sufficient to repress salr, we examined salr expression in CbxM1/+ wing discs in which Ubx is ectopically expressed along part of the DV boundary. In these wing discs salr expression is repressed in a cell autonomous fashion (Fig. 2F). Because sal/salr are required for the induction of vein development (Sturtevant et al. 1997), the selective repression of salr by Ubx suppresses part of the Dpp-mediated AP wing patterning program in the haltere.
As with the spatial patterning of wing veins, the pattern of intervein tissue is also determined by specific regulatory genes and critical for morphogenesis. The Drosophila Serum Response Factor (DSRF or blistered) gene is expressed in future intervein tissue and required for the adhesion of the dorsal and ventral surfaces of the flat wing (Montagne et al. 1996). The haltere, however, is more balloon-like and, interestingly, DSRF expression is absent from the haltere pouch except for two crescents at the extreme dorsal and ventral edges of the anterior compartment (Fig. 2G). This difference is caused by Ubx regulation because in Ubx− clones in the haltere disc, repression of DSRF is relieved and a pattern of DSRF expression homologous to that in the wing forms within the boundaries of the clone (Fig. 2H). Conversely, ectopic expression of Ubx in wing discs extinguishes DSRF expression in a cell-autonomous manner (Fig. 2I).
The dorsoventral axis: Ubx represses Wg in the posterior compartment and selectively represses genes along the DV boundary
It has been long assumed that the global coordinate systems in homologous appendages are the same and, indeed, the ap selector gene is expressed in the dorsal compartment of the haltere disc as in the wing (Fig. 3A,B). However, we found that Wg, which is expressed along both the anterior and posterior extent of the DV boundary in the wing disc (Fig. 3A), is not expressed in the posterior compartment of the haltere disc (Fig. 3B). Because Wg function along the DV boundary is required for growth and patterning of the wing disc (Couso et al. 1994; Diaz-Benjumea and Cohen 1995; Zecca et al. 1996; Neumann and Cohen 1997b), the absence of Wg in the posterior haltere disc probably contributes to its disproportionately smaller size in comparison to the anterior compartment. In posterior Ubx− clones in the haltere disc, Wg is expressed along the DV boundary (Fig. 3C), suggesting that Ubx represses the posterior portion of the Wg expression pattern. The activation of Wg along the DV boundary occurs via the Notch receptor signaling pathway (Diaz-Benjumea and Cohen 1995; Kim et al. 1995). This pathway also activates the “boundary” enhancer of the vg gene (Kim et al. 1996), which is activated along the entire anterior and posterior extent of the DV boundary in the haltere (Fig. 4A). These results demonstrate that the Notch pathway is active along the entire DV boundary but that Ubx selectively prevents Wg activation by this pathway in the posterior compartment.
Figure 3.
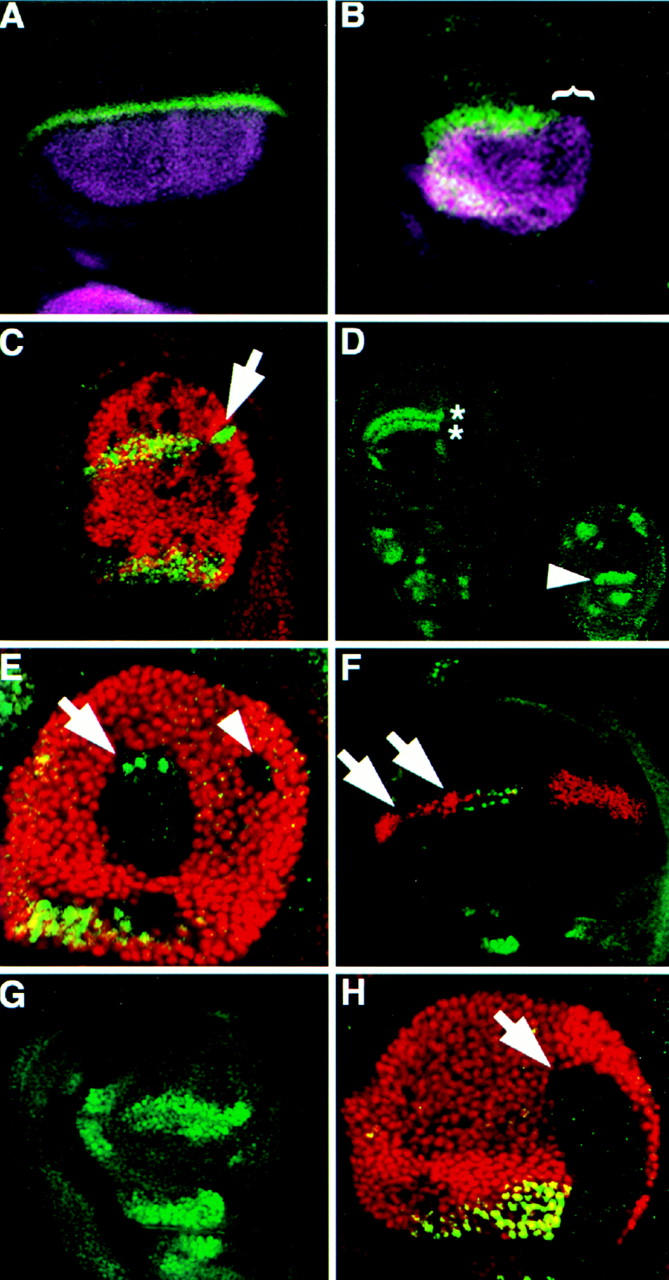
Ubx represses selected genes along the DV boundary of the haltere disc. (A–C) Antibody staining detecting Wg (green); Ap (purple), Ubx (red). (A,B) Ap and Wg are expressed in a similar domain in the haltere (B) as in the wing (A), but Wg expression is absent from the posterior haltere (bracket). (C) Haltere disc with several Ubx− clones (lack of red staining). A posterior clone, located along the DV boundary (arrow) shows derepression of Wg expression. (D–H) Antibody staining detecting Sc (green); Ubx (red). (D) Wild-type expression of Sc in the wing (left) and haltere (right) disc pouches. The double row of expression in sensory organ precursors along the future wing margin (asterisks) is absent from the haltere disc. In the haltere disc, Sc is also expressed in unique patterns including the pedicellular region (arrowhead). (E) Haltere disc with two dorsal Ubx− clones that each touch the DV boundary. The anterior (arrow) clone shows derepression of Sc expression; the posterior (arrowhead) clone shows no Sc expression as in the posterior of the wing. (F) Ubx expression along the DV boundary of a CbxM1/+ wing disc represses Sc expression along the presumptive anterior wing margin (arrows). (G) Ubx6.28/bx34e haltere disc showing ectopic Sc expression along the anterior DV boundary. (H) Ubx− clone (arrow) crossing into the pedicellar region of the haltere disc (see arrowhead in D) fails to activate the normal Sc expression there indicating that Ubx is necessary for activation of Sc in the pedicellar region of the haltere.
Figure 4.
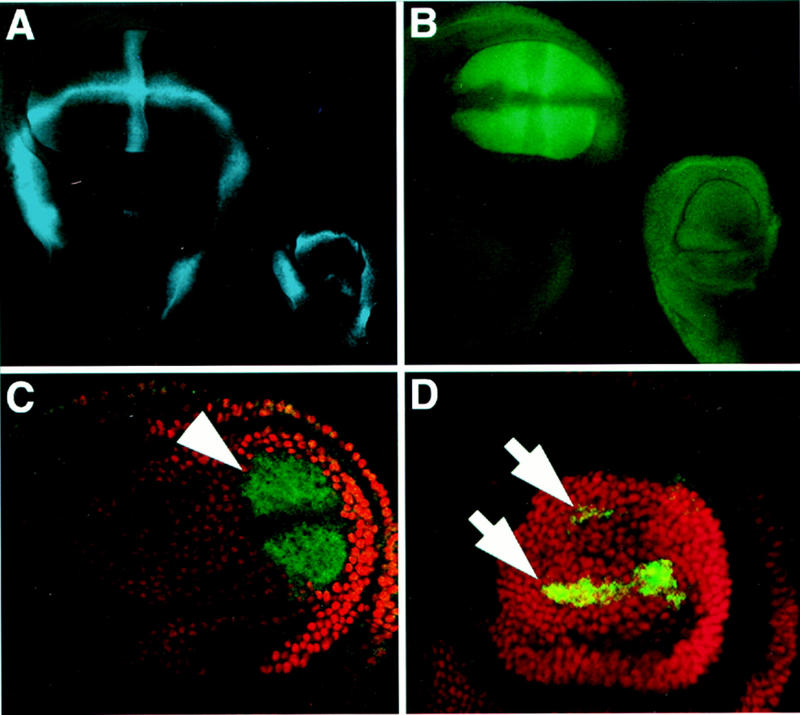
Ubx selectively regulates one enhancer of the vg gene. (A) The Notch-regulated vg boundary enhancer (blue, visualized by a lacZ reporter transgene) is activated along the DV boundary and hinge region in both the wing and haltere discs. (B–D) vg quadrant enhancer expression is visualized by lacZ (green) and antibody staining detecting Ubx (red). (B) vg quadrant enhancer expression fills the wing pouch (left) in a pattern complementary to the vg boundary enhancer, but is silent in the haltere (right). (C) Ubx6.28/+ haltere disc pouch showing derepression of the vg quadrant enhancer (arrows), indicating that repression of this enhancer is sensitive to Ubx gene dosage. (D) Ubx− clone in a haltere disc showing derepression of the vg quadrant pattern (arrowhead) that extends to the clone borders. The decreased expression along the DV boundary in the center of the clone is a normal feature of quadrant enhancer expression in the wing disc (see B).
Wg is expressed in the anterior compartment of the haltere disc, yet its phenotypic effects are markedly different than in the anterior of the wing disc. The most conspicuous difference is that in the wing, Wg activity along the DV boundary induces the formation of the prominent triple and double rows of bristles along the wing margin, whereas in the haltere it does not. The formation of margin bristles is regulated by Wg via the induction of the proneural achaete (ac) and scute (sc) target genes (Fig. 3D) and also requires the Cut transcription factor (Couso et al. 1994; Neumann and Cohen 1996). In the haltere disc, Cut is expressed along the anterior DV boundary (data not shown), whereas ac and sc are not induced (Fig. 3D).
To determine if Ubx represses ac/sc activation by Wg, we examined Ubx− clones. In the haltere disc, sc expression is derepressed in clones that touch or cross the anterior portion of the DV boundary (Fig. 3E). Conversely, sc expression is lost in anterior wing disc cells that ectopically express Ubx (Fig. 3F). This repression by Ubx is sensitive to the dosage of Ubx activity as ectopic ac/sc expression is observed in Ubx−/+ haltere discs (data not shown). This ectopic expression corresponds with ectopic bristles found on the halteres of Ubx−/+ adults. Further reductions of Ubx function in haltere discs causes greater derepression of sc on the DV boundary (Fig. 3G) and a corresponding emergence of triple row bristles on the adult haltere (Fig. 1E).
The haltere has several types of sense organs, including the proximally located pedicellular sensillae, that are not present on the wing. Correspondingly, sc is expressed in the presumptive pedicellar portion of the haltere disc but not in the equivalent part of the wing disc (Fig. 3D, arrowhead). Importantly, we found that in Ubx− clones in this region of the haltere disc, sc expression is lost (Fig. 3H). Therefore, Ubx is required to positively regulate sc in this unique pattern in the haltere disc. Together with the repression of sc along the DV boundary of the haltere, these observations suggest that Ubx acts upon two independent domains of the sc expression pattern, presumably via specific cis-regulatory elements controlling each aspect of sc gene expression.
The proximodistal axis: Ubx selectively represses one enhancer of the vestigial gene
We discovered a second and more dramatic example of the selective regulation of cis-regulatory elements by Ubx in the case of the vestigial (vg) gene. vg is expressed and required in the cells that will give rise to the distal appendage fields of the wing and haltere imaginal discs (Williams et al. 1994; Kim et al. 1996). vg expression in the wing field is regulated by two distinct enhancers that are activated by different signaling pathways. vg expression is first activated along the DV boundary of the wing disc by the Notch pathway through the boundary enhancer (Williams et al. 1994; Kim et al. 1996) and is later activated in the growing wing pouch by the Dpp and Wg signals through the “quadrant” enhancer (Kim et al. 1996; Zecca et al. 1996; Kim et al. 1997). The boundary enhancer is activated similarly in both the wing and haltere discs (Fig. 4A), however, the quadrant enhancer is silent in the haltere field (Fig. 4B).
The repression of the quadrant enhancer in the haltere is sensitive to the dosage of Ubx and is partially derepressed in Ubx−/+ haltere discs (Fig. 4C). More importantly, in Ubx− clones in the haltere disc, the quadrant enhancer is fully activated (Fig. 4D). These results show that Ubx selectively represses a portion of the native vg wing expression pattern in the haltere disc through the quadrant enhancer.
Ubx represses wing development through the independent regulation of target genes at multiple levels of regulatory hierarchies
We have identified in these experiments five genes whose function is necessary for the formation or patterning of various wing characters but whose expression is negatively regulated by Ubx in the haltere disc. For each gene, their repression in the haltere disc correlates with the absence of, or difference between, haltere characters and those in the serially homologous wing. One means by which to test the significance of the repression of these genes in the haltere disc is to determine what effects their derepression might have upon haltere morphology. It is crucial to recognize that the effects of expressing target genes in the haltere does not only depend upon the sufficiency of a given gene to induce a phenotype in the wing or at an ectopic site (legs, eyes, etc.) but also upon the architecture of the Ubx-regulated gene hierarchies in the haltere. There are three possible outcomes and interpretations for the ectopic expression of a differentially expressed gene. First, ectopic expression of individual genes in the haltere could be sufficient to induce a wing character. This result would show that the regulation of this gene by Ubx is the key event to determine the difference of that character in the wing and haltere. Second, there could be no effect on haltere morphology. Given that these genes are sufficient to induce ectopic phenotypes in the wing or elsewhere, this result could occur if downstream genes are independently regulated by Ubx and therefore prevented from being activated even when upstream activators are present. And third, one could induce haltere characters or structures with intermediate identity. This would imply that Ubx modifies the morphology of characters through other genes in addition to the ectopically expressed gene.
We first examined the effects of ectopic expression of the vg gene in the haltere and other tissues under the control of the GAL4/UAS system (Brand and Perrimon 1993; Kim et al. 1996). Whereas vg expression in all other appendages and tissues causes wing-like outgrowths (Kim et al. 1996), in the haltere we did not observe any significant change in adult appendage size or morphology. We did, however, observe striking differences between the morphology of the outgrowths formed on the second and third thoracic legs (Fig. 5). The former had clear wing-like morphology (Fig. 5A), whereas the latter had haltere-like morphology (Fig. 5B). The failure of ectopic vg expression to significantly alter haltere morphology and the distinct haltere-like character of the outgrowths formed in third thoracic legs suggests that Ubx acts on genes that are downstream of or parallel to vg in the genetic hierarchy.
Figure 5.
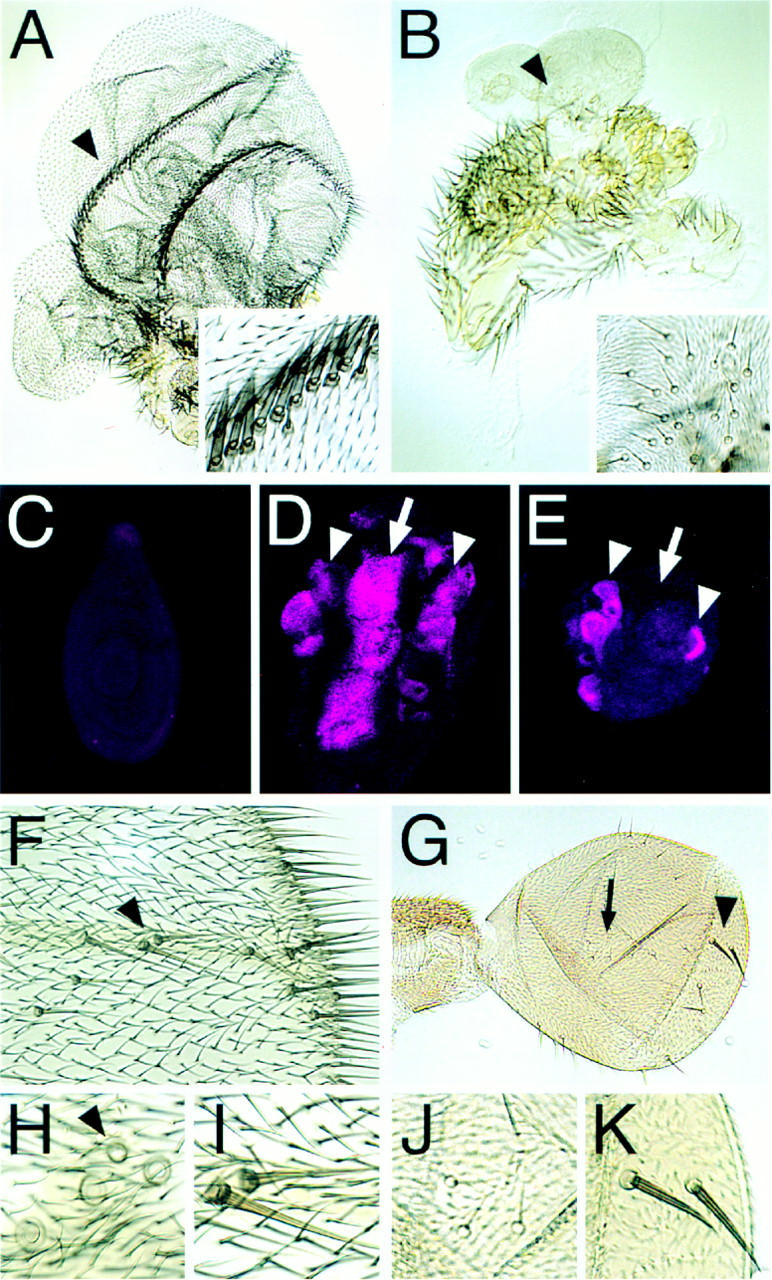
Targeted expression of Ubx-regulated genes. (A–E) Ectopic expression of Vg, (F–K) ectopic expression of Sc. (A) Transformation of a T2 leg to wing as a consequence of ectopic Vg expression. Two “wing margins” formed in this specimen showing the prominent triple row of margin bristles (region pointed out by arrowhead is magnified in inset). The hairs in the “blade region” are also of wing identity. (B) Transformation of a T3 leg towards haltere. Magnification is the same as in A. The ectopic structure is much smaller than that formed on the T2 leg and the hair morphology and density are like those found on a haltere. In addition, a cluster of what appears to be capitellar sensillae appear that are characteristic for the haltere (magnified in inset). (C) T2 leg imaginal disc of a wild-type third instar larva stained for Sal protein. No expression is detected. Sal is also not expressed in T1 and T3 leg discs (not shown). (D) Upon targeted expression of Vg, Sal is ectopically induced in T2 legs in a broad stripe along the AP axis (arrow) similar to its expression pattern in the developing wing pouch (compare to Fig. 2D). In addition, Sal is ectopically induced in smaller domains towards the periphery (proximal region) of the disc (arrowheads) that may correspond to Sal expression domains in the presumptive hinge and notal regions of the wing disc. (E) In contrast to the T2 leg disc, in T3 leg discs, ectopic Vg is unable to induce Sal expression along the AP axis. The corresponding pattern is normally repressed by Ubx in the developing haltere. Therefore, targeted expression of Vg is unable to override the Ubx-regulation of the downstream gene sal. (F) Distal part of a wing. Anterior is to the top, distal to the right. Extra bristles, resembling double row margin bristles developed along the distal AP boundary. The two bristles marked by the arrowhead are magnified in I. (H) More proximlly on the wing, mainly campaniform sensillae are induced. The region of the anterior crossvein where four instead of only one (arrowhead) campaniform sensilla developed. (G) Haltere; same orientation and magnification as F. On the proximal haltere, ectopic capitellar sensillae are induced (arrow, magnified in J); on the distal haltere, ectopic bristles resembling double row wing margin bristles developed (arrowhead, magnified in K).
To test whether Ubx regulates genes downstream of vg, we first searched for candidate genes whose expression depended upon Vg. We found that the sal (Fig. 5C,D) and DSRF (not shown) genes that are normally not expressed in leg imaginal discs are ectopically induced in first and second thoracic leg imaginal discs as a response to targeted expression of Vg and may thus be activated in the developing wing through some mechanism that is dependent upon Vg. The patterns of ectopic induction of sal and DSRF (not shown) in T1 and T2 leg discs are reminiscent of their normal expression patterns in wing discs (cf. Figs. 5D and 2D). In contrast to T3 leg discs, which also express Ubx, the central domains of ectopic induction of Sal and DSRF expression are suppressed (cf. Fig. 5E with Figs. 5D and 2D). These results demonstrate that downstream targets of Vg are also regulated by Ubx, independent of the Ubx regulation of Vg itself. The repression of these and other targets by Ubx would then suggest why the deregulation of Vg expression in the developing haltere is insufficient to reprogram haltere development towards wing development and to alter the morphology of the adult haltere.
Similarly, ectopic expression of the DSRF (not shown) or Sal (M. Averof, pers. comm.) transcription factors also do not alter haltere size, shape, or cell morphology. These results imply that there are genes downstream of DSRF and Sal whose expressions are necessary for the realization of a phenotype but which are repressed by Ubx in the haltere disc.
In contrast, ectopic expression of the sc gene in the developing haltere is sufficient to induce ectopic sensory organs (Fig. 5F). Interestingly, near the DV boundary, large bristles resembling those of the wing margin are induced (Fig. 5, cf. K with F and I), whereas in more proximal regions, sense organs characteristic of the haltere form (Fig. 5, cf. J and H). This result suggests that the repression of sensory organ formation by Ubx at the DV boundary is largely at the level of the sc gene, whereas the character of the proximal sense organs is modified by Ubx action downstream of or parallel to scute. Thus, all three outcomes outlined above are obtained in these ectopic expression experiments which reveal that Ubx acts independently upon the five genes we have identified as well as upon genes further downstream of or parallel to these regulators in the wing patterning hierarchy.
Discussion
The differentiation of the Drosophila haltere from the wing through the action of the Ubx gene is a classic example of Hox regulation of serial homology, and has served as the paradigm for understanding the nature of homeotic gene function (Lewis 1963; Garcia-Bellido 1975; Morata and Garcia-Bellido 1976; Lewis 1978). This study reveals several features of the control of haltere development by Ubx which, in principle, are likely to apply to the Hox-regulated differential development of other serially homologous structures in other animals. Specifically, we have shown that Ubx acts: (1) at many levels of regulatory hierarchies, upon long-range signaling proteins, their target genes, as well as genes further downstream, (2) selectively upon a subset of downstream target genes of signals common to both wing and haltere, and (3) independently upon these diverse targets. Below, we discuss these features of the Ubx-regulated gene hierarchy in the haltere and how they expand our general understanding of the Hox-regulated development of homologous structures.
The architecture of the Ubx-regulated gene hierarchy in the haltere
Ubx acts at many levels of wing patterning hierarchy
Unexpectedly, Ubx does not act solely on genes that are downstream of the global coordinate systems, but also regulates the expression of at least one global organizing signal. Along the DV boundary, Ubx represses the expression of the Wg signal in the posterior of the haltere field (Fig. 6). Ubx also regulates genes downstream of Wg, for example, the sc proneural gene, and downstream of the Dpp signal including salr and the vg quadrant enhancer (Fig. 6). Ubx must also control genes downstream of or parallel to Vg, DSRF, and Sal because the ectopic expression of these genes is not sufficient to alter haltere size or cell morphology (Fig. 6).
Figure 6.
The architecture of the Ubx-regulated gene hierarchy in the haltere. The products of the Ap and En selector genes and the Hh and Dpp signaling proteins are expressed similarly in the wing and haltere. Ser and Wg are expressed similarly in the anterior of the wing and haltere but not in the posterior. A selected subset of the target genes activated by Dpp, Wg, or other pathways are regulated by Ubx (shown boxed). Note that some target genes are also upstream activators or coactivators of genes which are also Ubx-regulated. The Salr, Vg, Sc, and DSRF products in turn affect the differentiation of veins, wing and haltere cells, sensory organs, and intervein cells. Ubx regulation can operate selectively upon different enhancers of the same gene. The vg quadrant enhancer (vgQ) is Ubx-regulated; the vg boundary enhancer (vgB) is not.
Ubx acts on a selected subset of genes downstream of the global organizing signals
We found that the Wg, Dpp, and Notch signal transduction pathways are active and competent throughout the haltere field and that Ubx selectively prevents activation of targets of these pathways. For example, Ubx prevents Notch-mediated Wg activation but not vg boundary activation on the DV boundary in the posterior of the haltere. Similarly, Ubx represses Dpp-mediated activation of salr and the vg quadrant enhancer, but not of the omb gene. Repression is therefore gene or enhancer-specific, not pathway-specific.
Ubx acts independently on target genes at different levels of wing patterning hierarchy
The picture emerging from this work is that there are different tiers of target genes that are regulated by Ubx independently of each other. For example, Ubx represses the expression of the vg quadrant enhancer and two downstream targets of Vg, salr and DSRF, in the haltere. However, the repression of salr and DSRF is independent of the repression of vg because ectopic expression of Vg cannot induce salr or DSRF when Ubx is present (Fig. 5). Similarly, the repression of scute in the anterior of the haltere is independent of the repression of Wg.
The independent regulation of these five genes or enhancers by Ubx may be either direct or indirect. Several observations are more consistent with direct control by Ubx. First, we have shown that the long-range signals and/or signal transduction pathways that are the activators of these genes operate in the haltere. The simplest explanation for the repression of wg, salr, DSRF, sc, and the vg quadrant enhancer is that Ubx is directly blocking their activation by these pathways. Second, the cell autonomy of the derepression of these target genes in Ubx− clones in the haltere and of their repression by ectopic Ubx in the wing disc show that Ubx is both necessary and sufficient for the differential regulation of these genes in individual cells. And third, the effects of reduced Ubx gene dosage on ac/sc and vg quadrant enhancer expression demonstrate that repression of these genes is operating near a threshold, which is also consistent with a direct control.
Regardless of whether Ubx regulation of any individual gene is direct or not, the independent regulation of these target genes by Ubx has several important implications. First, because there is independent regulation of genes at different levels of the same pathway, it reveals that Ubx is not acting on just a few genes at the top of regulatory hierarchies. Second, it explains why the deregulation of any individual Ubx target gene may be insufficient to transform particular haltere characters towards the wing. That is, it is difficult to break the grip of Ubx repression of wing characters because repression is operating on genes at multiple levels. And third, for repression to operate at these different levels, it implies that the evolution of the haltere progressed through the accumulation of a complex network of Ubx-regulated interactions.
Regulatory hierarchies and evolution
One unpredictable and very informative finding of this work was that the ectopic expression of certain Ubx-regulated genes that have fairly dramatic effects on other tissues did not perturb haltere development. One conclusion that might be drawn from these results is that the repression of these genes is not significant for haltere development. Yet, there is no doubt that the Ubx-regulated genes we have identified are developmentally significant in that they are required for the formation or patterning of major wing characters. Furthermore, the repression of their expression in the haltere disc correlates with the differences in size (Wg in the posterior, Vg in the “pouch”), venation (Salr), shape (DSRF), and sensory organs (Sc) between the Drosophila forewing and haltere. An alternative to the interpretation that the regulation of these genes is insignificant to the haltere is that some developmental pathways in the haltere are “canalized”. This concept, forwarded by Waddington in the 1940s (Waddington 1941, 1956, 1960), recognized that regulatory interactions in developmental processes may constrain the extent or direction of morphological change in response to environmental or genetic perturbation. In evolutionary terms, canalization is an example of “developmental constraint” for which there has been considerable comparative but relatively little experimental evidence (Maynard-Smith et al. 1985).
An explanation for the canalization of certain developmental pathways in the haltere may lie in the evolution and architecture of the Ubx-regulated hierarchies. The evolution of the haltere was a gradual transformation of a full-sized hindwing into a balancing organ, involving the modification, reduction, or elimination of many characters. If we consider just one feature, such as the relative size of the flight appendage, we can extrapolate from mutational studies to infer that there were many genes and pathways upon which selection could act to reduce the size of the hindwing. It is likely that the reduction of hindwing cell number and volume involved changes in the regulation of multiple genes acting at different developmental stages (with the vg and wg genes being two of many potentially affected genes). If Ubx regulation thus evolved at many loci, then we should find that perturbation of single genes in these networks may have no overt effects. Although it is not obvious why Ubx regulation would be maintained on targets whose derepression has no clear consequences, we must acknowledge that our resolution in these experiments is relatively low and we may not be able to perceive minor effects. Over evolutionary time selection against even the slightest deleterious effects that may arise from derepression of target genes would stabilize Ubx repression throughout a hierarchy.
Materials and methods
Clonal analysis and immunohistochemistry
The null allele (Kerridge and Morata 1982) Ubx6.28 was used to make mitotic clones in developing halteres. In Figures 2 and 3 clones were generated by heat-shock induction of FLP recombinase (Xu and Rubin 1993) in hsFLP122; P[ry+, hs-neo, FRT] 82B, Ubx6.28/P[ry+, hs-neo FRT] 82B, P[mini-w+, hsπM] 87E flies. The Ubx− clones in Figure 4 were generated by exposing vgquad/+; Ubx6.28/+ flies to δ-rays (4000 rads). Antibodies were provided by M. Affolter (DSRF) (Biozentrum, Basel, Switzerland), R. Bario (Sal-r) (EMBL, Heidelberg, Germany), S. Cumberledge (Wg) (University of Massachusetts, Amherst), N. Patel (En) (University of Chicago, IL), J. Skeath (Sc) (Washington University, St. Louis, MO), and R. White (Ubx) (Cambridge University, UK). Immunohistochemistry was performed as previously described (Carroll et al. 1995).
Targeted expression of regulatory genes
Vg and Sc were ectopically expressed by means of the GAL4 system (Brand and Perrimon 1993). Using a Distal-less (Dll)–GAL4 driver that directs ectopic expression in a large area of the developing leg including the region distal to the presumptive tibia ectopic, Vg expression was examined in developing leg discs. Ectopic expression of Sc in the wing and haltere was targeted by the Decapentaplegic (Dpp)-GAL4 driver that directs ectopic expression along the AP boundary of imaginal discs.
Acknowledgments
We thank M. Affolter, J. Montagne, R. Barrio, S. Cumberledge, N. Patel, J. Skeath, and R. White for antibodies and other reagents; M. Affolter for the UAS-SRF stock; G. Morata for the Dll-Gal4 stock; J. Skeath for the UAS-scute stock; J. Kennison for the slide of the four-winged fly; M. Hoffmann, J. Esch, and the Bloomington Stock Center for fly stocks; Steve Paddock for help with microscopy; Leanne Olds for artwork; Jen Grenier, Dave Keys, Allen Laughon, and Grace Panganiban for comments on the manuscript; and Jamie Wilson for help with its preparation. S.W. and A.H. are Predoctoral Fellows in Genetics, G.H. was supported by an EMBO fellowship, and S.B.C. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL sbcarrol@facstaff.wisc.edu; FAX (608) 262-9343.
References
- Beachy PA, Helfand SL, Hogness DS. Segmental distribution of bithorax complex proteins during Drosophila development. Nature. 1985;313:545–551. doi: 10.1038/313545a0. [DOI] [PubMed] [Google Scholar]
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Hedgehog signaling in Drosophila eye and limb development-conserved machinery, divergent roles? Curr Opin Neurobiol. 1997;7:55–61. doi: 10.1016/s0959-4388(97)80120-1. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Botas J, Garcia-Bellido A. Distribution of Ultrabithorax proteins in mutants of Drosophila bithorax complex and its transregulatory genes. Nature. 1985;318:569–571. [Google Scholar]
- Carroll S. Homeotic genes and the evolution of arthropods and chordates. Review. Nature. 1995;376:479–485. doi: 10.1038/376479a0. [DOI] [PubMed] [Google Scholar]
- Carroll S B, Gates J, Keys D, Paddock SW, Panganiban GF, Selegue J, Williams JA. Pattern formation and eyespot determination in butterfly wings. Science. 1994;265:109–114. doi: 10.1126/science.7912449. [DOI] [PubMed] [Google Scholar]
- Carroll S, Weatherbee S, Langeland J. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- Casanova J, Sánchez-Herrero E, Morata G. Prothoracic transformation and functional structure of the Ultrabithorax gene of Drosophila. Cell. 1985;42:663–669. doi: 10.1016/0092-8674(85)90123-0. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J, Micol J-L, García-Bellido A. Transvection in the Drosophila Ultrabithorax gene: A Cbx mutant allele induces ectopic expression of a normal allele in trans. Genetics. 1990;126:177–184. doi: 10.1093/genetics/126.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso J, Bishop S, Martinez-Arias A. The wingless signaling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- de Celis J, Barrio R, Kafatos F. A gene complex acting downstream of dpp in Drosophila wing morphogenesis. Nature. 1996;381:421–424. doi: 10.1038/381421a0. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F, Cohen S. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A. Genetic control of wing disc development in Drosophila. Ciba Found Symp. 1975;29:161–182. doi: 10.1002/9780470720110.ch8. [DOI] [PubMed] [Google Scholar]
- Gibson-Brown J, Agulnik SI, Chapman DL, Alexiou M, Garvey N, Silver LM, Papaioannou VE. Evidence of a role for T-box genes in the evolution of limb morphogenesis and the specification of forelimb/hindlimb identity. Mech Dev. 1996;56:93–101. doi: 10.1016/0925-4773(96)00514-x. [DOI] [PubMed] [Google Scholar]
- González-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development. 1995;121:2117–2125. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M, Micol J-L, García-Bellido A. Developmental genetic analysis of Contrabithorax mutations in Drosophila melanogaster. Genetics. 1990;126:139–155. doi: 10.1093/genetics/126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Pflugfelder G. Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science. 1996;271:1601–1604. doi: 10.1126/science.271.5255.1601. [DOI] [PubMed] [Google Scholar]
- Irvine KD, Vogt TF. Dorsal-ventral signaling in limb development. Curr Opin Cell Biol. 1997;9:867–876. doi: 10.1016/s0955-0674(97)80090-7. [DOI] [PubMed] [Google Scholar]
- Kerridge S, Morata G. Developmental effects of some newly induced Ultrabithorax alleles of Drosophila. J Embry Exp Morphol. 1982;8:211–234. [PubMed] [Google Scholar]
- Kim J, Irvine K, Carroll S. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell. 1995;82:795–802. doi: 10.1016/0092-8674(95)90476-x. [DOI] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch J, Kraus M, Vorwerk K, Magee J, Carroll S. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Kim J, Johnson K, Chen HJ, Carroll SB, Laughon A. MAD binds to DNA and directly mediates activation of vestigial by DPP. Nature. 1997;388:304–308. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Brook W, Ng M, Calleja M, Sun H, Cohen S. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- Lewis E. Some aspects of position pseudoallelism. Am Nat. 1955;89:73–89. [Google Scholar]
- ————— Genes and developmental pathways. Am Zool. 1963;3:33–56. [Google Scholar]
- ————— A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- ————— . Control of body segment differentiation in Drosophila by the bithorax gene complex. In: Burger M, editor. Embryonic development: Genes and cells. New York, NY: Alan R. Liss; 1982. pp. 269–288. [PubMed] [Google Scholar]
- Maynard-Smith, J., R. Burian, and S. Kauffman. 1985. Developmental constraints and evolution. Q. Rev. Biol. (Suppl.) 60: 265-287.
- Micol J, García-Bellido A. Genetic analysis of “transvection” effects involving Contrabithorax mutations in Drosophila melanogaster. Proc Natl Acad Sci. 1988;85:1146–1150. doi: 10.1073/pnas.85.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, Affolter M. The Drosophila serum response factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development. 1996;122:2589–2597. doi: 10.1242/dev.122.9.2589. [DOI] [PubMed] [Google Scholar]
- Morata G, Garcia-Bellido A. Developmental analysis of some mutants of the bithorax system of Drosophila. Wilhelm Roux’s Arch. 1976;179:125–143. doi: 10.1007/BF00848298. [DOI] [PubMed] [Google Scholar]
- Morata G, Kerridge S. Sequential functions of the bithorax complex of Drosophila. Nature. 1981;290:778–781. doi: 10.1038/290778a0. [DOI] [PubMed] [Google Scholar]
- Morata G, Lawrence P. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255:615–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range actions of a Dpp morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Neumann C, Cohen S. A hierarchy of cross-regulation involving Notch, wingless, vestigial, and cut organizes the dorsal/ventral axis of the Drosophila wing. Development. 1996;122:3477–3485. doi: 10.1242/dev.122.11.3477. [DOI] [PubMed] [Google Scholar]
- ————— Morphogens and pattern formation. BioEssays. 1997a;19:721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- ————— Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997b;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- Peterson R, Papenbrook T, Davada M, Awgulewitsch A. The murine Hoxc cluster contains five neighboring abdB-related Hox genes that show unique spatially coordinated expression in posterior embryonic subregions. Mech Dev. 1994;47:253–260. doi: 10.1016/0925-4773(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Serrano N, O’Farrell P. Limb morphogenesis: Connections between patterning and growth. Curr Biol. 1997;7:R186–R195. doi: 10.1016/s0960-9822(97)70085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant M, Biehs B, Marin E, Bier E. The spalt gene links the AP compartment boundary to a linear adult structure in the Drosophila wing. Development. 1997;124:21–32. doi: 10.1242/dev.124.1.21. [DOI] [PubMed] [Google Scholar]
- Waddington C. Canalization of development and the inheritance of acquired characteristics. Nature. 1941;150:563–565. [Google Scholar]
- Waddington C. Principles of embryology. New York, NY: Macmillan; 1956. [Google Scholar]
- Waddington C. Experiments. Genet Res. 1960;1:140–150. [Google Scholar]
- Wagner-Bernholz J, Wilson C, Gibson G, Schuh R, Gehring W. Identification of target genes of the homeotic gene Antennapedia by enhancer detection. Genes & Dev. 1991;5:2467–2480. doi: 10.1101/gad.5.12b.2467. [DOI] [PubMed] [Google Scholar]
- White R, Akam M. Contrabithorax mutations cause inappropriate expression of Ultrabithorax products in Drosophila. Nature. 1985;318:567–569. [Google Scholar]
- White R, Wilcox M. Regulation of the distribution of Ultrabithorax proteins in Drosophila. Nature. 1985a;318:563–567. doi: 10.1002/j.1460-2075.1985.tb03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Distribution of Ultrabithorax proteins in Drosophila. EMBO J. 1985b;4:2035–2043. doi: 10.1002/j.1460-2075.1985.tb03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Paddock S, Vorwerk K, Carroll S. Organization of wing formation and induction of a wing-patterning gene at a compartment boundary. Nature. 1994;368:299–305. doi: 10.1038/368299a0. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin G. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]