Abstract
Negative pressure pulmonary edema (NPPE) is a dangerous and potentially fatal condition with a multifactorial pathogenesis. Frequently, NPPE is a manifestation of upper airway obstruction, the large negative intrathoracic pressure generated by forced inspiration against an obstructed airway is thought to be the principal mechanism involved. This negative pressure leads to an increase in pulmonary vascular volume and pulmonary capillary transmural pressure, creating a risk of disruption of the alveolar–capillary membrane. The early detection of the signs of this syndrome is vital to the treatment and to patient outcome. The purpose of this review is to highlight the available literature on NPPE, while probing the pathophysiological mechanisms relevant in both the development of this condition and that involved in its resolution.
Keywords: Airway obstruction, negative pressure, negative pressure pulmonary edema, postoperative
INTRODUCTION
Negative pressure pulmonary edema (NPPE) or postobstruction pulmonary edema (POPE) is a clinical entity of great relevance in anesthesiology and intensive care. The presentation of NPPE can be immediate or delayed, which therefore necessitates immediate recognition and treatment by anyone directly involved in the perioperative care of a patient.[1,2] The incidence of NPPE has been reported to be 0.05%–0.1% of all anesthetic practices; however, it is suggested that it occurs more commonly than is generally documented.[2] According to one estimate, NPPE develops in 11% of all patients requiring active intervention for acute upper airway obstruction.[3] The Australian incident monitoring study of 4000 incidences of laryngospasm during anesthesia showed that NPPE occur in up to 4% of all incident reports of laryngospasm.[4] This disorder is classified as Type I or Type II.[5,6] Type I NPPE develops immediately after onset of acute airway obstruction and Type II NPPE develops after the relief of chronic upper airway obstruction. As Type I NPPE develops usually with upper airway acute obstruction or after manipulation of the airway surgically, some authors call it laryngeal spasm-induced pulmonary edema.[7] Other factors that increase the risk of Type I NPPE are hanging, strangulation, upper airway tumors, foreign bodies, epiglottitis, croup, chocking, migration of Folly's catheter balloon used to tamponade the nose in epistaxis, near drowning, endotracheal tube (ETT) obstruction, goitre, and mononucleosis [Table 1]. Type II NPPE can result after relief of upper airway obstruction caused by big tonsils, hypertrophic adenoids, or a redundant uvula [Table 1]. The incidence of developing Type I NPPE associated with acute postoperative upper airway obstruction is 9.6–12%, whereas the incidence of developing Type II NPPE is 44%.[8] In adults about 50% of NPPE occurrences are due to postoperative laryngospasm.[9]
Table 1.
Causes of negative pressure pulmonary edema

HISTORY OF NEGATIVE PRESSURE PULMONARY EDEMA
NPPE was first demonstrated in 1927 by Moore in spontaneously breathing dogs exposed to resistive load.[10] The first description of the pathophysiological correlation between creation of negative pressure and the development of pulmonary edema was in 1942 by Warren et al. the relationship between pulmonary edema and upper airway obstruction in two children, who had croup and epiglottitis was reported by Capitanio et al.[11] The report by Oswalt et al.[12] was the first showing the clinical significance of this phenomenon in three adult patients, who experienced the onset of pulmonary edema minutes to hours after severe acute upper airway obstruction. Since then, NPPE has been reported mainly by anesthetists as a consequence of postoperative laryngospasm.[2,13,14]
PATHOPHYSIOLOGY
The pathophysiology of NPPE has been extensively reviewed by several studies.[15–17] NPPE begins with a significant upper airway obstruction, inspiratory efforts to overcome the obstruction generate highly negative intrapleural and alveolar pressures, and the high pressure gradient causes fluid to move out of the pulmonary capillaries and into the interstitial and alveolar spaces[16,18] [Figure 1]. The pathophysiology of NPPE is attributed to four major mechanisms: Disturbances of pulmonary fluid homeostasis can be induced by four pathways that can lead to increased interstitial fluid—increased hydrostatic pressure in the pulmonary capillary bed (or conversely, decreased pressure in the interstitium), decreased osmotic pressure of plasma, increased permeability of the membrane, and decreased return of fluid to the circulation via lymphatics.[32,33]
Figure 1.
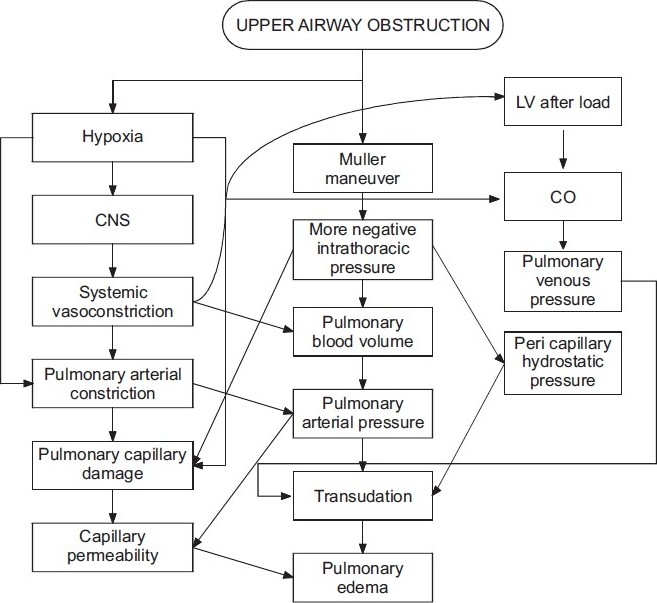
Postulated mechanisms of pulmonary edema secondary to upper airway obstruction
Creation of marked intrathoracic negative pressure of –50 to –100 cm H2O (normal –3 to 10 cm of H2O) results in a sudden increase of venous return of blood to the heart, which will expose the left ventricle to an after load stress and an increase in both end diastolic and end systolic ventricular volumes.[12,23,24] Because of the interdependent effect of both ventricles, the stress on the left ventricle will be excessive, leading to an increase of LVEDP. The sudden increase of pulmonary microvascular pressure, due to very low intrapulmonary pressure, in the face of the high LVEDP and low left ventricular compliance, will favor the formation of pulmonary edema.
The highly negative intrathoracic pressures cause a dramatic and immediate increase in systemic venous return to the heart with a simultaneous drop in cardiac output associated with the reduced pulmonary venous drainage to the left atrium. Pulmonary capillary pressures increase while intraalveolar pressures drop, and alveolar cell junctions are disrupted. Fluid moves rapidly into interstitial and alveolar spaces, and the pulmonary edema remains even after the airway obstruction is relieved.[19] When a critical quantity of edema fluid collects in the interstitial compartment, alveolar flooding occurs.[22]
The hypoxemia that results as a complication of upper airway obstruction, will increase pre- and post-capillary pulmonary vascular resistance in a nonuniform fashion,[25,26] increasing the pulmonary vascular resistance and capillary pressure and integrity precipitating a hyperadrenergic state, mimicking neurogenic pulmonary edema.[27] Hypoxemia also redistributes blood from the systemic veins to the pulmonary circulation, increasing by that the pulmonary capillary resistance.
In chronic upper airway obstruction there is a modest level of Auto positive end-expiratory pressure (PEEP) with an increase of end expiratory lung volume. When this chronic obstruction is relieved acutely, the Auto PEEP will disappear, the lung volumes and pressure return to normal, creating a negative intrapulmonary pressure, and if it is severe enough it will result in transudation of fluids in the lung interstitium and alveoli. This type of edema is called Type II NPPE.[11]
Cardiac anomalies may also predispose a patient to NPPE. Goldenberg et al.[23] indicate the strong association of NPPE with cardiac anomalies: they noted that 50% of patients with NPPE had such abnormalities (cardiomyopathy and valvular heart diseases) compared with 1% of the general population. Risk factors for NPPE include airway lesions, upper airway surgery, obesity, and obstructive sleep apnea. Besides postextubation laryngospasm, reported causes include foreign bodies, hanging, strangulation, croup, epiglottitis, obstructive sleep apnea, and artificial airway obstruction.[16]
The cause of NPPE II is less clear than that of NPPE I. It appears that the obstructing lesion produces a modest level of PEEP and increases end-expiratory lung volume. Relief of the obstruction removes the PEEP and returns lung volumes and pressures to normal. It is postulated that altered permeability and previously occult interstitial fluid do not resolve immediately. The sudden removal of the PEEP leads to interstitial fluid transudation and pulmonary edema.[24,25] NPPE II is much less commonly reported than NPPE I and predictive factors have not been clearly elucidated.
The symptoms of NPPE usually develop immediately after extubation, although sometimes the onset may be considerably delayed up to a few hours in the postoperative period. A possible explanation for this delayed manifestation is a positive pressure, created by forceful expiration against a closed glottis, opposing fluid transudation.[26] As airway obstruction relieves, increased venous return causes blood shift from peripheral to central circulation and hydrostatic transudation. Thus close postoperative observation must be continued for an extended time in patients experiencing respiratory difficulty.
Some information is available on the molecular mechanisms involved in increased endothelial barrier permeability in response to wall stress. When an acute increase in transmural pressure occurs, the radial expansion of the capillary wall translates into linear cellular stretch. Compared with shear stress from laminar flow, the response of endothelial cells to linear stretch is maladaptive.[27,28] Oxidative stress has been described as one of the mechanisms of injury that seems to be increased by the increased linear stretch. In fact, increasing levels of cyclic linear stretch result in upregulation of inducible nitric oxide synthase[29] and xanthine oxidoreductase, as has been shown by Abdulnour et al.[30] both of which have been repeatedly implicated in cellular injury and increased vascular permeability. Future studies will show whether these mechanisms of increased vascular permeability are clinically relevant in patients presenting with NPPE.
CLINICAL PRESENTATION
In clinical presentation, initial findings usually include decreased oxygen saturation, with pink frothy sputum and chest radiograph abnormalities.[8] Manifestations of the acute airway obstruction include stridor, suprasternal and supraclavicular retractions, urgent use of accessory muscles of inspiration, and panic in the facial expression. As NPPE develops, auscultation usually reveals crackles and occasionally wheezes. Pulmonary edema causes both impaired diffusion of oxygen and ventilation/perfusion mismatching, leading to sudden and possibly severe hypoxemia. The typical chest radiograph will show diffuse interstitial and alveolar infiltrates [Figure 2]. Although the radiographic findings associated with postextubation pulmonary edema have been described, there are minimal data regarding distribution of this postextubation edema within the lungs.[13] NPPE has a characteristic appearance in Computed tomography (CT). Unlike other forms of pulmonary edema, computed tomography sections displayed a striking preferential central and nondependent distribution of ground-glass attenuation (edema/hemorrhage), which parallels the pleural and interstitial pressure gradients. Both pressures tend to be more negative in the central and nondependent regions than in the dependent and peripheral lung regions, respectively, and those regional pressure differences tend to increase with inflation and inspiratory effort.[31] As a result, the interstitial and, therefore, perivascular pressures tend to decrease the most in the central and nondependent regions, and the transmural vascular pressure changes and capillary stress should be maximal in those regions.
Figure 2.
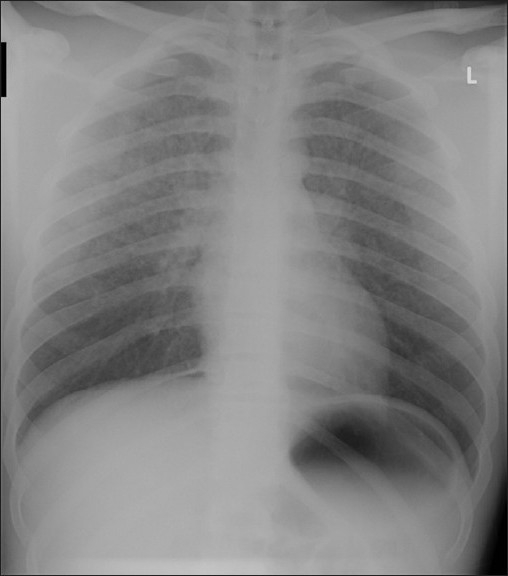
Chest radiograph of a patient who presented to intensive care department after a postoperative negative pressure pulmonary edema showing diffuse interstitial and alveolar infiltrates
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
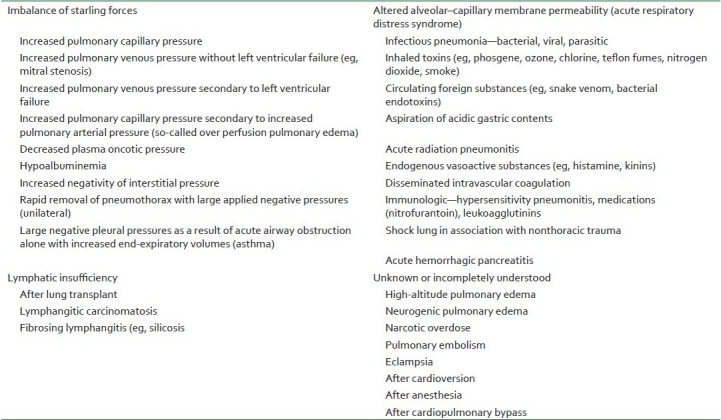
Diagnosis of NPPE is usually made on the basis of a history of a precipitating incident and symptoms. NPPE requires rapid intervention and may be confused with other causes of postoperative respiratory distress [Figure 3]. Although symptoms usually develop within 1 h of the precipitating event, delayed onsets have been reported.[12,23,34–36] The presence of agitation, tachypnea, tachycardia, frothy pink pulmonary secretions, rales, and progressive oxygen desaturation suggests the diagnosis of NPPE in the appropriate setting. Chest radiograph findings of pulmonary edema support the diagnosis.Other causes of pulmonary edema should be considered [Table 2]. Measurement of the pulmonary edema fluid/plasma protein ratio is a well-validated method to differentiate between hydrostatic pulmonary edema and increased permeability pulmonary edema.[21]
Figure 3.
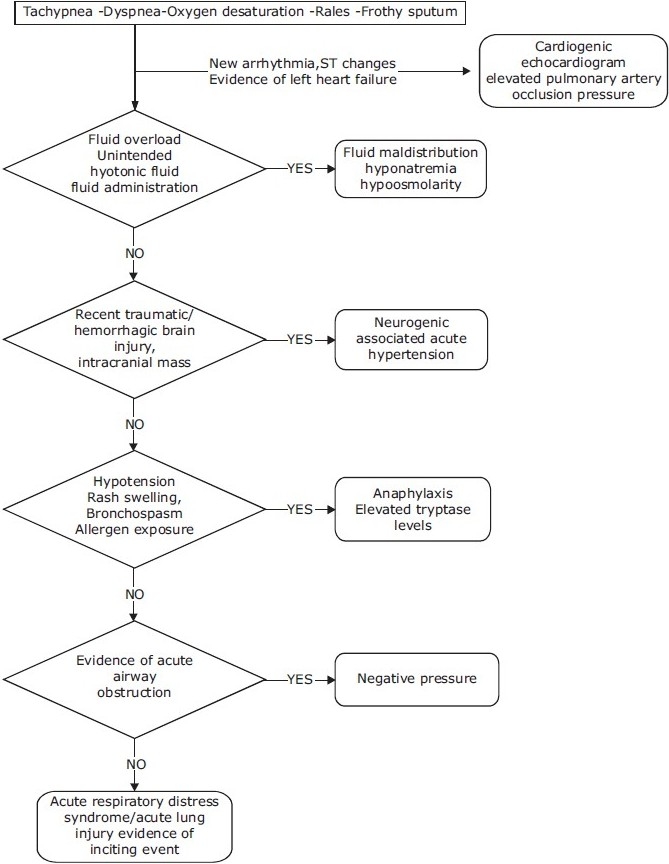
Flow chart showing a methord for diagnosing negative pressure pulmonary edema (NPPE)
Table 2.
Differential diagnosis for NPPE based on initiating mechanism of pulmonary edema
MANAGEMENT
The first treatment priority is relief of the airway obstruction and correction of hypoxemia. The next step is to address the pulmonary edema with a diuretic unless the patient is hypovolemic. Effective airway management and immediate treatment with oxygen and diuretics is sufficient in most cases of NPPE. Persistent airway obstruction may necessitate an artificial airway, and acute respiratory failure would require artificial ventilation with oxygen and appropriate levels of PEEP. If the airway obstruction is due to the patient biting down on the endotracheal tube, a dose of succinylcholine (0.1–0.2 mg/kg) may be needed to relax the jaw muscles, although controversial use of steroids in NPPE has been reported in different case reports.[13,18,37]
Diagnosis and rapid treatment are essential to alleviate this respiratory complication patients with suspected NPPE should have a longer period of observation in the postanaesthetic care unit. Most patients receive standard treatment that includes positive end-expiratory pressure and diuretics, however, the role of these interventions is unclear.[38] Continuous positive airway pressure is required in 9%–18% of all cases,[39] and 34%–46% of the patients require controlled mechanical ventilation via orotracheal intubation.[16,40] There have been reports of fatal evolution due to acute respiratory distress syndrome and multiple organ or system failure following upper airway obstruction.[41] Most patients respond quickly without further sequelae; however, there has been one reported case of a 43-year-old man with epiglottis developing postobstructive pulmonary edema that progressed to adult respiratory distress syndrome (ARDS) and resulted in death.[2,41]
An alternative to intubation is noninvasive respiratory support (ie, noninvasive positive pressure ventilation or treatment with continuous positive airway pressure). Recent data suggest that noninvasive respiratory support may be an important tool to prevent or treat acute respiratory failure while avoiding intubation. The aims of noninvasive respiratory support in the context of NPPE include the following: to partially compensate for the affected respiratory function by reducing the work of breathing; to improve alveolar recruitment with better gas exchange; and to reduce left ventricular after load, increasing cardiac output and improving hemodynamics.[42] Evidence suggests that noninvasive respiratory support may be an effective strategy to reduce intubation rates, intensive care unit and hospital lengths of stay, and morbidity and mortality in postoperative patients.[42,43] With prompt diagnosis and intervention, most patients can be treated without incident. It is important that patients who experience postanesthetic laryngospasm should be monitored for longer than the usual postoperative period.[18,44] The recommended postanesthetic monitoring period in this patient population ranges from 2 to 12 h.[12,45]
There is no intervention proven to prevent NPPE, but avoiding laryngeal irritation that leads to laryngospasm is likely to reduce the occurrence of NPPE. For this reason topical laryngotracheal anesthesia (of 2 mL each of 1% lidocaine and 2% tetacaine) is recommended,[23] careful oropharyngeal suctioning and extubation in stage 1 anesthesia not 2, when patients are more likely to go into laryngospasm.
CONCLUSION
With prompt diagnosis and therapeutic action, NPPE resolves generally within 24 h. However, when recognition is delayed, patients with NPPE have mortality rates ranging from 11% to 40%.[46] Therefore, early recognition of NPPE is crucial to decrease morbidity in these patients. A high index of suspicion for NPPE must be maintained for the patient who experiences postextubation laryngospasm.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Timby J, Reed C, Zeilender S, Glauser FL. “Mechanical” causes of pulmonary edema. Chest. 1990;98:973–9. doi: 10.1378/chest.98.4.973. [DOI] [PubMed] [Google Scholar]
- 2.McConkey PP. Postobstructive pulmonary oedema: A case series and review. Anaesthesia Intensive Care. 2000;28:72–6. doi: 10.1177/0310057X0002800114. [DOI] [PubMed] [Google Scholar]
- 3.Tami TA, Chu F, Wildes TO, Kaplan M. Pulmonary edema and acute upper airway obstruction. Laryngoscope. 1986;96:506–9. doi: 10.1288/00005537-198605000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Visvanathan T, Kluger MT, Webb RK, Westhorpe RN. Crisis management during anaesthesia: laryngospasm. Qual Saf Health Care. 2005;14:e3. doi: 10.1136/qshc.2002.004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dicpinigaitis PV, Mehta DC. Postobstructive pulmonary edema induced by endotracheal tube occlusion. Intensive Care Med. 1995;21:1048–50. doi: 10.1007/BF01700672. [DOI] [PubMed] [Google Scholar]
- 6.Anderson AF, Alfrey D, Lipscomb AB., Jr Acute pulmonary edema: An unusual complication following arthroscopy: A report of three cases. Arthroscopy. 1990;6:235–7. doi: 10.1016/0749-8063(90)90080-w. [DOI] [PubMed] [Google Scholar]
- 7.Guinard JP. Laryngospasm-induced pulmonary edema. Int J Pediatr Otorhinolaryngol. 1990;20:163–8. doi: 10.1016/0165-5876(90)90082-3. [DOI] [PubMed] [Google Scholar]
- 8.Lathan SR, Silverman ME, Thomas BL, Waters WC. Postoperative pulmonary edema. South Med J. 1999;92:313–5. doi: 10.1097/00007611-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Goli AK, Goli SA, Byrd RP, Jr, Roy TM. Spontaneous negative pressure changes: An unusual cause of noncardiogenic pulmonary edema. J Ky Med Assoc. 2003;101:317–20. [PubMed] [Google Scholar]
- 10.Moore RL, Binger CA. The response to respiratory resistance: A comparison of the effects produced by partial obstruction in the inspiratory and expiratory phases of respiration. J Exp Med. 1927;45:1065–80. doi: 10.1084/jem.45.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capitanio MA, Kirkpatrick JA. Obstructions of the upper airway in children as reflected on the chest radiograph. Radiology. 1973;107:159–61. doi: 10.1148/107.1.159. [DOI] [PubMed] [Google Scholar]
- 12.Oswalt CE, Gates GA, Holmstrom MG. Pulmonary edema as a complication of acute airway obstruction. JAMA. 1977;238:1833–5. [PubMed] [Google Scholar]
- 13.Cascade PN, Alexander GD, Mackie DS. Negative-pressure pulmonary edema after endotracheal intubation. Radiology. 1993;186:671–5. doi: 10.1148/radiology.186.3.8430172. [DOI] [PubMed] [Google Scholar]
- 14.Dolinski SY, MacGregor DA, Scuderi PE. Pulmonary hemorrhage associated with negative-pressure pulmonary edema. Anesthesiology. 2000;93:888–90. doi: 10.1097/00000542-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 15.Lorch DG, Sahn SA. Post-extubation pulmonary edema following anesthesia induced by upper airway obstruction: Are certain patients at increased risk? Chest. 1986;90:802–5. doi: 10.1378/chest.90.6.802. [DOI] [PubMed] [Google Scholar]
- 16.Butterell H, Riley RH. Life-threatening pulmonary oedema secondary to tracheal compression. Anaesthesia Intensive Care. 2002;30:804–6. doi: 10.1177/0310057X0203000615. [DOI] [PubMed] [Google Scholar]
- 17.Sofer S, Bar-Ziv J, Scharf SM. Pulmonary edema following relief of upper airway obstruction. Chest. 1984;86:401–3. doi: 10.1378/chest.86.3.401. [DOI] [PubMed] [Google Scholar]
- 18.Willms D, Shure D. Pulmonary edema due to upper airway obstruction in adults. Chest. 1988;94:1090–2. doi: 10.1378/chest.94.5.1090. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 20.Ware LB, Matthay MA. Clinical practice: Acute pulmonary edema. N Engl J Med. 2005;353:2788–96. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 21.Tarrac SE. Negative pressure pulmonary edema: A postanesthesia emergency. J Perianesth Nurs. 2003;18:317–23. doi: 10.1016/s1089-9472(03)00183-7. [DOI] [PubMed] [Google Scholar]
- 22.Zumsteg TA, Havill AM, Gee MH. Relationships among lung extravascular fluid compartments with alveolar flooding. J Appl Physiol. 1982;53:267–71. doi: 10.1152/jappl.1982.53.1.267. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg JD, Portugal LG, Wenig BL, Weingarten RT. Negative-pressure pulmonary edema in the otolaryngology patient. Otolaryngol Head Neck Surg. 1997;117:62–6. doi: 10.1016/S0194-59989770208-0. [DOI] [PubMed] [Google Scholar]
- 24.Guffin TN, Har-El G, Sanders A, Lucente FE, Nash M. Acute postobstructive pulmonary edema. Otolaryngol Head Neck Surg. 1995;112:235–7. doi: 10.1016/S0194-59989570242-3. [DOI] [PubMed] [Google Scholar]
- 25.Lang SA, Duncan PG, Shephard DA, Ha HC. Pulmonary oedema associated with airway obstruction. Can J Anaesth. 1990;37:210–8. doi: 10.1007/BF03005472. [DOI] [PubMed] [Google Scholar]
- 26.Westreich R, Sampson I, Shaari CM, Lawson W. Negative-pressure pulmonary edema after routine septorhinoplasty: Discussion of pathophysiology, treatment, and prevention. Arch Fac Plast Surg. 2006;8:8–15. doi: 10.1001/archfaci.8.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, et al. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol. 2003;285:L785–97. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 28.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304:40–9. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, et al. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:470–9. doi: 10.1164/rccm.200411-1547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, et al. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol. 2006;291:L345–53. doi: 10.1152/ajplung.00453.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lai-Fook SJ, Rodarte JR. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol. 1991;70:967–78. doi: 10.1152/jappl.1991.70.3.967. [DOI] [PubMed] [Google Scholar]
- 32.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiological reviews. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 33.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. The New England journal of medicine. 2005;353:2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 34.Travis KW, Todres ID, Shannon DC. Pulmonary edema associated with croup and epiglottitis. Pediatrics. 1977;59:695–8. [PubMed] [Google Scholar]
- 35.Glasser SA, Siler JN. Delayed onset of laryngospasm-induced pulmonary edema in an adult outpatient. Anesthesiology. 1985;62:370–1. doi: 10.1097/00000542-198503000-00034. [DOI] [PubMed] [Google Scholar]
- 36.Kollef MH, Pluss J. Noncardiogenic pulmonary edema following upper airway obstruction: 7 cases and a review of the literature. Medicine. 1991;70:91–8. doi: 10.1097/00005792-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Holmes JR, Hensinger RN, Wojtys EW. Postoperative pulmonary edema in young, athletic adults. Am J Sports Med. 1991;19:365–71. doi: 10.1177/036354659101900407. [DOI] [PubMed] [Google Scholar]
- 38.Koh MS, Hsu AA, Eng P. Negative pressure pulmonary oedema in the medical intensive care unit. Intensive Care Med. 2003;29:1601–4. doi: 10.1007/s00134-003-1896-7. [DOI] [PubMed] [Google Scholar]
- 39.Rocker GM, Mackenzie MG, Williams B, Logan PM. Noninvasive positive pressure ventilation: Successful outcome in patients with acute lung injury/ARDS. Chest. 1999;115:173–7. doi: 10.1378/chest.115.1.173. [DOI] [PubMed] [Google Scholar]
- 40.Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001;27:1718–28. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 41.Adolph MD, Oliver AM, Dejak T. Death from adult respiratory distress syndrome and multiorgan failure following acute upper airway obstruction. Ear Nose Throat Jr. 1994;73:324–7. [PubMed] [Google Scholar]
- 42.Pelosi P, Jaber S. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol. 2010;23:233–8. doi: 10.1097/ACO.0b013e328335daec. [DOI] [PubMed] [Google Scholar]
- 43.Jaber S, Chanques G, Jung B. Postoperative noninvasive ventilation. Anesthesiology. 2010;112:453–61. doi: 10.1097/ALN.0b013e3181c5e5f2. [DOI] [PubMed] [Google Scholar]
- 44.Wilson GW, Bircher NG. Acute pulmonary edema developing after laryngospasm: Report of a case. J Oral Maxillofac Surg. 1995;53:211–4. doi: 10.1016/0278-2391(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 45.Hamlin W, Schnobel L, Smith B. The patient with noncardiogenic pulmonary edema. J Post Anesth Nursing. 1991;6:43–9. [PubMed] [Google Scholar]
- 46.Mehta VM, Har-El G, Goldstein NA. Postobstructive pulmonary edema after laryngospasm in the otolaryngology patient. Laryngoscope. 2006;116:1693–6. doi: 10.1097/01.mlg.0000231762.91541.3a. [DOI] [PubMed] [Google Scholar]


