Abstract
Control of gene expression by stress-activated protein kinase (SAPK) cascades is crucial for combating cytotoxic stress. Elements of these cascades have been investigated in detail, but regulation of stress signal transduction from the cytoplasm to the nucleus is poorly understood. Herein are reported subcellular localization studies of fission yeast Spc1, a homolog of human p38 and budding yeast Hog1p SAPKs. Stress induces transient nuclear localization of Spc1. Nuclear translocation of Spc1 is coupled with disassociation from its activator kinase Wis1. However, Spc1 does not concentrate in the nucleus of Δwis1 cells; therefore Wis1 does not tether Spc1 in the cytoplasm. Unphosphorylatable forms of Spc1 are dispersed in the cytoplasm and nucleus, even in cells that also produce wild-type Spc1. Thus, Spc1 must be phosphorylated by Wis1 to localize in the nucleus. Nuclear retention of Spc1 requires Atf1, a transcription factor that is the key nuclear substrate of Spc1. Nuclear localization of Atf1 requires Pcr1, a heterodimerization partner of Atf1. These studies show that phosphorylation and association with Atf1 are required for nuclear localization of Spc1.
Keywords: Atf1, fission yeast, nuclear localization, Schizosaccharomyces pombe, Spc1, stress-activated protein kinase
The rapid integration of signals from the plasma membrane to the nucleus is crucial for the appropriate gene expression responses to a vast array of external stimuli (Karin 1994; Hill and Treisman 1995; Karin and Hunter 1995). Cumulative efforts of many laboratories over the past decade have led to the elucidation of several signaling pathways involved in the regulation of the gene expression in response to external stimuli. All of these pathways include three sequential protein kinases, the prototype being the mitogen-activated protein kinase (MAPK) cascade found in mammals (Seger and Krebs 1995). The cascade is composed of a MAPK, a serine–threonine kinase that is activated through phosphorylation at a pair of conserved threonine and tyrosine residues by a MAPK kinase (MAPKK or MEK), which is itself phosphorylated and activated by a MAPKK kinase (MAPKKK or MKK) (Marshall 1994; Cobb and Goldsmith 1995). The temporal regulation of the phosphorylation cascade, as well as the spatial distribution of the elements of the pathway, accounts for the rapid transmission of information from the membrane to the nucleus (Cano and Mahadevan 1995).
Investigations of the subcellular distribution of MAPK cascades have focused on extracellular signal-regulated kinase (ERK) cascades, which respond primarily to proliferation and differentiation signals (Nishida and Gotoh 1993). The MKK and MEK components of these cascades reside in the cytoplasm, whereas ERK translocates from the cytoplasm to the nucleus after it has been phosphorylated by MEK (Chen et al. 1992; Gonzalez et al. 1993; Lenormand et al. 1993; Zheng and Guan 1994). Cytoplasmic localization of MEK requires a nuclear exclusion signal (NES) located in the amino-terminal part of the protein (Fukuda et al. 1996). Translocation of ERK to the nucleus is apparently not dependent on ERK phosphorylation or activation, because ERK mutants that cannot be phosphorylated by MEK or are otherwise catalytically inactive translocate into the nucleus upon cellular activation (Sanghera et al. 1992; Seth et al. 1992; Lenormand et al. 1993). Thus, it appears that the cytoplasmic localization of ERK is dependent on its association with MEK, which acts as a cytoplasmic anchoring protein. Upon activation, ERK dissociates from MEK and translocates into the nucleus (Fukuda et al. 1997). However, ERK does not appear to have a nuclear localization signal (NLS); therefore, it is not known how ERK accumulates in the nucleus.
The extended MAPK family includes a group of protein kinases that are activated by cellular stress. These comprise the JNK and p38 stress-activated protein kinases (SAPKs) in mammals (Davis 1994; Kyriakis et al. 1994). SAPKs are activated by different types of signals such as UV irradiation, heat, inflammatory cytokines, oxidative stress, and high osmolarity (Derijard et al. 1994; Han et al. 1994; Rouse et al. 1994; Waskiewicz and Cooper 1995). Very little is known about the localization of these kinases or their upstream activators. UV was shown to stimulate nuclear translocation of JNK1 (Cavigelli et al. 1995), whereas p38 was found both in the cytoplasm and the nucleus and this pattern was unaffected by stress (Raingeaud et al. 1995). However, as noted by Raingeaud and coworkers, a caveat with the p38 studies is that they relied on ectopic overexpression and thus may not accurately reflect the distribution of endogenous p38.
Coping with stress is an ancient and universal challenge for eukaryotic cells; therefore, it is not surprising that even simple organisms such as yeasts have SAPK cascades that are homologous to the p38 pathway. Of particular interest is the cascade containing the SAPK Spc1 in the fission yeast Schizosaccharomyces pombe. Like its mammalian counterpart, Spc1 is activated by a wide range environmental insults, including high osmolarity, oxidative conditions, heat, UV, and starvation. Spc1, also known as Sty1 or Phh1 (Millar et al. 1995; Shiozaki and Russell 1995; Kato et al. 1996), is activated by the MEK homolog Wis1, which phosphorylates threonine-171 and tyrosine-173 of Spc1. Wis1 is activated by the MKK homolog Wis4, also known as Wik1 and Wak1 (Shiozaki and Russell 1996; Wilkinson et al. 1996; Samejima et al. 1997; Shiozaki et al. 1997). Spc1 is negatively regulated by tyrosine phosphatases Pyp1 and Pyp2 via direct dephosphorylation of tyrosine-173 (Millar et al. 1995; Shiozaki and Russell 1995; Degols et al. 1996). Interestingly, the Wis4–Wis1–Spc1 kinase cascade is linked to G2–M cell cycle control. In optimal growth conditions spc1− mutants exhibit a moderate delay of the onset of mitosis that is greatly exacerbated upon stress (Shiozaki and Russell 1995). The only substrate known for Spc1 is the transcription factor Atf1, which is responsible for Spc1-dependent regulation of gene expression in response to various forms of stress, although atf1 mutants do not exhibit the mitotic delay phenotype of spc1 cells (Shiozaki and Russell 1996; Wilkinson et al. 1996).
The discovery of a conserved stress response mechanism in fission yeast presents a unique opportunity to use genetic tools to investigate the spatial organization of a SAPK cascade. These studies are described in this report. These experiments were performed with wild-type and mutant proteins expressed at normal physiological levels and thus are not subject to the caveats and complications associated with the previous studies of mammalian ERK and SAPK cascades. At the most fundamental level our findings concur with the previous work of mammalian ERKs, in that they identify Spc1 nuclear translocation as the primary mechanism of signal transduction from the cytoplasm to the nucleus. However, our studies present an entirely different picture of how nuclear translocation of an SAPK is regulated.
Results
Nuclear translocation of Spc1 induced by stress
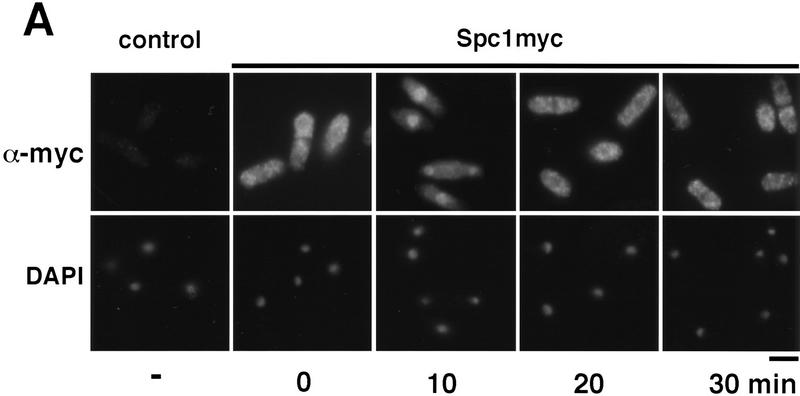
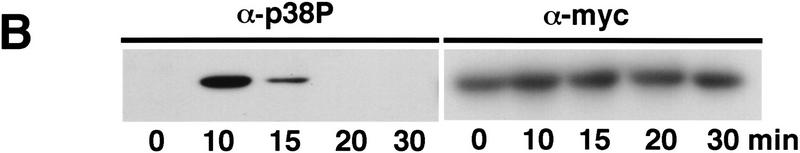
The subcellular distribution of Spc1 was examined by indirect immunofluorescence microscopy. These studies utilized strains in which the chromosomal copy of spc1 encoded a protein containing 12 tandem copies of the myc epitope (see Materials and Methods). These cells appeared identical to wild-type, therefore, the function of Spc1 was not impaired by the epitope tag. The myc-tagged Spc1 was detected using anti-myc monoclonal antibodies. Control experiments with wild-type cells encoding untagged Spc1 revealed no staining (Fig. 1A). Before stress, Spc1 was present in both the cytoplasm and nucleus, although in most cells the nuclear signal was weaker (Fig. 1A). Osmotic stress (0.6 m KCl) caused rapid relocalization of Spc1 into the nucleus. Nuclear accumulation of Spc1 was apparent within 5 min (F. Gaits and P. Russell, unpubl.) and maximal after ∼10 min of stimulation (Fig. 1A). The nuclear accumulation of Spc1 was remarkably transient. Spc1 was quite obviously excluded from the nucleus within 20 min after the initial exposure to stress (Fig. 1A). Evaluation of Spc1 activation, as assayed by tyrosine phosphorylation, revealed that activation paralleled nuclear localization, with maximum activation occurring at 10 min followed by dephosphorylation within 20 min (Fig. 1B). These findings suggest that stress-stimulated nuclear translocation of Spc1 is a major mechanism by which stress signals are transduced from the cytoplasm to the nucleus.
Figure 1.
Spc1 localization with stress. (A) Spc1 translocates to the nucleus upon stress. Strain GD1942, in which the genomic copy of spc1+ encodes an epitope-tagged form of Spc1 that has 12 copies of a myc tag at the carboxyl terminus, was grown to mid-log phase at 30°C in YES medium. Aliquots were harvested before and after osmostress in YES + 0.6 m KCl at the indicated time and fixed in −80°C methanol. After permeabilization, cells were incubated with the anti-myc antibody and Cy3 goat anti-mouse as a secondary antibody to visualize Spc1myc. The nuclei were stained with DAPI. Bar, 10 μm. (B) In parallel, some aliquots were frozen in liquid nitrogen and total cellular homogenates were prepared to evaluate Spc1 phosphorylation. Proteins were subjected to SDS-PAGE and electroblotted, and immunodetection of Spc1 was achieved using the anti-p38 phosphorylated antibody (α-p38P). The level of proteins in each lane was evaluated using the anti-myc antibody (α-myc).
Wis1, the kinase that activates Spc1, is located in the cytoplasm
Wis1 is the sole activator kinase of Spc1 in vivo. We therefore determined the localization of Wis1. This study used a strain that expressed myc-tagged Wis1 from the wis1+ genomic locus. This strain appeared identical to wild-type; therefore, the epitope tag had no effect on Wis1 function in vivo. Wis1 localized in the cytoplasm; no significant Wis1 signal was detected in the nucleus before or during osmotic stress (Fig. 2A). These observations suggest that Wis1 may be actively excluded from the nucleus. Wis1 has several sequences that closely match the NES recently characterized in a mammalian MEK.
Figure 2.
(A) Localization of Wis1 MAPKK. Strain GD1892, bearing a genomic copy of wis1+ tagged with 12 copies of the myc epitope at the carboxyl terminus, was grown to mid-log phase at 30°C in YES medium. Cells were harvested before and after stress in YES + 0.6 m KCl at the indicated time and fixed in cold methanol. Wis1 was visualized by immunofluorescence using anti-myc antibody. Bar, 10 μm. (B) Strain FG2150 bearing a genomic copy of spc1+ tagged with two copies of the HA-epitope and six consecutive histidine residues on its carboxyl terminus, as well as a genomic copy of wis1+ tagged with 12 copies of the myc epitope, was grown to mid-log phase and harvested before and after KCl stress at the indicated times. Total cell homogenates were then prepared under native conditions, and Spc1 was purified using Ni2+–NTA–agarose beads. The precipitates were analyzed by immunoblotting after SDS-PAGE. Wis1 was detected with the anti-myc and Spc1 with anti-HA epitope antibodies.
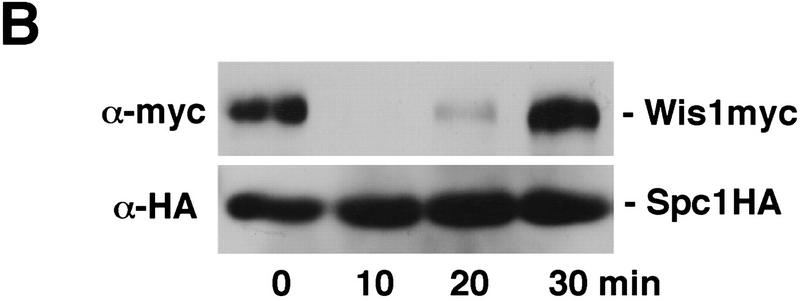
Nuclear relocation of Spc1 correlates with disassociation from Wis1 in the cytoplasm
Next we investigated physical interactions involving Wis1 and Spc1. We constructed a strain bearing a chromosomal copy of wis1 expressing myc-tagged Wis1 protein and a chromosomal copy of spc1 expressing Spc1 tagged with the HA epitope followed by six histidine residues. Spc1HA6his was purified under native conditions with Ni+-NTA-agarose. Wis1 co-precipitated with Spc1 before stress (Fig. 2B). Upon activation of the pathway by osmotic stress, the amount of Wis1 associated with Spc1 decreased dramatically. This change was quite transient with Wis1 becoming fully reassociated with Spc1 within 30 min of the initial stress. This process paralleled the changes in Spc1 phosphorylation and localization (Fig. 1). These findings indicate that the affinity between Spc1 and Wis1 is reduced in response to stress. The decreased association between the two proteins may allow translocation of Spc1 into the nucleus.
Spc1 is predominantly cytoplasmic in Δwis1 cells
Our findings were consistent with a model in which Wis1 acts as a cytoplasmic anchor for Spc1. In this model Spc1 has a tendency to localize in the nucleus, perhaps because of an interaction with another protein that transports Spc1 into the nucleus. Stress causes Spc1 to be released from Wis1, allowing translocation of Spc1 into the nucleus. This model makes a number of important predictions. One is that there should be roughly equal amounts of Wis1 and Spc1, assuming that each Wis1 protein interacts with a single Spc1 protein. This prediction was tested by performing immunoblots of strains that expressed myc-tagged Spc1 and Wis1 from their respective genomic loci. This analysis revealed that Spc1 is much more abundant than Wis1 (Fig. 3A). These findings are inconsistent with a simple mechanism of cytoplasmic retention of Spc1 by Wis1, although they cannot exclude a more complex model in which each Wis1 protein anchors many Spc1 proteins.
Figure 3.
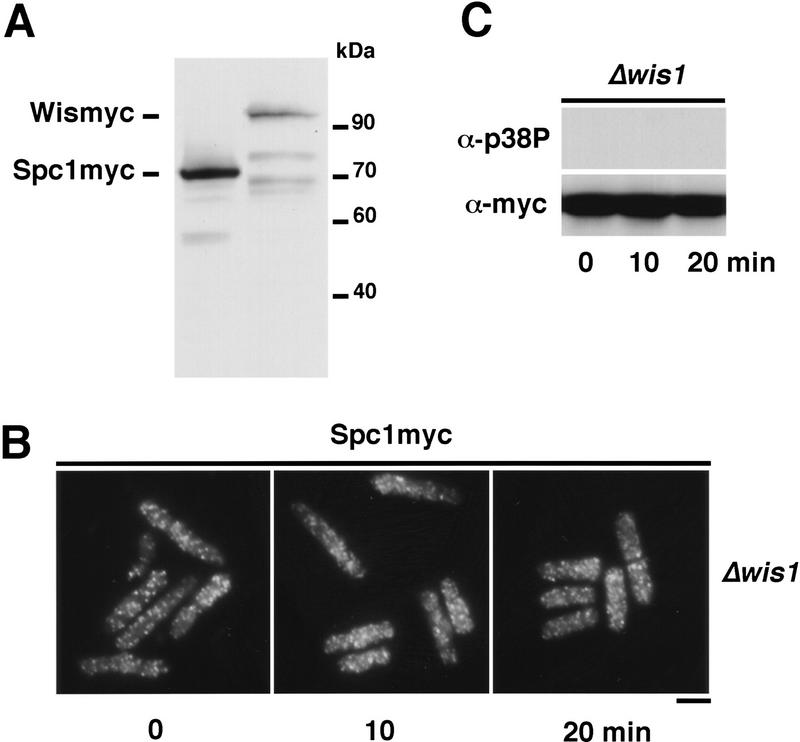
Wis1 activity is necessary to translocate Spc1 to the nucleus. (A) Spc1 is more abundant than Wis1. Total lysate of strains bearing myc-tagged genomic copies of Spc1 or Wis1, respectively, strains GD1942 and GD1892, were prepared and proteins separated by SDS-PAGE. The levels of Spc1 and Wis1 in the extract were analyzed by immunoblotting with the anti-myc antibody, the loading control being realized by amidoblack staining of the membrane prior to blotting. (B) Strain FG2151 (bearing the myc-tagged genomic copy of spc1+ and deleted for wis1) was grown to mid-log phase in YES medium and cells were harvested before and after stress with 0.6 m KCl. They were fixed in cold methanol, and Spc1 localization was examined by immunofluorescence using the anti-myc antibody. Bar, 10 μm. (C) Aliquots of cells were collected before and after KCl stress, and the phosphorylation status of Spc1 was analyzed by immunoblotting with the anti-p38P after SDS-PAGE. The loading control was realized by probing the membranes with anti-myc antibody.
A more definitive prediction of the model is that elimination of Wis1 should cause Spc1 to concentrate in the nucleus. We therefore investigated the behavior of Spc1 in a strain in which wis1 was deleted. Contrary to the model, we found that Spc1 was evenly distributed between the nucleus and cytoplasm in a Δwis1 mutant (Fig. 3B). This pattern of Spc1 localization was unchanged following exposure to osmotic stress (Fig. 3B). Consistent with previous studies, no tyrosine phosphorylation of Spc1 was detected in the Δwis1 mutant (Fig. 3C). These findings argue against a model in which the cytoplasmic localization of Spc1 is dependent on Wis1 acting as a cytoplasmic anchor.
Activating phosphorylation is necessary for nuclear accumulation of Spc1
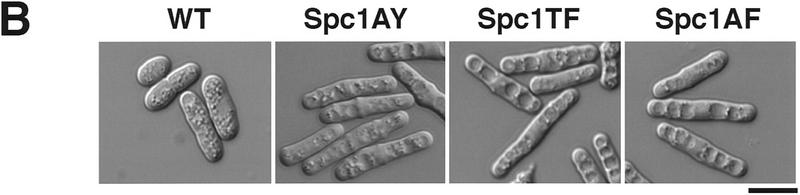
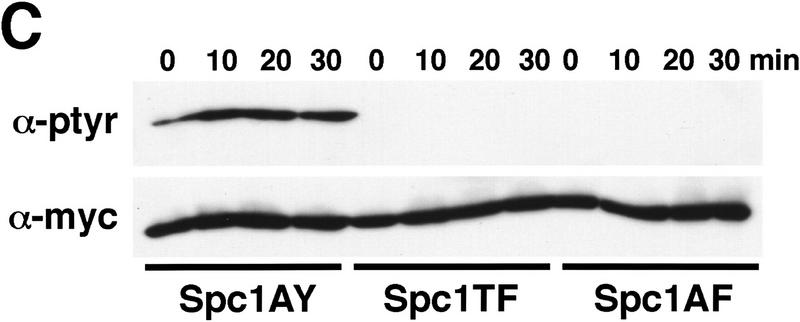
Our findings showed that Wis1 activity is required for stress-induced nuclear relocation of Spc1. Like all of the members of the greater MAPK family, Spc1 possesses conserved threonine and tyrosine residues that are phosphorylated by its cognate MEK. Wis1 phosphorylates threonine-171 and tyrosine-173 in the motif TGY (Millar et al. 1995; Shiozaki and Russell 1995). To investigate further the role of these phosphorylations in regulating the localization of Spc1, three spc1 mutations were engineered to encode the following proteins: Spc1AY (T171A), Spc1TF (Y173F), and Spc1AF (T171A, Y173F). Chromosomal replacements were performed with the myc-tagged spc1 mutant genes, and the resulting phenotypes evaluated. Cells were streaked on standard YES (yeast extract and glucose) medium or medium supplemented with 1 m KCl. Wild-type, Δspc1, and the mutants expressing unphosphorylatable forms of Spc1 all grew well on YES medium. However, on YES + KCl the spc1AY, spc1TF, spc1AF, and Δspc1 mutants exhibited strong osmotic sensitivity and were unable to form colonies (Fig. 4A). Microscopic observation showed that the spc1AY, spc1TF, and spc1AF mutants displayed an elongated morphology phenotype that was identical to Δspc1 cells (Fig. 4B). Interestingly, Spc1AY became phosphorylated on tyrosine in response to stress, showing that the T171A mutation did not disrupt interaction with Wis1 (Fig. 4C). These findings confirm that Spc1 activation requires phosphorylation on both threonine-171 and tyrosine-173.
Figure 4.
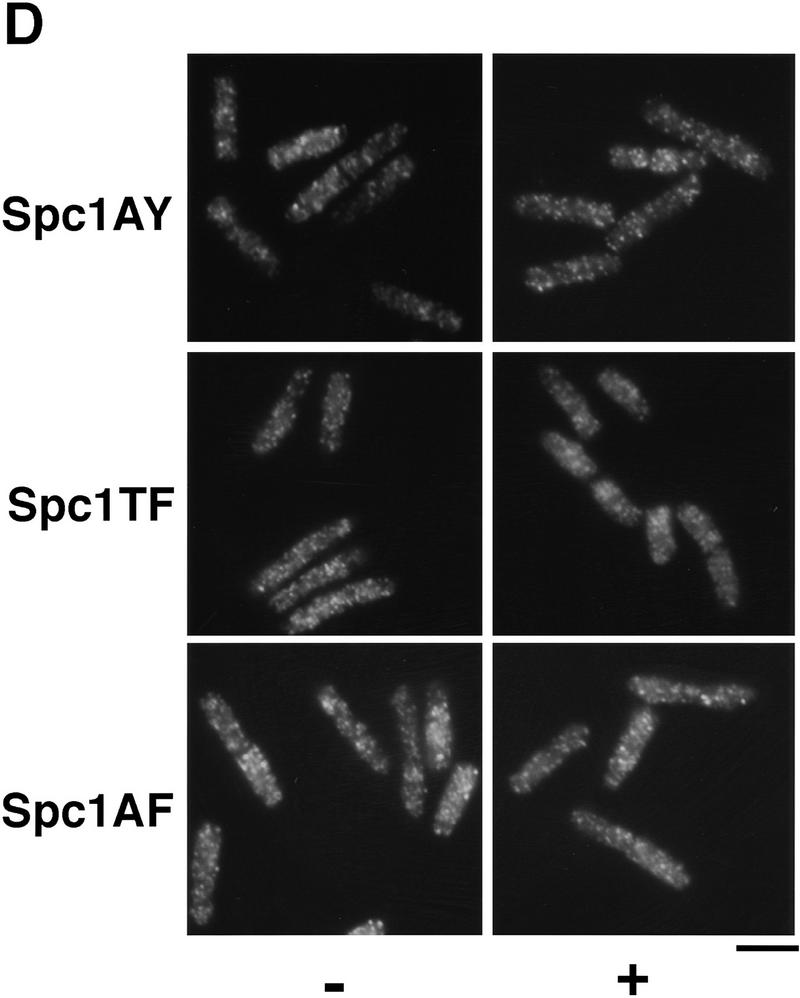
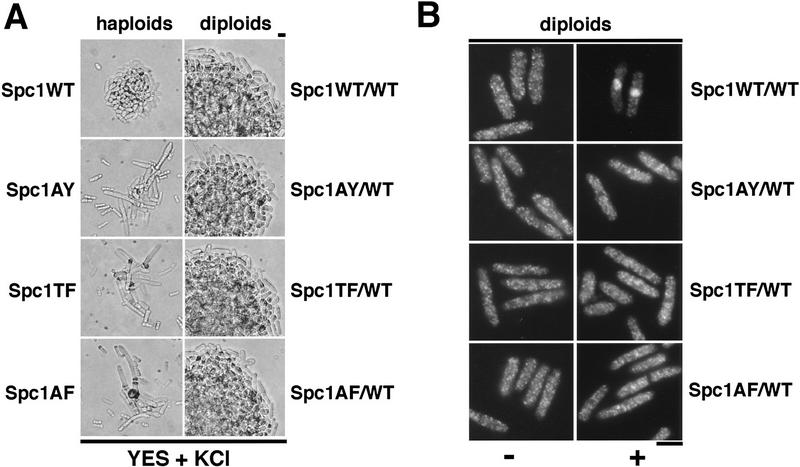
Spc1 phosphorylation mutants are sensitive to high osmolarity and unable to relocate to the nucleus upon osmotic stress. (A) Strains FG2153 (genomic spc1+ replaced by spc1T171Amyc), FG2154 (genomic spc1+ replaced by spc1Y173Fmyc), FG2155 (genomic spc1+ replaced by spc1T171A Y173Fmyc), KS1366 (Δspc1), and PR109 (wild type) were streaked out onto YES agar plates supplemented with or without 1 m KCl, and incubated for 4 days at 32°C to test their ability to form colonies. (B) FG2153, FG2154, FG2155, and PR109 cells were streaked out on EMM2 agar plates. The phenotype of the cells was then analyzed by phase contrast microscopy after 2 days at 32°C. Bar, 10 μm. (C) The Spc1 mutant strains FG2153, FG2154 and FG2155 were grown to mid-log phase in YES medium and stressed with 0.6 m KCl before being harvested and frozen in liquid nitrogen. Total lysates were then prepared, and the phosphorylation status of the Spc1 mutants was analyzed by immunoblotting after SDS-PAGE using the anti-phosphotyrosine antibody (α-pTyr). The loading was checked using the anti-myc antibody (α-myc). (D) Strains FG2153 (Spc1AY), FG2154 (Spc1TF), FG2155 (Spc1AF) were grown to mid-log phase in YES medium, harvested before or after 10 min of incubation in YES supplemented by 0.6 m KCl, and fixed in cold methanol. The cellular localization of the mutant Spc1 was followed by indirect immunofluorescence with the anti-myc antibody. Bar, 10 μm.
Next we determined the localization of Spc1AY, Spc1TF, and Spc1AF. These proteins failed to accumulate in the nucleus after osmotic stress (Fig. 4D), even at late time points (F. Gaits and P. Russell, unpubl.). The localization pattern of the mutant Spc1 proteins appeared identical to wild-type Spc1 in Δwis1 cells (Fig. 3B). These findings show that Wis1-catalyzed phosphorylation on both threonine-171 and tyrosine-173 is somehow required for nuclear localization of Spc1.
Restoration of Spc1 activity does not rescue the localization defect of unphosphorylatable Spc1
One interpretation of our data was that Spc1 activity is required for nuclear translocation. For example, Spc1 may phosphorylate a protein that promotes nuclear translocation of Spc1. An alternative explanation is Spc1 must be phosphorylated on threonine-171 and tyrosine-173 to localize in the nucleus. For example, phosphorylation of these residues may promote association with a nuclear transport system or stabilize interaction with a nuclear anchor. To distinguish between these hypotheses, the localization of mutant Spc1 was monitored in heterozygous diploid strains containing mutant and wild-type spc1 alleles. A prediction of the first hypothesis is that restoration of Spc1 activity should rescue the localization defect of unphosphorylatable Spc1. Conversely, the second hypothesis predicts that unphosphorylatable forms of Spc1 should be unable to concentrate in the nucleus of cells that express wild-type Spc1.
Diploid strains having one copy of wild-type spc1+ and one copy of the mutant genes were not sensitive to osmotic stress when compared to homozygous wild-type diploid strains (Fig. 5A), indicating that the spc1AY, spc1TF, and spc1AF alleles behaved as typical recessive mutations and the stress-activated kinase cascades were fully operable. In the homozygous wild-type diploid (spc1+/spc1+:myc), myc-tagged Spc1 concentrated in the nucleus in response to osmotic stress (Fig. 5B). In contrast, in the heterozygous diploids containing one copy of myc-tagged mutant spc1 and one copy of the wild-type spc1+, the mutant forms of Spc1 failed to accumulate in the nucleus in response to stress (Fig. 5B). These data establish that Spc1 must be phosphorylated on threonine-171 and tyrosine-173 to accumulate in the nucleus.
Figure 5.
Restoration of Spc1 activity does not rescue the localization defect of the unphosphorylatable mutants. (A) The haploid strains FG2153 (Spc1AY), FG2154 (Spc1TF), and FG2155 (Spc1AF), and the diploid strains FG2201 (Spc1WT/WT), FG2202 (Spc1AY/WT), FG2203 (Spc1TF/WT), and FG2204 (Spc1AF/WT) were streaked out onto YES agar plates supplemented with 1 m KCl and incubated for 3 days at 32°C. The phenotype of the cells was then analyzed by phase-contrast microscopy. (B) The diploid strains FG2201 (Spc1WT/WT), FG2202 (Spc1AY/WT), FG2203 (Spc1TF/WT), and FG2204 (Spc1AF/WT) were grown to mid-log phase in YES medium. Cells were then harvested before and after 10 min of KCl stress and fixed with cold methanol. The localization of the Spc1 proteins was then determined as described previously. Bar, 10 μm.
Transcription factor Atf1 is a nuclear anchor for Spc1
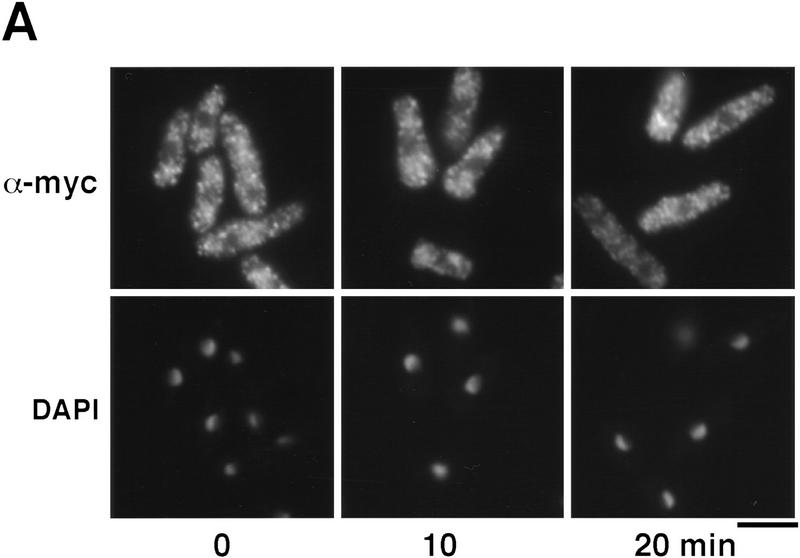
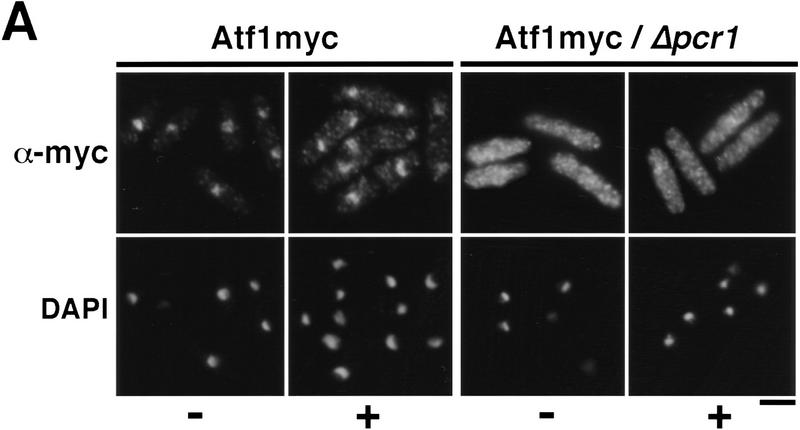
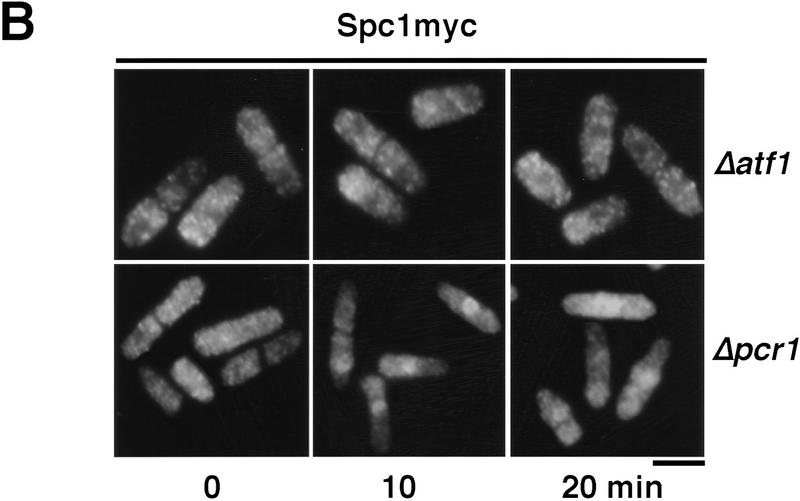
Having established the requirement of Wis1-catalyzed phosphorylation for nuclear localization of Spc1, we then turned our attention to the role of the transcription factor Atf1, a key substrate of Spc1 (Shiozaki and Russell 1996; Wilkinson et al. 1996). First, using the strategies described above, we determined the localization of Atf1. The myc-tagged Atf1 was constitutively localized in the nucleus, both before and during exposure to osmotic stress (Fig. 6A). As already shown at the transcriptional level (Shiozaki and Russell 1996; Wilkinson et al. 1996), immunofluorescence indicates that the quantity of Atf1 increases after KCl treatment (Fig. 6A). We then examined the localization of Spc1 in cells lacking Atf1. Osmotic stress failed to induce the accumulation of Spc1 in the nucleus of Δatf1 cells (Fig. 6B). Importantly, Spc1 became tyrosine-phosphorylated in Δatf1 cells at a level comparable with wild type (Fig. 6C). These findings are most simply interpreted to indicate that the transient nuclear localization of activated Spc1 is dependent on an association with Atf1.
Figure 6.
Atf1 acts as a nuclear anchor for Spc1. (A) The localization of Atf1 was determined by immunofluorescence performed on strain FG2156 in which the genomic atf1+ is replaced by a myc-tagged copy, and in the FG2157 (Δpcr1 and bearing the genomic myc-tagged atf1+). Cells were harvested before and after 10 min of KCl stress and fixed in cold methanol. The immunofluorescence was realized using the anti-myc antibody. (B) The strain GD1952 (Δatf1 and bearing the genomic myc-tagged copy of spc1+) and FG2158 (Δpcr1 and bearing the myc-tagged genomic spc1+) were grown to mid-log phase, and cells were harvested before and after KCl stress. For each time point, an aliquot was fixed in cold methanol for immunofluorescence analysis. The localization of Spc1 was determined with the anti-myc antibody. (C) The phosphorylation status of Spc1 in strains GD1952 and FG2158 was followed with the anti-p38P and the loading checked with the anti-myc antibodies, as described previously. Bar, 10 μm.
Nuclear localization of Atf1 requires its cofactor Pcr1
Atf1 forms a heterodimer with the transcription factor Pcr1 (Watanabe and Yamamoto 1996). Δpcr1 and Δatf1 strains exhibit similar levels of stress sensitivity, and Δpcr1 cells are defective in the expression of most genes that are dependent on Atf1 for expression. In an attempt to gain some initial insights into the mechanism by which Atf1 is retained in the nucleus, we investigated whether Pcr1 was required for the nuclear accumulation of Atf1. In Δpcr1 cells, Atf1 was equally distributed between the nucleus and cytoplasm (Fig. 6A). As was the case for Δatf1 cells, Spc1 was tyrosine phosphorylated in Δpcr1 cells (Fig. 6C). The phosphorylation of Spc1 was slighty prolonged, reflecting the difficulty of cells to adapt to stress due to the defect of transcription of stress–response genes (Shiozaki and Russell 1996; Wilkinson et al. 1996). Nuclear accumulation of Spc1 was greatly reduced in Δpcr1 cells, although a weak nuclear signal was detected in some cells during osmotic stress (Fig. 6B). These findings suggest that Atf1 nuclear retention requires dimerization with Pcr1. Moreover, the presence of Atf1 is not in itself sufficient to localize Spc1 in the nucleus because Spc1 is excluded from the nucleus after stress when the quantity of Atf1 is maximal (Figs. 1A and 6A). Instead, after activation of the cascade, Atf1 is required in the nucleus for nuclear retention of Spc1.
Discussion
Our studies have explored the spatial regulation of three sequential elements of the stress signal transduction system: The kinases Wis1 and Spc1 and the transcription factor Atf1. We have established that Wis1 is detected completely in the cytoplasm, whereas Atf1 is found exclusively in the nucleus. These patterns of localization are unaffected by stress. In contrast, stress induces a rapid relocalization of Spc1 from the cytoplasm to the nucleus. Spc1 nuclear import closely coincides with activation of Spc1 as assayed by tyrosine phosphorylation, as well as with transcriptional induction of stress response genes. These observations suggest strongly that the rapid nuclear import of activated Spc1 is the primary mechanism by which the stress signal is transmitted from the cytoplasm to the nucleus.
We have also addressed the mechanism regulating the localization of Spc1. Our studies have tested the hypothesis that Wis1 acts as a cytoplasmic anchor for Spc1. Generation of this hypothesis was based on studies of vertebrate ERKs. In mammalian cells these kinases are translocated into the nucleus following growth factor stimulation (Sanghera et al. 1992; Seth et al. 1992; Lenormand et al. 1993). However, high expression of ERKs, accomplished either by plasmid transfection or by protein microinjection, leads to nuclear localization by a mechanism that is independent of growth factors. Nuclear localization of ERKs was prevented by microinjection of large amounts of a protein containing the NES and MAPK-binding region of a cognate MEK (Fukuda et al. 1997). These findings led to the hypothesis that MEK is required to retain inactive ERK in the cytoplasm. This hypothesis generates a number of predictions, the most crucial being that elimination of cognate MEKs should cause nuclear localization of ERKs. This prediction is not easily tested with mammalian cells, but the genetical advantages of fission yeast provided an ideal system for testing the model as it pertains to the SAPK cascade. Contrary to the key prediction of the MEK–anchor model, we found that Spc1 was distributed throughout the cell in Δwis1 strains. Spc1 did not appear to be excluded from the nucleus, but the majority of the Spc1 protein was in the cytoplasm. Thus, in unstressed cells, the cytoplasmic localization of the majority of Spc1 protein does not depend on an interaction with Wis1. It is very unlikely that Spc1 is anchored in the cytoplasm through interactions with another MAPKK homolog, because Spc1 tyrosine phosphorylation is abolished in a Δwis1 cell.
How is Wis1 required for nuclear accumulation of Spc1? Wis1 activates Spc1 by phosphorylating threonine-171 and tyrosine-173; therefore, we asked whether nuclear accumulation of Spc1 requires phosphorylation of these sites. We found that Spc1 mutant proteins that had substitutions at either or both phosphorylation sites failed to concentrate in the nucleus in response to stress. The Spc1 localization patterns of these mutants were very similar to the staining pattern of Spc1 protein in Δwis1 cells. These findings led to the obvious conclusion that Spc1 protein fails to accumulate in the nucleus of Δwis1 cells because it cannot be phosphorylated.
Phosphorylation of threonine-171 and tyrosine-173 is required for Spc1 kinase activity, suggesting that Spc1 kinase activity is required for nuclear import of Spc1. In such a scenario one could propose that a substrate of Spc1 is involved in regulating the nuclear import of Spc1. If this model was correct, we would have expected that nuclear import of the Spc1AY, Spc1TF, and Spc1AF mutants be restored by coexpression of active Spc1. However, our studies showed clearly that this prediction was incorrect. The mutant forms of Spc1 did not accumulate in the nucleus of diploid cells in which half of the Spc1 was expressed from a wild-type copy of spc1+, even though these cells appeared wild type in terms of cell size and sensitivity to osmotic stress. These findings led to the conclusion that Spc1 protein must be phosphorylated on threonine-171 and tyrosine-173 to localize in the nucleus. This fact contrasts sharply with studies of MAPK localization in mammalian cells, which have shown that unphosphorylatable forms of ERK2 localize in the nucleus, at least when they are overexpressed (Lenormand et al. 1993).
Why is phosphorylation of threonine-171 and tyrosine-173 essential for nuclear localization of Spc1? One possible explanation is that phosphorylation of these sites stabilizes an interaction of Spc1 with a nuclear anchor protein. Atf1 is a key substrate of Spc1 and is localized in the nucleus; therefore, we asked whether Atf1 was required for nuclear localization of Spc1. We found that nuclear accumulation of Spc1 was abolished in an Δatf1 strain, showing that Atf1 is required for retention of Spc1 in the nucleus. In this sense Atf1 is a nuclear anchor for activated Spc1. These findings suggest a model in which Wis1-catalyzed phosphorylation of Spc1 enhances the ability of Spc1 to associate with Atf1. Thus, it suggests that the stabilized association of phosphorylated Spc1 with Atf1 causes the nuclear accumulation of Spc1.
Little is known about the role of activating phosphorylation of MAPK homologs in regulating association with substrate transcription factors. There is no evidence suggesting that phosphorylation stimulates substrate binding; many of the in vitro studies performed to identify interaction domains have been carried out with unphosphorylated MAPK homologs. However, a careful analysis of the effect of phosphorylation on binding affinities has not been reported. Studies of the mammalian JNK1 and JNK2 stress-activated kinases identified a small region near the catalytic pocket that accounted for a ∼25-fold difference in binding affinity for the amino-terminal domain of c-Jun (Kallunki et al. 1994). However, structure determinations of the dephosphorylated and phosphorylated forms of ERK2 suggest that the binding groove of JNKs does not change as a consequence of phosphorylation (Canagarajah et al. 1997; Wang et al. 1997). These observations have prompted speculation that transcription factors such as c-Jun should bind to cognate SAPKs independent of the state of phosphorylation. In our system, the phosphorylation mutants Spc1AY, Spc1TF, and Spc1AF associate with the GST–Atf1 fusion protein in vivo (F. Gaits and P. Russell, unpubl.), indicating that phosphorylation of Spc1 is not required for binding to Atf1.
An alternative interpretation of our observations is that phosphorylation of threonine-171 and tyrosine-173 stimulates nuclear translocation of Spc1, at which point Atf1 is required to retain Spc1 in the nucleus. In this model phosphorylation might be required for (1) release of Spc1 from a cytoplasmic anchor; (2) association of Spc1 with a nuclear import protein; or (3) shielding of Spc1 from a nuclear export system. The first explanation appears unlikely because Spc1 does not localize in the nucleus when highly overexpressed in Δwis1 cells (F. Gaits and P. Russell, unpubl.); therefore, a cytoplasmic anchor, if it exists, cannot be saturated by very large amounts of Spc1. The other explanations cannot be excluded and thus deserve attention in future studies.
These studies raise an additional important point. The stress-induced relocalization of Spc1 from the cytoplasm to the nucleus can be very transient, with Spc1 becoming quite clearly absent from the nucleus 10–20 min after the peak nuclear signal is detected. This change correlates closely with the tyrosine dephosphorylation of Spc1 and the reduction of stress-induced gene expression. These observations raise the question of the connection between export of Spc1 from the nucleus and dephosphorylation of Spc1. Export of Spc1 from the nucleus may lead to dephosphorylation by cytoplasmic phosphatases. Alternatively, dephosphorylation of Spc1 by nuclear phosphatases may induce the export of Spc1 from the nucleus. Experiments to clarify the interrelation between phosphatase activity and Spc1 relocalization are currently under way.
Materials and methods
Media, strains, and general techniques
S. pombe strains used in this study are listed in Table 1. They are derivatives of 972 h− and 975 h+ (Mitchison 1970). Growth media and basic genetic and biochemical techniques for fission yeast have been described (Alfa et al. 1993). Yeast extract medium (YES) and synthetic minimal medium (EMM2) were used in growing S. pombe cells.
Table 1.
S. pombe strains used in this study
| Strains
|
Genotype
|
Source or reference
|
|---|---|---|
| PR109 | h− | lab stock |
| KS1366 | h− spc1::ura4+ | Shiozaki and Russell (1995) |
| GD1942 | h− spc1-12 myc (ura4+) | this study |
| GD1952 | h− spc1-12 myc (ura4+) atf1::ura4+ | this study |
| GD1892 | h− wis1-12 myc (ura4+) | this study |
| FG2150 | h− spc1 HA6H (ura4+) wis1-12 myc (ura4+) | this study |
| FG2151 | h− spc1-12 myc (ura4+) wis1::ura4+ | this study |
| FG2153 | h+ spc1AY-12 myc (ura4+) | this study |
| FG2154 | h+ spc1TF-12 myc (ura4+) | this study |
| FG2155 | h+ spc1AF-12 myc (ura4+) | this study |
| FG2156 | h− atf1-12 myc (ura4+) | this study |
| FG2157 | h− his7-366 atf1-12 myc (ura4+) pcr1::his7+ | this study |
| FG2158 | h+ his7-366 spc1-12 myc (ura4+) pcr1::his7+ | this study |
| FG2201 | h−/h+ ade6–M210/ade6–M216 his7-366/his7-366 spc1 HA6H (ura4+/spc1-12 myc (ura4+) | this study |
| FG2202 | h−/h+ ade6–M210/ade6–M216 his7-366/his7-366 spc1 HA6H (ura4+)/spc1AY-12 myc (ura4+) | this study |
| FG2203 | h−/h+ ade6–M210/ade6–M216 his7-366/his7-366 spc1 HA6H (ura4+)/spc1TF-12 myc (ura4+) | this study |
| FG2204 | h−/h+ ade6–M210/ade6–M216 his7-366/his7-366 spc1 HA6H (ura4+)/spc1AF-12 myc (ura4+) | this study |
All strains are leu1-32 ura4-D18.
Construction of the myc-tagged alleles
To introduce an epitope tag at the carboxyl terminus of the proteins, the pRIP-12myc vector was designed as follows. A cassette containing 12 tandem copies of the myc epitope was amplified by PCR using a 5′ primer containing a KpnI site. This DNA fragment was cloned into the SmaI site of the pRIP42 vector (Basi et al. 1993). The resultant plasmid was digested with PstI and NdeI and religated to eliminate the nmt1 promoter sequence, producing pRIP-12myc. Sequences encoding the carboxyl terminus of different proteins were amplified by PCR and cloned in this vector as BamHI or BamHI–KpnI fragments, except for the atf1 fragment, which was cloned as a BglII–KpnI fragment. The following oligonucleotides were used: spc1 5′ primer CGCGGATCCATGGCAGAATTTATTCGTACA-C and 3′ primer CGGGGTACCTTGGATTGCAGTTCATTATCCATG; wis1 5′ primer GGTCAGACTTGGCAGATCTACGTCCAG and 3′ primer CGGGGTACCTTGCTTCTTTTTTCACCTTTCTCTTTAAGAGCG; atf1 5′ primer GGAAGATCTATGTCCCCGTCTCCCGTCAATACTTCC and 3′ primer CGGGGTACCACGTACCCTAAATTGATTCTTTGAGC. The resultant plasmids were used for integrative transformation of wild-type cells (PR109) after linearization at the NruI site of spc1, the HpaI site of wis1, or the AflII site of atf1.
Construction of spc mutants
Mutations in the spc1 sequence were made as follows. The 5′ end of spc1 (codons 1–177) was amplified by PCR with the 5′ primer used for the myc-tagged constructs and the mutagenic 3′ primers: CGAGTAGAAACATAGCCAGCCATTTGAGG for the T171A mutant; CGAGTAGAAACGAAGCCCGTCATTTGAGG for the Y173F mutant; and CGAGTAGAAACGAAGCCAGCCATTTGAGG for the T171A Y173F double mutant. The underlined sequence indicates the mutated codons. The 3′ end of spc1 (codons 168–341) was amplified with the 3′ primer used for the myc-tagged construct and the mutagenic 5′ primers: CCTCAAATGGCTGGCTATGTTTCTACTCG for the T171A mutant; CCTCAAATGACGGGCTTCGTTTCTACTCG for the Y173F mutant; and CCTCAAATGGCTGGCTTCGTTTCTACTCG for the T171A Y173F double mutant. Then, for each mutant, the 5′ and 3′ PCR products were mixed in equal ratios and amplified with the 5′ and 3′ primers used for the myc-tagged constructions. The mutated spc1 sequences were then subcloned in the pRIP-12myc plasmid after digestion with BamHI and KpnI and sequenced. The resulting plasmids were then linearized with BglII and used to transform wild-type cells (PR110).
Stress treatment of cells
Cells were grown to early logarithmic phase (OD600 = ∼0.5) at 30°C. High-osmolarity stress was achieved by adding a one-third volume of prewarmed medium containing 2.4 m KCl to the culture to obtain a final KCl concentration of 0.6 m. All experiments used YES medium unless otherwise indicated. For the immunoblotting analysis, cells were harvested by filtration and immediately frozen in liquid nitrogen.
Immunoblotting of Spc1
Cells (10 OD600) were lysed in 0.2 ml of lysis buffer (50 mm Tris at pH 8.0, 150 mm NaCl, 5 mm EDTA, 10% glycerol, 50 mm NaF, 1 mm Na3VO4, 1 μg/ml each of leupeptin, aprotinin, and pepstatin, 1 mm PMSF) by breaking cells with glass beads under vigorous agitation. After centrifugation (10,000x for 10 min), the protein concentration in the supernatant was estimated by measuring A280. About 150 μg of total protein was loaded for each sample and resolved by SDS-PAGE. The tyrosine phosphorylation status of Spc1 was determined by immunoblotting with the anti-phosphotyrosine p38 (tyr-182) MAPK antibody (New England Biolabs) or the anti-phosphotyrosine 4G10 (Upstate Biotechnology) antibody. The amount of Spc1–myc loaded was evaluated with the anti-myc 9E10 (BabCo) antibody. Immunoreactive bands were revealed with horseradish peroxidase-conjugated secondary antibodies and the SuperSignal Western blotting detection system (Pierce).
Purification of Spc1 under native conditions
About 20 OD600 cells of strain FG2150, bearing a chromosomal copy of spc1 encoding Spc1 tagged with two HA epitopes and 6 histidine residues, and a chromosomal copy of wis1 encoding Wis1 protein tagged with 12myc were harvested and lysed in lysis buffer containing 50 mm Tris at pH 8.0, 150 mm NaCl, 5 mm EGTA, 10 mm imidazole, 0.2% NP-40, 10% glycerol, 50 mm NaF, 1 mm Na3VO4, 1 μg/ml each of leupeptin, aprotinin, and pepstatin, and 1 mm PMSF. After centrifugation, the supernatants were incubated with Ni2+–NTA–agarose beads, resolved by SDS-PAGE, and the purified proteins were detected by immunoblotting with either the anti-HA 12CA5 antibody for Spc1 or the anti-myc 9E10 antibody for Wis1.
Indirect immunofluorescence microscopy
Mid-log phase cells were harvested by filtration on glass-fiber filters and fixed by immersion in −80°C methanol for 10 min. After three washes in PEM buffer (100 mm PIPES, 1 mm EGTA, 1 mm MgSO4 at pH 6.9), the cell wall was digested at 37°C for 10 min with 0.5 mg/ml Zymolyase 100T (Seikagaku America, Rockville, MD) in PEMS buffer (PEM buffer supplemented with 1 m sorbitol), followed by permeabilization with 1% Triton X-100. After washes, cells were blocked for 30 min at room temperature in PEMBAL buffer (PEM buffer at pH 6.9 supplemented with 1% BSA, 0.1% NaN3, 100 mm l-lysine monohydrochloride). Primary antibodies, anti-myc 9E10 antibody (BabCo), and anti-GST antibody were added to PEMBAL and incubated overnight at room temperature. After washing three times in PEMBAL, cells were incubated for 5 hr at room temperature in PEMBAL containing CY3-conjugated anti-mouse IgG (Jackson Laboratories) or FITC-conjugated anti-rabbit IgG (Zymed) as secondary antibodies. Cells were photographed using a Nikon Eclipse E800 microscope equipped with a Photometrics Quantix CCD camera. Images were acquired with IPLab Spectrum software (Signal Analytics Corporation).
Acknowledgments
We thank Odile Mondesert for providing expert technical assistance and the Scripps Cell Cycle groups for support and encouragement. F.G. was supported by the Leukemia Society of America, G.D. was supported by an American Cancer Society International Cancer Research Fellowship, and K.S. was supported by a California Division–American Cancer Society Fellowship. This research was supported by National Institutes of Health grant GM41281 awarded to P.R.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL prussell@scripps.edu; FAX (619) 784-2265.
References
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R-H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Davis RJ. MAPKs: New JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 Mitogen-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun Activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: Mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Kallunki T, Su B, Tsigelny I, Sluss H, Derijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes & Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- Karin M. Signal transduction from the cell surface to the nucleus through the phosphorylation of transcription factors. Curr Opin Cell Biol. 1994;6:415–424. doi: 10.1016/0955-0674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Karin M, Hunter T. Transcriptional control by protein phosphorylation: Signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- Kato T, Okazaki K, Murakami H, Stettler P, Fantes P, Okayama H. Stress signal, mediated by a HOG1-like MAP kinase controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis J, Banerjee P, Nikolakaki E, Dai T, Rubie E, Ahmad M, Avruch J, Woodgett J. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pagès G, L’Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes & Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–146. [Google Scholar]
- Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch R, Davis R. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange MA-L, Zamanillo D, Hunt T, Nebreda A. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera JS, Peter M, Nigg EA, Pelech SL. Immunological characterization of avian MAP kinases: Evidence for nuclear localization. Mol Biol Cell. 1992;3:775–787. doi: 10.1091/mbc.3.7.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Seth A, Gonzalez FA, Gupta S, Raden DL, Davis RJ. Signal transduction within the nucleus by mitogen activated protein kinase. J Biol Chem. 1992;267:24796–24804. [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;377:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- ————— Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase via Atf1 transcription factor in fission yeast. Genes & Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Harkins PC, Ulevitch RJ, Han J, Cobb MH, Goldsmith EJ. The structure of mitogen-activated protein kinase p38 at 2.1 A resolution. Proc Natl Acad Sci. 1997;94:2327–2332. doi: 10.1073/pnas.94.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Yamamoto M. Schizosaccharomyces pombe pcr1+ encodes a CREBS/ATF protein in regulation of gene expression for sexual development. Mol Cell Biol. 1996;16:704–711. doi: 10.1128/mcb.16.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh J-C, Toda T, Millar JBA, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes & Dev. 1996;18:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. Cytoplasmic localization of the mitogen-activated protein kinase activator MEK. J Biol Chem. 1994;269:19947–19952. [PubMed] [Google Scholar]