SUMMARY
Survivable injuries are a common yet costly experience. The ability to sense and respond to noxious stimuli is an almost universal trait, and prolonged behavioral alterations, including sensitization to touch and other stimuli, may function to ameliorate fitness costs associated with injury. Cephalopods can modify their behavior by learned association with noxious electric shock, but non-associative alterations of behavioral responses after tissue injury have not been studied. The aim of this study was to make the first systematic investigations in any cephalopod of behavioral responses and alterations elicited by explicit, minor injury. By testing responsiveness in the longfin squid, Loligo pealeii, to the approach and contact of an innocuous filament applied to different parts of the body both before and after injury to the distal third of one arm, we show that a cephalopod expresses behavioral alterations persisting for at least 2 days after injury. These alterations parallel forms of nociceptive plasticity in other animals, including general and site-specific sensitization to tactile stimuli. A novel finding is that hyper-responsiveness after injury extends to visual stimuli. Injured squid are more likely to employ crypsis than escape in response to an approaching visual stimulus shortly after injury, but initiate escape earlier and continue escape behaviors for longer when tested from 1 to 48 h after injury. Injury failed to elicit overt wound-directed behavior (e.g. grooming) or change hunting success. Our results show that long-lasting nociceptive sensitization occurs in cephalopods, and suggest that it may function to reduce predation risk after injury.
KEY WORDS: cephalopod, crypsis, escape, nociception, pain, sensory
INTRODUCTION
Sublethal injury is a common occurrence, arising from predatory interactions, intraspecific conflicts and damage from physical environmental features (Vermeij, 1987; Juanes and Smith, 1995). Injury costs are reflected in increased predation risk (Wilson, 1992), and reduction both in growth rate (Wilbur and Semlitsch, 1990) and reproductive output (Bernardo and Agosta, 2005). Because nonlethal injuries are common and often costly and slow to repair, selection should favor individuals that can adapt their behavior and physiology for relatively long periods to compensate for injury and thereby reduce their mortality risk. One potentially adaptive mechanism that may drive behavioral changes that enhance survival after injury is long-lasting nociceptive sensitization, defined operationally as increased sensitivity to test stimuli following the application of a noxious stimulus (one that causes tissue damage or would if sufficiently prolonged) (Sherrington, 1906).
Nociceptive sensitization after tissue injury is a widespread and presumably ancient form of plasticity (Walters, 1991; Walters, 1994), which has been studied in detail in a few invertebrates in a search for fundamental neural mechanisms of learning [e.g. Aplysia californica (Kandel, 2001); Hirudo medicinalis (Sahley, 1995)] but only rarely with respect to its function or putative adaptive significance (but see Walters et al., 2001). Some cellular and molecular mechanisms of nociceptive sensitization are highly conserved among both invertebrates and vertebrates (Walters, 1994; Walters and Moroz, 2009); however, little is known about the incidence of nociceptive sensitization across different taxa and the extent to which behavioral patterns of sensitization vary across organisms with diverse lifestyles and different degrees of neural complexity.
Unlike the invertebrates in which cellular mechanisms of nociceptive sensitization have been examined, cephalopods have large brains and exhibit impressive perceptual and learning abilities (Hanlon and Messenger, 1996; Hochner et al., 2006; Crook et al., 2009; Nixon and Young, 2003). In previous studies of learning and memory, aversive conditioning has been produced with an artificial stimulus – electric shock – resulting in long-lasting associative memory (Boycott and Young, 1956; Young, 1961; Darmillaq et al., 2004; Shomrat et al., 2008). Octopus (Octopus joubini) withdraw from hermit crabs protected by stinging anemones (Brooks, 1988), but there are no reports of nociception or either non-associative or associative behavioral changes after tissue injury in any cephalopod.
Squid are a major food source for many marine species and exhibit complex defenses specific to different types of predator (Staudinger et al., 2011), but the extent to which these behaviors are modifiable by injurious experience has not been examined. Loligo pealeii employs a primary defense of crypsis (Hanlon and Messenger, 1996) followed by secondary defenses including release of ink pseudomorphs which may confuse or obscure the vision of a pursuing predator, fleeing and protean behaviors (Humphries and Driver, 1970) such as erratic swimming with rapid directional changes (Moynihan and Rodaniche, 1982; Hanlon and Messenger, 1996; Adamo et al., 2006; Langridge, 2009). An interesting question is whether injury alters the relative reliance on primary and secondary defenses. For example, injury might reduce the efficacy of crypsis if wounds produce olfactory cues or if camouflage is compromised visually by damage to skin. If so, earlier deployment of secondary defenses might decrease encounter rates with predators. Alternatively, if speed or maneuverability necessary for effective escape is compromised by injury, squid might rely for longer periods on crypsis during a predatory encounter.
In squid, vigilance, hunting and social behaviors are primarily visually mediated (Hanlon and Messenger, 1996); thus, long-lasting sensitization of responses to visual stimuli after injury may be highly adaptive. To our knowledge, such sensitization after injury has not been described previously. Studies of nociceptive sensitization in mammals have been carried out by investigators of pain mechanisms, who have focused almost exclusively on tactile sensitization (Mogil, 2009). Long-lasting sensitization in invertebrates after injury has only been described in animals possessing very limited visual capabilities – the sea hare Aplysia (Walters, 1987), leeches (Sahley, 1995), moth larvae (Walters et al., 2001) and fly larvae (Babcock et al., 2009) – and thus these studies were also restricted to tactile sensitization.
Here we describe tests of the following hypotheses in L. pealeii: (1) that sensitization of behavioral responses to looming visual stimuli develops rapidly and remains for days after injury; (2) that the relative reliance on primary and secondary defensive behaviors is altered by injury; and (3) that long-lasting sensitivity to tactile stimulation increases throughout the body after injury (general sensitization), but is greatest in the injured region (site-specific sensitization).
MATERIALS AND METHODS
Animals
Adult L. pealeii (standard length: males 17–24 cm, females 13–19 cm) were collected from waters around Woods Hole, MA, USA. From large holding tank populations we selected only healthy, active individuals that showed no evidence of prior injury to any part of the body. Acceptable squid were transferred to the experimental tank and allowed to acclimate for 24 h before behavioral trials. Each squid was fed daily with a small live fish (Fundulus spp., ∼40 mm total length). Fish remains and any surviving fish were removed from the tank the following morning before experiments began.
Experimental arena
The experimental arena was a large, shallow fiberglass tank, 365×90 cm, filled with running seawater (∼18°C) to a depth of 33 cm. The bottom of the tank was covered in a layer of beach sand mixed with small pebbles, and interior walls were painted in a naturalistic mottled pattern of white and grey. There were two overhead fluorescent lights approximately 1.6 m above the water surface containing a total of eight tubes, while windows on two sides of the tank provided additional daylight. Illumination from multiple sources ensured that no shadows were cast over the tank surface by experimenters or equipment. Overnight the tank room was usually unlit but at some points red night-lights were used by experimenters working in the dark phase. The tank was divided into five equal bays of 90×73 cm using brown knotless netting as dividers – this provided physical but not visual or chemical separation of individuals in adjacent bays. During experimental trials and videotaping water flow was stopped, but was otherwise continuous.
Behavioral tests
Squid (N=18, 8 male, 10 female; 8 injured, 10 sham treated) were housed singly in each bay of the tank. After acclimation, behavioral testing began 2 h after lights-on the following morning. Before experiments began we made a close visual inspection of each squid and excluded any animals with evidence of skin scratches or any other tissue damage that was not apparent previously. Squid deemed unsuitable were returned to the holding tanks to be used by other investigators.
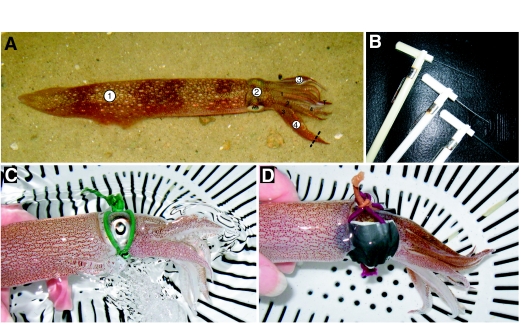
Free-swimming squid were tested with a single Semmes–Weinstein filament (Stoelting, Wood Dale, IL, USA) attached to a thin handle (10×4×800 mm) brought into contact with four points on the body of the squid. Contact was made with each body area by pressing downward on the body surface until the filament bent, although in some cases squid moved away before the filament bent. After each touch the filament was withdrawn to a designated position on the side of the experimental bay either until the animal resumed behavior that was occurring prior to the pursuit or until at least 30 s had elapsed, whichever was longer; the next body area was then approached and stimulated. Squid were always contacted on the dorsal surface in the same sequence: midline of the posterior mantle, center of the head between the eyes, halfway along the length of the third right or left arm, halfway along the length of the contralateral third arm (Fig. 1A).
Fig. 1.
(A) Adult male Loligo pealeii. Numbered circles show sites and sequence of tactile stimulation – 1, central mantle; 2, head; 3 and 4, paired third arms. Broken black line on right arm 3 indicates amputation point on a randomly selected third arm for injured squid. Right arms are numbered in black from 1 to 4 and T indicates tentacles retracted beneath arms. (B) An ascending series of Semmes–Weinstein filaments was used for testing thresholds for defensive behaviors. Bending forces from top to bottom: 4 g, 15 g, 60 g (0.6 g filament not shown). (C) Pseudo-blindfolded, sham-injured squid – blindfolds were constructed from fabric-covered elastic with or without duct tape eye patches. Pseudo-blindfolds control for the tactile sensation of the blindfold and the handling procedure required for application. This squid is attempting to right itself by jetting (water turbulence visible below mantle) after being turned on its side to display its blindfold. During testing squid were not handled. (D) Blindfolded, sham-injured squid.
After conclusion of the free-swimming tests at each time point, each animal was corralled separately into a shallow holding container (a white plastic kitchen colander), which was submerged to a depth of approximately 4 cm. After the squid settled in the container we applied an ascending series of filaments (bending forces of 0.6 g, 4.0 g, 15.0 g, 60.0 g; Fig. 1B) to the four body areas that we tested previously. Filaments were applied by hand from directly above the animal, which in some cases produced anticipatory escape behaviors. Only responses that occurred after contact with the filament were included in the analysis; however, these almost certainly included visual and tactile components. We chose not to apply stiffer filaments in the cases where no response was observed, to avoid puncture or abrasion injuries during repeated testing, potentially confounding the effects of the experimental injury. Animals were tested ∼30 min prior to the tissue injury procedure and again at 10 min, 1 h, 6 h, 24 h and 48 h after injury.
Arm injury
After pre-injury baseline testing was complete each squid was corralled in a net (∼35 cm wide) and one of the two third arms (see Fig. 1A) was grasped at the tip with toothed forceps and the distal third of the arm (∼4–8 mm of tissue) was removed using surgical scissors. Immediately after injury the animal was released and allowed to swim freely until the 10 min post-test began. In sham-injury procedures a squid was corralled in the same way and the forceps were pressed for 1 s against a randomly selected third arm. Post-tests followed in an identical fashion for injured and sham-treated animals. Squid were not anesthetized during injury for several reasons. Primarily, we aimed to approximate a relatively natural and minor injurious experience. In addition, in another mollusc, local or general anesthesia at the time of tissue injury can block development of behavioral sensitization by preventing neural activity at the injury site (Walters, 1987). Finally, the standard anesthesia for cephalopods is immersion in MgCl2 solution (Messenger et al., 1985; Mooney et al., 2010), which causes muscle relaxation. The possibility of sustained loss of hemolymph after injury would have been increased without normal muscle contraction, potentially increasing mortality and morbidity. Each experimental block consisted of only injured or only sham-treated squid, as we anticipated olfactory cues from injured animals might influence the behavior of sham-injured animals.
Ethical note
In the US invertebrates are not covered by IACUC regulations; thus, no welfare protocol was required for this study. Nevertheless, we followed guidelines similar to those for vertebrates undergoing potentially painful experimental procedures. We chose an injury procedure that was minor, fast and repeatable, minimizing both distress to the individual and variance in the procedure itself. Our samples sizes were small, as we considered failure to detect weak effects preferable to testing more animals. Mortality in the experimental population was low (3 of 48 squid died during the observation period and one squid was killed, see details below), and the incidence was not higher in the injured group than in the uninjured group (one injured squid and two control squid died, and one injured squid was killed); thus, the injury itself did not increase mortality risk. We observed animals in the holding tank population with similar and more severe degrees of injury to arms and fins; therefore, the injury we inflicted experimentally was probably similar to survivable injuries experienced in the wild. Because we could not ascertain the age of these natural injuries and the injury extent was highly variable, we did not utilize squid with existing injuries. Injured arms were inspected twice daily for signs of infection, parasite presence or tissue necrosis, and the general health of all squid was monitored closely throughout the experiment. Any squid that was unresponsive to visual cues, showed an impaired righting response or evidence of tissue damage not associated with the wound site, was killed immediately by immersion in ice-cold isotonic MgCl2.
Squid were monitored for up to 48 h after injury and then killed to ensure that any distress was not prolonged. Uninjured squid were returned to the holding tanks to be used by other investigators. The relatively brief period of observation after injury meant we were not able to determine the total duration of behavioral sensitization, or examine how its temporal characteristics relate to tissue healing. We chose to focus on acute effects of injury both because we expect negative fitness consequences to be greatest in the hours and days after an injury is first sustained and because this permitted us to curtail any ongoing pain or distress in our subjects. In the first experiment conducted (non-blindfolded squid) we monitored animals for 48 h. In later experiments with blindfolded and pseudo-blindfolded squid we stopped the experiment at 24 h as behavioral measurements were clearly stable (see Results) by this point.
Feeding with live fish was conducted in accordance with the Marine Biological Laboratory's guidelines for treatment of live food animals. Fish that were not consumed were returned to holding tanks and were not re-offered.
Feeding trials
At the conclusion of testing on each day we presented each squid with a small live food fish. At the conclusion of the 6 h post-test we tracked the response to fish presentation for 3 min or until the fish was successfully captured by the squid. We recorded whether the squid attempted to strike at the fish within the 3 min period, and how many unsuccessful strikes were made prior to capture.
Visual deprivation during tactile stimulation
In initial experiments squid could see the approaching filament and a portion of the experimenter standing alongside the tank, and showed clear visual responses to approach. To assess the role of visual input in sensitized behavior, we blindfolded squid during experimental procedures identical to those described above. To control for handling and ongoing tactile stimulation we included a ‘pseudo-blindfold’ group that wore the same head-gear, but without the eye patches (Fig. 1C and D). We used males only for this experiment because our blindfolds fitted larger animals better, males are larger, and males habituated more rapidly to handling and blindfolding.
Blindfolds were constructed from fabric-covered elastic bands (hair-ties, Scünci brand, Stamford, CT, USA) and duct tape. Blindfolds were applied 1 h prior to behavioral testing and remained on for baseline, injury, 10 min and 1 h post-tests, then were removed immediately after the 1 h tests and reapplied 1 h prior to the 6 h and 24 h post-tests. We did not include a 48 h test in this group as results from the previous experiment suggested behaviors were stable at 24 h.
Data acquisition and analysis
Trials were videotaped with a Digital-8 handycam (DCR-TRV460, Sony, New York, NY, USA). Video analysis was performed and blind cross-checked by two observers (R.J.C. and T.L.), who showed 89% agreement in their assessment of three randomly selected behavioral measures from 20 randomly selected trials (number of ink jets, body lengths and latency to resume crypsis).
To assess visual sensitization, we recorded the number of jets and ink plumes (anticipatory responses) produced during the ‘pursuit’ stage, prior to contact being made with the filament but after the experimenter was actively attempting to touch the animal, and the distance in body lengths between the closest part of the squid's body and the end of the approaching filament when the first response to approach was apparent. After contact, we recorded the number of body lengths traveled and the total time after each contact for the animal to resume cryptic behavior (latency to crypsis). We define crypsis as either settling on the substrate while showing disruptive body patterns or hovering in the water column in uniform tan coloration. For sensory threshold testing, we recorded the lightest force filament that produced any noticeable behavioral change, which was typically an increase in fin movement or change in coloration. We recorded the next highest value filament (100 g) as the response threshold when none of the filaments produced a response.
Statistical analyses were conducted using SAS 9.1 (IBM, Cary, NC, USA) and SPSS 16.0 (Somers, NY, USA). Continuous data met the assumptions of normal distribution and were analyzed first with factorial ANOVA to detect main effects followed by repeated measures ANOVA with post hoc paired, Bonferroni-corrected t-tests to detect changes in continuous variables over time, and mixed model ANOVA partitioned by time post-injury to detect differences between treatments, with treatment and blindfold type as fixed factors and experimental block, injury site (left L or right R arm) and sex as random factors. We analyzed ordinal data following the identification of main effects on continuous data. Bending forces were log(x+1) transformed and analyzed using non-parametric statistics. Categorical data (feeding motivation and hunting success/failure) were analyzed with Fisher's exact tests.
RESULTS
Immediate response to injury
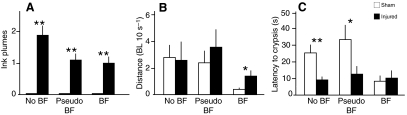
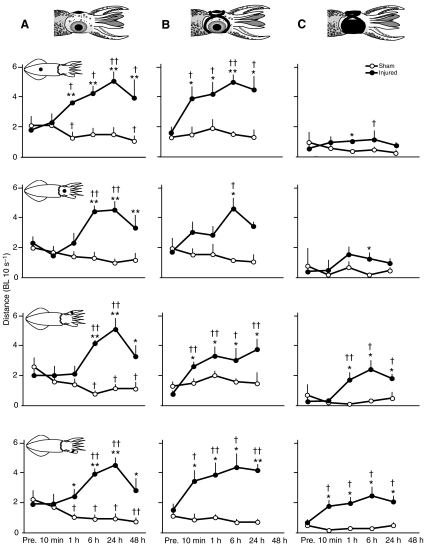
All animals in the three experimental groups (no blindfold, pseudo-blindfold and blindfold) responded to the arm injury with escape jetting and ink release. Significantly more episodes of ink release occurred during the 30 s period after injury than in the corresponding sham-injured controls (Fig. 2A). Blindfolded injured animals traveled slightly farther after injury than blindfolded, sham-injured squid but there were no significant differences in the non-blindfolded and pseudo-blindfolded groups (Fig. 2B). By contrast, the latency to settle and resume crypsis after injury was significantly shorter among injured animals in the two sighted groups, but there was no difference in latency between injured and sham-injured blindfolded squid, which both showed similar latency to that of injured, non-blindfolded squid (Fig. 2C). The observation that non-blindfolded, injured squid traveled approximately the same distances as the uninjured squid immediately after injury (Fig. 2B), but spent considerably less time locomoting before crypsis occurred (Fig. 2C), gives an indication of the large but brief increase in velocity of locomotion evoked by the arm injury. At no time following the injury did squid appear to attend to or groom the wound. The absence of post-injury attention to the injured arm was unlikely to result from an inability to reach or manipulate the injured area with their other arms because the arms were often observed manipulating their blindfolds, which were close to the injury site.
Fig. 2.
Arm injury evokes immediate defensive responses. (A) Injured squid released more ink plumes compared with controls in the 30 s interval after injury. (B) Blindfolded injured squid traveled further after injury than blindfolded, sham-treated squid, but there were no differences in the other groups. BL, body lengths. (C) The latency to resume crypsis after injury was shorter among injured animals in the two sighted groups, but there was no difference between injured and sham-injured blindfolded squid. Bars show means + 1 s.e.m. Pairwise comparisons made with post hoc independent samples t-tests after mixed model ANOVA. *P<0.05, **P<0.01. No BF, squid not wearing blindfolds (N=8 injured, N=10 sham treated). Pseudo BF, squid wearing blindfold frame with no eye patches (N=5 sham injured, N=5 injured). BF, squid wearing opaque blindfold patches (N=7 sham injured, N=8 injured).
Injury-induced alterations of primary and secondary defensive responses revealed in free-swimming squid
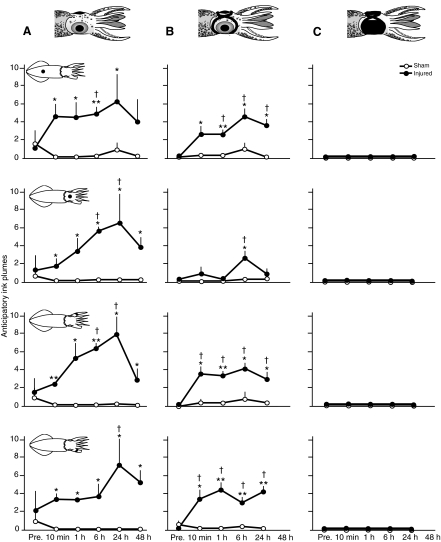
An initial screen of data from all experiments on free-swimming animals identified treatment (injury or sham treatment) as the main effect both on anticipatory behaviors occurring prior to touch with the filament and on behaviors measured after touch. Behavioral measurements taken from free-swimming squid prior to touch showed pronounced effects (mean number of ink plumes: factorial ANOVA, F1,178=63.63, P<0.0001; mean number of escape jets: F1,178=21.91, P<0.0001; distance between filament and squid at earliest behavioral response: F1,41=26.32, P<0.0001), indicating enhanced secondary defensive responses to visual stimuli after injury (Figs 3, 4, 5). Behaviors measured after touch (Figs 6, 7, 8) showed the same general pattern. Differences in mean latency to resume cryptic behavior after touch were affected most strongly by treatment (F1,178=63.63, P<0.0001), as was mean number of body lengths traveled after touch (F1,178=170.96, P<0.0001). As expected, there were significant effects of blindfolding (no blindfold, pseudo-blindfold and blindfold groups) on anticipatory behaviors (anticipatory jets: F1,178=19.59, P<0.0001; anticipatory ink releases: F1,178=14.65, P=0.0002; distance at first response; F1,178=9.69, P=0.001), as well as weaker effects on behaviors post-touch (latency to crypsis, F1,178=4.41, P=0.03; mean body lengths, F1,178=3.51, P=0.01). This indicates a strong effect of injury on visual responsiveness, but the significant differences in response to touch between injured and sham groups of blindfolded squid show that tactile sensitization also occurs. There were no significant effects of sex on any of the behaviors (settling latency: F1,178=1.29, P=0.25; body lengths: F1,178=2.51, P=0.11; anticipatory jets: F1,178=0.45, P=0.50; inking: F1,178=0.40, P=0.53) so we pooled data from males and females. The interaction of experimental blocks and blindfolds was significant only for body lengths traveled (F1,178=4.31, P=0.002); thus, we included blocks as a random factor in subsequent mixed-model ANOVA for this experiment. No other significant interactions were identified.
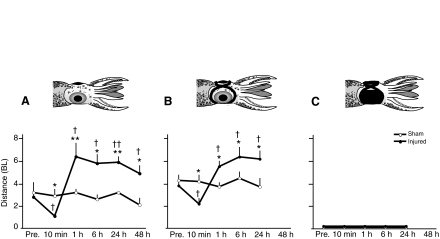
Fig. 3.
Arm injury produces a biphasic change in distance from the filament when the first response occurs. First response is defined as any change in movement or coloration after the filament is first introduced into the experimental bay for tests of free-swimming behaviors at each test interval. The first approach is directed toward the posterior mantle as this was always the first body area tested. (A) Non-blindfolded, injured squid (N=8) allowed the filament to approach significantly closer before responding compared with sham-treated animals (N=10) 10 min post-injury, but this trend reversed by 1 h and remained stable up to 48 h after injury. (B) There was a similar pattern of responses for pseudo-blindfolded squid (N=5 injured, N=5 sham treated). (C) Blindfolded squid (N=8 injured, N=7 sham injured) responded only once the filament touched their skin, suggesting that visual rather than hydrodynamic cues were used to detect approach. BL, body lengths; Pre., pre-test baseline recorded ∼30 min prior to the injury procedure. Points show means + 1 s.e.m. Asterisks show significant differences between injured and sham-injured groups at each time point (*P<0.05, **P<0.01). Daggers indicate significant differences from within-group baseline (†P<0.05, ††P<0.01).
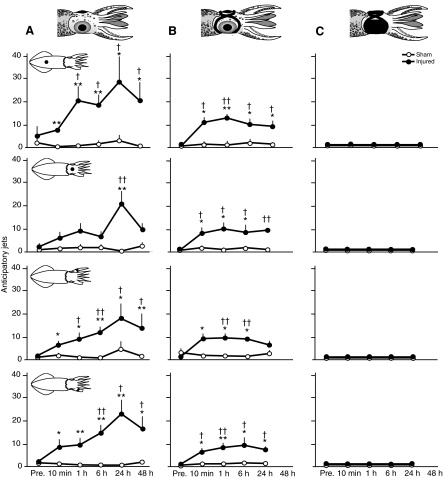
Fig. 4.
Escape jets made by free-swimming squid prior to the touch of the filament reveal sensitization to visual stimuli in injured squid. (A) In non-blindfolded squid (N=8 injured, N=10 sham treated) there was an elevation in jetting propensity among injured squid apparent by 10 min after injury that persisted up to 48 h after injury. (B) In pseudo-blindfolded squid (N=5 injured, N=5 sham treated) the same general pattern was apparent although the magnitude of the response was somewhat smaller. (C) Blindfolded squid (N=8 injured, N=7 sham treated) produced no anticipatory behaviors. Squid schematic diagrams show the site of stimulation for each test. Pre., pre-test prior to injury.
Fig. 5.
Number of ink plumes produced by free-swimming squid prior to touch reveals sensitization to visual stimuli in injured squid. (A) In non-blindfolded squid (N=8 injured, N=10 sham injured) there was an elevation in ink release among injured squid apparent by 10 min after injury that persisted up to 48 h after injury. (B) In pseudo-blindfolded squid (N=5 sham injured, N=5 injured) the same general pattern was apparent. (C) Blindfolded squid (N=8 injured, N=7 sham injured) produced no anticipatory inking responses. Squid schematic diagrams show the site of stimulation for each test. Pre., pre-test prior to injury.
Fig. 6.
Distance in body lengths (BL, averaged across three 10 s intervals) traveled after touch in free-swimming squid indicates differences in the development of tactile and visual sensitization. (A) In non-blindfolded squid (N=8 injured, N=10 sham treated) there was an increase in distance among injured squid apparent between 1 and 6 h after injury, depending on the body area touched. (B) In pseudo-blindfolded squid (N=5 injured, N=5 sham treated) the same general pattern was apparent. (C) In blindfolded squid (N=8 injured, N=7 sham treated) touch of the injured arm produced an elevated response 10 min after injury, indicating site-specific tactile sensitization arising earlier near the site of injury than at other sites. Squid schematic diagrams show the site of stimulation for each test. Pre., pre-test prior to injury.
Fig. 7.
Latency to return to crypsis or settled behavior in free-swimming squid after being touched by the filament indicates tactile sensitization after injury. (A) In non-blindfolded squid (N=8 injured, N=10 sham treated) there was an increase in settling latency among injured squid apparent 1 h after injury that persisted for at least 48 h. (B) In pseudo-blindfolded squid (N=5 sham injured, N=5 injured) response after touch on the injured arm was elevated by 10 min post-injury. (C) In blindfolded squid (N=8 injured, N=7 sham injured) touch on the injured arm produced an elevated response at 10 min after injury, indicating site-specific tactile sensitization arising earlier near the site of injury than at other sites. Squid schematic diagrams show the site of stimulation for each test. Pre., pre-test prior to injury.
Fig. 8.
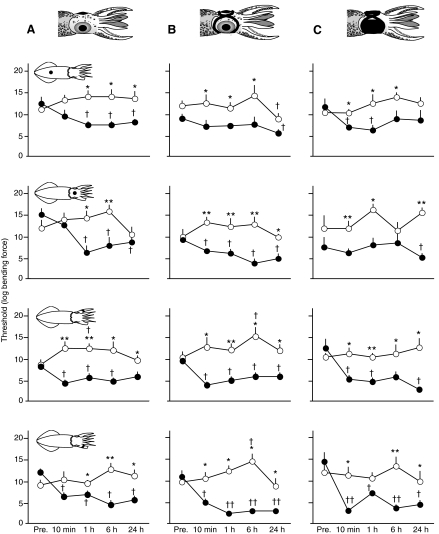
Thresholds for eliciting a defensive response to touch on the four body areas in semi-restrained squid held in a plastic colander indicate tactile sensitization in injured animals appears shortly after injury and persists for at least 24 h. Filament bending forces were log(x+1) transformed. (A) In non-blindfolded squid (N=8 injured, N=10 sham) response threshold decreased compared with pre-injury tests (Pre.) among injured squid from 10 min to 48 h after injury. (B) In pseudo-blindfolded squid (N=5 sham injured, N=5 injured), and blindfolded squid (N=8 injured, N=7 sham treated) the same general pattern was apparent. Squid schematic diagrams show the site of stimulation for each test. Asterisks show significant differences in threshold between injured and sham-treated groups at each time point (Mann–Whitney U-tests, *P<0.05, **P<0.01). Daggers indicate significant differences from within-group baseline (Wilcoxon signed-ranks tests, †P<0.05, ††P<0.01).
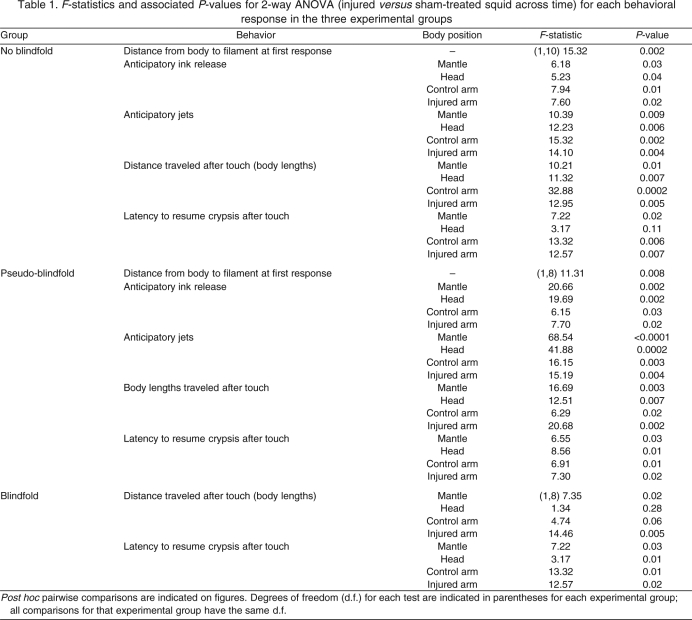
Behavioral variables were recorded before and for up to 2 days after injury. Each behavior was charted over six time intervals (baseline 30 min prior to injury, and 10 min, 1 h, 6 h, 24 h and 48 h after injury) after touch on four body areas (mantle, head, contralateral third arm, injured or sham-injured third arm), except distance at first response which was recorded only upon the first introduction of the filament into the experimental bay, which culminated in a touch on the mantle. Statistics for between-group comparisons are summarized in Table 1 and indicated in Figs 3, 4, 5, 6, 7, 8.
Table 1.
F-statistics and associated P-values for 2-way ANOVA (injured versus sham-treated squid across time) for each behavioral response in the three experimental groups
In both sighted groups, the distance between the approaching filament and the squid's body, at the first introduction of the filament during each test interval, followed a biphasic curve where this distance was significantly less for injured animals shortly after injury but increased at later times (Fig. 3A and B, Table 1). Thus, when tested 10 min after injury, squid employed secondary defense of escape swimming for shorter distances than did sham-treated squid before returning to their primary defense of cryptic behavior. When tested between 1 and 48 h after injury, injured squid swam longer distances than did sham-treated squid before crypsis occurred. Blindfolded squid did not respond (Fig. 3C) until they were touched.
In the two sighted groups the secondary defenses of anticipatory jets (Fig. 4A and B, Table 1) and anticipatory inking (Fig. 5A and B, Table 1) were greatly increased among injured animals prior to touch at each of the body regions contacted. Blindfolded squid showed no anticipatory behaviors in either the sham or the injured groups (Fig. 4C, Fig. 5C). In both the sighted groups and in the blindfolded group, post-contact secondary defensive behaviors were more sustained among injured squid compared with controls (Fig. 6, Fig. 7, Table 1).
Post hoc pairwise comparisons of free-swimming behaviors (Figs 3, 4, 5, 6, 7) at each time interval compared with pre-test baseline and between injured and sham-injured groups at each time point showed increases in the magnitude of secondary responses in injured animals apparent 1 h after injury compared with 10 min after injury, with the increases lasting for at least 48 h after injury. In contrast, sham-injured animals showed significant decreases in response magnitude over this time period, suggesting habituation to the test conditions.
Injury-induced alterations of tactile sensitivity revealed in partially restrained squid
Tests of the defensive response threshold to mechanical stimulation were performed on squid confined briefly in a shallow colander. There was a significant decline in tactile threshold among injured animals (Friedman test, W=37.4, d.f.=4, P<0.001) and a small but significant increase in threshold among sham-treated animals (W=15.4, d.f.=4, P=0.004), similar to results from free-swimming squid (Fig. 8). The significant enhancement of responsiveness to tactile stimulation in blindfolded squid after injury (Figs 6, 7, 8) demonstrates that tactile sensitization occurred, expressed both as general tactile sensitization at test sites distant from the injury and as the earlier appearance (at 10 min) of enhanced responses to stimulation of the injured arm, indicating that some site-specific sensitization also occurred.
At the conclusion of the 6 h post-test in the non-blindfolded group we conducted an experimental feeding trial to assess changes both in motivation to hunt and in hunting success. Injured and sham-injured animals oriented to fish released into their enclosures at the same rate (5 of 8 injured animals oriented to the fish, compared with 7 of 10 sham-inured squid; Fisher's exact test, P=0.94), and 3 of 5 injured animals and 6 of 7 sham-injured squid oriented and captured their fish on the first strike, indicating similar hunting success (Fisher's exact test, P=0.63). Thus, arm injury caused little or no interference with effective hunting behavior several hours after injury.
DISCUSSION
Behavioral responses to injury and long-term sensitization of defensive responses
Although defensive responses have been examined in cephalopods (e.g. Hanlon and Messenger, 1996), behavioral effects of injury have not been reported for squid or any other cephalopod. We found here that squid respond to minor injury with long-lasting enhancement of defensive responses to visual and tactile stimuli. This long-term hyper-responsiveness was expressed as general sensitization of active responses to touch distributed across the entire dorsal body surface and as site-specific sensitization to touch in the injured region. Long-term sensitization is likely to depend primarily upon non-associative mechanisms, although it is possible that the changes observed also included associative contributions. Squid might have associated some aspect of the experimental context (aquarium, nearby humans) with injury; however, the stimuli specific to the injurious event (net and surgical scissors) were never present during testing, and the mechanical test stimulus was not present during injury of the arm, so these could not have been associative cues. The general context of the arena or the presence of an experimenter should not have been effective associative cues because of the prolonged pre-exposure to both prior to injury. Moreover, the tactile sensitization expressed in blindfolded squid cannot be explained by conditioning to visual cues. While conditioning to the olfactory context remains possible, this too should have been blocked at least partially by prolonged pre-exposure before injury.
Shortly after injury (at 10 min), injured squid initiated escape from looming visual stimuli later than sham-injured squid. In contrast, injured squid abandoned crypsis and initiated escape earlier than sham-injured squid when tested 1–48 h after injury (see Fig. 3). Both sets of results are consistent with the likelihood that effective wound closure takes time and during the period when the wound may still be open (or easily opened), active escape responses might be maladaptive (e.g. by interfering with hemostatic processes or by distributing chemical evidence of injury), temporarily making prolonged crypsis a preferred tactic. More generally, transient inhibition of active responses to threats occurring shortly after noxious stimulation has been described in many animals, including molluscs (Mackey et al., 1987; Marcus et al., 1988; Illich et al., 1994; Walters, 1994). In contrast, after a wound has been effectively sealed, earlier initiation of escape in response to potentially threatening stimuli may be more adaptive than prolonged crypsis, particularly where predators inspect and selectively target injured prey.
In blindfolded squid, tactile sensitization close to the wound site was present earlier and expressed more strongly than regional or general sensitization, indicating site-specific sensitization similar to that described in the gastropod mollusc Aplysia californica (Walters, 1987). This pattern was not present in non-blinded or sham-blinded groups, suggesting that visually mediated general sensitization is as strong as site-specific tactile sensitization is in the injured area, although the possibility remains that more pronounced sensitization occurred closer to the injury site (arm test stimuli were not applied closer than 1 cm from the injury site). This long-lasting general sensitization following injury differs from nociceptive tactile sensitization that has been reported in mammals, which is expressed most dramatically as primary hyperalgesia and allodynia close to a wound (Treede et al., 1992) and from site-specific tactile sensitization of defensive responses in opisthobranch molluscs, which is far more prominent after an injury or single session of noxious electric shock than is general tactile sensitization (Walters, 1987). Robust general sensitization in the squid bears some similarity to the general nociceptive sensitization after injury described in moth (Walters et al., 2001) and possibly fruitfly (Babcock et al., 2009) larvae. An interesting possibility is that squid exhibit two long-lasting sensitization processes following injury: (1) site-specific tactile hypersensitivity near the injury that involves mechanisms of increased sensitivity in cutaneous sensory neurons similar to those described in Aplysia and mammals (see Walters and Moroz, 2009), and (2) priming of defensive response systems so that specific motor patterns (inking, jetting) are triggered more readily by diverse stimuli, including visual stimuli, after injury (Erickson and Walters, 1988; Walters, 1994).
Fitness consequences of visual versus tactile sensitization of defensive behavior
If one role of sensitization is to reduce mortality risk from future predation attempts, then differences among sensitizing responses in different species should be related to differing anti-predator defenses. Predation can be divided into three discrete phases: (1) detection and recognition of prey by a predator, (2) pursuit and (3) subjugation (Vermeij, 1987; Endler, 1991). Changes to defensive behavior should be of most value when they occur in the stage where the highest frequency of escape behavior occurs normally. Among molluscs, cephalopods have the greatest visual acuity and the fastest locomotion; thus, anti-predator defenses differ from those of other molluscs. For example, the sea hare Aplysia, the subject of many studies of nociceptive sensitization, lacks image-forming eyes so its primary means of defense (directed release of ink, balling up to make grasping more difficult and escape locomotion) usually do not occur until there has been noxious contact with a predator (Nolen et al., 1995; Walters and Erickson, 1986). Physical contact is required for the behavioral expression of tactile sensitization; thus, this form of sensitization necessarily functions during the most vulnerable stage of a predation encounter. In contrast, squid can react visually to predators at a distance, and defensive tactics progress from the primary defense of crypsis to the secondary defense of deimatic (startle, threat) and protean behaviors (erratic, unpredictable escape patterns) that help squid avoid close encounters with predators (Hanlon and Messenger, 1996; Staudinger et al., 2011). Hence, enhanced responsiveness to visual stimuli after injury has advantages over enhanced sensitivity to touch in injured areas by facilitating escape before a predatory encounter reaches the subjugation phase, where escape without further injury is unlikely.
The behavioral changes we observed in squid after injury may also have negative fitness consequences. For example, an increased propensity to flee from threatening stimuli can draw the attention of predators and lead to higher encounter rates, increasing rather than decreasing predation risk. This is suggested by observations of lizards with autotomized tails, which are more likely to flee from a model snake predator, making themselves more conspicuous even though their escape speed is slower than that of normal lizards (Downes and Shine, 2001). Various ecological studies have demonstrated survival costs of injuries, e.g. in tadpoles (Semlitsch, 1990; Figiel and Semlitsch, 1991) and larval damselflies (Stoks, 1998), but we know of no studies of the consequences of injury-induced behavioral sensitization for survival or predation. While it seems likely that experiencing an injury greatly increases mortality risk, the value of long-term nociceptive sensitization for reducing these costs remains unclear.
Does prolonged nociceptive sensitization imply pain-like responses in squid?
Tissue injury in humans and probably most mammals causes immediate pain and often long-lasting hypersensitivity. Pain is defined as an ‘unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (Merskey and Bogduk, 1994). In mammalian pain studies, long-term sensitization of defensive responses is often used as an indicator of persisting pain, although this link has been questioned because of the lack of evidence for an emotional component in these response measures (e.g. Mogil, 2009). Whether cephalopods are capable of experiencing the affective component required for pain is a matter of ongoing debate (see Mason, 2011; Carere et al., 2011). Our demonstration of long-term nociceptive sensitization in a cephalopod is interesting because these molluscs have the largest brains and perhaps the most sophisticated behavioral capacities among the invertebrates (Nixon and Young, 2003), and these features have been cited as support for the claim that cephalopods can experience vertebrate-like pain (Carere, 2011; Mather, 2008), despite the absence of a logical necessity for this link (e.g. Mason, 2011). Indeed, the cephalopod nervous system differs substantially from that of vertebrates, so it is difficult to conclude that, simply because the brain is large and complex, its sensory and motivational processes are similar to those of vertebrates. We observed interesting differences in post-injury behavior in squid compared with patterns typical of mammals. Specifically, sustained attention to an injured region, combined with prolonged changes in activity and avoidance of contexts associated with noxious experience, are often considered evidence for ongoing pain in vertebrates. We did not observe similar behaviors in squid suggestive of ongoing pain or distress after arm injury. Evidence for behaviors suggestive of pain-like sensations in any invertebrate is limited. In hermit crabs, Pagurus bernhardus, electric shocks delivered to the abdominal surface produce long-term behavioral and motivational alterations with parallels to changes commonly proposed as evidence for pain in vertebrates (Appel and Elwood, 2009; Elwood and Appel, 2009). Shrimp (Paelamon elegans) with injured antennae display sustained grooming and other attentive behaviors directed at the injury site that suggest ongoing sensitivity to tissue damage (Barr et al., 2008). Conversely, a formal study (Puri and Faulkes, 2010) and numerous published anecdotes have described a lack of pain-like responses in various invertebrates, including cephalopods (Walters, 1994; Crook and Walters, 2011). While our results do not provide evidence that prolonged nociceptive sensitization in squid is associated with the motivational/emotional components central to the definition of pain, neither do they exclude this possibility.
ACKNOWLEDGEMENTS
We thank the staff of the Marine Resources Center at the Marine Biological Laboratory for supplying healthy squid throughout the summer and assisting with animal care and tank maintenance. We are grateful for the assistance of Ivan Anastassov during the development of our blindfolding procedure and with videography.
FOOTNOTES
This study was supported by the Baxter Research Award and the Bang Summer Research Fellowship from the Marine Biological Laboratory to R.J.C., by the Sholley Foundation to R.T.H., and by National Institutes of Health grant NS35979 to E.T.W. Deposited in PMC for release after 12 months.
REFERENCES
- Adamo S. A., Ehgoetz K., Sangster C., Whitehorne I. (2006). Signaling to the enemy? Body pattern expression and its response to external cues during hunting in the cuttlefish Sepia officinalis (Cephalopoda). Biol. Bull. 210, 192-200 [DOI] [PubMed] [Google Scholar]
- Appel M., Elwood R. W. (2009). Motivational trade-offs and potential pain experience in hermit crabs. App. An. Behav. Sci. 119, 120-124 [Google Scholar]
- Babcock D. T., Landry C., Galko M. J. (2009). Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. 19, 799-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr S., Laming P. R., Dick J. T. A., Elwood R. W. (2008). Nociception or pain in a decapod crustacean? An. Behav. 75, 745-751 [Google Scholar]
- Bernardo J., Agosta S. J. (2005). Evolutionary implications of hierarchical impacts of nonlethal injury on reproduction, including maternal effects. Biol. J. Linnean Soc. 86, 309-331 [Google Scholar]
- Boycott B. B., Young J. Z. (1956). Reactions to shape in Octopus vulgaris Lamarck. Proc. Zool. Soc. Lond. 126 491-547 [Google Scholar]
- Brooks W. R. (1988). The influence of the location and abundance of the sea anemone Calliactis tricolor (Le Sueur) in protecting hermit crabs from octopus predators. J. Exp. Mar. Biol. Ecol. 116, 15-21 [Google Scholar]
- Carere C., Wood J. B., Mather J. (2011). Species differences in captivity: where are the invertebrates? Trends Ecol. Evol. 26, 211 [DOI] [PubMed] [Google Scholar]
- Crook R. J., Walters E. T. (2011). Nociceptive behavior and physiology in molluscs: animal welfare implications. ILAR J. 52, 185-195 [DOI] [PubMed] [Google Scholar]
- Crook R. J., Hanlon R. T., Basil J. A. (2009). Memory of visual and topographical features suggests spatial learning in Nautilus (Nautilus pompilius L.). J. Comp. Psych. 123, 264-274 [DOI] [PubMed] [Google Scholar]
- Darmaillacq A. S., Dickel L., Chichery M. P., Agin V., Chichery R. (2004). Rapid taste aversion learning in adult cuttlefish, Sepia officinalis. An. Behav. 68, 1291-1298 [Google Scholar]
- Downes S., Shine R. (2001). Why does tail loss increase a lizard’s later vulnerability to snake predators? Ecology 82, 1293-1303 [Google Scholar]
- Elwood R. W., Appel M. (2009). Pain experience in hermit crabs? An. Behav. 77, 1243-1246 [Google Scholar]
- Endler J. A. (1991). Interactions between predators and prey. In Behavioral Ecology: an Evolutionary Approach (ed. Krebs J. R., Davies N. B.), pp. 169-196 Oxford: Blackwell Press; [Google Scholar]
- Figiel C. R., Semlitsch R. D. (1991). Effects of nonlethal injury and habitat complexity on predation in tadpole populations. Can. J. Zool. 69, 830-834 [Google Scholar]
- Hanlon R. T., Messenger J. B. (1996). Cephalopod Behavior. Cambridge: Cambridge University Press; [Google Scholar]
- Hochner B., Shomrat T., Fiorito G. (2006). The octopus: a model for a comparative analysis of the evolution of learning and memory mechanisms. Biol. Bull. 210, 308-317 [DOI] [PubMed] [Google Scholar]
- Humphries D. A., Driver P. M. (1970). Protean defence by prey animals. Oecologia 5, 285-302 [DOI] [PubMed] [Google Scholar]
- Juanes F., Smith L. D. (1995). The ecological consequences of limb damage and loss in decapod crustaceans: a review and prospectus. J. Exp. Mar. Biol. Ecol. 193, 197-223 [Google Scholar]
- Kandel E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030-1038 [DOI] [PubMed] [Google Scholar]
- Langridge K. V. (2009). Cuttlefish use startle displays, but not against large predators. An. Behav. 77, 847-856 [Google Scholar]
- Mason G. J. (2011). Species differences in response to captivity: stress, welfare and the comparative method. Trends Ecol. Evol. 25, 713-721 [DOI] [PubMed] [Google Scholar]
- Merskey H. M., Bogduk N. (1994). Classification of Chronic Pain, 2nd edn. Seattle: IASP Press; [Google Scholar]
- Messenger J. B., Nixon M., Ryan K. P. (1985). Magnesium chloride as an anaesthetic for cephalopods. Comp. Biochem. Physiol. 82C, 203-205 [DOI] [PubMed] [Google Scholar]
- Mogil J. S. (2009). Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283-294 [DOI] [PubMed] [Google Scholar]
- Mooney T. A., Lee W., Hanlon R. T. (2010). Long duration anesthetization of squid (Doryteuthis pealeii). Mar. Freshw. Behav. Physiol. 43, 297-303 [Google Scholar]
- Moynihan M., Rodaniche A. F. (1982). The behavior and natural history of the Caribbean reef squid, Sepioteuthis sepioidea: with a consideration of social, signal, and defensive patterns for difficult and dangerous environments. In Advances in Ethology (ed. Wickler W., Bochmun E. C.), pp. 1-151 Berlin: Paul Parey Press; [Google Scholar]
- Nolen T. G., Johnson P. M., Kicklighter C. E., Capo T. (1995). Ink secretion by the marine snail Aplysia californica enhances its ability to escape from a natural predator. J. Comp. Physiol. A. 176, 239-254 [Google Scholar]
- Puri S., Faulkes Z. (2010). Do decapod crustaceans have nociceptors for extreme pH? PLoS ONE 5, e10244 doi:10.1371/journal.pone.0010244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahley C. L. (1995). What we have learned from the study of learning in the leech. J. Neurobiol. 27, 434-445 [DOI] [PubMed] [Google Scholar]
- Semlitsch R. D. (1990). Effects of body size, sibship, and tail injury on the susceptibility of tadpoles to dragonfly predation. Can. J. Zool. 68, 1027-1030 [Google Scholar]
- Sherrington C. (1906). The Integrative Action of the Nervous System. New York: Charles Scribner and Sons; [Google Scholar]
- Shomrat T., Zarrella I., Fiorito G., Hochner B. (2008). The octopus vertical lobe modulates short-term learning rate and uses LTP to acquire long-term memory. Cur. Biol. 18, 337-342 [DOI] [PubMed] [Google Scholar]
- Staudinger M. D., Hanlon R. T., Juanes F. (2011). Primary and secondary defenses of squid to cruising and ambush fish predators: variable tactics and their survival value. An. Behav. 81, 585-594 [Google Scholar]
- Stoks R. (1998). Effect of lamellea autonomy on survival and foraging success of the damselfly Lestes sponsa (Odonata: Lestidae). Oecologia 117, 443-448 [DOI] [PubMed] [Google Scholar]
- Treede R. D., Meyer R. A., Raja S. N., Campbell J. N. (1992). Peripheral and central mechanisms of cutaneous hyperalgesia. Prog. Neurobiol. 38, 397-421 [DOI] [PubMed] [Google Scholar]
- Vermeij G. J. (1987). Evolution and Escalation. Princeton: Princeton University Press; [Google Scholar]
- Walters E. T. (1987). Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J. Neurosci. 7, 400-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E. T. (1991). A functional, cellular, and evolutionary model of nociceptive plasticity in Aplysia. Biol. Bull. 180, 241-251 [DOI] [PubMed] [Google Scholar]
- Walters E. T. (1994). Injury-related behavior and neuronal plasticity: an evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int. Rev. Neurobiol. 36, 325-427 [DOI] [PubMed] [Google Scholar]
- Walters E. T., Erickson M. T. (1986). Directional control and the functional organization of defensive responses in Aplysia. J. Comp. Physiol. A 159, 339-351 [DOI] [PubMed] [Google Scholar]
- Walters E. T., Moroz L. L. (2009). Molluscan memory of injury: evolutionary insights into chronic pain and neurological disorders. Brain Behav. Evol. 74, 206-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E. T., Illich P. A., Weeks J. C., Lewin M. R. (2001). Defensive responses of larval Manduca sexta and their sensitization by noxious stimuli in the laboratory and field. J. Exp. Biol. 204, 457-469 [DOI] [PubMed] [Google Scholar]
- Wilbur H. M., Semlitsch R. D. (1990). Ecological consequences of tail injury in Rana tadpoles. Copeia 1990, 18-24 [Google Scholar]
- Wilson B. S. (1992). Tail injuries increase the risk of mortality in free-living lizards (Uta stansburiana). Oecologia 92, 145-152 [DOI] [PubMed] [Google Scholar]
- Young J. Z. (1961). Learning and discrimination in the octopus. Biol. Rev. 36, 32-95 [DOI] [PubMed] [Google Scholar]