SUMMARY
The ability to control the bandwidth, amplitude and duration of echolocation pulses is a crucial aspect of echolocation performance but few details are known about the neural mechanisms underlying the control of these voice parameters in any mammal. The basal ganglia (BG) are a suite of forebrain nuclei centrally involved in sensory-motor control and are characterized by their dependence on dopamine. We hypothesized that pharmacological manipulation of brain dopamine levels could reveal how BG circuits might influence the acoustic structure of bat echolocation pulses. A single intraperitoneal injection of a low dose (5 mg kg–1) of the neurotoxin 1-methyl-4-phenylpyridine (MPTP), which selectively targets dopamine-producing cells of the substantia nigra, produced a rapid degradation in pulse acoustic structure and eliminated the bat's ability to make compensatory changes in pulse amplitude in response to background noise, i.e. the Lombard response. However, high-performance liquid chromatography (HPLC) measurements of striatal dopamine concentrations revealed that the main effect of MPTP was a fourfold increase rather than the predicted decrease in striatal dopamine levels. After first using autoradiographic methods to confirm the presence and location of D1- and D2-type dopamine receptors in the bat striatum, systemic injections of receptor subtype-specific agonists showed that MPTP's effects on pulse acoustics were mimicked by a D2-type dopamine receptor agonist (Quinpirole) but not by a D1-type dopamine receptor agonist (SKF82958). The results suggest that BG circuits have the capacity to influence echolocation pulse acoustics, particularly via D2-type dopamine receptor-mediated pathways, and may therefore represent an important mechanism for vocal control in bats.
KEY WORDS: hypophonia, dysarthria, voice, echolocation, dopamine, Lombard response
INTRODUCTION
Echolocating bats precisely regulate the acoustic properties of their echolocation pulses to maximize the efficiency of their sonar behavior (Chiu et al., 2009; Obrist, 1995; Schnitzler and Kalko, 2001). Like other bats, free-tailed bats (Tadarida brasiliensis) constantly adjust the spectral and temporal parameters of their pulses in response to auditory feedback and ever-changing behavioral and environmental contexts (Gillam and McCracken, 2007; Gillam et al., 2007; Jarvis et al., 2010; Ratcliffe et al., 2004; Schwartz et al., 2007; Simmons et al., 1978; Tressler and Smotherman, 2009; Ulanovsky et al., 2004). In this regard the vocal plasticity displayed by echolocating bats is uncommon among mammals because it represents a cognitive rather than limbic control of the vocal motor circuitry. Limited examples of mammalian vocal plasticity have also been reported for primates and cetaceans (Brumm et al., 2004; Cleveland and Snowdon, 1982; Egnor and Hauser, 2006; Egnor et al., 2006; Egnor et al., 2007; Ghazanfar et al., 2002; Insley and Southhall, 2005; Scheifele et al., 2005) but the neural pathways guiding these behaviors have yet to be explored.
Previous work has highlighted the role of the midbrain as a substrate for audio-vocal integration in bats (Metzner and Schuller, 2007) but there is also evidence of a cortical network regulating bat vocalizations (Gooler and O'Neill, 1986). Several lines of evidence from studies of birdsong and human speech (Alm, 2004; Doupe and Kuhl, 1999; Jarvis, 2004; Kuhl, 2003) have suggested that a suite of forebrain nuclei known collectively as the basal ganglia (BG) may play an important role in vocal plasticity among vertebrates. In songbirds, the BG are essential for vocal learning but also provide a substrate for sensory and contextual influences on vocalizing in adults (Doupe et al., 2005; Kao et al., 2005; Woolley and Doupe, 2008). In humans, pathological dysfunctions of the BG contribute to many of the most common human speech motor disorders, such as stuttering (Alm, 2004) and dysarthrias (Goberman and Blomgren, 2003; Ho et al., 1999; Holmes et al., 2000; Louis et al., 2001; Sanabria et al., 2001). It therefore appears that the BG have the potential to contribute to some general aspects of mammalian vocal plasticity but their precise functions remain unclear.
Consistent levels of striatal dopamine are necessary for the proper functioning of motor control circuits (Alexander and Crutcher, 1990). In rats, unilateral lesions of the dopamine-producing cells of the substantia nigra reduced mating call amplitudes and bandwidths in a manner similar to the symptoms of Parkinsonian dysarthria in humans (Ciucci et al., 2009; Ciucci et al., 2007). An alternative method for knocking down dopamine production is through the use the neurotoxin 1-methyl-4-phenylpyridine (MPTP). MPTP can cross the blood–brain barrier and exerts its primary effects through metabolic neurotoxicity of dopaminergic cells in the substantia nigra pars compacta (SNc) (Bradbury et al., 1986). It has been widely used to generate animal models of Parkinson's disease (Smeyne and Jackson-Lewis, 2005) but its effects on the mammalian vocal motor pathways have not been described. MPTP has, so far, been shown to be a potent catecholaminergic neurotoxin in primates, cats, rats, mice and pigs but its efficacy and behavioral effects were found to vary due to species-specific differences in metabolic processing and neuronal uptake of MPTP and its metabolites (Riachi et al., 1988).
We hypothesized that if MPTP was potent in bats, the resulting reduction of striatal dopamine could reveal the extent to which dopaminergic circuits contributed to the spontaneous production, modulation and variability of echolocation pulses. We predicted that reduced dopamine levels caused by MPTP administration would lead to voice changes similar to those typical of Parkinsonian dysarthria, namely decreased voice amplitude, duration and bandwidth. To test whether the loss of striatal dopamine levels had an effect on audio-vocal integration, we measured the bats' ability to make compensatory changes in voice loudness in response to background noise (i.e. the Lombard response) before and after MPTP treatment. Although our acoustic measurements supported our hypotheses, subsequent measurements of striatal dopamine concentrations revealed that MPTP increased rather than decreased striatal dopamine. This led us to test whether administration of dopamine receptor subtype-specific agonists could mimic the effects of MPTP, which would allow an assignment of MPTP's effects on pulse acoustics to a particular dopamine receptor subtype. The results of these experiments provide evidence that dopamine and the BG may be involved in the control of bat echolocation pulse acoustics.
MATERIALS AND METHODS
Animal husbandry
A total of 37 [8 for MPTP, 9 for autoradiography, 15 for high-performance liquid chromatography (HPLC), and 5 for in vivo receptor pharmacology] male Mexican free-tailed bats, Tadarida brasiliensis mexicana (I. Geoffroy 1824), were caught wild from a year-round roost on the campus of Texas A&M University and housed in the Texas A&M Department of Biology vivarium facility. All animal care and handling procedures and experimental protocols were approved by the Texas A&M animal care and use committee, and all acoustic behavioral assays were performed exactly as described previously (Jarvis et al., 2010; Schwartz et al., 2007; Tressler and Smotherman, 2009). Animals receiving neurotoxin injections were subsequently housed separately from the main colony.
Acoustic methods
All experiments were performed in a sound-isolated room lined with sound-absorbing 4″ acoustic foam (model UNX-4; Sonex©, San Jose, CA, USA), with the lights off. Recordings were made using a Brüel & Kjaer Free-field ¼″ microphone (Type 4939; Nærum, Germany), which has an inherent noise floor of approximately 30 dB (re. 20 μP) and a dynamic range of 35–164 dB. Bats hung from the top of a 14 cm (length) × 14 cm (width) × 5 cm (height) ¼″ wire mesh cage, and their position and behavior were monitored remotely by infrared video throughout the experiment. The distance and orientation of the bat relative to the microphone were consistent because the bats always preferred to hang from the top of the cage and face downwards towards the microphone, which was centered 10 cm from the bottom of the cage. Slight changes in signal strength resulting from head movements and beam projection paths can be averaged out across conditions as long as a sufficient number of calls are recorded, in this case at least 100 calls over approximately 1–2 min. Recorded intensity of acoustic stimuli was minimized by surrounding the microphone with acoustic foam on all sides except that facing the bat, which facilitated the digital extraction of echolocation pulses from the background noise. We used the methods of Penna and colleagues (Penna et al., 2005) to subtract the contributions of stimulus amplitude on the measurements of pulse amplitude by empirically measuring the average effect of the noise stimulus on the measured amplitude of an artificially generated echolocation pulse. Incoming signals were digitized with a National Instruments DAQmx analog-to-digital converter (NI PCI-6251; 16 bit, 200 kHz, Austin, TX, USA), and viewed with an Avisoft Recorder v.3.0 (Berlin, Germany). For acoustic analyses of the bats' echolocation pulses, 100 echolocation pulses from each bat for each dose, acoustic condition and time point were selected at random from the recordings, and the pulse acoustic parameters bandwidth, duration and intensity were extracted automatically using Avisoft SASLab Pro v4.39 as described in Tressler and Smotherman (Tressler and Smotherman, 2009). For temporal analyses, we used 256-point fast Fourier transforms (FFTs) with 93.75% overlap, providing 976 Hz spectral and 0.064 ms temporal resolutions. For spectral analysis 1024-point FFTs provided 244 Hz spectral and 0.256 ms temporal resolutions. The bats' normal echolocation pulses ranged in intensity from 80 dB to 115 dB sound pressure level (SPL).
Broadband noise stimuli were generated digitally with Tucker-Davis Technology (TDT, Alachua, FL, USA) system III hardware and the openEX software v5.4. The broadband noise was digitally filtered to present a total signal bandwidth spanning a range of 15–100 kHz, which covered the entire range of the two loudest harmonic components of the echolocation pulses of Tadarida brasiliensis mexicana. Stimuli were played through a Sony amplifier (model #STR-DE598; Tokyo, Japan) driving a four-speaker array composed of two Pioneer Ribbon Tweeters (ART-55D/301080; Kawasaki, Japan) and two Pioneer Rifle Tweeters (ART-59F/301081). Each speaker provided a flat (±3 dB) output at 85 dB SPL across the principal frequency range of interest, roughly 15–60 kHz.
The effect of broadband noise on the subject's echolocation pulse parameters (the Lombard response) was determined by subtracting the mean value of each acoustic parameter in the presence of noise from the mean value in the absence of noise for each parameter for each subject. The effect of MPTP on the Lombard response was determined by subtracting the mean Lombard response of individuals treated with MPTP from the same individuals treated with saline for each parameter for each subject.
Pharmacology
All drugs were obtained from Sigma Chemical Co. (St Louis, MO, USA). Solid MPTP powder (catalog #M0896) was dissolved in physiological saline at a concentration by which a 0.1 ml intraperitoneal injection resulted in final doses of 1 mg kg–1, 5 mg kg–1 or 10 mg kg–1. For systemic manipulation of specific dopamine receptor subtypes, we used the D1-type dopamine receptor agonist SKF82958 (3-allyl-6-chloro-1-phenyl-1,2,4,5-tetrahydro-3-benzazepine-7,8-diol; Sigma Chemical Co. #C130), the D1 antagonist SCH23390 (7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol; Sigma Chemical Co. #D054), the D2-type dopamine receptor agonist Quinpirole [(4aR,8aR)-5-propyl-4,4a,5,6,7,8,8a,9-octahydro-1H-pyrazolo[3,4-g]quinolone; Sigma Chemical Co. #Q102] and the D2 antagonist Eticlopride (3-chloro-5-ethyl-N-{[(2S)-1-ethylpyrrolidin-2-yl]methyl}-6-hydroxy-2-methoxybenzamide; Sigma Chemical Co. #E101) dissolved in physiological saline. Saline injections of equivalent volume were used for controls.
The dosage and administration of MPTP was based on a review of mouse models of Parkinson's disease (Sedelis et al., 2001). In mice a broad spectrum of dosages and administration schedules has been reported to produce variable results depending in part on strain, age, sex and laboratory. Key factors in our choice of dose and schedule were that: (1) we could not afford high mortality rates because we were working with wild-caught animals; and (2) free-tailed bats echolocate more freely when they are able to move and actively explore their environment. We therefore devised an administration schedule predicted to produce mild chronic effects that accumulated over time. In mice, acute effects of MPTP injections (30 mg kg–1 and higher) have been reported to occur within 1 h post-injection (Sedelis et al., 2001), and significant behavioral deficits as well as the loss of dopaminergic neurons of the substantia nigra became apparent after 24 h and plateaued within four to seven days (Jackson-Lewis et al., 1995). Some reports indicated mortality rates as high as 60% after one day at doses as low as 30 mg kg–1 (Sikiric et al., 1999), and our own preliminary trials with a single 20 mg kg–1 dose in four bats resulted in 50% mortality and a severe akinesia in the two surviving bats that lasted more than 48 h and precluded conducting any vocalization studies. We therefore carried out experiments at three lower doses of 1 mg kg–1, 5 mg kg–1 and 10 mg kg–1 administered by a single injection of 0.1 ml of physiological saline once a week for four weeks. For each MPTP dose tested, three individual Mexican free-tailed bats were selected at random and housed separately from the captive colony. One day before MPTP administration (–1 day) each bat received a saline injection, and 1 h later their performance on the motor control assays was assessed and their mean pulse acoustics and Lombard response were quantified. After 24 h the same individuals were then administered with an MPTP injection. Post-MPTP administration, acoustic recordings and motor control assessments were taken for each bat 1 h, 1 day and 7 days after MPTP injection (+1 h, +1 day and +7 days, respectively). This paradigm was repeated four times for each dose. No effect of repeated administration was found and the results of all trials were combined.
For each of the four dopamine receptor-specific drugs tested, five individual bats received a 0.1 ml intraperitoneal injection of either one of the four drugs or saline and were immediately placed alone in the recording cage and their spontaneous vocalizations continuously recorded for up to 90 min. Treatment order was randomized and balanced across bats. Mean pulse acoustic parameters were assessed by successive time bins within each trial to characterize the time course of any potential drug effects. Visual inspection of all records confirmed that only echolocation pulses and not communication calls were included in the analyses.
HPLC
Whole brain tissue was harvested from 10 treated (5 mg kg–1 MPTP; 5 at +1 h and 5 at +1 day) and 5 untreated (saline injected) bat brains and flash frozen and stored at –80°C until dopamine analyses. Frozen brains were blocked, mounted on a freezing microtome stage and then sectioned at 150 μm through the striatum. Tissue sections were placed on glass slides onto a freezing plate and the striatum was micropunched under a surgical microscope using 0.75 mm-diameter metal core punches (Model 15072; Ted Pella, Redding, CA, USA). Tissue concentrations of dopamine were determined using HPLC (Kramer et al., 2007; Sved, 1989). Just prior to assay, the tissue samples were weighed and sonicated in perchloric acid containing dihydrobenzylamine (DHBA) (an internal standard, 100 ng ml–1; ESA, Chelmsford, MA, USA). Each sample was centrifuged for 20 min at 3200 g at 4°C. The supernatant was passed through a low-volume nylon 0.2 micron filter (Model 8110; Fisher Scientific, Houston, TX, USA) and the resulting supernatant was injected onto a reversed phase C-18 column (Shiseido, 5 cm, Model #A3RE01176; ESA). The sample amines were eluted using a filtered and degassed mobile phase (MDTM-II; ESA) and then quantified by electrochemistry (Coulochem II; ESA) using a microdialysis cell (Model 5014B; ESA). Sample dopamine peak heights were compared with external standard peak heights (Sigma Chemical Co.) that were processed in a manner similar to that of the samples. Sample values were expressed as amine concentrations (pg) per mg wet mass of sample tissue.
Dopamine receptor autoradiography
To confirm the presence and location of D1- and D2-type dopamine receptors in the Mexican free-tailed bat brain, we used slight modifications of previously described protocols for dopamine receptor autoradiography (Kim et al., 2000; Lidow et al., 1991). All slides were pre-incubated in assay buffer (50 mmol l–1 Tris HCl buffer with 120 mmol l–1 NaCl, 5 mmol l–1 KCl, 2 mmol l–1 CaCl2, 1 mmol l–1 MgCl2, pH 7.4) for 20 min. To map the distribution of D1-type dopamine receptors, the experimental slide sets from five non-injected bats were first incubated for 1 h at 23°C in assay buffer with 1 nmol l–1 N-methyl-3H-SCH23390 (Perkin-Elmer Corp., Foster City, CA, USA), and 5 μmol l–1 of the 5-HT2 serotonin receptor antagonist ketanserin was added to prevent binding of the radiolabeled ligand to serotonin receptors as described in Kim et al. (Kim et al., 2000). Control slide sets were processed similarly but with the addition of 5 μmol l–1 of fluphenazine, a D1/D2 dopamine receptor antagonist, which prevented binding of the radiolabeled receptor antagonist to D1-type dopamine receptors and thereby provided quantitative determination of non-specific binding. To map D2-type dopamine receptors, the experimental slide sets from four bats were incubated in assay buffer with 1 nmol l–1 methoxy-3H-raclopride (Perkin-Elmer Corp.) for 45 min at 23°C. Control slide sets were processed similarly but with the addition of 10 μmol l–1 butaclamol, a D1/D2 dopamine receptor antagonist, to determine non-specific binding. All slide sets were then transferred to ice-cold assay buffer for two rinses of 20 s each, followed by an ice-cold water rinse of 10 s. Finally, all slide sets were then dried and apposed to BioMax Maximum Resolution autoradiographic film (Kodak) with a calibrated 14C standard for 17–19 weeks at –80°C.
All D1 and D2 films were developed and optical density measurements from five sections on the experimental slide set were measured and averaged for each brain area for each animal using NIH Image J (Rasband, 1997). All sections were subsequently stained with Cresyl Violet to aid in brain structure identification. Areas exhibiting binding were also measured in control brains to provide non-specific binding control data. The threshold for binding in the autoradiography experiments was determined by adjusting background level for each image according to the non-specific binding levels obtained from the control slide sets. Any area with binding higher than the non-specific binding levels was analyzed. A standard curve was generated using the optical densities from the calibrated standards. 3H-SCH23390 and 3H-raclopride binding (nCi mg–1 protein) from each brain region was calculated using the values from the standard curve. Non-specific binding was subtracted from total binding to determine specific binding for each brain region.
Motor control assays
In order to determine if MPTP injections were having an effect on gross motor control, motor control assays based on similar studies in rodents were adapted for bats and performed at each experimental session (Muralikrishnan and Mohanakumar, 1998; Sikiric et al., 1999). Under normal circumstances an untreated Mexican free-tailed bat placed at the center of the bottom of a cage will quickly move to the side of the chamber and climb to the highest point in the cage. For these experiments, treated and untreated subjects were placed individually into a 14×14×5 cm wire mesh arena, and the time it took the subject to: (1) move all four limbs; and (2) climb onto the wall of the arena were recorded. Additionally, the presence and degree of tremor was scored by two observers on a 0–4 scale. The bats' ability to fly, avoid obstacles, land safely, and eat, drink and groom were checked periodically throughout the study but we did not force treated bats to fly.
Statistical analysis
The effect of MPTP on each bat was first tested using the raw recorded values of each parameter. In order to more accurately compare the effect between bats and gain an understanding of the general effect of MPTP, two derived values were computed for each parameter, i.e. the absolute mean and its relative difference from that obtained following saline injections. The deviation from saline was computed for pulses emitted in silence for each bat for each pulse parameter by subtracting each bat's mean parameter value after saline injection from that bat's mean parameter value after MPTP injection for each time point. The effect of MPTP on the Lombard response was calculated by subtracting the change caused by noise after saline injection from the change caused by noise after MPTP injection for each bat at each time point.
All statistical procedures were performed utilizing SAS-JMP v.7.0.7 at the α=0.05 level. Data were normally distributed with the exception of the effect of MPTP on the mean Lombard response. Overall effects of MPTP on echolocation pulse parameters were analyzed by multivariate analysis of variance (MANOVA). If significant, individual parameters were compared with analysis of variance (ANOVA). Differences between individual treatments within a parameter were tested with a Kruskal–Wallis one-way ANOVA for the effects of MPTP on the Lombard response and a Student's t-test for all others, α=0.05. Motor control assays were analyzed by comparing pre- vs post-treatment scores using a non-parametric χ2 test. Results listed in the text are given as means ± s.d.
RESULTS
Dose dependency of response to MPTP
The 1 mg kg–1 dose of MPTP did not produce measurable signs of extrapyramidal symptoms nor any significant change in any echolocation call parameters or their response to broadband noise (Fig. 1; N=3; MANOVA, P=0.1056). However, the 10 mg kg–1 dose proved fatal within 2 h of the injection in the first two bats tested and was not repeated a third time. Within 30 min after injections of the 10 mg kg–1 dose of MPTP, both treated bats exhibited profound akinesia, a postural rigidity of the head and trunk, and brief periodic episodes of violent wing tremors before suddenly expiring. The 5 mg kg–1 dose of MPTP had a significant and obvious effect on all echolocation pulse acoustic parameters (MANOVA, P<0.0001) (illustrated in Fig. 1) without causing symptoms or extrapyramidal side effects similar to those caused by the 10 mg kg–1 dose. All further analysis pertains only to the 5 mg kg–1 dose.
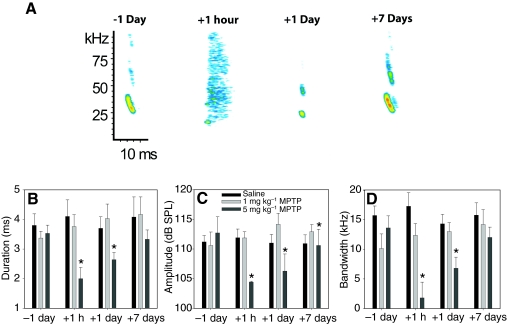
Fig. 1.
(A) Representative examples of how 1-methyl-4-phenylpyridine (MPTP) altered the acoustic structure of spontaneous echolocation pulses of one bat. All bats received saline injections as pre-treatment controls one day before drug administration (–1 day) and their echolocation pulses were recorded 1 h afterwards. +1 hour reflects calls recorded 1 h after receiving either a drug or saline injection on the day of treatment; +1 day reflects calls recorded 24 h after injection; +7 days corresponds to calls recorded one week after treatment. Panels B–D provide the mean ± s.e.m. values for the echolocation pulse acoustic parameters duration (B), amplitude (C) and bandwidth (D) for three bats receiving injections of either saline, 1 mg kg–1 MPTP or 5 mg kg–1 MPTP as their primary treatment once a week for four weeks. Asterisks indicate a significant within-group difference from pre-treatment (–1 day) measurements (P<0.05).
Effect of MPTP on behavioral motor assays
There was no significant effect of the 5 mg kg–1 dose of MPTP on either the time taken to move four limbs (P=0.5165), time to cross the arena (P=0.4860) or on the presence of tremor (P=0.4317). Only one bat showed the presence of tremor on the day of and the day after the first injection. At this dosage, no other visible signs of MPTP-induced motor deficits were observed. Bats remained able to self-feed and water, as well as climb and fly throughout the course of the experiment.
Effect of MPTP on echolocation call parameters
All three bats displayed a similar pattern of change in response to MPTP treatment for all acoustic parameters (Fig. 2A). The effect of MPTP on call parameters was significant (P<0.0001 for all) for each bat. The complete list of acoustic parameter measurements for each bat is presented in Table 1. The mean deviation from saline is an accurate representation of the individual response patterns (Fig. 2B) and will be the focus of further discussion.
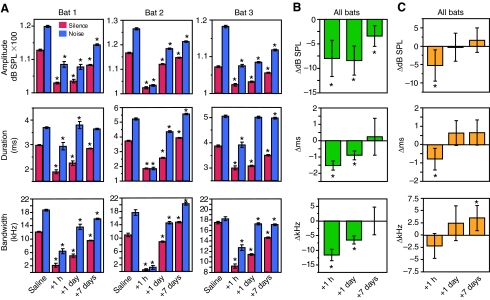
Fig. 2.
Relative effect of 1-methyl-4-phenylpyridine (MPTP) on echolocation pulse acoustics. (A) The effect of MPTP on the acoustic parameters of each of the three bats receiving a 5 mg kg–1 dose. Red bars correspond to pulses uttered in silence; blue in the presence of broadband noise. Asterisks (*) denote a significant difference from measurements obtained following saline injections the day before. (B) Mean effect of MPTP on pulse parameters in silence. Each bar denotes the mean of each bat's deviation from saline for each parameter. (C) The effect of MPTP on the Lombard response. Each bar represents the mean change in the response to noise of the three bats (B,C). Asterisks (*) denote a significant difference from zero change. Error bars are constructed with ± 2 s.e.m.
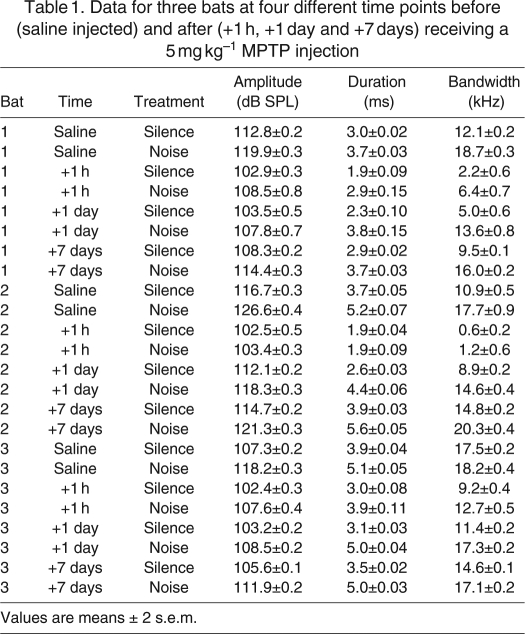
Table 1.
Data for three bats at four different time points before (saline injected) and after (+1 h, +1 day and +7 days) receiving a 5 mg kg–1 MPTP injection
Mean echolocation pulse amplitude in silence 1 h after MPTP injection was 104.8±3.2 dB SPL, a significant decrease (P=0.0049) of 7.9±6.4 dB SPL. One day after injection the mean amplitude was 105.5±5.3 dB SPL, and the mean decrease of 8.4±5.1 dB SPL was still significantly less than pre-treatment recordings (P=0.0005). By the seventh day, the mean pulse amplitude was still significantly less than pre-treatment levels by an average of 3.4±3.3 dB SPL (P=0.0059) but had returned to near normal levels at 110.6±5.5 dB SPL (Fig. 2B).
One hour after injection the mean duration of pulses uttered in silence had decreased significantly by 43.4% to a value of 2.0±0.4 ms (P<0.0001). One day after injection the mean pulse duration was 2.6±0.4 ms, still significantly less than the mean call duration obtained with saline injections by 25.9% (P<0.0001). No significant change from baseline was found 7 days after injection (P=0.6836), when pulse duration was only 5.5% less than saline levels at 3.3±0.7 ms (Fig. 2B).
MPTP significantly decreased pulse bandwidth 1 h and 1 day after injection (P<0.0001) but there was no longer a significant difference 7 days after injection (P=0.9984). Echolocation pulse bandwidth in silence was 1.8±2.8 kHz 1 h after injection, 6.8±3.3 kHz 1 day after injection and 12.0±4.1 kHz 7 days after injection, a decrease of 11.8±3.3 kHz, 6.7±2.5 kHz and 1.8±5.4 kHz relative to saline injections, respectively (Fig. 2B).
Effect of MPTP on the Lombard response
MPTP had a significant effect on how bats altered their echolocation pulses in response to broadband noise (MANOVA, P=0.0072). The baseline response to noise for saline injections was an increase in amplitude of 9.7±2.2 dB SPL, an increase in duration of 1.1±0.4 ms and a bandwidth increase of 4.7±3.4 kHz.
The noise-induced change in pulse amplitude was significantly affected by administration of MPTP (P=0.0473). Following MPTP injection, the mean echolocation pulse amplitude in broadband noise was 108.3±6.3 dB SPL 1 h after injection, 111.8±7.8 dB SPL 1 day after injection and 119.8±5.3 dB SPL 7 days after injection. The net effect on the response to broadband noise was a decrease of 5.3±7.3 dB SPL after 1 h, a decrease of 0.3±6.6 dB SPL after 1 day, and an increase of 1.6±5.3 dB SPL on day 7 (Fig. 2C).
MPTP significantly affected the change in duration caused by broadband noise (P=0.0042). Mean echolocation duration in broadband noise was significantly reduced to 2.4±1.0 ms 1 h after injection (48.9% less than controls), 3.8±1.3 ms 1 day after injection (17.4% less) and increased to 5.0±0.7 ms 7 days after injection (7.5% greater) (Fig. 2C), a significant change from the baseline response (P=0.0042).
The noise-evoked change in bandwidth was also significantly affected by MPTP (P=0.0228). One hour after injection the mean pulse bandwidth in noise was reduced to 3.8±4.9 kHz, then partially recovered to 11.7±7.4 kHz on day 1, and finally recovered to 18.1±3.7 kHz on day 7. These values corresponded to a relative change in the response to broadband noise of –2.2±4.4 kHz after 1 h, 2.4±6.1 kHz after 1 day and 3.5±4.0 kHz on day 7 (Fig. 2C).
Effect of MPTP on striatal dopamine concentration
Bats receiving a 5 mg kg–1 dose of MPTP and killed 1 h later had significantly (P=0.001, α=0.05) higher concentrations of striatal dopamine (21.127±7.024 pg mg–1, N=5) relative to bats receiving saline injections (5.760±1.236 pg mg–1, N=5). One day after MPTP injections striatal dopamine was still elevated (26.496±4.538 pg mg–1, N=5) and was not significantly different from results obtained 1 h after injection (P=0.387). In the MPTP-treated bats, we confirmed that echolocation pulses exhibited all the acoustic changes described above at the time the animals were killed. As the behavioral and acoustic measurements indicated that the effects of MPTP only waned with time and did not change in nature, we did not repeat this experiment at additional time points.
Dopamine receptor autoradiography
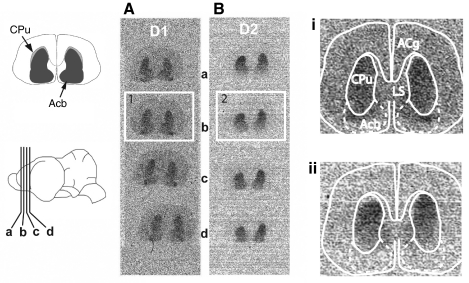
Both D1- and D2-type dopamine receptors were found co-localized throughout the nucleus accumbens and the caudate putamen of the Mexican free-tailed bat BG (Fig. 3). Receptor distributions appeared homogenously distributed throughout the striatum, although binding was higher for both receptor subtypes in the caudate putamen (D1: 0.00131±0.000193 μCi g–1, N=5; D2: 0.00159±0.000249, N=4) than in the nucleus accumbens (D1: 0.000984±0.000141 μCi g–1, N=5; D2: 0.00118±0.000381, N=4). No other brain areas showed binding for either drug that was significantly above background levels.
Fig. 3.
Autoradiographic localization of D1- and D2-type dopamine receptors in the Mexican free-tailed bat brain. (A) Representative autoradiograph showing binding in the striatum in four adjacent brain sections (shown inset on the bottom left) incubated with 3H-SCH23390, a D1-type receptor antagonist. (B) Binding in the striatum for four adjacent brain sections incubated with 3H-raclopride, a D2-type receptor antagonist. Panels i and ii are enlarged to the right and overlaid with approximate boundaries of the caudate/putamen (CPu) and nucleus accumbens (Acb) based on Cresyl Violet-stained adjacent sections (shown inset on the top left). LS, lateral septum; Acg, anterior cingulate cortex.
Effects of dopamine receptor subtype-specific drugs on pulse acoustics
Administration of the D1-type dopamine receptor agonist SKF82958 at doses of 0.1 mg kg–1, 1.0 mg kg–1, 10 mg kg–1 and 100 mg kg–1 caused no significant change in the emission rate or acoustic parameters of spontaneously emitted echolocation pulses. Testing the D1-type receptor antagonist SCH23390 proved difficult because doses as low as 100 μg kg–1 caused a rapid generalized akinesia, which consequently eliminated all pulse emission. We ultimately carried out experiments using the highest doses that produced only mild akinesia, which were 1.0 μg kg–1 and 10 μg kg–1. However, at these doses we saw no significant changes in any call acoustic parameter. Pulse duration, a reliable indicator of any changes in Mexican free-tailed bat pulse acoustics, did not vary significantly across time (P=0.9465) regardless of drug type (D1 agonist or antagonist) or with dose (P=0.1151). Likewise, neither SKF82958 nor SCH23390 caused mean pulse amplitudes to vary significantly over time (P=0.3702) regardless of treatment (P=0.5439).
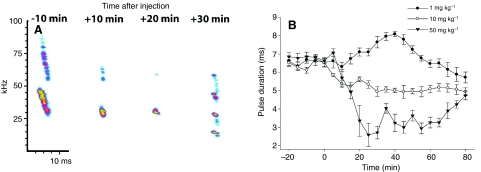
We tested the D2-type dopamine receptor agonist Quinpirole at doses of 1 mg kg–1, 10 mg kg–1 and 50 mg kg–1. After initial recordings revealed the emergence of very short, narrow bandwidth pulses similar to those seen following MPTP treatment (Fig. 4A), an analysis of co-variance (ANCOVA) with dose as a factor, bat as a block and time after injection as a covariate confirmed a significant effect of this drug on call durations (F2,305=185, P<0.0001). There was a significant interaction effect between dose and time and the time effect varied with dose (F2,305=42.1, P<0.0001). The lowest dose caused a brief increase in call durations between 20 min and 40 min after injection (Fig. 4B), while the two higher doses resulted in proportionally greater and more rapid reductions in pulse duration (Fig. 4B). Changes in pulse duration were a reliable reflection of concurrent changes in bandwidth and amplitude as illustrated in Fig. 4A. This highest dose of Quinpirole reduced but did not eliminate regular pulse emissions. Conversely, the D2-type dopamine receptor antagonist Eticlopride had no significant effects on the acoustic parameters of spontaneous pulse emissions at any of the four doses tested, 0.1 mg kg–1, 1.0 mg kg–1, 10 mg kg–1 and 100 mg kg–1 (P=0.1014). These doses were chosen based on the literature and were predicted to achieve greater than 80% receptor occupancy in the striatum at the lowest dose.
Fig. 4.
(A) Representative example of how a 50 mg kg–1 dose of Quinpirole acutely altered the acoustic structure of spontaneous echolocation pulses. (B) The effect of three doses of Quinpirole on echolocation pulse duration in five bats. Pulse durations were binned and averaged over successive 5 min periods beginning 20 min before drug injection (mean ± s.e.).
DISCUSSION
The action of MPTP on the dopamine-producing cells of the SNc in mammals is well established (Jakowec et al., 2004; Smeyne and Jackson-Lewis, 2005). In the brain MPTP is metabolized to 1-methyl-4-phenylpyridinium (MPP+) by the enzyme monoamine oxidase B. MPP+ selectively enters dopamine-producing cells via their dopamine transporter (DAT). As the highest densities of DAT are located in the midbrain substantia nigra and ventral tegmental area, these neurons are preferentially impacted by MPTP injection. Once inside the neuron MPP+ interferes with complex 1 of the electron transport chain in mitochondria leading to the depletion of ATP levels (Suzuki et al., 1990). Reduced cellular respiration is not suspected of being the imminent cause of cell death (Smeyne and Jackson-Lewis, 2005) but ATP depletion has been linked to the abnormal release of dopamine from intracellular stores into the striatum (Ofori and Schorderet, 1987; Rollema et al., 1988). It appears likely that in our experiments MPTP did not acutely kill catecholaminergic neurons within the same time frame that dramatic changes in pulse acoustics appeared. Instead, MPTP administration appears to have caused an efflux of dopamine into the striatum that led to significant reductions in echolocation pulse duration, bandwidth and loudness. Since the effects of MPTP on pulse acoustics gradually rescinded over the ensuing week after injection, we assume that this was the time course over which MPTP and its metabolites were cleared and neurons recovered normal functioning. However, in mice an 80 mg kg–1 dose of MPTP (four injections over eight hours) caused a reduction in striatal dopamine that reached its nadir only after three to seven days (Jakowec et al., 2004). We therefore cannot rule out the possibility that the acute hyperdopaminergic conditions were not subsequently replaced by cell death and hypodopaminergic conditions at some point during the week after injection. That question could be addressed with more HPLC measurements but it goes beyond the immediate goals of this project.
In our initial experimental design we tested for the effects of repeated low-dose administration of MPTP hoping to achieve a mild chronic change in vocalizations. However, four weeks of repeated injections produced no significant cumulative effects, although each subsequent injection produced changes quantitatively similar to the preceding injections. Had we shortened the time between injections to once a day or every other day for four consecutive days, as has been done in primates to achieve chronic effects (Albanese et al., 1993; Jenner et al., 1984), we may have achieved hypodopaminergic rather than hyperdopaminergic results. However, these experiments were constrained by a limited number of animals and apparently a fine threshold dose beyond which the drug appeared to be rapidly lethal. Although the 1 mg kg–1 dose had no measurable acoustic or behavioral effects, the 5 mg kg–1 dose dramatically altered echolocation pulse acoustics without evidence of any other significant motor deficits, and the 10 mg kg–1 dose caused severe deficits that were almost instantly lethal. The 5 mg kg–1 dose may have caused motor deficits that were not adequately reflected by our simple motor assays. More sensitive assays possibly applied in conjunction with different drug administration schedules would be required to completely address the effects of MPTP on the bat's behavior and neurophysiology.
The hypothesis that MPTP interfered with echolocation pulse production via a hyperdopaminergic mechanism is supported by the observation that high doses of the D2-type dopamine receptor agonist Quinpirole resulted in similarly distorted echolocation pulse structures. Our observation that the lowest dose of Quinpirole caused a slight increase in call durations while the two higher doses caused decreases in call duration is consistent with previous reports of Quinpirole evoking biphasic dose responses due to its actions at pre-synaptic autoreceptors, which have a higher affinity for the drug than post-synaptic D2 receptors (Lin and Walter, 1994; Plantje et al., 1987). Activation of D2 autoreceptors has the net effect of suppressing post-synaptic activity at D2 synapses, and if that was the case here then the results imply that D2 receptors actively influence the acoustic structure of spontaneously emitted echolocation pulses. However, as the D2 antagonist Eticlopride had no measureable effect on pulse acoustics, further studies are needed to clarify this issue.
Dopamine's effects on echolocation pulse acoustics
In humans, speech disorders can arise from either increases or decreases in striatal dopamine concentrations, resulting in what are known as hyperkinetic or hypokinetic dysarthrias, respectively (Darley et al., 1969). In rats, Ciucci and colleagues found that striatal dopamine depletions produced symptoms similar to hypokinetic dysarthria, namely reduced call loudness and bandwidth (Ciucci et al., 2009). Those symptoms can be accounted for by reduced laryngeal and respiratory muscle tone. In our case, however, although we observed similar changes in pulse acoustics, these symptoms were more likely attributable to hypertonicity of the vocal musculature. In humans, a condition known as hypertonic dysphonia sometimes presents as a breathy and harsh whispering voice with frequent interruptions resulting from an inability to articulate correctly. These symptoms have been linked to abnormal increases in BG dopamine levels (Duffy, 2005). Striatal dopamine levels regulate the output of thalamocortical feedback pathways, which in turn control activity in descending motor pathways (Herrero et al., 2002), and elevated dopamine levels driving over activity of the pyramidal speech motor pathways is thought to cause the hypertonicity of the laryngeal musculature. In bats, it is not yet known whether BG circuits are modulating the vocal motor pathway via thalamocortical projections as in humans or through an alternative BG output pathway that projects from the substantia nigra pars reticulata to the midbrain components of the vocal motor pathway (Grillner et al., 2005; Hikosaka, 2007; Sinha and Moss, 2007).
Changes in echolocation pulse loudness, duration and bandwidth are likely to be interdependent consequences of biomechanical constraints of the bat's laryngeal apparatus. We have previously shown in free-tailed bats that any changes in pulse duration and bandwidth are positively correlated with amplitude (Tressler and Smotherman, 2009). Any change in subglottic pressure is expected to alter the frequency content of echolocation pulses due to the mechanical properties of the larynx (Suthers and Fattu, 1973). The primary effect of MPTP is hypothesized to be a reduction in voice loudness, the biophysical consequences of which were concomitant changes in call duration and bandwidth. Bats need high subglottic pressures to generate high-frequency vocalizations. As MPTP interfered with the bats' ability to generate high subglottic pressures, the high starting frequency of the downward frequency modulation sweep should have been most sensitive to this change, and this is what we observed (Figs 2 and 4). It remains unclear however if the effects of MPTP on call structure are attributable solely to changes in respiratory drive or whether the drug also degraded the bats' ability to articulate the laryngeal apparatus in a complex way. Ciucci and colleagues speculated that striatal dopamine depletion may have constrained fine sensorimotor processes needed for the articulation of more complex broadband vocalizations in rats (Ciucci et al., 2009). It may also be the case that hypertonic laryngeal conditions similarly interfere with articulatory mechanisms in both bats and humans.
Dopamine agonists can suppress vocalization emission rates in rodents (Cuomo et al., 1987; Dastur et al., 1999), and D2 agonists can generally suppress motor activity in rats (Tidey and Miczek, 1992). Cuomo and colleagues reported that early chronic exposure to the D1 antagonist SCH23390 increased isolation call durations and loudness and decreased frequency parameters, while the D2 antagonist sulpiride reduced isolation call amplitudes (Cuomo et al., 1987). However, these results were attributed to changes in motivational and reward circuitry and not necessarily to changes in vocal motor control. Systemic administration of dopaminergic agonists may alter vocalizing for several reasons, including changes in motivation, activity levels or physiological responses, such as a change in body temperature (Dastur et al., 1999). The advantage of testing the effects of these drugs on bat echolocation pulses is that while motivation may be important for overall emission rates, the acoustic structure and precise timing of echolocation pulse emissions is dictated by cognitive rather than limbic factors. Bats must use echolocation pulses to navigate similarly regardless of emotive or motivational context. For this reason, the observed changes in echolocation pulse acoustics caused by hyperdopaminergic conditions can be reliably assigned to changes in the properties of the motor control circuitry rather than the perceptual or motivating conditions driving vocalizing. Given that the behavioral assays showed no major motor defects and that the bats were capable of normal locomotion (including flight), the change in dopamine seems to have had a larger impact on the bats' ability to regulate the vocal control musculature than the locomotor muscles, although it should be noted that muscle force amplitude was not directly measured for any motor system during these experiments. It is possible that very small changes in respiratory or laryngeal muscle force may have a more pronounced effect on vocalizing than similar changes in other motor systems due to the ballistic nature of animal vocalizations.
MPTP effect on the Lombard response
One potential advantage of using bat echolocation performance for this study was that we could exploit their compensatory changes in pulse loudness in the presence of background noise as a means of testing the role of dopamine in audio-vocal integration. In stationary bats MPTP treatment appeared to strongly suppress the Lombard response, although in two of three bats the noise stimuli continued to evoke a small increase in pulse loudness. This evidence supports the hypothesis that the BG may be making an important contribution to the integration of auditory sensory cues into the volitional vocal motor commands but also leaves open the possibility that other mechanisms also contribute to the behavior. For example, it was previously shown that decerebrate cats retained some evidence of a Lombard response (Nonaka et al., 1997), indicating that the Lombard response may depend upon brainstem reflexes. In an intact animal such reflexes may be regulated by BG outputs (Hikosaka, 2007). In the MPTP-treated bats, the Lombard response appeared to recover in conjunction with the return of normal pulse structures, raising some questions about whether in fact this was a reliable test of the sensorimotor control circuitry. If the bats could not produce normal echolocation pulses then it is difficult to know whether or not the sensorimotor circuitry might have been performing normally in the background. Audio-vocal feedback might have been correctly delivered to but not implemented by the vocal motor apparatus.
Seven days after receiving an MPTP injection the noise-induced changes in one acoustic parameter, pulse bandwidth, had rebounded to a value significantly greater than the pre-treatment response to noise, indicating that the bats exhibited a relatively stronger Lombard response following recovery from the MPTP treatment. The mean values for all three acoustic parameters appeared to return to values slightly exceeding their original measurements. Interestingly, the Lombard response seems to have recovered within one day after MPTP injections while baseline pulse acoustics and the striatal dopamine levels remained significantly elevated. This may reflect an adaptive response to the suppressive effects of a hyperdopaminergic condition, which will have to be addressed with future work.
Localization of dopamine receptor subtypes
In this study, both D1 and D2 receptors were found primarily in the caudate putamen and nucleus accumbens, showing a similar distribution to those reported in other mammals. This indicates that the effects of MPTP and Quinpirole are probably the result of changes in striatal dopamine concentrations. In the rat, dopamine receptors were found throughout the forebrain with autoradiography, with the highest levels in the caudate putamen, nucleus accumbens and olfactory tubercle (Boyson et al., 1986). Lower levels were detected in areas of the cortex, amygdala, globus pallidus and hippocampus, among others. There could be D1 and D2 receptors in other areas of the Mexican free-tailed bat brain that were too low in density to find with this technique; Fig. 3 offers some indication that D1 receptors may be broadly distributed throughout the cortex at low densities. D1 receptor density was at least threefold higher than D2 receptor density in all areas in the rat, while in the bat we found approximately the same densities of the two receptor types within the striatum. Humans, monkeys and cats have a similar distribution and D1/D2 ratio to that seen in the rat (Camps et al., 1989; Lidow et al., 1991; Richfield et al., 1987). It was suggested that the higher ratio was due to the fact that an antagonist was used for the D1 receptor type while an agonist was used for the D2 receptor type because dopamine antagonists bind both high and low affinity states of receptors while agonists only label the high affinity states (Camps et al., 1990). This study used antagonists for both receptor types, which may explain the nearly 1:1 ratio reported here. Also, another analysis in humans using SCH23390 and raclopride reported a ratio similar to the bat, further supporting the suggestion that the radioligands used might account for discrepancies in the measurements of receptor densities (Hall et al., 1988).
CONCLUSIONS
The BG are suspected of playing an important role in the regulation of human speech production (Alm, 2004; Duffy, 2005) but their precise functions in normal speech or any other mammalian vocalization remain poorly understood. In spontaneously echolocating bats, increasing striatal dopamine concentrations or selectively activating D2-type dopaminergic synapses dramatically but reversibly altered the acoustic structure and loudness of echolocation pulses. Importantly, results presented here qualitatively mimicked some of the symptoms of hyperkinetic dysarthria observed in humans, which may have important implications for understanding how pathological disruption of the BG circuitry impacts the speech motor control circuitry. These changes indicate that the dopaminergic tone in the BG is an important factor for the correct production of bat echolocation pulses. Further work is needed to define the precise contributions of the BG in the broader context of both mammalian vocalizations and bat echolocation behavior.
ACKNOWLEDGEMENTS
We are grateful to the Texas A&M Athletic Department for granting us access to the bats living in their facilities. We thank Barb Earnest for her assistance in maintaining the animals. We thank Drs Kirsten Bohn, L. Rene Garcia, Mark Zoran and Jim Grau for helpful discussions, advice and comments.
FOOTNOTES
This study was funded by Texas A&M University and NIH NIDCD Grant No. DC007962 to M.S. Deposited in PMC for release after 12 months.
REFERENCES
- Albanese A., Granata R., Gregori B., Piccardi M. P., Colosimo C., Tonali P. (1993). Chronic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to monkeys: behavioral, morphological and biochemical correlates. Neuroscience 55, 823-832 [DOI] [PubMed] [Google Scholar]
- Alexander G. E., Crutcher M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266-271 [DOI] [PubMed] [Google Scholar]
- Alm P. A. (2004). Stuttering and the basal ganglia circuits: a critical review of possible relations. J. Commun. Disord. 37, 325-369 [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. (1986). Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci. 6, 3177-3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury A. J., Costall B., Domeney A. M., Jenner P., Kelly M. E., Marsden C. D., Naylor R. J. (1986). 1-methyl-4-phenylpyridine is neurotoxic to the nigrostriatal dopamine pathway. Nature 319, 56-57 [DOI] [PubMed] [Google Scholar]
- Brumm H., Voss K., Köllmer I., Todt D. (2004). Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 207, 443-448 [DOI] [PubMed] [Google Scholar]
- Camps M., Cortés R., Gueye B., Probst A., Palacios J. M. (1989). Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience 28, 275-290 [DOI] [PubMed] [Google Scholar]
- Camps M., Kelley P. H., Palacios J. M. (1990). Autoradiographic localization of dopamine D1 and D2 receptors in the brain of several mammalian species. J. Neural Transm. 80, 105-127 [DOI] [PubMed] [Google Scholar]
- Chiu C., Xian W., Moss C. F. (2009). Adaptive echolocation behavior in bats for the analysis of auditory scenes. J. Exp. Biol. 212, 1392-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci M. R., Ma S. T., Fox C., Kane J. R., Ramig L. O., Schallert T. (2007). Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav. Brain Res. 182, 284-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci M. R., Ahrens A. M., Ma S. T., Kane J. R., Windham E. B., Woodlee M. T., Schallert T. (2009). Reduction of dopamine synaptic activity: degradation of 50 kHz ultrasonic vocalization in rats. Behav. Neurosci. 123, 328-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland J., Snowdon C. T. (1982). The complex vocal repertoire of the adult cotton-top tamarin (Saguinus oedipus oedipus). Z. Tierpsychol. 58, 231-270 [Google Scholar]
- Cuomo V., Gagiano R., Renna G., De Salvia M. A., Racagni G. (1987). Ultrasonic vocalizations in rat pups: effects of early postnatal exposure to SCH23390 (A DA1-receptor antagonist) and sulpipride (A DA2-receptor antagonist). Neuropharmacology 26, 701-705 [DOI] [PubMed] [Google Scholar]
- Darley F. L., Aronson A. E., Brown J. R. (1969). Differential diagnostic patterns of dysarthria. J. Speech Hear. Res. 12, 249-269 [DOI] [PubMed] [Google Scholar]
- Dastur F. N., McGregor I. S., Brown R. E. (1999). Dopaminergic modulation of rat pup ultrasonic vocalizations. Eur. J. Pharmacol. 382, 53-67 [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Kuhl P. K. (1999). Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567-631 [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Perkel D. J., Reiner A., Stern E. A. (2005). Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 28, 353-363 [DOI] [PubMed] [Google Scholar]
- Duffy J. R. (2005). Motor Speech Disorders. St Louis: Elsevier Mosby. [Google Scholar]
- Egnor S. E., Hauser M. D. (2006). Noise-induced vocal modulation in cotton-top tamarins (Saguinus oedipus). Am. J. Primatol. 68, 1183-1190 [DOI] [PubMed] [Google Scholar]
- Egnor S. E., Iguina C. G., Hauser M. D. (2006). Perturbation of auditory feedback causes systematic perturbation in vocal structure in adult cotton-top tamarins. J. Exp. Biol. 209, 3652-3663 [DOI] [PubMed] [Google Scholar]
- Egnor S. E., Wickelgren J. G., Hauser M. D. (2007). Tracking silence: adjusting vocal production to avoid acoustic interference. J. Comp. Physiol. A 193, 477-483 [DOI] [PubMed] [Google Scholar]
- Ghazanfar A. A., Smith-Rohrberg D., Pollen A. A., Hauser M. D. (2002). Temporal cues in the antiphonal long-calling behaviour of cottontop tamarins. Anim. Behav. 64, 427-438 [Google Scholar]
- Gillam E. H., McCracken G. F. (2007). Variability in the echolocation of Tadarida brasiliensis: effects of geography and local acoustic environment. Anim. Behav. 74, 277-286 [Google Scholar]
- Gillam E. H., Ulanovsky N., McCracken G. F. (2007). Rapid jamming avoidance in biosonar. Proc. Biol. Sci. 274, 651-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberman A. M., Blomgren M. (2003). Parkinsonian speech disfluencies: effects of L-dopa-related fluctuations. J. Fluency Disord. 28, 55-70 [DOI] [PubMed] [Google Scholar]
- Gooler D. M., O’Neill W. E. (1986). The central control of biosonar signal production in bats demonstrated by microstimulation of anterior cingulate cortex in the echolocating bat, Pteronotus parnelli parnelli. In The Physiological Control of Mammalian Vocalization (ed. Newman J. D.), pp. 153-184 New York: Plenum Press; [Google Scholar]
- Grillner S., Hellgren J., Menard A., Saitoh K., Wikstrom M. A. (2005). Mechanisms for selection of basic motor programs-roles for the striatum and pallidum. Trends Neurosci. 28, 364-370 [DOI] [PubMed] [Google Scholar]
- Hall H., Farde L., Sedvall G. (1988). Human dopamine receptor subtypes – in vitro binding analysis using 3H-SCH 23390 and 3H-raclopride. J. Neural Transm. 73, 7-21 [DOI] [PubMed] [Google Scholar]
- Herrero M. T., Barcia C., Navarro J. M. (2002). Functional anatomy of thalamus and basal ganglia. Childs Nerv. Syst. 18, 386-404 [DOI] [PubMed] [Google Scholar]
- Hikosaka O. (2007). GABAergic output of the basal ganglia. Prog. Brain Res. 160, 209-226 [DOI] [PubMed] [Google Scholar]
- Ho A. K., Bradshaw J. L., Iansek R., Alfredson R. (1999). Speech volume regulation in Parkinson’s disease: effects of implicit cues and explicit instructions. Neuropsychologia 37, 1453-1460 [DOI] [PubMed] [Google Scholar]
- Holmes R. J., Oates J. M., Phyland D. J., Hughes A. J. (2000). Voice characteristics in the progression of Parkinson’s disease. Int. J. Lang. Commun. Disord. 35, 407-418 [DOI] [PubMed] [Google Scholar]
- Insley S. J., Southhall B. L. (2005). Source levels of northern elephant seal vocalizations in-air. J. Acoust. Soc. Am. 118, 2018-2019 [Google Scholar]
- Jackson-Lewis V., Jakowec M., Burke R. E., Przedborski S. (1995). Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4, 257-269 [DOI] [PubMed] [Google Scholar]
- Jakowec M., Nixon K., Hogg E., McNeill T., Petzinger G. M. (2004). Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurdegeneration of the mouse nigrostriatal pathway. J. Neurosci. Res. 76, 539-550 [DOI] [PubMed] [Google Scholar]
- Jarvis E. D. (2004). Learned birdsong and the neurobiology of human language. Ann. N. Y. Acad. Sci. 1016, 749-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis J., Bohn K. M., Tressler J., Smotherman M. (2010). A mechanism for antiphonal echolocation by free-tailed bats. Anim. Behav. 79, 787-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P., Rupniak N. M. J., Rose S., Kelly E., Kilpatrick G., Lees A., Marsden C. D. (1984). 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the commone marmoset. Neurosci. Lett. 50, 85-90 [DOI] [PubMed] [Google Scholar]
- Kao M. H., Doupe A. J., Brainard M. S. (2005). Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433, 638-643 [DOI] [PubMed] [Google Scholar]
- Kim D. S., Szczypka M. S., Palmiter R. D. (2000). Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J. Neurosci. 20, 4405-4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer P. R., Guan G., Wellman P. J., Bellinger L. L. (2007). Nicotine’s attenuation of body weight involves the perifornical hypothalamus. Life Sci. 81, 500-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P. K. (2003). Human speech and birdsong: communication and the social brain. Proc. Natl. Acad. Sci. USA 100, 9645-9646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow M. S., Goldman-Rakic P. S., Gallager D. W., Rakic P. (1991). Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone, and [3H]SCH23390. Neuroscience 40, 657-671 [DOI] [PubMed] [Google Scholar]
- Lin M.-Y., Walter D. E. (1994). Dopamine D2 autoreceptors in rats are behaviorallly functional at 21 but not 10 days of age. Psychopharmacology 114, 262-268 [DOI] [PubMed] [Google Scholar]
- Louis E. D., Winfield L., Fahn S., Ford B. (2001). Speech dysfluency exacerbated by levodopa in Parkinson’s disease. Mov. Disord. 16, 562-565 [DOI] [PubMed] [Google Scholar]
- Metzner W., Schuller G. (2007). Vocal control in echolocating bats. In Handbook of Mammalian Vocalizations (ed. Brudzynski S. M.), pp. 403-415 Amsterdam: Elsevier; [Google Scholar]
- Muralikrishnan D., Mohanakumar K. P. (1998). Neuroprotection by bromocriptine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. FASEB J. 12, 905-912 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Takahashi R., Enomoto K., Katada A., Unno T. (1997). Lombard reflex during PAG-induced vocalization in decerebrate cats. Neurosci. Res. 29, 283-289 [DOI] [PubMed] [Google Scholar]
- Obrist M. K. (1995). Flexible bat echolocation-the influence of individual, habitat and conspecifics on sonar signal design. Behav. Ecol. Sociobiol. 36, 207-219 [Google Scholar]
- Ofori S., Schorderet M. (1987). Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on dopamine synthesis and release in the rabbit retina in vitro. Neuropharmacology 26, 1607-1610 [DOI] [PubMed] [Google Scholar]
- Penna M., Pottstock H., Velasquez N. (2005). Effect of natural and synthetic noise on evoked vocal responses in a frog of the temperate austral forest. Anim. Behav. 70, 639-651 [Google Scholar]
- Plantje J. E., Steinbusch H. W., Schipper J., Dijcks F. A., Verheijden P. F. H. M., Stoof J. C. (1987). D-2 dopamine-receptors regulate the release of [3H]-dopamine in rat cortical regions showing dopamine immunoreactive fibers. Neuroscience 20, 157-168 [DOI] [PubMed] [Google Scholar]
- Rasband W. S. (1997). ImageJ, http://rsb.info.nih.gov/ij/ Bethesda, MD: U. S. National Institutes of Health; [Google Scholar]
- Ratcliffe J. M., ter Hofstede H. M., Avila-Flores R., Fenton M. B., McCracken G. F., Biscardi S., Blasko J., Gillam E., Orprecio J., Spanjer G. (2004). Conspecifics influence call design in the Brazilian free-tailed bat, Tadarida brasiliensis. Can. J. Zool. 82, 966-971 [Google Scholar]
- Riachi N. J., Harik S. I., Kalaria R. N., Sayre L. M. (1988). On the mechanisms underlying 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity: II Susceptibility among mammalian species correlates with the toxin’s metabolic patterns in brain microvessels and liver. J. Pharmacol. Exp. Ther. 244, 443-448 [PubMed] [Google Scholar]
- Richfield E. K., Young A. B., Penney J. B. (1987). Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J. Comp. Neurol. 262, 446-463 [DOI] [PubMed] [Google Scholar]
- Rollema H., Kuhr W. G., Kranenborg G., De Vries J., Van Den Berg C. (1988). MPP+-induced eflux of dopamine and lactate from rat striatum have similar time courses as shown by in vivo brain dialysis. J. Pharmacol. Exp. Ther. 245, 858-866 [PubMed] [Google Scholar]
- Sanabria J., Ruiz P. G., Gutierrez R., Marquez F., Escobar P., Gentil M., Cenjor C. (2001). The effect of levodopa on vocal function in Parkinson’s disease. Clin. Neuropharmacol. 24, 99-102 [DOI] [PubMed] [Google Scholar]
- Scheifele P. M., Andrew S., Cooper R. A., Darre M., Musiek F. E., Max L. (2005). Indication of a Lombard vocal response in the St Lawrence River Beluga. J. Acoust. Soc. Am. 117, 1486-1492 [DOI] [PubMed] [Google Scholar]
- Schnitzler H.-U., Kalko E. K. V. (2001). Echolocation by insect-eating bats. BioScience 51, 557-569 [Google Scholar]
- Schwartz C., Tressler J., Keller H., Vanzant M., Ezell S., Smotherman M. (2007). The tiny difference between foraging and communication buzzes uttered by the Mexican free-tailed bat, Tadarida brasiliensis. J. Comp. Physiol. A 193, 853-863 [DOI] [PubMed] [Google Scholar]
- Sedelis M., Schwarting R. K. W., Huston J. P. (2001). Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 125, 109-122 [DOI] [PubMed] [Google Scholar]
- Sikiric P., Marovic A., Matoz W., Anic T., Buljat G., Mikus D., Stancic-Rokotov D., Separovic J., Seiwerth S., Grabarevic Z., et al. (1999). A behavioural study of the effect of pentadecapeptide bpC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J. Physiol. Paris 93, 505-512 [DOI] [PubMed] [Google Scholar]
- Simmons J. A., Lavender W. A., Lavender B. A., Childs J. E., Hulebak K., Rigden M. R., Sherman J., Woolman B., O’Farrell M. J. (1978). Echolocation by free-tailed bats (Tadarida). J. Comp. Physiol. A 125, 291-299 [Google Scholar]
- Sinha S. R., Moss C. F. (2007). Vocal premotor activity in the superior colliculus. J. Neurosci. 27, 98-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne R. J., Jackson-Lewis V. (2005). The MPTP model of Parkinson’s disease. Brain Res. Mol. Brain. Res. 134, 57-66 [DOI] [PubMed] [Google Scholar]
- Suthers R. A., Fattu J. M. (1973). Mechanisms of sound production by echolocating bats. Am. Zool. 13, 1215-1226 [Google Scholar]
- Suzuki K., Mizuno Y., Yoshida M. (1990). Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-like compounds on mitochondrial respiration. Adv. Neurol. 53, 212-218 [PubMed] [Google Scholar]
- Sved A. F. (1989). PNMT-containing neurons are not necessarily adrenergic. Brain Res. 481, 113-118 [DOI] [PubMed] [Google Scholar]
- Tidey J. W., Miczek K. A. (1992). Effects of SKF 38393 and quinpirole on aggressive, motor and schedule-controlled behaviors in mice. Behav. Pharmacol. 3, 553-566 [PubMed] [Google Scholar]
- Tressler J., Smotherman M. (2009). Context-dependent effects of noise on echolocation pulse characteristics in free-tailed bats. J. Comp. Physiol. A 195, 923-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N., Fenton M. B., Tsoar A., Korine C. (2004). Dynamics of jamming avoidance in echolocating bats. Proc. Biol. Sci. 271, 1467-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley S. C., Doupe A. J. (2008). Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 6, e62 [DOI] [PMC free article] [PubMed] [Google Scholar]