Abstract
In eukaryotic species from yeast to human, stress-activated protein kinases (SAPKs), members of a MAP kinase (MAPK) subfamily, regulate the transcriptional response to various environmental stress. It is poorly understood how diverse forms of stress are sensed and transmitted to SAPKs. Here, we report the heat shock regulation of the fission yeast Spc1 SAPK, a homolog of human p38 and budding yeast Hog1p. Although osmostress and oxidative stress induce strong activation of the Wis1 MAPK kinase (MEK), which activates Spc1 through Thr-171/Tyr-173 phosphorylation, activation of Wis1 upon heat shock is relatively weak and transient. However, in heat-shocked cells, Pyp1, the major tyrosine phosphatase that dephosphorylates and inactivates Spc1, is inhibited for its interaction with Spc1, which leads to strong activation of Spc1. Subsequently, Spc1 activity is rapidly attenuated by Thr-171 dephosphorylation, whereas Tyr-173 remains phosphorylated. Thr-171 dephosphorylation is compromised in a strain lacking functional type 2C serine/threonine phosphatases (PP2C), Ptc1 and Ptc3. Moreover, Ptc1 and Ptc3 can dephosphorylate Thr-171 of Spc1 both in vivo and in vitro. These observations strongly suggest that PP2C enzymes play an important role in the attenuation of Spc1 activity in heat-shocked cells. Thus, transient activation of Spc1 upon heat shock is ensured by differential regulation of threonine and tyrosine phosphorylation.
Keywords: Heat shock, protein phosphatase 2C, protein tyrosine phosphatase, Schizosaccharomyces pombe, Spc1, stress-activated protein kinase
A MAP kinase (MAPK) cascade is an intracellular signaling module ubiquitous among eukaryotes. The basic mechanism of signal transduction appears to be very similar in all MAPK cascades (Marshall 1995); MAPK is activated by dual phosphorylation of neighboring threonine and tyrosine residues within protein kinase subdomain VIII, which is carried out by a threonine/tyrosine-kinase, MAPK kinase (MEK). MEK is activated in turn by an upstream MEK kinase (MEKK) also through phosphorylation of the conserved serine/threonine residues in subdomain VIII. Thus, signals are transmitted by sequential activation of the protein kinases, MEKK → MEK → MAPK. Cells from yeast to human utilize multiple MAPK cascades to transmit diverse extracellular stimuli to the nucleus (Waskiewicz and Cooper 1995). MAPKs dedicated for stress signaling are generically called SAPKs (stress-activated protein kinases). SAPKs include highly homologous Hog1p in budding yeast Saccharomyces cerevisiae (Brewster et al. 1993), Spc1 (also known as Sty1 or Phh1) in fission yeast Schizosaccharomyces pombe (Millar et al. 1995; Shiozaki and Russell 1995b; Kato et al. 1996), and mammalian p38 (Han et al. 1994).
S. pombe spc1 (suppressor of phosphatase 2C) was first identified as a suppressor mutation of the phenotypes caused by loss of type 2C serine/threonine phosphatases (PP2Cs) encoded by ptc1+ and ptc3+ (Shiozaki and Russell 1995a). The same genetic screen also isolated mutations in wis1+, which encodes a MEK homolog implicated in cell cycle regulation (Warbrick and Fantes 1991). Subsequent genetic and biochemical analyses demonstrated that Wis1 is the MEK that phosphorylates Thr-171/Tyr-173 of Spc1 (Millar et al. 1995; Shiozaki and Russell 1995b). Wis1 activity is counteracted by tyrosine-specific phosphatases, Pyp1 and Pyp2 (Millar et al. 1995; Shiozaki and Russell 1995b). Pyp1 accounts for the major cellular activity that dephosphorylates Spc1 Tyr-173, with Pyp2 having a minor role (Degols et al. 1996; Samejima et al. 1997). Because MAPK activation requires both threonine and tyrosine phosphorylation (Cobb and Goldsmith 1995), dephosphorylation of Tyr-173 is sufficient to inactivate Spc1. Simultaneous deletion of pyp1+ and pyp2+ brings about Spc1 hyperactivation lethal to the cell (Millar et al. 1995; Shiozaki and Russell 1995b), indicating that dephosphorylation of Tyr-173 by Pyp1/Pyp2 is central to negative regulation of Spc1. On the other hand, Thr-171 dephosphorylation of Spc1 has never been carefully examined. Whereas inactivation of MAPKs by tyrosine phosphatases has also been reported in budding yeast and mammals (Keyse 1998), many MAPK phosphatases belong to the dual-specificity phosphatase family, which dephosphorylates both the threonine and tyrosine residues (Clarke 1994; Keyse 1995).
Like mammalian p38 (Kyriakis and Avruch 1996), Spc1 is activated in response to diverse stress stimuli including high osmolarity, oxidative stress, and heat shock (Millar et al. 1995; Shiozaki and Russell 1995b; Degols et al. 1996). Δspc1 mutants are supersensitive to these stress conditions, indicating that the function of Spc1 is crucial for cellular resistance to multiple forms of stress. Spc1 is not phosphorylated at all in Δwis1 mutants under any stress conditions, which suggests that Wis1 is the only MEK for Spc1 (Millar et al. 1995; Shiozaki and Russell 1995b; Degols et al. 1996). However, it has yet to be determined whether Wis1 is activated by all the stress conditions that are known to activate Spc1. To examine whether various stress stimuli are transmitted to the Wis1 MEK through MEKKs, we previously performed mutational analyses of the MEKK phosphorylation sites in Wis1 (Shiozaki et al. 1998). Ser-469 and Thr-473 of Wis1, which correspond to the MEKK phosphorylation sites conserved among various MEKs, were substituted with alanine and aspartic acid to create wis1AA and wis1DD mutants, respectively. As in the Δwis1 strain, Spc1 phosphorylation was not detectable in the wis1AA mutant under various stress conditions, demonstrating that Ser-469 and Thr-473 are crucial for Wis1 activation. In wis1DD mutant cells, Spc1 phosphorylation was higher than in wild-type cells even in the absence of stress, which suggests that the wis1DD mutation partially stimulates Wis1 activity presumably by mimicking phosphorylation of Ser-469/Thr-473. However, high osmolarity and oxidative stress failed to induce further activation of Spc1 in wis1DD cells. These results suggest that osmostress and oxidative stress signals are mediated by MEKKs through phosphorylation of Ser-469/Thr-473 of Wis1, and substitution of these sites with unphosphorylatable residues abolishes signaling to Spc1. To our surprise, heat shock induced strong activation of Spc1 in wis1DD cells as well as in wild-type cells, indicating that heat shock signals can be transmitted to Spc1 independently of MEKKs. It should be noted, however, that heat shock-induced activation of Spc1 still requires Wis1 MEK activity, as Spc1 is not activated at all in Δwis1 and wis1AA mutants even after heat shock (Degols et al. 1996; Shiozaki et al. 1998).
With the aim of understanding how heat shock stimuli are transmitted to Spc1, we developed an in vitro assay for Wis1 to examine how the catalytic activity of Wis1 and Wis1DD is regulated in response to heat shock. We found that Wis1 activation upon heat shock is relatively weak and transient, which contrasts with strong Wis1 activation induced by osmostress and oxidative stress. The activity of Wis1DD is constitutive and not affected by heat shock, suggesting that heat shock-induced activation of Spc1 in the wis1DD strain is not mediated by an increase of Wis1DD activity. On the other hand, the tyrosine phosphatase activity that dephosphorylates Spc1 Tyr-173 is significantly inhibited in heat-shocked cells. We found that heat shock impairs the interaction of the Pyp1 tyrosine phosphatase with Spc1. These results strongly suggest that Spc1 activation upon heat shock is partly mediated by inhibition of the tyrosine phosphatases dephosphorylating Spc1. Importantly, Spc1 activated in heat-shocked cells is rapidly attenuated by Thr-171 dephosphorylation, whereas Tyr-173 remains phosphorylated. Genetic and biochemical data strongly suggest that PP2C enzymes, Ptc1 and Ptc3, dephosphorylate Spc1 Thr-171. Thus, transient activation of the Spc1 SAPK upon heat shock is ensured by differential regulation of Thr-171 and Tyr-173 phosphorylation by PP2C and tyrosine-specific phosphatases.
Results
Stress-induced activation of the Wis1 MEK
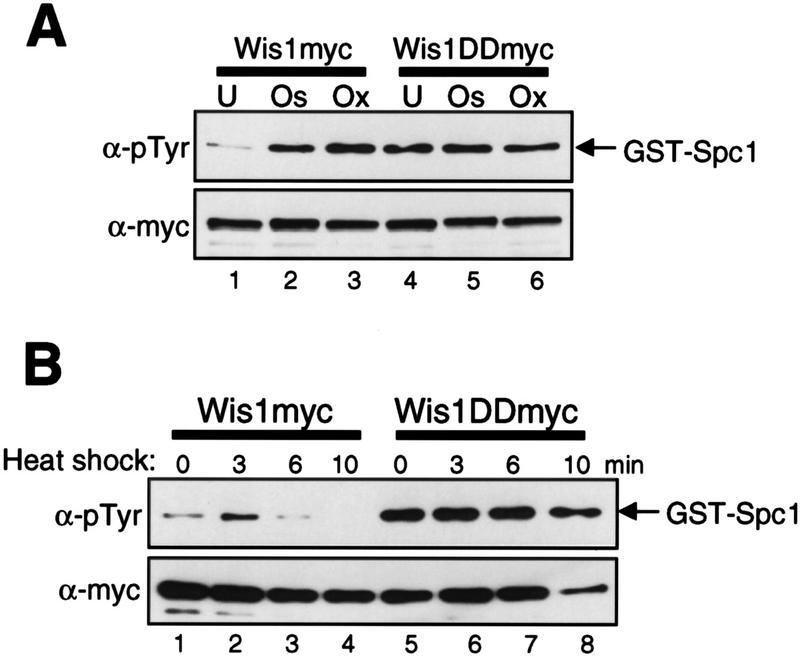
To determine whether the catalytic activity of Wis1 is regulated in response to various stress stimuli that activate Spc1, we developed an in vitro assay for Wis1. Wis1 was purified by immunoprecipitation from a strain in which chromosomal wis1+ is tagged with the sequence encoding the myc epitope (Shiozaki et al. 1998). Activity of Wis1 was measured using the glutathione S-transferase (GST)–Spc1 fusion protein as substrate (see Materials and Methods). Anti-phosphotyrosine (pTyr) immunoblotting showed weak phosphorylation of GST–Spc1 by Wis1 isolated from unstressed cells (Fig. 1A, lane 1). On the other hand, GST–Spc1 was strongly phosphorylated when incubated with Wis1 purified from cells exposed to osmostress by 0.6 m KCl (Fig. 1A, lane 2) or oxidative stress by 0.3 mm H2O2 (lane 3) for 10 min. This result indicates that osmostress and oxidative stress induce activation of the Wis1 MEK.
Figure 1.
Activity of the wild-type and constitutively active mutant of the Wis1 MEK under stress conditions. Strains carrying the wis1:myc (KS1878) or wis1DD:myc (KS2125) alleles were grown to mid-log phase at 30°C in YES medium and exposed to various forms of stress. Wis1myc and Wis1DDmyc, which have 12 copies of the myc tag at the carboxy-terminus, were purified by anti-myc immunoprecipitation, and their catalytic activity was measured with GST–Spc1 as substrate. Phosphorylation of GST–Spc1 was detected by immunoblotting with anti-phosphotyrosine (α-pTyr) antibodies. Anti-myc (α-myc) immunoblotting shows the amount of immunoprecipitated Wis1myc and Wis1DDmyc in the assay. (A) Activity of Wis1myc and Wis1DDmyc purified from unstressed cells (U) and cells exposed to osmotic (0.6 m KCl for 10 min; Os) or oxidative (0.3 mm H2O2 for 10 min; Ox) stress. (B) Activity of Wis1myc and Wis1DDmyc after the culture temperature was shifted from 30°C to 48°C at time 0. No significant change in Wis1DD activity was detected after the stress treatments in repeated experiments.
In addition to osmostress and oxidative stress, Spc1 is also activated when cells are exposed to heat shock by shifting the growth temperature from 30°C to 48°C (Degols et al. 1996). Under this condition, Δwis1 and Δspc1 mutants lose viability more quickly than wild-type cells, indicating that Spc1 activity is crucial for thermotolerance (Degols et al. 1996; Kato et al. 1996). Using the assay described above, we measured the activity of Wis1 isolated from cells heat-shocked by the temperature shift from 30°C to 48°C. Compared with Wis1 activation by osmostress and oxidative stress, relatively weak, transient activation of Wis1 was observed in response to heat shock (Fig. 1B, lanes 1–4). Wis1 activity became undetectable within 10 min after the temperature shift.
We also assayed the activity of Wis1DD, which has aspartic acid substitutions at Ser-469/Thr-473, the conserved phosphorylation sites by MEKKs (Shiozaki et al. 1998). The Wis1DD protein was immunoprecipitated with anti-myc antibodies before and after the stress treatments from a strain in which chromosomal wis1+ was replaced with the wis1DD gene tagged with the myc epitope sequence (Shiozaki et al. 1998). Wis1DD from unstressed cells showed high activity comparable to stress-activated Wis1, but Wis1DD activity did not increase after osmostress, oxidative stress (Fig. 1A, lanes 4–6), and heat shock (Fig. 1B, lanes 5–8). Thus, aspartic acid substitutions of the MEKK phosphorylation sites make Wis1 constitutively active and unresponsive to the stress conditions tested. These results strongly suggest that the catalytic activity of the Wis1 MEK is regulated by MEKKs in response to stress stimuli.
Tyrosine dephosphorylation of Spc1 is inhibited in heat-shocked cells
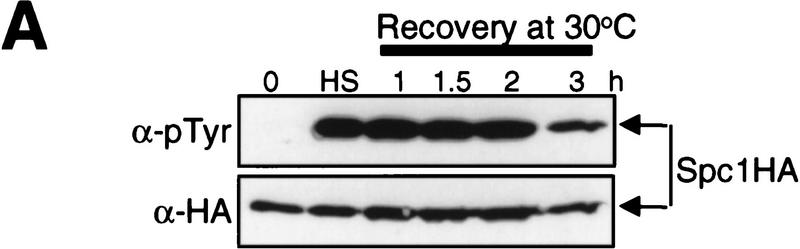
The results described above indicate that activity of Wis1DD does not change significantly upon stress stimuli, which is consistent with the observation that the level of Spc1 activation in the wis1DD mutant strain is not affected by osmostress and oxidative stress (Shiozaki et al. 1998). In contrast, we found previously that tyrosine phosphorylation of Spc1 increases significantly upon heat shock in both wild-type and wis1DD mutant strains (Shiozaki et al. 1998; see Fig. 3C). One hypothesis to explain the induced tyrosine phosphorylation of Spc1 without a significant increase in Wis1DD activity is that the tyrosine phosphatases dephosphorylating Spc1 are inhibited upon heat shock. To test this hypothesis, tyrosine dephosphorylation of Spc1 after heat shock was examined with a strain in which chromosomal spc1+ is tagged with the HA6H sequence encoding two copies of hemagglutinin (HA) and six histidine residues (Shiozaki and Russell 1995b). Cells grown at 30°C were exposed to heat shock at 48°C for 10 min to induce tyrosine phosphorylation of Spc1 (Fig. 2A, lane labeled HS). The culture was then shifted back to 30°C, and tyrosine phosphorylation of Spc1 was monitored by anti-phosphotyrosine immunoblotting. The level of Spc1 tyrosine phosphorylation remained high until some decrease was detected after 3 hr of recovery at 30°C (Fig. 2A). Wis1 activity was undetectable after 10 min of heat shock and the temperature shift to 30°C did not significantly activate Wis1 (data not shown). This result contrasts with rapid dephosphorylation of Spc1 after osmostress; tyrosine phosphorylation of Spc1 returns to the basal level within 50 min even under continuous high osmolarity stress (Gaits et al. 1997). Therefore, this result indicates that tyrosine dephosphorylation of Spc1 is inhibited in cells treated by a brief heat shock, which is consistent with the idea that inhibition of the tyrosine phosphatases for Spc1 contributes to Spc1 activation in response to heat shock.
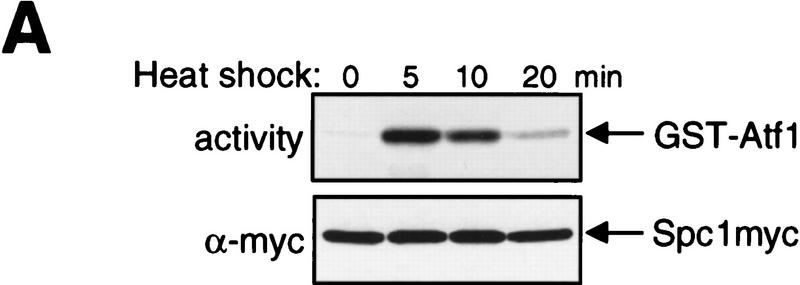
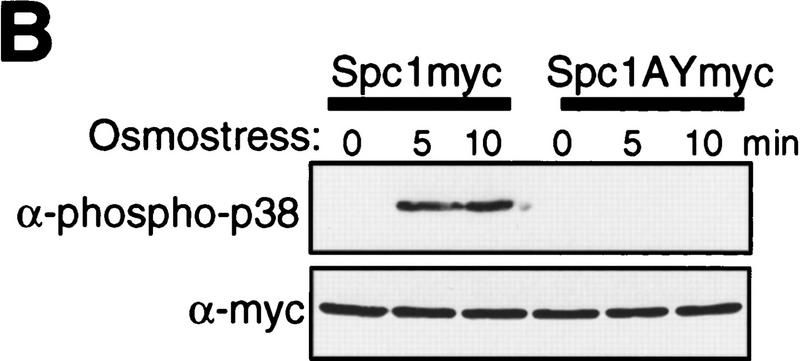
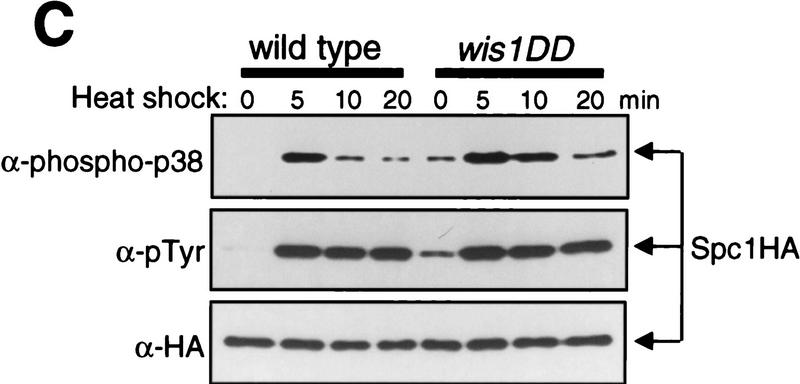
Figure 3.
The dual-phosphorylated, active form of Spc1 accumulates only transiently in response to heat shock. (A) Transient activation of Spc1 after heat shock. Strain GD1942, in which chromosomal spc1+ is tagged with the sequence encoding the myc epitope, was grown to mid-log phase in YES medium at 30°C and then shifted to 48°C at time 0. Aliquots of cells were harvested at the indicated time points, and Spc1 was purified by anti-myc immunoprecipitation. Activity of Spc1 was assayed by incubation with the substrate GST–Atf1 in the presence of Mg2+ and [γ-32P]ATP, and was analyzed by SDS-PAGE and autoradiography (top). Anti-myc immunoblotting shows the amount of immunoprecipitated Spc1 in the assays (bottom). (B) Antibodies against dual-phosphorylated mammalian p38 cross react with Spc1 phosphorylated on both Thr-171 and Tyr-173. Strains expressing the myc-tagged wild-type Spc1 (GD1942) or Spc1AY (CA140), which has Thr-171 substituted with unphosphorylatable alanine, were grown in YES medium at 30°C and then exposed to osmostress by 0.6 m KCl. The wild-type and mutant Spc1 in the cell lysates was probed with anti-phospho-p38 (top) and anti-myc (bottom) antibodies. (C) Spc1 phosphorylated on both Thr-171 and Tyr-173 accumulates only transiently after heat shock in wild-type and wis1DD cells. KS2096 (wild type) and KS2086 (wis1DD) strains carrying the spc1:HA6H allele were grown to mid-log phase at 30°C in YES medium and shifted to 48°C at time 0. Aliquots of cells were harvested at the indicated time points, and Spc1 was purified by Ni–NTA chromatography, which was followed by immunoblotting with anti-phospho-p38, anti-phosphotyrosine, and anti-HA antibodies.
Figure 2.
Dephosphorylation of Spc1 Tyr-173 is inhibited in heat-shocked cells. (A) Wild-type strain (KS2096) carrying chromosomal spc1+ tagged with the HA6H sequence was grown to mid-log phase in YES medium at 30°C. The culture was shifted to 48°C for 10 min (heat shock, HS) to induce Spc1 tyrosine phosphorylation and then shifted back to 30°C (Recovery). Aliquots of cells were harvested at the indicated time points, and Spc1 was purified by Ni–NTA chromatography. The level of Spc1 tyrosine phosphorylation was examined by immunoblotting with anti-phosphotyrosine antibodies. No tyrosine dephosphorylation of Spc1 was detected >2 hr after the temperature shift down. (B) Overexpression of the Pyp1 tyrosine phosphatase cannot suppress Spc1 activation induced by heat shock. Wild-type strain (KS1376) carrying chromosomal spc1+ tagged with the HA6H sequence was transformed with pREP1–pyp1HA6H, which expresses Pyp1 with a carboxy-terminal HA6H tag under the control of the thiamine-repressible nmt1 promoter. The transformant was grown in EMM2 medium at 30°C in the presence (+B1) and absence (−B1) of thiamine (vitamin B1) for 18 hr and exposed to osmostress by 0.6 m KCl for 10 min (Os) or heat shock at 48°C for 5, 10, and 20 min. Spc1 and Pyp1 were purified by Ni–NTA chromatography under denaturing conditions and analyzed by immunoblotting with anti-phosphotyrosine and anti-HA antibodies. In the absence of thiamine, overexpressed Pyp1 completely suppressed Spc1 tyrosine phosphorylation upon osmostress but showed little activity in heat-shocked cells. (C) The Pyp1 tyrosine phosphatase rapidly loses its ability to interact with Spc1 after heat shock. Strain CA187 has chromosomal pyp1+ replaced with pyp1–C470S:myc to express the catalytically inactive Pyp1C470S with a carboxy-terminal myc epitope tag. This strain was transformed with pREP1-GST–Spc1 and grown at 30°C in EMM2 medium without thiamine to induce expression of GST–Spc1 from the thiamine-repressible nmt1 promoter. Cells were exposed to heat shock at 48°C (left) or osmostress by 0.6 m KCl (right), and aliquots of cells were harvested at the indicated time points. Cell lysates were absorbed to GSH–Sepharose beads, and, after extensive washes, proteins bound to the beads (GSH-Beads) were analyzed by immunoblotting with anti-myc and anti-GST antibodies. The amount of Pyp1C470S detected in the crude lysates (Lysate) did not change significantly after the stress treatments.
Spc1 is dephosphorylated by two tyrosine-specific phosphatases, Pyp1 and Pyp2, with Pyp1 having the major activity (Millar et al. 1995; Shiozaki and Russell 1995b; Degols et al. 1996). Therefore, we examined whether Pyp1 function is inhibited in response to heat shock. In strains overexpressing pyp1+ from a strong promoter, Spc1 tyrosine phosphorylation is suppressed completely even after osmostress (Millar et al. 1995; Shiozaki and Russell 1995b). A spc1:HA6H strain was transformed with the pREP1–pyp1HA6H plasmid (Shiozaki and Russell 1995b), which expresses Pyp1 with a carboxy-terminal HA6H tag under the control of the thiamine (vitamin B1)-repressible nmt1 promoter (Maundrell 1990). The transformant was grown at 30°C in the presence and absence of thiamine and treated with osmostress by 0.6 m KCl or heat shock at 48°C. Spc1 and Pyp1 were purified by Ni–NTA chromatography under denaturing conditions for immunoblotting with anti-phosphotyrosine and anti-HA antibodies. Pyp1 was highly overexpressed under thiamine depletion (−B1), which completely inhibited Spc1 tyrosine phosphorylation upon osmostress (Fig. 2B, lanes labeled Os). In contrast, overexpressed Pyp1 showed little effect on Spc1 phosphorylation induced by heat shock (Fig. 2B). This observation suggests that dephosphorylation of Spc1 by Pyp1 is strongly inhibited in heat-shocked cells.
To explore further the inhibition of Pyp1 by heat shock, the in vivo interaction of Pyp1 with Spc1 was probed by use of the pyp1–C470S mutation before and after heat shock. Pyp1 with Cys-470 substituted with serine (Pyp1C470S) is catalytically inactive but stably interacts with the substrate, Spc1 (Shiozaki and Russell 1995b). The chromosomal pyp1+ locus was replaced with the pyp1–C470S mutant gene, which was tagged with the sequence encoding the myc epitope for detection with anti-myc antibodies. Phenotypes of the control strain expressing wild-type Pyp1 tagged with the myc epitope were indistinguishable from those of cells expressing untagged Pyp1, indicating that the Pyp1 function was not disturbed by the myc epitope (data not shown). The GST–Spc1 fusion protein was expressed from the pREP1–GST–Spc1 plasmid in this pyp1–C470S:myc strain, and cell lysates were incubated with glutathione (GSH)–Sepharose beads. The Pyp1C470S protein was copurified with GST–Spc1 on GSH–beads (Fig. 2C, time 0) as reported previously (Shiozaki and Russell 1995b). When this strain was exposed to heat shock, the amount of Pyp1C470S bound to GST–Spc1 decreased significantly within 5 min and became undetectable by 20 min after heat shock (Fig. 2C, left). On the other hand, osmostress showed no effect on the amount of Pyp1C470S coprecipitated with GST–Spc1 (Fig. 2C, right). Thus, heat shock, but not osmostress, inhibits the interaction between Pyp1 and Spc1. We also found that the GST–Spc1 fusion protein immobilized on GSH–beads can precipitate Pyp1C470S from lysates of unstressed cells but not from those of heat-shocked cells (data not shown). Taken together, these results strongly suggest that heat shock impairs the ability of Pyp1 to interact with its substrate, Spc1.
Spc1 activity is rapidly attenuated by Thr-171 dephosphorylation in heat-shocked cells
As shown in Figure 2B, the level of activating tyrosine phosphorylation of Spc1 remains high >20 min after heat shock. To our surprise, only transient induction of the catalytic activity of Spc1 was observed in response to heat shock (Fig. 3A). Spc1 was purified by anti-myc immunoprecipitation from cells in which the chromosomal spc1+ gene is tagged with the sequence encoding the myc epitope (Gaits et al. 1998). Spc1 activity was measured by incubation with the substrate GST–Atf1 (Shiozaki and Russell 1996; Wilkinson et al. 1996) in the presence of Mg2+ and [γ-32P]ATP. Strong activation of Spc1 was detected 5 min after heat shock and rapidly diminished at the indicated time points (Fig. 3A). This finding, with the result in Figure 2B, indicates that heat shock-induced Spc1 activation is rapidly attenuated independently of Tyr-173 phosphorylation.
Like other MAPKs, Spc1 activation requires phosphorylation on both Thr-171 and Tyr-173 (Gaits et al. 1998), which prompted us to determine whether attenuation of Spc1 activity is brought about by dephosphorylation of Thr-171 in heat-shocked cells. Anti-phospho-p38 antibodies recognize phosphorylated Thr-180/Tyr-182 of mammalian p38 SAPK as well as the homologous sequence of Spc1 phosphorylated on both Thr-171 and Tyr-173 (Shiozaki and Russell 1997; Gaits et al. 1998). Immunoblotting with the anti-phospho-p38 antibodies detected wild-type Spc1 activated upon osmostress but not mutant Spc1 with Thr-171 → Ala (Spc1AY, Fig. 3B) or Tyr-173 → Phe (Spc1TF) substitutions (Gaits et al. 1998). By use of anti-phospho-p38 and anti-phosphotyrosine antibodies, Spc1 phosphorylation after heat shock was examined in wild-type and wis1DD mutant strains. Dual-phosphorylated Spc1 accumulated only transiently after the temperature shift from 30°C to 48°C in both wild-type and wis1DD strains (Fig. 3C). The peak of dual phosphorylation was observed ∼5 min after heat shock, decreasing rapidly at the following time points, which correlates to the kinetics of Spc1 activation after heat shock (Fig. 3A). On the other hand, probing the same samples with anti-phosphotyrosine antibodies showed that the level of Spc1 tyrosine phosphorylation remained high >20 min after heat shock (Fig. 3C, middle). Therefore, these data strongly suggest that Spc1 activated by heat shock is rapidly attenuated by dephosphorylation of Thr-171.
PP2C enzymes dephosphorylate Thr-171 of Spc1
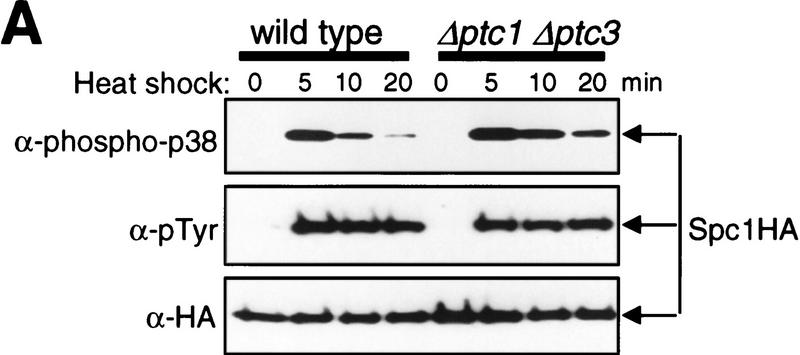
What is the threonine phosphatase that dephosphorylates Spc1 after heat shock? Three genes encoding PP2Cs, ptc1+, ptc2+, and ptc3+, have been identified in S. pombe (Shiozaki et al. 1994; Shiozaki and Russell 1995a).Previous genetic data suggested that ptc1+ and ptc3+ counteract the wis1+ gene function: wis1 mutations were isolated as suppressors of the Δptc1 Δptc3 defects (Shiozaki and Russell 1995a). In addition, Δptc1 Δptc3 shows a synthetic lethal phenotype with Δpyp1, which is rescued by the Δwis1 mutation (Gaits et al. 1997). These observations suggest that the Ptc phosphatases may negatively regulate the Spc1 pathway. To test whether the Ptc phosphatases are involved in dephosphorylation of Spc1 Thr-171, we examined Spc1 phosphorylation after heat shock in various ptc mutants. Δptc1, Δptc2, and Δptc3 mutations were introduced into the spc1:HA6H strain, and Spc1 phosphorylation was probed with anti-phospho-p38 and anti-phosphotyrosine antibodies after temperature shift from 30°C to 48°C. None of the single mutations of Δptc1, Δptc2, and Δptc3 showed significant effect on the Spc1 phosphorylation before and after heat shock (data not shown). However, in the Δptc1 Δptc3 double mutant, the dual-phosphorylated form of Spc1 decreased more slowly after the initial activation upon heat shock than in wild-type cells (Fig. 4A, top), which was reproducible in repeated experiments. Because tyrosine dephosphorylation of Spc1 was not detected after heat shock in both strains (Fig. 4A, middle), these results indicate that Thr-171 dephosphorylation of Spc1 is compromised in the Δptc1 Δptc3 mutant. The kinetics of Spc1 dephosphorylation in the Δptc1 Δptc2 Δptc3 triple mutant were similar to those in the Δptc1 Δptc3 double mutant (data not shown), which suggests that Ptc2 may not be important for Spc1 dephosphorylation. It is known that type 2A serine/threonine phosphatases (PP2As) dephosphorylate and inactivate mammalian MAPKs (Gómez and Cohen 1991; Alessi et al. 1995). However, mutations in the S. pombe PP2A genes ppa1+ and ppa2+ (Kinoshita et al. 1990), showed no apparent effect on the phosphorylation state of Spc1 both before and after heat shock (data not shown).
Figure 4.
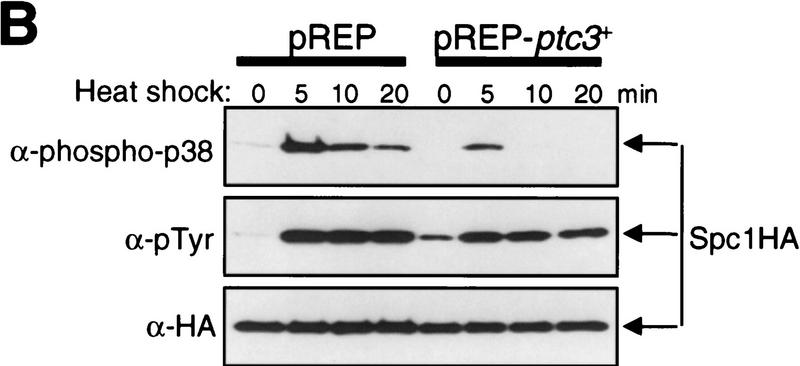
Ptc1 and Ptc3 phosphatases regulate phosphorylation of Spc1 Thr-171. (A) Wild-type (KS1376) and Δptc1Δptc3 mutant (CA135) strains carrying the spc1:HA6H allele were grown at 30°C to mid-log phase and shifted to 48°C at time 0. Cells were harvested at the indicated time points and Spc1 was purified on Ni–NTA beads for immunoblotting with anti-phospho-p38, anti-phosphotyrosine, and anti-HA antibodies. After the initial Spc1 activation, dual-phosphorylated Spc1 decreased more slowly in Δptc1 Δptc3 cells than in wild-type cells, a result that was reproduced in repeated experiments. (B) Wild-type spc1:HA6H strain (KS1376) was transformed with the pREP vector and the pREP-ptc3+ plasmid to express ptc3+ under the regulation of the thiamine-repressible nmt1 promoter. The transformants were grown at 30°C for 18 hr in EMM2 medium without thiamine to induce expression from the nmt1 promoter before the temperature shift to 48°C at time 0. Cells harvested at the indicated time points were subjected to analyses as described in A.
If Ptc1 and Ptc3 dephosphorylate Thr-171 of Spc1, overexpression of these phosphatases should lead to a decrease in the level of Thr-171 phosphorylation. ptc3+ was overexpressed from the pREP-ptc3+ plasmid by use of the nmt1 promoter, and phosphorylation of Spc1 after heat shock was examined. Compared with the strain transformed with the pREP vector alone, the level of doubly phosphorylated Spc1 was reduced dramatically in the Ptc3-overexpressing strain (Fig. 4B, top). A very similar result was obtained with a strain overexpressing Ptc1 (data not shown). On the other hand, Ptc3 overproduction exhibited little effect on tyrosine phosphorylation of Spc1 upon heat shock, except to cause higher tyrosine phosphorylation in the absence of stress when compared with the control strain (Fig. 4B, middle, time 0). It is possible that the Wis1 MEK is up-regulated or the Pyp phosphatases are down-regulated to compensate inactivation of Spc1 by Ptc3.
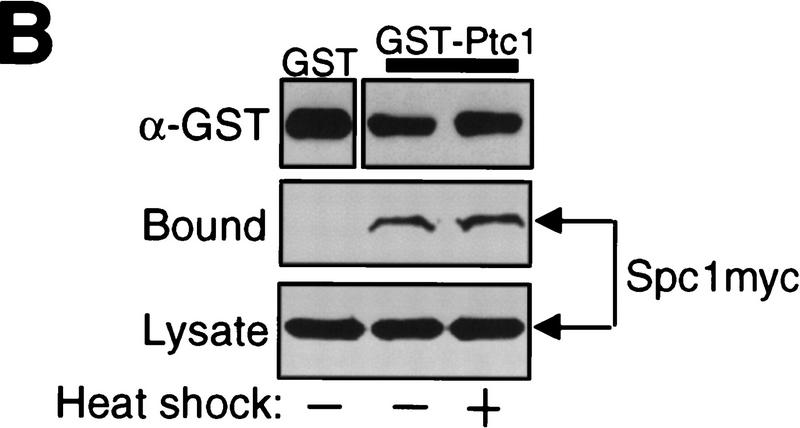
These results strongly suggest that PP2C enzymes Ptc1 and Ptc3 are involved in dephosphorylation of Spc1 Thr-171 to attenuate heat shock-activated Spc1. Consistent with this model, it was observed that Ptc1 also dephosphorylated Thr-171 of Spc1 in vitro. GST–Ptc1 fusion protein expressed in Escherichia coli was purified and incubated with phosphorylated GST–Spc1 in the presence and absence of Mg2+, because, like other PP2C enzymes, Ptc1 requires Mg2+ for its activity (Shiozaki et al. 1994). By anti-phospho-p38 immunoblotting, dephosphorylation of GST–Spc1 by GST–Ptc1 was observed in a Mg2+-dependent manner (Fig. 5A, top), whereas no tyrosine dephosphorylation was observed during the experiment (bottom). Furthermore, specific interaction between the Spc1 and Ptc1 proteins was also detected by the experiment shown in Figure 5B. GST and GST–Ptc1 immobilized on GSH–beads were incubated with lysates prepared from cells expressing myc-tagged Spc1. Spc1 was coprecipitated with GST–Ptc1 beads but not with GST beads, indicating specific interaction between Spc1 and Ptc1. No apparent difference was observed between unstressed and heat-shocked cells in the affinity of Spc1 for GST–Ptc1 (Fig. 5B).
Figure 5.
(A) In vitro dephosphorylation of Spc1 Thr-171 by Ptc1. GST–Ptc1 was expressed in E. coli DH5α and purified by GSH–Sepharose chromatography. Wild-type (PR109) S. pombe strain carrying the pREP1-GST–Spc1 plasmid was grown in EMM2 medium without thiamine to induce expression of GST–Spc1. After a 10-min osmostress by 0.6 m KCl, cells were harvested, and phosphorylated GST–Spc1 was purified on GSH–Sepharose beads. GST–Ptc1 and GST–Spc1 were incubated in the presence (+) or absence (−) of 20 mm MgCl2, and the phosphorylation state of GST–Spc1 was examined by immunoblotting with anti-phospho-p38 and anti-phosphotyrosine antibodies. (B) Physical interaction between Spc1 and the Ptc1 phosphatase. Strain GD1942 expressing the myc-tagged Spc1 was grown to mid-log phase in YES medium and harvested before and after a 5-min heat shock at 48°C. Cell lysates were incubated with bacterially produced GST and GST–Ptc1, which were immobilized on GSH–Sepharose beads. After extensive washes, proteins bound to the beads (top and middle) were detected by immunoblotting with anti-GST and anti-myc antibodies. (bottom) Anti-myc immunoblotting of the total lysates.
These in vivo and in vitro data strongly suggest that Ptc1 and Ptc3 are involved in dephosphorylation of Spc1 Thr-171 to attenuate heat shock-induced activation of Spc1. However, some level of Thr-171 dephosphorylation was still observed in Δptc1 Δptc3 cells (Fig. 4A), implying that other threonine phosphatases may also dephosphorylate Spc1.
Discussion
The S. pombe Spc1 SAPK plays crucial roles in the cellular responses to survive severe environmental conditions. Spc1 is strongly activated when cells are exposed to heat shock, and Δspc1 mutant cells lose viability more quickly than wild-type cells at 48°C (Degols et al. 1996; Kato et al. 1996), indicating that Spc1 is essential for cellular thermotolerance. Activated Spc1 phosphorylates the Atf1 transcription factor to induce various stress response genes (Shiozaki and Russell 1996; Wilkinson et al. 1996) including tps1+ involved in trehalose synthesis, which is known to be important in the cellular heat shock response (Blázquez et al. 1994; De Virgilio et al. 1994). In this study, we have explored the regulation of Spc1 in response to heat shock. Our results strongly suggest that heat shock brings about inhibition of the Pyp1 tyrosine phosphatase, which dephosphorylates and inactivates Spc1 (Fig. 6). Because Tyr-173 dephosphorylation by Pyp1 is a major mechanism for Spc1 inactivation, inhibition of Pyp1 is expected to play a key role in activation of Spc1 upon heat shock. Moreover, we have discovered a mechanism that allows only transient activation of Spc1 after heat shock: Thr-171 of Spc1 is rapidly dephosphorylated after the initial activation induced by heat shock, whereas Tyr-173 remains phosphorylated. This is the first example showing that MAPK activity is modulated by differential regulation of threonine and tyrosine phosphorylation in response to stimuli.
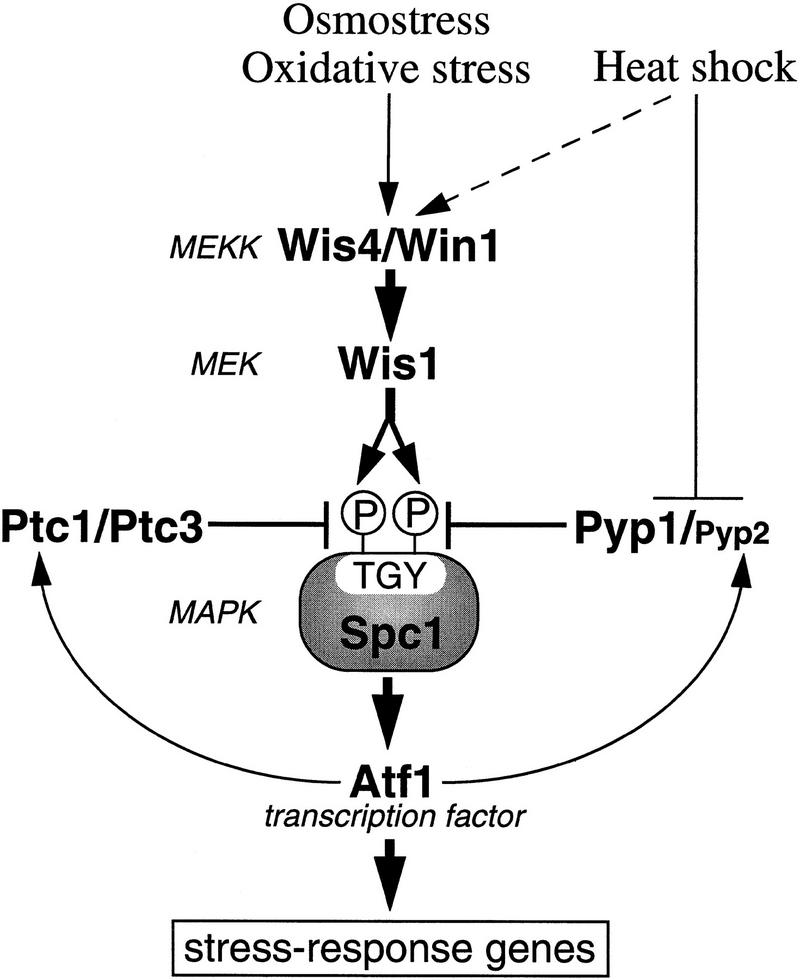
Figure 6.
Regulation of the Spc1 SAPK by PP2C and the Pyp tyrosine phosphatases. Spc1 is activated by various forms of stress including high osmolarity stress, oxidative stress, and heat shock. The Wis1 MEK activates Spc1 by phosphorylating Thr-171 and Tyr-173. Osmostress and oxidative stress activate Wis1 through phosphorylation of Ser-469/Thr-473, which is carried out by MEKKs, Wis4 and Win1. Heat shock brings about weak activation of Wis1 in a MEKK-dependent manner as well as inhibition of Pyp1 and presumably Pyp2, the Spc1 Tyr-173 phosphatases, which results in strong activation of Spc1. Although Tyr-173 remains phosphorylated in heat-shocked cells because of inhibition of the Pyp phosphatases, Spc1 activity is attenuated by Thr-171 dephosphorylation, which is carried out by Ptc1, Ptc3, and other threonine phosphatases. Transcription of pyp2+ and ptc1+ is induced by the Spc1–Atf1 pathway in response to stress stimuli, which constitutes dual loops of negative feedback. Expression of pyp1+ and ptc3+ is constitutive; however, they might be subjected to post-transcriptional regulation.
Stress regulation of the Wis1 MEK
Previous studies demonstrated that Wis1 is the only MEK for Spc1 (Millar et al. 1995; Shiozaki and Russell 1995b; Degols et al. 1996), suggesting that Wis1 phosphorylates and activates Spc1 under various stress conditions. However, it had never been examined whether the catalytic activity of Wis1 is increased in response to stress stimuli that induce Spc1 activation. In this study, an in vitro assay for Wis1 activity was developed, and this assay has demonstrated that Wis1 is strongly activated upon osmostress and oxidative stress, whereas only weak, transient activation of Wis1 is detected after heat shock. Modulation of Wis1 activity in response to these stress stimuli is dependent on the MEKK phosphorylation sites, Ser-469/Thr-473, of Wis1, suggesting that MEKKs mediate stress signals to Wis1 through phosphorylation of these sites. So far, two MEKKs that phosphorylate and activate Wis1 have been identified, Wis4 (also called Wik1 or Wak1) and Win1 (Samejima et al. 1997, 1998; Shieh et al. 1997; Shiozaki et al. 1997). However, how these MEKKs are regulated in response to stress signals remains unknown. Genetic studies have identified Mcs4 upstream of the Wis4 MEKK (Cottarel 1997; Shieh et al. 1997; Shiozaki et al. 1997); Mcs4 is a protein closely related to the budding yeast Ssk1p, a homolog of the response regulator member of bacterial two-component systems (Maeda et al. 1994). In the S. cerevisiae HOG1 cascade, the Ssk2p MEKK is regulated in response to osmostress by a variation of the two-component system, multistep phosphorelay composed of the Sln1p histidine kinase, Ypd1p, and Ssk1p (Posas et al. 1998). Therefore, it is likely that regulators homologous to Sln1p and Ypd1p also exist upstream of the Spc1 cascade. However, relatively weak activation of Wis1 upon heat shock implies that those upstream regulators might not be fully operational under extreme environment such as high temperature. It is also possible that a sensor dedicated for heat shock stimuli might never have evolved upstream of the SAPK cascades.
Regulation of MAPKs by threonine- and tyrosinespecific phosphatases
Evidence presented in this report strongly suggests that Pyp1, the major tyrosine phosphatase for Spc1, is inhibited upon heat shock. First, tyrosine phosphorylation of Spc1 becomes very stable in heat-shocked cells, which contrasts with rapid dephosphorylation of Spc1 after osmostress. Second, heat shock induces strong activation of Spc1 even in the strain overexpressing Pyp1. Third, experiments with Pyp1C470S indicate that the interaction of Pyp1 with Spc1 is rapidly lost after heat shock. Recently, we found that Pyp1 is a soluble protein in unstressed cells but the majority of Pyp1 becomes insoluble within 10 min when cells are exposed to heat shock (A.N. Nguyen and K. Shiozaki, unpubl.). This change in the solubility of Pyp1 is not accompanied by an alteration of its cellular localization: By immunofluorescence microscopy, Pyp1 is a cytoplasmic protein, and Pyp1 localization is not affected by heat shock (A.N. Nguyen and K. Shiozaki, unpubl.). One possible explanation for these results is that, upon heat shock, Pyp1 protein is subjected to changes in conformation, modification, or interaction with other proteins. The Pyp2 protein also becomes insoluble in heat-shocked cells (A.N. Nguyen and K. Shiozaki, unpubl.), which may suggest that Pyp2 is also inactivated after heat shock. Previously, Samejima et al. (1997) also suggested Pyp1 inhibition as a mechanism for Spc1 activation upon heat shock, which was based on their failure to detect heat shock-induced activation of Spc1 in Δpyp1 mutants. However, we and others have demonstrated that heat shock and other forms of stress can bring about Spc1 activation in Δpyp1 cells (Shieh et al. 1998; Shiozaki et al. 1998). It is likely that, in Δpyp1 mutants, weak activation of Wis1 and inhibition of Pyp2 cause further Spc1 activation upon heat shock.
In unstressed cells, Spc1 is kept inactive mainly through dephosphorylation of Tyr-173, which is carried out by two tyrosine-specific phosphatases, Pyp1 and Pyp2; first, mutational inactivation of Pyp1 brings about phenotypes similar to those of the strain expressing the constitutively active Wis1DD (Shiozaki et al. 1998). Second, simultaneous deletion of the pyp1+ and pyp2+ genes induces hyperactivation of Spc1, which is lethal to the cell (Millar et al. 1995; Shiozaki and Russell 1995b). Third, we observed that mutational inactivation of pyp1+ is sufficient to induce a significant increase of dual-phosphorylated, active Spc1 (data not shown). These observations may suggest that unstressed cells have only limited activity to dephosphorylate Spc1 Thr-171. Thus, when cells are exposed to heat shock, inhibition of the Pyp phosphatases, combined with transient, moderate activation of Wis1, induces strong activation of Spc1. In contrast, osmostress and oxidative stress induce Spc1 activation solely in a MEKK-dependent manner (Shiozaki et al. 1998). SAPK activation by inhibition of the SAPK phosphatase has also been proposed for As3+-induced activation of JNK [c-Jun amino (N)-terminal kinase; Cavigelli et al. 1996], although the identity of the JNK phosphatase remains unknown. We found that, like heat shock, As3+ also impairs the interaction of Pyp1 with Spc1, resulting in Spc1 activation (A.N. Nguyen and K. Shiozaki, unpubl.). An attractive model would be that SAPK phosphatases sensitive to certain types of stress can serve as stress sensors.
Following the initial activation induced by heat shock, Spc1 is rapidly inactivated by Thr-171 dephosphorylation. Our data strongly suggest that PP2C enzymes encoded by ptc1+ and ptc3+ are involved in this attenuation mechanism. However, dephosphorylation of Spc1 Thr-171 is not completely abolished in the Δptc1 Δptc3 mutant, suggesting that other threonine phosphatases may also be involved in Spc1 inactivation after heat shock. Ptc1, Ptc2, and Ptc3 do not account for all the cellular PP2C activity in S. pombe (Shiozaki and Russell 1995a), and Spc1 dephosphorylation may involve additional PP2C enzymes that are not yet identified. Transcription of ptc1+ is regulated by the Spc1–Atf1 pathway (Gaits et al. 1997), and ptc1+ expression is induced in response to heat shock (Shiozaki et al. 1994), which may be important for swift attenuation of Spc1 activity (Fig. 6). It has been reported recently that PP2C inhibits the mammalian p38 and JNK pathways in transient transfection assays and that PP2C dephosphorylates the threonine residue of p38 in vitro (Takekawa et al. 1998). Although the physiological role of SAPK inhibition by PP2C in mammalian cells is unknown, these observations suggest that the PP2C function in SAPK inhibition is evolutionarily conserved. However, our findings do not exclude the possibility that PP2C also regulates events downstream of the SAPKs (Gaits et al. 1997).
Dual-specificity phosphatases that can dephosphorylate both threonine and tyrosine residues are known to inactivate many members of the MAPK family (Hunter 1995; Keyse 1995), including SAPKs (Liu et al. 1995; Raingeaud et al. 1995; Gupta et al. 1996; Hirsch and Stork 1997). Data presented here, however, clearly demonstrate that transient activation of Spc1 upon heat shock is brought about through differential regulation of Thr-171 and Tyr-173 phosphorylation. MAPK regulation by tyrosine-specific phosphatases has also been reported in budding yeast (Jacoby et al. 1997; Wurgler-Murphy et al. 1997; Zhan et al. 1997) and mammalian cells (Alessi et al. 1995; Ogata et al. 1999). Dual phosphorylation is a common activation mechanism for all MAPKs, and differential dephosphorylation of the threonine and tyrosine residues allows two distinct inputs to a MAPK. Therefore, the regulatory mechanism we described here could prove to be widespread in other MAPK pathways of lower and higher eukaryotes.
Materials and methods
Yeast strains and general techniques
S. pombe strains used in this study are listed in Table 1. They are derivatives of 972 h− and 975 h+ (Mitchison 1970). Growth media and basic genetic and biochemical techniques for S. pombe have been described (Moreno et al. 1991; Alfa et al. 1993). S. pombe cells were grown in yeast extract medium YES and synthetic minimal medium EMM2.
Table 1.
S. pombe strains used in this study
|
Strain
|
Genotype
|
Source or reference
|
|---|---|---|
| PR109 | lab stock | |
| JM544 | wis1∷ura4+ | lab stock |
| KS1366 | spc1∷ura4+ | lab stock |
| CA140 | his7 spc1AY:12myc(ura4+) | Gaits et al. (1998) |
| KS1376 | spc1:HA6H(ura4+) | Shiozaki and Russell (1995) |
| KS1878 | spc1∷ura4+ wis1:12myc(ura4+) | Shiozaki et al. (1998) |
| GD1942 | spc1:12myc(ura4+) | Gaits et al. (1998) |
| GD1953 | pyp1:12myc(ura4+) | Gaits and Russell (1999) |
| KS2088 | spc1:HA6H(ura4+) wis1DD:12myc(ura4+) | Shiozaki et al. (1998) |
| KS2096 | spc1:HA6H(ura4+) wis1:12myc(ura4+) | Shiozaki et al. (1998) |
| KS2125 | spc1∷ura4+ wis1DD:12myc(ura4+) | Shiozaki et al. (1998) |
| CA98 | ppa2∷ura4+ spc1:HA6H(ura4+) | this study |
| CA129 | ppa1∷ura4+ spc1:HA6H(ura4+) | this study |
| CA135 | his7-366 ptc1∷LEU2 ptc3∷his7+ spc1:HA6H(ura4+) | this study |
| CA142 | ptc2∷ura4+ spc1:HA6H(ura4+) | this study |
| CA143 | ptc1∷LEU2 spc1HA6H(ura4+) | this study |
| CA145 | ptc3∷his7+ spc1:HA6H(ura4+) | this study |
| CA148 | his7-366 ptc1∷LEU2 ptc2∷ura4+ ptc3∷his7+ spc1:HA6H(ura4+) | this study |
| CA171 | pyp1∷ura4+ spc1:HA6H(ura4+) | this study |
| CA187 | pyp1-C470S:12myc(ura4+) | this study |
All strains are h− leu1-32 ura4-D18.
In vitro assay for Wis1 activity
In strain KS1878 and KS2125, the chromosomal wis1+ gene is replaced by the wild-type wis1 and wis1DD mutant genes, respectively, which are tagged with sequence encoding 12 copies of the myc epitope (Shiozaki et al. 1998). These strains were grown to early logarithmic phase in YES medium at 30°C and then treated with 0.6 m KCl for 10 min (osmostress), 0.3 mm H2O2 for 10 min (oxidative stress), or shifted to 48°C for heat shock (Shiozaki et al. 1997). Cells were lysed in IP lysis buffer [50 mm Tris-HCl (pH 7.2), 5 mm EDTA, 150 mm NaCl, 1 mm 2-mercaptoethanol, 10% glycerol, 50 mm NaF, 0.1 mm Na3VO4] containing a protease inhibitor cocktail. The homogenate was centrifuged at 12,000g for 10 min, and the supernatant was incubated for 40 min at 4°C with polyclonal anti-myc antibodies (Santa Cruz Biotechnology) conjugated to protein A–Sepharose. After extensive washes with IP lysis buffer supplemented with 0.5% Triton X-100, immunoprecipitated Wis1myc and Wis1DDmyc were incubated with GST–Spc1 in kinase reaction buffer [25 mm Tris-HCl (pH 7.2), 10 mm MgCl2, 50 mm NaCl, 50 μm ATP, 10 mm glutathione, 50 μg/ml BSA, 0.1 mm Na3VO4] at 30°C for 10 min. Purification of unphosphorylated GST–Spc1 from a Δwis1 strain (JM544) has been described previously (Shiozaki and Russell 1995b). The samples were analyzed by SDS-PAGE followed by immunoblotting with monoclonal anti-phosphotyrosine (Upstate Biotechnology) and anti-myc (BAbCO) antibodies. Under the experimental conditions used, we observed a linear increase of GST–Spc1 phosphorylation by Wis1DD along the time course of reaction up to 20 min.
Purification and detection of the Spc1HA6H protein
KS1376, KS2088 (wis1DD:myc), KS2096 (wis1:myc), CA98 (Δppa2), CA129 (Δppa1), CA135 (Δptc1 Δptc3), CA142 (Δptc2), CA143 (Δptc1), CA145 (Δptc3), and CA148 (Δptc1 Δptc2 Δptc3) carry chromosomal spc1+ tagged with the HA6H sequence encoding two copies of the HA epitope and six consecutive histidine residues (Shiozaki and Russell 1995b, 1997). Spc1HA6H protein was purified on Ni–NTA–agarose beads under denaturing conditions and analyzed by immunoblotting with anti-HA (12CA5), anti-phosphotyrosine, and anti-phospho-p38 MAPK (New England Biolabs) antibodies (Shiozaki and Russell 1997). Stress treatments of S. pombe cells by KCl, H2O2, and heat shock were performed as described previously (Shiozaki et al. 1997). Cells were harvested by rapid filtration (Shiozaki and Russell 1997), which appears to be less stressful to the cells than centrifugation (Shiozaki et al. 1998).
Construction of the pyp1-C470S:myc allele
The pyp1-C470S sequence (Shiozaki and Russell 1995b) was amplified by PCR and cloned into the pRIP–12myc vector as described previously (Gaits et al. 1998). The plasmid was linearized at the MscI site of pyp1-C470S and used to transform a wild-type strain (PR109). Integration of pyp1-C470S:12myc to the pyp1+ locus was confirmed by Southern hybridization. The resultant pyp1-C470S:12myc strain (CA187) was transformed with pREP1-GST–Spc1 (Shiozaki and Russell 1995b), and the transformant was grown at 30°C in EMM2 medium without thiamine to induce expression of GST–Spc1 from the thiamine-repressible nmt1 promoter (Maundrell 1990). Cells were exposed to heat shock at 48°C or 0.6 m KCl for osmostress, harvested by filtration and frozen in liquid nitrogen. GST–Spc1 was collected on GSH–Sepharose beads from cell lysates as described (Shiozaki and Russell 1995b). After extensive washes, proteins bound to the beads were analyzed by immunoblotting with anti-GST and anti-myc antibodies.
In vitro assay for Spc1 activity
spc1:myc cells (GD1942) were grown to early logarithmic phase in YES medium at 30°C and then shifted to 48°C for heat shock. Harvested cells were lysed in IP lysis buffer and Spc1myc was purified by immunoprecipitation with polyclonal anti-myc antibodies (Santa Cruz Biotechnology) conjugated to Protein A–Sepharose. As substrate, GST–Atf1 was expressed in the Δspc1 strain (KS1366) and unphosphorylated GST–Atf1 was purified on GSH–Sepharose beads (Shiozaki and Russell 1997). Then, Spc1myc and GST–Atf1 were incubated in KA buffer [25 mm Tris-HCl (pH 7.2), 10 mm MgCl2, 0.1 mm Na3VO4, 10 mm glutathione] containing 50 μm [γ-32P]ATP at 25°C for 10 min. Samples were subjected to SDS-PAGE, which was followed by autoradiography and immunoblotting with anti-myc antibodies.
In vitro dephosphorylation of Spc1 by GST–Ptc1
GST and GST–Ptc1 were expressed in E. coli DH5α cells and purified on GSH–Sepharose beads as described previously (Shiozaki et al. 1994). Wild-type S. pombe cells (PR109) transformed with pREP1-GST–Spc1 were grown in EMM2 medium without thiamine to induce expression of GST–Spc1. Then, cells were stressed with 0.6 m KCl in EMM2 for 10 min, and phosphorylated GST–Spc1 was purified on GSH–Sepharose beads (Shiozaki and Russell 1995b). GST–Ptc1 and GST–Spc1 bound to the beads were mixed and incubated in Solution A [50 mm Tris-HCl (pH 7.0), 0.1 mm EGTA, 1 mg/ml BSA, 10 mm glutathione] in the presence of 20 mm MgCl2 or 1 mm EDTA at 30°C for 30 min. Samples were analyzed by immunoblotting with anti-phospho-p38 MAPK and anti-phosphotyrosine antibodies. For in vitro binding assays between Ptc1 and Spc1, the spc1:12myc strain (GD1942) was grown in YES medium at 30°C, and aliquots were harvested before and after a 5-min heat shock at 48°C. Cells were lysed in IP lysis buffer containing 0.5% Triton X-100 and centrifuged at 12,000g for 10 min at 4°C. The supernatant was incubated with GST or GST–Ptc1 bound to GSH–Sepharose beads at 4°C for 25 min on a rocking platform. The beads were then washed four times with IP lysis buffer containing 1% Triton X-100, and proteins bound to the beads were analyzed by immunoblotting with anti-GST and anti-myc antibodies.
Acknowledgments
We thank Albert Lee for technical assistance, Frédérique Gaits, Geneviève Degols, Paul Russell, and Mitsuhiro Yanagida for strains, Jodi Nunnari, Chester Price, Paul Russell, and Mitsuhiro Yanagida for helpful discussion and critical reading of the manuscript. This research was supported in part by grant IRG-95-125-04 from the American Cancer Society.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL kshiozaki@ucdavis.edu; FAX (530) 752-9014.
References
- Alessi DR, Gómez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast: A laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Blázquez MA, Stucka R, Feldmann H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- Clarke PR. Switching off MAP kinases. Curr Biol. 1994;4:647–650. doi: 10.1016/s0960-9822(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Cottarel G. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics. 1997;147:1043–1051. doi: 10.1093/genetics/147.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio C, Hottiger T, Dominguez J, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1994;219:179–186. doi: 10.1111/j.1432-1033.1994.tb19928.x. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Russell P. Active nucleocytoplasmic shuttling required for function and regulation of stress-activated kinase Spc1/Sty1 in fission yeasts. Mol Biol Cell. 1999;10:1395–1407. doi: 10.1091/mbc.10.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Shiozaki K, Russell P. Protein phosphatase 2C acts independently of stress-activated kinase cascade to regulate the stress response in fission yeast. J Biol Chem. 1997;272:17873–17879. doi: 10.1074/jbc.272.28.17873. [DOI] [PubMed] [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes & Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N, Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991;353:170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hirsch DD, Stork PJ. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. J Biol Chem. 1997;272:4568–4575. doi: 10.1074/jbc.272.7.4568. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Jacoby T, Flanagan H, Faykin A, Seto AG, Mattison C, Ota I. Two protein-tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J Biol Chem. 1997;272:17749–17755. doi: 10.1074/jbc.272.28.17749. [DOI] [PubMed] [Google Scholar]
- Kato TJ, Okazaki K, Murakami H, Stettler S, Fantes PA, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Keyse SM. An emerging family of dual specificity MAP kinase phosphatases. Biochim Biophys Acta. 1995;1265:152–160. doi: 10.1016/0167-4889(94)00211-v. [DOI] [PubMed] [Google Scholar]
- ————— Protein phosphatases and the regulation of MAP kinase activity. Semin Cell Dev Biol. 1998;9:143–152. doi: 10.1006/scdb.1997.0219. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Ohkura H, Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990;63:405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: Protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun amino-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes & Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–146. [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Ogata M, Oh-hora M, Kosugi A, Hamaoka T. Inactivation of mitogen-activated protein kinases by a mammalian tyrosine-specific phosphatase, PTPBR7. Biochem Biophys Res Commun. 1999;256:52–56. doi: 10.1006/bbrc.1999.0278. [DOI] [PubMed] [Google Scholar]
- Posas F, Takekawa M, Saito H. Signal transduction by MAP kinase cascades in budding yeast. Curr Opin Microbiol. 1998;1:175–182. doi: 10.1016/s1369-5274(98)80008-8. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch R, Davis R. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Warbrick E, Weisman R, Fantes PA. The fission yeast mitotic regulator win1+ encodes a MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol Biol Cell. 1998;9:2325–2335. doi: 10.1091/mbc.9.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JBA. The Mcs4 response regulator coordinately controls the stress-activated Wak1–Wis1–Sty1 MAP kinase pathway and fission yeast cell cycle. Genes & Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shieh JC, Martin H, Millar JBA. Evidence for a novel MAPKKK-independent pathway controlling the stress activated Sty1/Spc1 MAP kinase in fission yeast. J Cell Sci. 1998;111:2799–2807. doi: 10.1242/jcs.111.18.2799. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Counteractive roles of protein phosphatase 2C and a MAP kinase kinase homolog in the osmoregulation of fission yeast. EMBO J. 1995a;14:492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Cell-cycle control linked to the extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995b;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- ————— Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes & Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- ————— Stress-activated protein kinase pathway in cell cycle control of fission yeast. Methods Enzymol. 1997;283:506–520. doi: 10.1016/s0076-6879(97)83040-6. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Akhavan-Niaki H, McGowan CH, Russell P. Protein phosphatase 2C encoded by ptc1+ is important in the heat shock response of fission yeast. Mol Cell Biol. 1994;14:3743–3751. doi: 10.1128/mcb.14.6.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Heat stress activates fission yeast Spc1/Sty1 MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M, Maeda T, Saito H. Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E, Fantes PA. The wis1 protein is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991;10:4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh J-C, Toda T, Millar JBA, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes & Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Wurgler-Murphy SM, Maeda T, Witten EA, Saito H. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol Cell Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan XL, Deschenes RJ, Guan KL. Differential regulation of FUS3 MAP kinase by tyrosine-specific phosphatases PTP2/PTP3 and dual-specificity phosphatase MSG5 in Saccharomyces cerevisiae. Genes & Dev. 1997;11:1690–1702. doi: 10.1101/gad.11.13.1690. [DOI] [PubMed] [Google Scholar]