Abstract
Effective preventive measures against HIV must function near the time of virus transmission to prevent the establishment of a chronic infection. Low-dose SIV/SHIV infections by multiple routes lead to remarkably rapid systemic dissemination of virus and large numbers of infected cells during the initial weeks of the acute infection. Here we describe the narrow time-frame during which potent post-exposure interventions such as anti-retroviral therapy or the administration of high-titered neutralizing antibodies can block the establishment of the in vivo infection. This short window of opportunity is applicable to HIV infections and represents a formidable challenge for developing effective chemoprophylaxis and vaccine approaches.
Introduction
Because once an HIV-1 infection is established it cannot be eradicated, the period immediately following exposure to virus becomes a most critical phase of the infection. This interval may be the only time when preventive measures can completely block the process of “virus acquisition”, the current focus of HIV vaccine and pre-exposure chemoprophylaxis approaches [1,2]. For practical reasons, it has been difficult to determine this “window of opportunity” for HIV because so few patients, with an unequivocally determined time of exposure, have been monitored by frequent sampling of clinical specimens. Historically, numerous temporal parameters of early HIV-1 infection have been measured including the onset of symptoms, seroconversion, p24 antigenemia, virus isolation, and more recently, determinations of plasma viral RNA and cell-associated viral DNA. The results of many studies have given rise to the notion that the “eclipse” period for HIV-1, during which virus is undetectable in the blood, lasts for 10 days and that the peak of viremia may not occur for more than three weeks following HIV-1 transmission [3–5].
Simian immunodeficiency virus (SIV) and SIV/HIV chimeric viruses (SHIVs) have been extensively used as surrogates for HIV-1 to study the dynamics of the acute virus infection. Unlike exposed humans, the properties of the virus inoculum, the precise time of virus exposure, dose size, and route of transmission are all known ahead of time. Depending on the research goal, each of these parameters can be altered to investigate specific questions relevant to the transmission, establishment, and kinetics of the acute HIV-1 infection in humans. In this regard, there are at least two critical issues pertaining to the “window of opportunity” for intervention during the acute infection: 1) the identity of the initial cell type(s) targeted by the incoming virus and their activation status and 2) the rate of systemic virus spread, as monitored by the total number of infected CD4+ T lymphocytes in an exposed individual at different times following virus transmission. By evaluating these parameters, the SIV/SHIV macaque model has provided important answers to both questions.
Transmission route and inoculum size considerations
Traditionally, experimental animals have been inoculated with viruses by the intravenous (IV), intramuscular, and the oral routes. For SIV and SHIV studies, the IV route has been extensively used in natural history and pathogenesis experiments and is the most reliable and direct way to establish infections and to disseminate virus to tissues/organs throughout the body. IV inoculated SIVs and SHIVs are rapidly delivered to secondary lymphoid tissues and the GI tract where virus replication proceeds exponentially, heralding the onset of the systemic infection. The IV route has been used to model drug user/blood transfusion, and possibly, needle-stick transmissions of HIV-1.
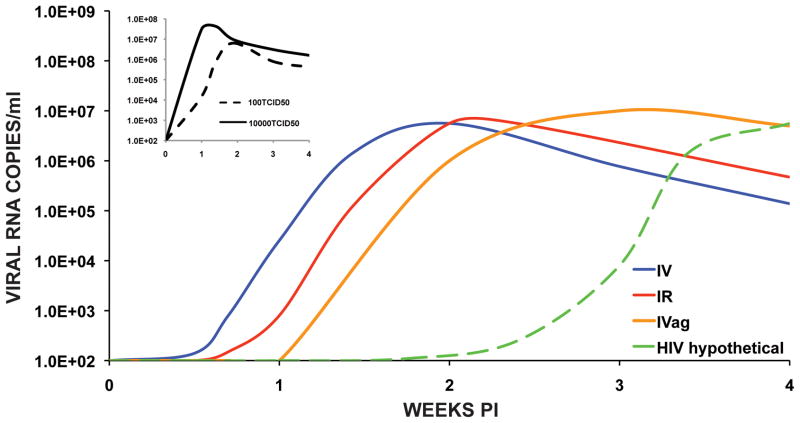
Mucosal inoculation of SIVs and SHIVs has become the route of choice for vaccine experiments, although it is now appreciated that virus can rapidly cross the mucosal barrier [6]. Following the penetration of the epithelial cell layer, “founder infections” are established in CD4+ memory T cells residing in the submucosa where the progeny virus population expands [7,8]. Translocation of virus and infected cells to draining lymphoid tissue/lymph nodes ushers in the systemic phase of the acute infection, resulting in an exponential increase of progeny virion production. Intrarectal inoculation of SIV and SHIV, modeling male to male transmission, typically results in a modest delay in the appearance of peak plasma viremia versus inoculation by the IV route, when comparable inoculum sizes are administered (see below). This delay presumably reflects the additional time required to penetrate the colonic columnar epithelial barrier and to establish a local infection in the submucosa. Vaginal transmission of SIV and SHIV is thought by some to be slower than when virus inoculation occurs by the IV and intrarectal routes [9]. This additional delay could reflect the relative paucity of lymphoid tissue adjacent to submucosal founder infections and/or suppressive effects of innate immune responses in the female genitourinary tract [10,11]. Representative patterns of SIV replication following low dose inoculation by different routes are shown in Fig. 1.
Figure 1.
Representative SIV infectivity profiles in rhesus macaques following low dose inoculations by different routes. The curves shown are based on reports for inoculation of SIV by the IV (100 TCID50) [14], IR (930 TCID50) [16], and IVag (1000 TCID50) [20] routes. The hypothetical HIV curve is based on an eclipse period of 10 days and peak levels of plasma viremia occuring on day 25 post exposure [3,17]. The inset depicts the levels of plasma viremia measured in macaques inoculated with 100 or 10,000 TCID50 of SIVmac239 by the IV route [14,15].
Inter-comparisons of dose effects following inoculation by parenteral and non-parenteral routes can be problematic. First, dose size based on infectivity (viz. TCID50) is dependent on the cell types used for: 1) preparation of the virus stock; and 2) the assay used to measure infectivity. Dose size based on viral RNA copy number or Gag protein content will be affected by the amount of defective virions present in the challenge stock. Second, passage through a mucosal barrier can be affected by multiple factors compared to intravenous inoculation, where a clear relationship exists between inoculum size and the rate at which virus is disseminated systemically [12,13].
When rhesus macaques are inoculated IV with a relatively low dose (viz. 100 TCID50) of SIVmac239, peak plasma viremia occurs between days 10 to 14 PI, whereas inoculation of 10,000 TCID50 accelerates this phase of the infection by 3 or 4 days (Fig. 1 inset) [14,15]. A study of SIVmac251 titration by the IR route (106 down to 103 TCID50) reported that plasma viral RNA was initially detected 4 to 8.5 days PI, depending on the inoculum size [16]. In the 2 of 6 animals that became infected at the dilution endpoint (103 TCID50), peak plasma viremia occurred at day 14 PI. It is worth noting that inoculation of macaques with as little as 1 TCID50 of SIV by the IV route can establish infections leading to AIDS, indicating the inefficiency of transmission by the rectal route [13]. Another study, evaluating repeated low dose IR inoculation of SIVsmE660 or SIVmac239, reported that peak plasma viral RNA loads were attained within 1 to 2 weeks of the last unsuccessful inoculation [17]. Single genome analyses of virus transmitted by repeated low dose rectal inoculation of uncloned SIVsmE660 or SIVmac251 revealed the presence of one or a few viruses in plasma prior to peak viremia, a result similar to that found in humans exposed to HIV-1 by mucosal routes [17,18]. Taken together, these results suggest that once the mucosal barrier is penetrated by an infectious particle, the replication kinetics and systemic spread of SIV are similar to those reported for low dose inoculation by the IV route (Fig. 1).
Less data is available relating inoculum size and SIV infection kinetics following intravaginal (IVag) inoculation. When analyzed in the absence of hormones that cause thinning of the vaginal epithelium, inoculation with 105 or 104 TCID50 led to the establishment of SIVmac251 infection in 20 of 20 animals, whereas macaques receiving 103 TCID50 only became transiently viremic (intermittent virus isolations from PBMC and no detectable cell-associated viral DNA) [19]. Based on inoculum size alone, this result would make SIV transmission by the IVag route 10-fold less efficient than virus administered rectally [17]. In a study examining repeated low dose (1000 TCID50) IVag inoculation of SIVmac251, rhesus monkeys became infected 2 to 3 weeks following a prior unsuccessful challenge [20]. This is somewhat slower than the kinetics reported for low dose inoculation of SIV by the IR route. Some low dose IVag inoculated animals experienced the transient plasma viremia described above before developing a prototypical SIV systemic infection.
The dynamics of virus replication and systemic dissemination during the acute infection
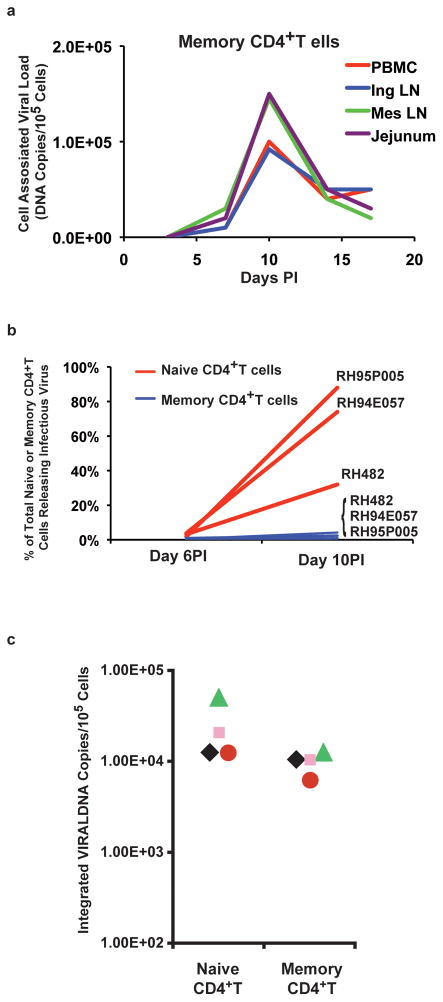
Because of obvious logistic difficulties in obtaining clinical specimens in a timely fashion, very little is known about virus and infected cell dynamics during the early phase of acute HIV-1 infections. Nonetheless, studies using the SIV/macaque model had revealed that the gastrointestinal tract was a major site of virus replication and CD4+ T cell loss during acute SIV infections [21,22]. This result stimulated several groups, including our own, to examine the effects of SIV and SHIV on CD4+ T cell subset loss as well as the levels of plasma viral RNA and cell-associated viral DNA during the initial weeks of the acute infection. Not unexpectedly, high numbers of infected cells were detected in the blood between days 7 to 10 post inoculation. The results from one study reported that the frequency of SIV infected memory CD4+ T cells, recovered from the blood, inguinal and mesenteric lymph nodes, and jejunal mucosa, peaked at day 10 PI [23]. Surprisingly, the fraction of memory CD4+ T cells from each of these tissues carrying SIV DNA was extraordinarily high -- ranging from 30 to 60% of the total memory cells analyzed (Fig. 2a). This unexpected massive infection of memory CD4+ T cells was associated with a corresponding rapid systemic depletion of this T cell subset during the acute infection.
Figure 2.
Massive numbers of CD4+ T lymphocytes become infected systemically by SIV and SHIV during the acute infection. (a) Cell-associated viral DNA present in memory CD4+ T cells, recovered and sorted from the indicated tissues of SIVmac 251 infected animals (adapted from reference [23]. (b) Percentage of naïve and memory CD4+ T cells releasing infectious particles (limiting-dilution/co-cultivation with uninfected PBMC) on days 6 and 10 following IV SHIVDH12R inoculation (adapted from reference [30]). (c) Frequency of naïve and memory CD4+ T cells containing integrated viral DNA in 4 monkeys at day 10 post SHIVDH12R infection (adapted from reference [32]).
Unlike SIV and HIV-1, pathogenic X4 SHIVs exclusively utilize the CXCR4 chemokine receptor during infections of rhesus macaques [24]. The expression of CXCR4 on virtually all naïve and a significant fraction of memory CD4+ T lymphocytes makes both subsets the target of SHIVs and explains the characteristic rapid, irreversible, and complete depletion of CD4+ T cells that occurs in virus infected animals [25–27]. Live cell sorting followed by limiting-dilution cocultivation with macaque PBMC was used to determine the frequencies of naïve or memory CD4+ T cells releasing infectious SHIV during the acute infection. The results obtained in three infected animals were quite striking (Fig. 2b). On day 10 PI, 30 to 90% of total circulating naïve CD4+ T cells were producing infectious particles.
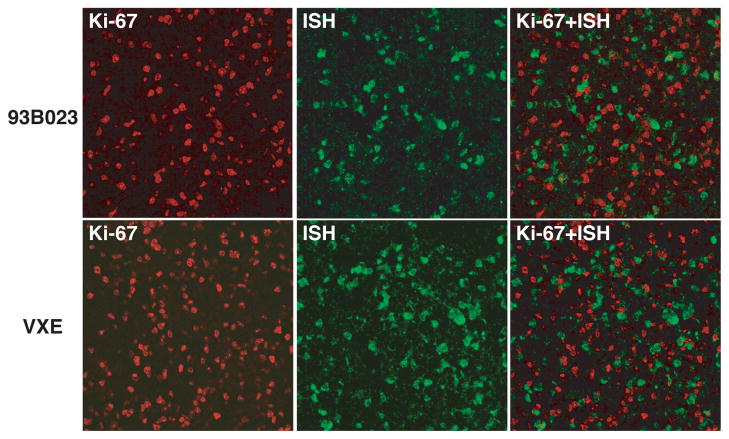
For many years, a prevailing dogma was the HIV and SIV were unable to replicate in quiescent CD4+ T cells [28,29]. Because naïve CD4+ T cells are prototypical “resting” cells and the frequency of SHIV-infected naïve CD4+ T lymphocytes releasing infectious particles at day 10 PI was enormous, it became imperative to verify the activation status of these virus-producing population. Immunophenotyping by flow cytometery revealed that during the period of prodigious virus production and systemic dissemination, naïve CD4+ T cells did not express the Ki-67, HLA-DR, or CD69 activation markers [30]. This result was also confirmed by combined in situ hybridization/immunohistochemistry, which indicated that virtually none of the SHIV producing cells in the mesenteric lymph node was co-expressing the Ki-67 activation marker (Fig. 3). These results were also concordant with reports demonstrating that during the acute SIV infection, virus producing memory CD4+ T cells in lymph nodes or colon submucosa exhibited a non-activated phenotype [8,31].
Figure 3.
Virus producing lymph node cells from macaques are not activated during the acute infection. Mesenteric lymph node sections from two SHIVDH12R infected monkeys (day 10 PI) were analyzed by combined immunostaining for Ki-67 (red) and in situ hybridization (green) (adapted from reference [30]). Superimposed images are shown in the right panels.
Another dogma involving HIV and resting CD4+ T lymphocytes is that integration of viral DNA is blocked in quiescent cells. Limiting-dilution Alu-LTR PCR was therefore used to ascertain the integration status of viral DNA in the large population of infected, non-activated CD4+ T cells during the acute SHIV infection [32]. As shown in Fig. 2c, a remarkably high proportion of these cells were found to contain integrated viral DNA. Taken together, the results presented in the three panels of Fig. 2 use independent assays, measuring different parameters, to show that enormous numbers of resting CD4+ T cells are infected by SIV and SHIVs during acute infections of rhesus monkeys. These include CD4+ T lymphocytes circulating in the blood, present in secondary lymphoid tissues, such as lymph nodes, spleen, and tonsils, and mucosal associated locations such as the GI tract. Based on the high frequencies of SIV/SHIV infected cells in the blood and tissues by days 7 to 10 PI, a conservative estimate would predict that 109 to 1010 CD4+ T cells (corresponding to 1% and 10% of total body CD4+ T lymphocytes, respectively, in a 5 kg macaque) might be infected at this time [33–35].
Can acute SIV or SHIV infections of macaques be blocked?
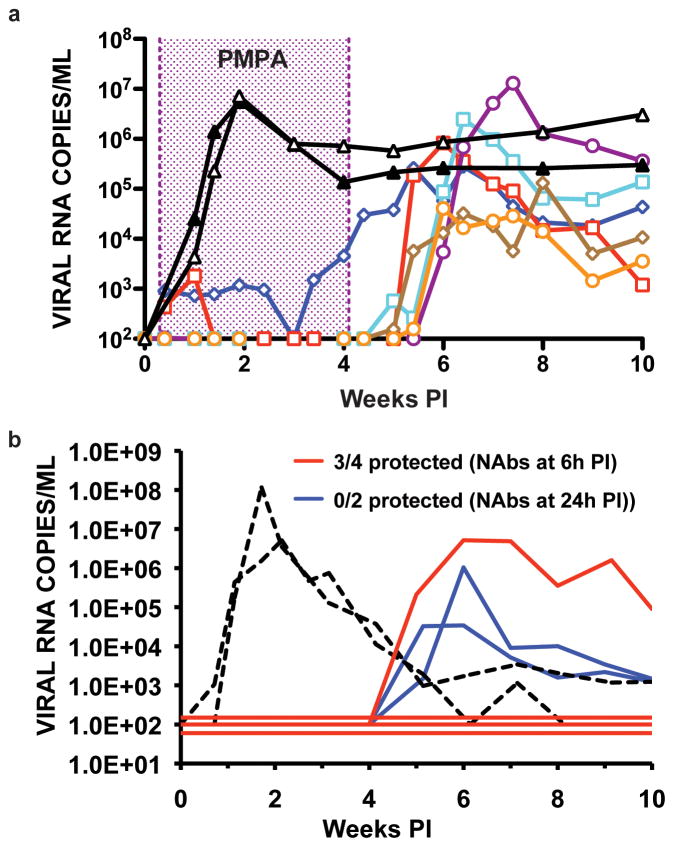
A previous study reported that initiating a 28-day course of a potent anti-retroviral therapy (ART) at 24h PI prevented SIV infections in rhesus macaques [36]. In contrast, extending the time of treatment start from 24h to 72h post SIV inoculation resulted in subsequent uncontrolled virus replication and disease development. In a follow-up study, ART was delayed until 48h post intravenous inoculation (100 TCID50 SIVmac239) to allow a “controlled” establishment of the viral infection and possibly enable an immune system, sensitized to low levels of virus replication, to suppress SIV when treatment was discontinued [14]. As shown in Fig. 4a, plasma viral RNA levels were below detection limits in four of the six macaques during the 4 weeks of ART. In addition, memory CD4+ T cells in the blood and at an effector site (lungs) were maintained at pre-infection levels during the period of drug administration. Unfortunately, plasma viremia became detectable in the treated animals immediately following the cessation of ART. All six of these SIV infected macaques eventually developed immunodeficiency and were euthanized between weeks 34 and 174 PI. These results indicate that the prevention of virus induced injury to the immune system, by potently suppressing SIV replication beginning at 48 PI for one month, did not result in subsequent control of the virus infection following cessation of treatment.
Figure 4.
Post-exposure administration of an anti-retroviral drug or NAbs. (a) A 4-week course of the reverse transcriptase inhibitor, tenofovir, was initiated in 6 animals, 48h following IV inoculation of 100 TCID50 of SIVmac239 (colored curves). Two untreated monkeys (black curves) served as controls (adapted from reference [41]). (b) Polyclonal anti-viral NAbs were transferred at 6h (four macaques) or 24h (2 macaques) post IV inoculation of SHIVDH12 (75 TCID50). The dashed curves represent two recipients of non-neutralizing antibodies (adapted from reference [41]).
Passive transfer of antiviral neutralizing antibodies (NAbs) to macaques can confer sterilizing protection when present at sufficient titers prior to an SHIV challenge [37–40]. To determine how soon following virus exposure NAbs must be present to block the establishment of a virus infection, high-titered anti-SHIV IgG, known to prevent virus acquisition, was administered to macaques at 6 or 24h following the intravenous inoculation of 75 TCID50 of virus [41]. Sterilizing protection was achieved in three of four animals receiving neutralizing IgG 6 hours following SHIV inoculation, as monitored by DNA and RT-PCR of circulating PBMC and plasma, respectively, and attempted virus isolations from plasma, PBMC and lymph node specimens (Fig. 4B). Although virus replication was suppressed for more than 4 weeks in the fourth animal, plasma viremia and cell-associated viral DNA subsequently became detectable. In contrast to these results, transfer of NAbs to two monkeys 24h post exposure delayed, but did not block, SHIV infection in either animal. Compared to macaques receiving post-exposure ART (protection up to 24h post virus exposure), the time interval during which SHIV acquisition can be prevented by administering NAbs was significantly reduced (up to 6h PI).
How open is the “window of opportunity” for blocking the establishment of an HIV infection?
The answer to this question has always been problematic because the time of HIV exposure is rarely precisely known. However, is it reasonable to think that HIV has an eclipse period lasting for 10 days or that the peak level of plasma viremia is delayed for more than 3 weeks in a recently exposed individual [3,17]? A hypothetical HIV replication profile incorporating these two parameters is presented in Fig. 1. One could argue that SIV/SHIV and HIV have fundamentally different biological properties, which affect virus acquisition, or that the very short intervention times for preventing the establishment of SIV/SHIV infections by administering post-exposure NAbs and ART are inappropriate because the virus challenges were intravenous. Route dependent transmission differences are not the critical factors explaining the purported long delay in establishing HIV infections. Rather, the fundamental determinant that would mitigate this supposed difference is the guiding principle of virology: an infection is initiated when a single infectious particle enters a susceptible cell. For HIV sexual transmission, this occurs when an infectious virion successfully penetrates the vaginal/rectal mucosa and enters a CD4+ T lymphocyte to establish a founder infection [7,8]. Subsequent spread to secondary lymphoid tissues leads to the systemic phase of the acute infection, the time when plasma viremia first becomes detectable in exposed individuals. In this regard, there is no a priori reason to believe that infectious SIV/SHIV particles in the submucosa would interact with dendritic and/or CD4+ T cells and be transferred to regional lymph nodes in a fundamentally different manner than HIV. Furthermore, although higher exponential growth rates and reproduction ratios have been proposed for SIV versus HIV [42,43], the “ramp up” virus replication periods (lasting 12 to 14 days), measured in recently HIV exposed individuals [3,44], are similar to the values for SIV/SHIV in macaques.
A Swedish study of primary HIV-1 infections (transmission by homosexual and heterosexual intercourse/needle sharing) in patients with single and determinable exposure times is consistent with relatively short virus eclipse and exponential expansion phases. In this cohort, 12 of 15 individuals experienced clinical symptoms 13 to 15 days following exposure and plasma viral RNA initially became detectable during the week preceding the onset of illness [45,46]. When considered together with the exponential HIV-1 replication rates reported for recently infected persons cited above [3,44], these results are not that different from those observed for SIV/SHIV. The HIV infectivity profile during acute infection shown in Fig. 1, based on hypothetical eclipse phases and time of peak plasma viremia, should therefore be shifted much closer to the time of virus transmission.
Conclusion
Currently there are no data describing the kinetics of systemic virus dissemination following exposure to HIV-1. However, given the likelihood that the total number of infected CD4+ T cells increases from 0 to more than 1010 in SIV/SHIV infected macaques during the first 10 days of the acute infection, the critical time frame for interdicting HIV-1 acquisition may also be quite narrow – perhaps as short as one or two replication cycles after transmission, depending on the local density of CD4+ T cell targets at the founder infection site. This would be consistent with the recommendation to initiate post-exposure HIV prophylaxis as soon as possible following needle stick injury. Treatment beginning at 72h following exposure is unlikely to be of benefit [47]. The rapid systemic dissemination of virus in exposed individuals therefore presents a formidable challenge for developing an effective prophylactic HIV vaccine.
Highlights.
Kinetics of SIV/SHIV infection are dependent on dose and transmission route.
Exponential virus replication rates are independent of transmission routes
During the acute infection, 30–90% of CD4 T cells become productively infected.
The time frame for blocking SIV/SHIV acquisition is 6h to 24 h post exposure
This narrow window of opportunity must be considered in HIV vaccine development
Acknowledgments
Our work is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest published within the period of review have been highlighted as:
* of special interest
** of outstanding interest
- 1**.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. This study describes the first successful anti-HIV microbicide to prevent virus transmission in women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Gasper-Smith N, Crossman DM, Whitesides JF, Mensali N, Ottinger JS, Plonk SG, Moody MA, Ferrari G, Weinhold KJ, Miller SE, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–7710. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. This report describes the establishment of SIV founder infections in the female genital tract following mucosal transmission of virus. [DOI] [PubMed] [Google Scholar]
- 5.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 9.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 10.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo Y, Igarashi T, Nishimura Y, Buckler C, Buckler-White A, Plishka R, Dimitrov DS, Martin MA. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J Virol. 2000;74:6935–6945. doi: 10.1128/jvi.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 14*.Kubo M, Nishimura Y, Shingai M, Lee W, Brenchley J, Lafont B, Buckler-White A, Igarashi T, Martin MA. Initiation of antiretroviral therapy 48 hours after infection with simian immunodeficiency virus potently suppresses acute-phase viremia and blocks the massive loss of memory CD4+ T cells but fails to prevent disease. J Virol. 2009;83:7099–7108. doi: 10.1128/JVI.02522-08. This study shows that initiation of a 28 day course of potent anti-retroviral therapy at 48 hours post infection fails to prevent SIV acquisition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura Y, Igarashi T, Buckler-White A, Buckler C, Imamichi H, Goeken RM, Lee WR, Lafont BA, Byrum R, Lane HC, et al. Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in Simian immunodeficiency virus-infected macaques. J Virol. 2007;81:893–902. doi: 10.1128/JVI.01635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. This study demonstrates how low-dose intrarectal SIV inoculations affect the eclipse period of the acute infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. This study uses single genome analysis to show that low-dose mucosal transmission leads to one or a few viruses in plasma prior to peak viremia, in agreement with results previously reported for HIV in humans [ref16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, Hendrickx AG. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma ZM, Abel K, Rourke T, Wang Y, Miller CJ. A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J Virol. 2004;78:14048–14052. doi: 10.1128/JVI.78.24.14048-14052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 22.Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura Y, Igarashi T, Donau OK, Buckler-White A, Buckler C, Lafont BA, Goeken RM, Goldstein S, Hirsch VM, Martin MA. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc Natl Acad Sci U S A. 2004;101:12324–12329. doi: 10.1073/pnas.0404620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, et al. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc Natl Acad Sci U S A. 1999;96:14049–14054. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I, Pinson DM, McClure HM, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 29.Zagury D, Bernard J, Leonard R, Cheynier R, Feldman M, Sarin PS, Gallo RC. Long-term cultures of HTLV-III--infected T cells: a model of cytopathology of T-cell depletion in AIDS. Science. 1986;231:850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura Y, Brown CR, Mattapallil JJ, Igarashi T, Buckler-White A, Lafont BA, Hirsch VM, Roederer M, Martin MA. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc Natl Acad Sci U S A. 2005;102:8000–8005. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 32*.Nishimura Y, Sadjadpour R, Mattapallil JJ, Igarashi T, Lee W, Buckler-White A, Roederer M, Chun TW, Martin MA. High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc Natl Acad Sci U S A. 2009;106:8015–8020. doi: 10.1073/pnas.0903022106. Measures yet another parameter demonstrating the remarkably rapid increase of virus infected cells during the first weeks of the acute primate lentivirus infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Mascio M, Paik CH, Carrasquillo JA, Maeng JS, Jang BS, Shin IS, Srinivasula S, Byrum R, Neria A, Kopp W, et al. Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood. 2009;114:328–337. doi: 10.1182/blood-2008-12-192203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007;28:514–518. doi: 10.1016/j.it.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig. 1992;70:539–544. doi: 10.1007/BF00184787. [DOI] [PubMed] [Google Scholar]
- 36.Lifson JD, Rossio JL, Arnaout R, Li L, Parks TL, Schneider DK, Kiser RF, Coalter VJ, Walsh G, Imming RJ, et al. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura Y, Igarashi T, Haigwood N, Sadjadpour R, Plishka RJ, Buckler-White A, Shibata R, Martin MA. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura Y, Igarashi T, Haigwood NL, Sadjadpour R, Donau OK, Buckler C, Plishka RJ, Buckler-White A, Martin MA. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc Natl Acad Sci U S A. 2003;100:15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowak MA, Lloyd AL, Vasquez GM, Wiltrout TA, Wahl LM, Bischofberger N, Williams J, Kinter A, Fauci AS, Hirsch VM, et al. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stafford MA, Corey L, Cao Y, Daar ES, Ho DD, Perelson AS. Modeling plasma virus concentration during primary HIV infection. J Theor Biol. 2000;203:285–301. doi: 10.1006/jtbi.2000.1076. [DOI] [PubMed] [Google Scholar]
- 44.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindback S, Karlsson AC, Mittler J, Blaxhult A, Carlsson M, Briheim G, Sonnerborg A, Gaines H. Viral dynamics in primary HIV-1 infection. Karolinska Institutet Primary HIV Infection Study Group. AIDS. 2000;14:2283–2291. doi: 10.1097/00002030-200010200-00009. [DOI] [PubMed] [Google Scholar]
- 46.Lindback S, Thorstensson R, Karlsson AC, von Sydow M, Flamholc L, Blaxhult A, Sonnerborg A, Biberfeld G, Gaines H. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. Karolinska Institute Primary HIV Infection Study Group. AIDS. 2000;14:2333–2339. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 47.Katz MH, Gerberding JL. Management of occupational and nonoccupational postexposure HIV prophylaxis. Curr HIV/AIDS Rep. 2004;1:159–165. doi: 10.1007/s11904-004-0025-8. [DOI] [PubMed] [Google Scholar]