Abstract
Premature infants are at risk for intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL) theorized to be a result from fluctuations in cerebral blood flow. Monitoring cerebral oxygenation offers a method to observe changes in cerebral blood flow that may be beneficial for detecting and preventing IVH and PVL. This article explains the potential for cerebral oxygenation monitoring in detecting IVH and PVL using cerebral oximetry, reviews current knowledge known about cerebral oxygenation, and describes current challenges for cerebral oxygenation to be the next neuroprotective vital sign.
Keywords: cerebral oxygenation, premature infant, intraventricular hemorrhage, periventricular hemorrhage
Every year approximately 500,000 infants are born prematurely in the United States.1 Advances in technology have increased premature infants’ chances of survival and subsequent discharge from the hospital to home. However, as many as 50% of premature infants go home with neurological deficits 2 related to prematurity that result in motor impairments and learning disabilities.3–6 As compared to their full-term counterparts, premature infants are at increased risk for these neurological complications due to the immaturity of their nervous system and its undeveloped cerebrovascular control.5,7–9 Prevention of neurological complications could promote neurodevelopment comparable to the full-term infant.
Intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL) are two prevalent neurological complications in premature infants resulting from fluctuations in cerebral blood flow and cerebral ischemic episodes, respectively. To detect and prevent IVH or PVL, a continuous method to monitor the premature infants’ nervous system via cerebral blood flow is necessary. Cerebral oxygenation is a potential biomarker to assess an infant’s nervous system that can be monitored using cerebral oximetry. Cerebral oxygenation is the amount of oxygen present in arteries, veins, and capillaries of the brain.10 Since cerebral blood flow is the mechanism by which oxygen is delivered to the brain, than measuring cerebral oxygenation offers a method to observe changes in cerebral blood flow. Thus, preferred over invasive methods, cerebral oxygenation monitoring would provide neuroprotection at the bedside to observe and potentially detect in real-time the development of IVH and PVL. The aim of this article is to explain the potential for cerebral oxygenation monitoring in detecting IVH and PVL using cerebral oximetry, review current knowledge known about cerebral oxygenation, and describe current challenges for cerebral oxygenation to be the next neuroprotective vital sign.
Vulnerability to IVH and PVL
As a protective mechanism, blood flow in the brain is regulated to maintain adequate circulation. Autoregulation is the ability to maintain cerebral blood flow despite changes in cerebral perfusion pressure11; cerebral vessels constrict when pressure rises and dilate when pressures decrease.12 The ability of premature infants to autoregulate, however, is not clear.13–18 Absence of autoregulation or severe changes in cerebral blood flow is hypothesized to cause central nervous system complications including cerebral white matter damage, PVL, and intraventricular hemorrhage (IVH).2,19
IVH is characterized as bleeding in the germinal matrix primarily during the first week of life20 and it occurs in 1 in 4 premature infants due to fluctuations in cerebral blood flow.21 Marked areas of necrotic cerebral tissue distinguish PVL from other neurological diagnoses and results from episodes of ischemia often due to low blood pressure.22 No real-time measure exists to identify infants with impaired autoregulation. Therefore, these two neurological diagnoses are commonly discovered via head ultrasound or MRI after the first week of life when neurological damage has already occurred. Cerebral oxygenation as a proxy to monitor changes in cerebral blood flow related to IVH or PVL may serve as a viable measure to noninvasively screen for impairment in autoregulation.
Development of cerebral blood flow and oxygenation
As infants grow and develop, cerebral blood flow increases.23–26 At birth, all infants transition to inhaling oxygen into the lungs and transporting the oxygen by a beating heart to the entire body because the placenta is no longer attached. Decreased pulmonary pressure allows the lungs to inflate and increased systemic pressure results from increased blood flow.27 Once the placenta is removed from circulation, an infant must maintain temperature and meet metabolic demands that require two to three times as much oxygen than in utero.28 Because cerebral blood flow supplies the brain with oxygen and nutrients, cerebral oxygenation is considered an appropriate measurement for cerebral blood flow as long as adequate oxygen can reach the circulation.
Research has shown that cerebral blood flow decreases for approximately the first 2 hours after birth and then remains stable at this low flow for the next 24 hours, though for unknown reasons.29 After the first day of life, cerebral blood flow gradually increases inconsistently over time, possibly due to postnatal growth, increased blood pressure, blood viscosity, or oxygenation.26 Yet how cerebral oxygenation develops overtime is unknown. The fluctuations in cerebral blood flow place premature infants at risk for IVH21, particularly in the first week of life, and PVL. As cerebral blood flow increases oxygen delivery to meet higher oxygen demands, cerebral oxygenation will also increase. In addition to changes related to chronological age significant changes of circulation and adaptation expand. Patent ductus arteriosus,30 respiratory distress requiring respiratory support, 29 or infection can also effect cerebral blood flow.31 As a result, cerebral oxygenation levels may continue to change after the first week of life as premature infants continue to stabilize.
Measurement of cerebral blood flow and cerebral oxygenation
Actual cerebral blood flow is difficult to measure in premature infants because the most accurate measurement methods including single-photon emission computed tomography, positron emission tomography, and radionuclide angiography32 require radioactive material that is not recommended for use in infants.33 Doppler ultrasound and cerebral oximetry are non-invasive, indirect measures of cerebral blood flow in infants.33 Doppler ultrasound focuses on provider selected cerebral arteries or veins to measure cerebral blood flow21, but it requires an ultrasound technician at the bedside to gather data that must then be interpreted by a radiologist.34 In addition, studies utilizing Doppler ultrasound do not provide guidance for continuous clinical decision-making. Cerebral oximetery measures cerebral oxygenation without use of radioactive materials33,35 or requiring additional personnel for interpretation at the bedside.
Cerebral Oximetry
Cerebral oximeters obtain continuous, noninvasive cerebral oxygenation values36 using near-infrared spectroscopy (NIRS) technology. A cerebral oximeter set-up consists of an oximeter probe attached to a monitor cable that is connected to a cerebral oximeter monitor. In general, most cerebral oximeters can support 2 to 4 oximeter probes with respective monitor cables. Oximeter probes can be placed anywhere on the head, but most commonly the forehead where there is the least amount of hair. The oximeter probe includes a fiber optic light source and light detector(s).37 Depending on the cerebral oximeter, fiber optic strands release LASER (light amplification by stimulated emission of radiation)38 or LED (light emitting diodes) light.39 Emitted light wavelengths are sent from the light source penetrating the skull and cerebrum and the light detector(s) receives the light not absorbed during the light pathway through the skull and cerebrum. The amount of oxygen present in the brain is the difference between the amount of light sent and received by the probe, which is suggested by the percentage of oxygen displayed on the monitor screen. However, each cerebral oximeter measures cerebral oxygenation slightly different due to the number of light wavelengths utilized and whether the oximeter measures trends or absolute values.
Trend cerebral oxygenation monitoring focuses more on the amount of change from an established baseline cerebral oxygenation value whereas absolute cerebral oxygenation monitoring focuses more on the meaning of the cerebral oxygenation value. For example, a decrease in cerebral oxygenation from 80% to 70% might be of concern for trend monitoring because there was a drop in cerebral oxygenation by 10%. However, if monitored by an absolute cerebral oximeter, a change in cerebral oxygenation by 10% may not be of concern if an established normal range for this patient is 65–80%.
Cerebral oximeters calculate cerebral oxygenation using NIRS technology based on a modified light absorbent theory called the Beer-Lambert law. According to the Beer-Lambert law, an amount of a substance or compound in this case, oxygen, can be determined by how much light the substance absorbs.40 In theory, a light source will decrease in intensity when an absorbing substance mediates the light source pathway and the more light absorbed by a substance, the more a substance is present.41 The length the light travels from the light source to the light detector(s) determines the light pathway distance. This value is fixed based on the cerebral oximeter probe size and manufacturer.
Oxygen can be bound or unbound to hemoglobin also known as oxygenated hemoglobin and deoxygenated hemoglobin respectively, with each hemoglobin type absorbing different light wavelength amounts.40 Oxygenated and deoxygenated hemoglobin found in vessels and tissue oxygen concentrations, reflecting cellular oxygenation, are thought to comprise all sources of oxygen in the brain.36 These values are unknown at a given time and must be calculated along with weighted algorithm values for arterial, venous, and capillary oxygen42,43 to acquire cerebral oxygenation values. Light wavelengths within the near infrared light spectrum (650 to 900 nm) are the only light wavelengths strong enough to go through skull bone and capture the presence of cerebral tissue oxygenation.40 Additionally, oxygenated hemoglobin, deoxygenated hemoglobin, and tissue oxygenation (cytochrome aa3) are the only substances in the brain with the capacity to change light absorption when oxygenation levels change.44 Lastly, proprietary formulas calculate the differences between light absorbed by oxygenated hemoglobin, deoxygenated hemoglobin, and tissue oxygen to display a percentage of cerebral oxygen present in that particular cerebral light source pathway. This equation divides the amount of oxygenated hemoglobin by the total hemoglobin to calculate a percentage of cerebral oxygenation (see Table 1.).40
Table 1.
Cerebral Oxygenation Equation
| Cerebral Oxygenation (%) = HbO2/Total Hb |
| where: Total Hb= (Hb + HbO2) |
| Hb = deoxygenated hemoglobin |
| HbO2 = oxygenated hemoglobin |
There are an increasing number of cerebral oximeters for infants50 around the world and domestic prototypes for research use only. Currently there are two cerebral oximeters FDA approved in the United States for use in the infant population, the INVOS® System by Somanetics® Corporation and the FORE-SIGHT® Cerebral Oximeter by CAS Medical Systems. Suitable probes sized for infant heads and FDA approval, if used for clinical care, will increase the device opitons. The INVOS® is a trend cerebral oxygenation monitor whereas the FORE-SIGHT® is regarded as measuring absolute measures of cerebral oxygenation. Cerebral oxygenation measured by the FORE-SIGHT™ Cerebral Oximeter is considered to be comprised of 70% venous and 30% arterial blood42 while the INVOS® uses a 75% venous and 25% arterial blood ratio.51 To validate these cerebral oximeters, a comparison of serum blood values was required so it would be known whether or not cerebral oxygenation reflected similar oxygen quantities in the blood. Also, due to the mixed-vascular nature45 of cerebral oxygenation 42,43, cerebral oxygenation validation incorporated blood samples from both vessels, which were easiest to access from infants on extracorporeal membrane oxygenation (ECMO).46 A small number of studies test validity and reliability in these two cerebral oximeter machines.45–48,52 However, these studies clearly demonstrate these two devices are not sensitive to the same changes in cerebral oxygenation. Therefore, caution is highly advised when comparing cerebral oxygenation values between these and other oximeters53 because it appears that values are not similar.
Advantages and Disadvantages of Cerebral Oxygenation Monitoring
The advantages of using a cerebral oximeter include providing noninvasive, real-time data to the clinician at the bedside for immediate care decisions and immediate observations of the effects of interventions (see Table 2.). Cerebral oxygenation monitoring provides continuous information with little to no hindrance of nursing or medical care.54,55 Care decisions not only have the potential to improve patient outcomes by reducing neurological complications, but also decrease hospital costs by up to 400%.56
Table 2.
Cerebral Oxygenation Advantages and Disadvantages.
| Advantages | Disadvantages |
|---|---|
|
|
Disadvantages also exist for cerebral oxygenation monitoring. First of all, cerebral oxygenation values do not provide information on how much oxygen reaches the brain nor can it be inferred how the brain metabolizes oxygen.57 Some studies have established equations to determine cerebral metabolism of oxygen by fractional tissue oxygenation exchange (FTOE), but these values are not provided as part of the commercial cerebral oximeter output.58,59 Therefore, changes seen in cerebral oxygenation values could be misleading as it may not directly relate to physiological processes occurring in the brain, but instead blood flow prior to or immediately after the brain, as in cerebral venous congestion.
Another disadvantage relates to the cerebral oxygenation probe placement. Regardless of how many cerebral oximeter probes used, a large amount of brain tissue will remain unmonitored and only the brain tissue underlying each probe will be examined for the presence of oxygenation (see Table 2.). This is important to keep in mind because in order to capture in real-time the unfolding of IVH or PVL, the cerebral oximeter probe may have to be strategically placed, especially with PVL since it effects multiple sites in the brain. Currently, the best landmark(s) for the cerebral oximeter probe to monitor neurological complications has not been identified.
In addition to location, how to attach the probe is also of concern knowing that skin integrity is an issue when caring for premature infants. Careful consideration must be taken to protect the skin when attaching a cerebral oximeter probe to the head. Non-adhesive probes secured by headbands seem to be the least irritating to the skin, but using hydrogel tape or Tegaderm™ are also possibilities depending on the age of the infant. Manufacturer’s suggest using a new probe every 24 hours and following unit policies for skin assessment.60
Current Knowledge about Cerebral Oxygenation
Although cerebral oximetry is not a standard instrument in the NICU, several studies have examined cerebral oxygenation in infants. What we know thus far about cerebral oxygenation is limited to infants on ECMO 42,61,62, infants undergoing cardiac surgery63,64, or during care activities such as suctioning65–67, surfactant administration65,68, indomethacin dosing69–71, and blood transfusions.72,73
Limited knowledge exists to identify IVH using cerebral oxygenation monitoring. Through the use of optical tomography, total hemoglobin levels, particularly deoxygenated hemoglobin, are higher on the ipsilateral side of an IVH.74 As mentioned earlier, the simplistic formula for calculating cerebral oxygenation divides the amount of oxygenated hemoglobin by the total hemoglobin (see Table 1.). If deoxygenated hemoglobin increases in the vicinity of an IVH than the amount of measurable cerebral oxygenation will decrease. As a result, cerebral oxygenation values will decrease compared to unaffected cerebral locations (see Table 3.).
Table 3.
Hypothesized Cerebral Oxygenation Values with IVH
IVH Present =
 Cerebral Oxygenation= HbO2/ Cerebral Oxygenation= HbO2/
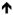 Hb + HbO2 Hb + HbO2
|
| Vs. |
IVH Absent =
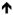 Cerebral Oxygenation= HbO2/ Cerebral Oxygenation= HbO2/
 Hb + HbO2 Hb + HbO2
|
This logic is confirmed in a prospective study that examined 73 premature infants in the first day of life.75 Results showed infants without IVH ranged from 67.7% to 82.1% and those with IVH from 53.3% to 86.4%. As the severity in IVH increased, cerebral oxygenation was shown to decrease. From this one study during the first day of life infants who develop an IVH appear to have lower cerebral oxygenation levels. To continue to confirm this assumption, infant age and cerebral oxygenation monitoring beyond the first day of life are needed.
Cerebral oxygenation monitoring related to PVL diagnosis is also unclear. Ischemic episodes are theorized to cause outcomes of PVL. Cells during an ischemic episode revert to anaerobic metabolism as the nutrients derived from blood flow is diminished or inhibited resulting in what some call an “energy failure”.54 “Energy failure” will show as lower tissue oxygenation values and the amount of cerebral oxygenation available to be measured is reduced. Timing, however, is essential, as these ischemic events are not anticipated. The ability to identify these particular low cerebral oxygenation levels at the correct time and know when an infant is at risk for PVL remains unknown. Further research will need to identify cerebral oxygenation probe placement in order to capture these lower levels of tissue oxygenation that are indicative of PVL.
Although infant cerebral oxygenation research is fragmented, it is important to begin to connect individual studies to the bigger picture in caring for premature infants. For example, when an anemic infant receives a blood transfusion, cerebral oxygenation values will increase due to the increase in oxygenated hemoglobin. However, infants who receive an exchange transfusion will dilute their blood and show lower levels of cerebral oxygenation.73 These notations will help scrutinize between an expected change in cerebral oxygenation and unexpected changes that may lead to neurological complications. As a result, a better understanding can be extracted for how care activities and medical decisions effect health outcomes such as IVH or PVL that can be reduced in the future.
Challenges
Currently neonatal intensive care units across the country have begun to introduce cerebral oximeters to measure cerebral oxygenation levels. Although there has been an explosion of research with premature infants using near-infrared spectroscopy to measure cerebral oxygenation, normative values for cerebral oxygenation measurements at each post-menstrual age or postnatal age are not available for premature infants. Current values for the first day of life come from two studies, 55.1% to 96.4%76 and 67.7% to 82.1%.75 Although these values provide a good starting point, narrower ranges taking into account age and time beyond the first day of life are necessary. In addition to normative values, percentage of change or thresholds may also need to be determined to identify impending neurological injury.57 Care decisions and the assessment of infants at higher risk for IVH and PVL will remain unknown until normative values are better established for each cerebral oximeter. Therefore further research to establish normal cerebral oxygenation ranges need to be examined longitudinally to capture the development of cerebral oxygenation as premature infants mature.
Current vital signs of heart rate, respiratory rate, peripheral oxygenation, and blood pressure may indicate cerebral blood flow and the brain’s receipt of oxygen-rich blood 77,78. Although, cerebral oxygenation may be a more sensitive indicator of reduced blood flow to the brain, in-depth evaluation is necessary to compare current vital signs with cerebral oxygenation. Before neonatal practice decides to add another probe to the bodies of these tiny humans, its imperative that the science establishes certain cause for the addition of another monitored vital sign. Future research will need to determine that cerebral oxygenation values are not embedded or apparent in currently monitored vital signs. As a result, before cerebral oxygenation becomes a new vital sign examination in comparison to currently measured vital signs is needed.
Discussion
Premature infants’ neurodevelopment is vulnerable to alterations compared to full-term infants because of an immature nervous system and early exposure to the neonatal intensive care environment 79. Since premature infants are nonverbal, vital signs are one of the primary methods to examine the effects of underlying neurological damage. Cerebral oximeters measure cerebral oxygenation in a noninvasive manner, based upon the Beer-Lambert law. A cerebral oximeter machine may show value at the bedside for the high-risk premature infant population in the neonatal intensive care unit by providing real-time changes in cerebral oxygenation values that may one day be used as an instrument for neuroprotection in premature infants. Detection of altered cerebral oxygenation using cerebral oximetry may help to identify subtle, abnormal neurological activity before behavioral changes are manifested.
Currently, neonatal providers rely on non-specific measurements to indicate adequate cerebral perfusion in premature infants. Cerebral oxygenation measurements would provide a more direct assessment of the percentage of oxygen in the brain and in the future, have predictive ability to identify infants at-risk for cerebral damage. However, before cerebral oxygenation can be accepted as a standard of care, normative values based on age and over the course of hospitalization are needed as well as complete understanding of its benefits over the current vital sign measures. As more knowledge is gained through research, the potential for cerebral oxygenation monitoring to reduce neurological complications will be revealed to provide optimal care for premature infants.
Acknowledgments
The preparation of this paper was supported by Grant F31NR011269 from the National Institute for Nursing Research, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Heather E. Elser, Email: hem4@duke.edu, Duke University School of Nursing, DUMC 3322 Durham, NC 27710, 919-684-9198, fax: 919-681-8899.
Diane Holditch-Davis, Marcus E. Hobbs Distinguished Professor of Nursing and Associate Dean for Research Affairs, Duke University School of Nursing.
Debra H. Brandon, Duke University School of Nursing.
References
- 1.Hamilton BE, Martin JA, Ventura SJ. [Accessed December 5, 2009];Births: Preliminary data for 2007. 2009 http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf.
- 2.Cole C, Binney G, Casey P, et al. [Accessed May 6, 2010];Criteria for determining disability in infants and children: Low birth weight. 2002 http://www.ahrq.gov/clinic/epcsums/lbwdissum.htm. [PMC free article] [PubMed]
- 3.Donohue PK, Graham EM. Earlier markers for cerebral palsy and clinical research in premature infants. J Perinatol. 2007;27:259–261. doi: 10.1038/sj.jp.7211741. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32:51–58. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:241–248. doi: 10.1002/mrdd.10049. [DOI] [PubMed] [Google Scholar]
- 6.Maitre NL, Marshall DD, Price WA, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124:e1153–1160. doi: 10.1542/peds.2009-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawke J. Neurological outcomes following preterm birth. Seminars in Fetal and Neonatal Medicine. 2007;12:374–382. doi: 10.1016/j.siny.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. The Journal of the American Medical Association. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 9.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 10.Bruns AR, Norwood BR, Bosworth GA, et al. Update for nurse anesthetists--Part 1--The cerebral oximeter: What is the efficacy? AANA J. 2009;77:137–144. [PubMed] [Google Scholar]
- 11.Chillon JM, Baumbach GL. Autoregulation: Arterial and intracranial pressure. In: Edvinsson L, Krause DN, editors. Cerebral blood flow and Metabolism. Philadelphia: Lippincott, Williams, & Wilkins; 2002. pp. 395–412. [Google Scholar]
- 12.Kontos HA, Wei EP, Navari RM, et al. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- 13.Boylan GB, Young K, Panerai RB, et al. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000;48:12–17. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. The Journal of Pediatrics. 1979;94:118–121. doi: 10.1016/s0022-3476(79)80373-x. [DOI] [PubMed] [Google Scholar]
- 15.Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;114:1591–1596. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 16.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 17.Tyszczuk L, Meek J, Elwell C, et al. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics. 1998;102:337–341. doi: 10.1542/peds.102.2.337. [DOI] [PubMed] [Google Scholar]
- 18.Wong FY, Leung TS, Austin T, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121:e604–611. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- 19.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Madan A, Hamrick SE, Ferriero DM. Central nervous system injury and neuroprotection. In: Taeusch HW, Ballard RA, Gleason CA, editors. A very’s diseases of the newborn. 8. Philadelphia: Saunders Elsevier; 2005. pp. 965–992. [Google Scholar]
- 21.Inder TE, Volpe JJ. Pathophysiology of intraventricular hemorrhage in the neonate. In: Polin RA, WFW, Abman SH, editors. Fetal and neonatal physiology. 3. Vol. 2. Philadelphia: W.B. Saunders Co; 2004. pp. 1757–1772. [Google Scholar]
- 22.Volpe JJ. Neurology of the Newborn. 5. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 23.Kehrer M, Blumenstock G, Ehehalt S, et al. Development of cerebral blood flow volume in preterm neonates during the first two weeks of life. Pediatr Res. 2005;58:927–930. doi: 10.1203/01.PDR.0000182579.52820.C3. [DOI] [PubMed] [Google Scholar]
- 24.Meek JH, Tyszczuk L, Elwell CE, et al. Cerebral blood flow increases over the first three days of life in extremely preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1998;78:F33–37. doi: 10.1136/fn.78.1.f33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuji M, Saul JP, Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 26.Volpe JJ. The developing nervous system: A series of review articles. Pediatr Res. 2001;50:553–562. [Google Scholar]
- 27.Cifuentes J, Carlo WA. Respiratory system. In: Kenner C, Lott JW, editors. Comprehensive neonatal care: An interdisciplinary approach. 4. St. Louis: Saunders Elsevier; 2007. pp. 1–17. [Google Scholar]
- 28.Avery ME. The lung and its disorders. 3. Philadelphia: WB Saunders; 1974. [Google Scholar]
- 29.Cooke RW, Rolfe P, Howat P. Apparent cerebral blood flow in newborns with respiratory disease. Dev Med Child Neurol. 1979;21:154–160. [Google Scholar]
- 30.Kurtis PS, Rosenkrantz TS, Zalneraitis EL. Cerebral blood flow and EEG changes in preterm infants with patent ductus arteriosus. Pediatr Neurol. 1995;12:114–119. doi: 10.1016/0887-8994(94)00150-z. [DOI] [PubMed] [Google Scholar]
- 31.Perlman JM, Volpe JJ. Episodes of apnea and bradycardia in the preterm newborn: Impact on cerebral circulation. Pediatrics. 1985;76:333–338. [PubMed] [Google Scholar]
- 32.Serna-Fonseca ST. Brain death in infants and children. Crit Care Nurse. 2006;26:117–124. 126–118. [PubMed] [Google Scholar]
- 33.Perlman JM. Cerebral blood flow in premature infants: Regulation, measurement, and pathophysiology of intraventricular hemorrhage. In: Polin RA, Fox WW, Abman SH, editors. Fetal and neonatal physiology. 3. Vol. 2. Philadelphia: Saunders; 2004. pp. 1745–1757. [Google Scholar]
- 34.Phillips Electronics. General imaging: Ultrasound. 2007. [Accessed April 15, 2008]. [Google Scholar]
- 35.Watzman HM, Kurth CD, Montenegro LM, et al. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93:947–953. doi: 10.1097/00000542-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Toet MC, Lemmers PM. Brain monitoring in neonates. Early Hum Dev. 2009;85:77–84. doi: 10.1016/j.earlhumdev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Brazy JE, Vander-Vliet FJ. Clinical application of near infrared spectroscopy to neonatal intensive care. In: Kim Y, Spelman FA, editors. Images of the Twenty-First Century: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Seattle, Washington November 9–12 1989. Vol. 11. NY: IEEE; 1989. pp. 337–338. [Google Scholar]
- 38.CAS Medical Systems. [Accessed August 3, 2009];Patient monitoring: FORE-SIGHT. 2008 http://www.casmed.com/foresight.html.
- 39.Somanetics Corporation. [Accessed August 3, 2009];Invos System. 2009 http://www.somanetics.com/invos.asp.
- 40.Owen-Reece H, Smith M, Elwell CE, et al. Near infrared spectroscopy. Br J Anaesth. 1999;82:418–426. doi: 10.1093/bja/82.3.418. [DOI] [PubMed] [Google Scholar]
- 41.Schubert D, Leyba J. Chemistry and physics for anesthesia: A student centered approach. Wichita, KS: Newman University; 2008. Electrical circuits; pp. 265–285. [Google Scholar]
- 42.Benni PB, Chen B, Dykes FD, et al. Validation of the CAS neonatal NIRS system by monitoring vv-ECMO patients: preliminary results. Adv Exp Med Biol. 2005;566:195–201. doi: 10.1007/0-387-26206-7_27. [DOI] [PubMed] [Google Scholar]
- 43.Ito H, Kanno I, Iida H, et al. Arterial fraction of cerebral blood volume in humans measured by positron emission tomography. Ann Nucl Med. 2001;15:111–116. doi: 10.1007/BF02988600. [DOI] [PubMed] [Google Scholar]
- 44.Jobsis FF. Non-invasive, infra-red monitoring of cerebral O2 sufficiency, bloodvolume, HbO2-Hb shifts and bloodflow. Acta Neurol Scand Suppl. 1977;64:452–453. [PubMed] [Google Scholar]
- 45.MacLeod D, Ikeda K, Keifer JC, et al. International Anesthesia Research Society. Vol. 102. San Francisco, CA: Anesthesia and Analgesia; 2006. Validation of the CAS adult cerebral oximeter during hypoxia in healthy volunteers; p. S-162. [Google Scholar]
- 46.Rais-Bahrami K, Rivera O, Short BL. Validation of a noninvasive neonatal optical cerebral oximeter in veno-venous ECMO patients with a cephalad catheter. J Perinatol. 2006;26:628–635. doi: 10.1038/sj.jp.7211573. [DOI] [PubMed] [Google Scholar]
- 47.MacLeod D, Ikeda K, Moretti E, et al. Using the CAS cerebral oximeter to estimate cerebral venous oxygen saturation. Anesthesiology. 2005;2005:A16. www.asaabstracts.com.
- 48.Knirsch W, Stutz K, Kretschmar O, et al. Regional cerebral oxygenation by NIRS does not correlate with central or jugular venous oxygen saturation during interventional catheterisation in children. Acta Anaesthesiol Scand. 2008;52:1370–1374. doi: 10.1111/j.1399-6576.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 49.MacLeod D, Ikeda K, Vacchiano C. Simultaneous Comparison of FORE-SIGHT and INVOS Cerebral Oximeters to Jugular Bulb and Arterial Co-Oximetry Measurements in Healthy Volunteers. Anesth Analg. 2009;108:1–104. [Google Scholar]
- 50.Wolf M, Greisen G. Advances in near-infrared spectroscopy to study the brain of the preterm and term neonate. Clin Perinatol. 2009;36:807–834. vi. doi: 10.1016/j.clp.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Somanetics Corporation. [Accessed January 10, 2011];Somanetics Invos 5100 Cerebral Oximeter 510(K) Premarket Notification. 2000 http://www.accessdata.fda.gov/cdrh_docs/pdf/k001842.pdf.
- 52.MacLeod D, Ikeda K, Vacchiano C. Absolute and trending accuracy of FORE-SIGHT and INVOS cerebral oximeters in healthy volunteers. Paper presented at: Annual Meeting of the American Society Anesthesiologists; 2009. [Google Scholar]
- 53.Nollert G. Cerebral oxygenation measured by near-infrared spectroscopy. Ann Thorac Surg. 1997;63:291–292. author reply 292–293. [PubMed] [Google Scholar]
- 54.Reynolds EO, McCormick DC, Roth SC, et al. New non-invasive methods for the investigation of cerebral oxidative metabolism and haemodynamics in newborn infants. Ann Med. 1991;23:681–686. doi: 10.3109/07853899109148103. [DOI] [PubMed] [Google Scholar]
- 55.Brazy JE. Cerebral oxygen monitoring with near infrared spectroscopy: clinical application to neonates. J Clin Monit. 1991;7:325–334. doi: 10.1007/BF01619354. [DOI] [PubMed] [Google Scholar]
- 56.Austin EH, 3rd, Edmonds HL, Jr, Auden SM, et al. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 1997;114:707–715. 717. doi: 10.1016/S0022-5223(97)70074-6. discussion 715–706. [DOI] [PubMed] [Google Scholar]
- 57.Brazy JE, Lewis DV, Mitnick MH, et al. Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics. 1985;75:217–225. [PubMed] [Google Scholar]
- 58.Verhagen EA, Keating P, ter Horst HJ, et al. Cerebral oxygen saturation and extraction in preterm infants with transient periventricular echodensities. Pediatrics. 2009;124:294–301. doi: 10.1542/peds.2008-2057. [DOI] [PubMed] [Google Scholar]
- 59.van Hoften JC, Verhagen EA, Keating P, et al. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed. 2010;95:F352–358. doi: 10.1136/adc.2009.163592. [DOI] [PubMed] [Google Scholar]
- 60.Somanetics Corporation. [Accessed November 20, 2010];INVOS Cerebral/Somatic Oximeter Quick Reference Guide for Pediatric Use. n.d http://lane.stanford.edu/portals/cvicu/HCP_Equipment/NIRS-INVOS_Reference_Guide.pdf.
- 61.Liem KD, Hopman JC, Oeseburg B, et al. Cerebral oxygenation and hemodynamics during induction of extracorporeal membrane oxygenation as investigated by near infrared spectrophotometry. Pediatrics. 1995;95:555–561. [PubMed] [Google Scholar]
- 62.Liem KD, Kollee LA, Klaessens JH, et al. Disturbance of cerebral oxygenation and hemodynamics related to the opening of the bypass bridge during veno-arterial extracorporeal membrane oxygenation. Pediatr Res. 1995;38:124–129. doi: 10.1203/00006450-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 63.du Plessis AJ, Johnston MV. The pursuit of effective neuroprotection during infant cardiac surgery. Semin Pediatr Neurol. 1999;6:55–63. doi: 10.1016/s1071-9091(99)80047-3. [DOI] [PubMed] [Google Scholar]
- 64.Van Bel F, Zeeuwe PE, Dorrepaal CA, et al. Changes in cerebral hemodynamics and oxygenation during hypothermic cardiopulmonary bypass in neonates and infants. Biol Neonate. 1996;70:141–154. doi: 10.1159/000244359. [DOI] [PubMed] [Google Scholar]
- 65.Skov L, Hellstrom-Westas L, Jacobsen T, et al. Acute changes in cerebral oxygenation and cerebral blood volume in preterm infants during surfactant treatment. Neuropediatrics. 1992;23:126–130. doi: 10.1055/s-2008-1071327. [DOI] [PubMed] [Google Scholar]
- 66.Mosca FA, Colnaghi M, Lattanzio M, et al. Closed versus open endotracheal suctioning in preterm infants: effects on cerebral oxygenation and blood volume. Biol Neonate. 1997;72:9–14. doi: 10.1159/000244460. [DOI] [PubMed] [Google Scholar]
- 67.Shah AR, Kurth CD, Gwiazdowski SG, et al. Fluctuations in cerebral oxygenation and blood volume during endotracheal suctioning in premature infants. J Pediatr. 1992;120:769–774. doi: 10.1016/s0022-3476(05)80246-x. [DOI] [PubMed] [Google Scholar]
- 68.Dorrepaal CA, Benders MJ, Steendijk P, et al. Cerebral hemodynamics and oxygenation in preterm infants after low-vs. high-dose surfactant replacement therapy. Biol Neonate. 1993;64:193–200. doi: 10.1159/000243989. [DOI] [PubMed] [Google Scholar]
- 69.Mosca F, Bray M, Lattanzio M, et al. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr. 1997;131:549–554. doi: 10.1016/s0022-3476(97)70060-x. [DOI] [PubMed] [Google Scholar]
- 70.Liem KD, Hopman JC, Kollee LA, et al. Effects of repeated indomethacin administration on cerebral oxygenation and haemodynamics in preterm infants: combined near infrared spectrophotometry and Doppler ultrasound study. Eur J Pediatr. 1994;153:504–509. doi: 10.1007/BF01957006. [DOI] [PubMed] [Google Scholar]
- 71.Benders MJ, Dorrepaal CA, van de Bor M, et al. Acute effects of indomethacin on cerebral hemodynamics and oxygenation. Biol Neonate. 1995;68:91–99. doi: 10.1159/000244223. [DOI] [PubMed] [Google Scholar]
- 72.Murakami Y, Yamashita Y, Nishimi T, et al. Changes of cerebral hemodynamics and oxygenation in unstable septic newborns during exchange transfusion. Kurume Med J. 1998;45:321–325. doi: 10.2739/kurumemedj.45.321. [DOI] [PubMed] [Google Scholar]
- 73.Liem KD, Hopman JC, Oeseburg B, et al. The effect of blood transfusion and haemodilution on cerebral oxygenation and haemodynamics in newborn infants investigated by near infrared spectrophotometry. Eur J Pediatr. 1997;156:305–310. doi: 10.1007/s004310050606. [DOI] [PubMed] [Google Scholar]
- 74.Austin T, Gibson AP, Branco G, et al. Three dimensional optical imaging of blood volume and oxygenation in the neonatal brain. Neuroimage. 2006;31:1426–1433. doi: 10.1016/j.neuroimage.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 75.Sorensen LC, Maroun LL, Borch K, et al. Neonatal cerebral oxygenation is not linked to foetal vasculitis and predicts intraventricular haemorrhage in preterm infants. Acta Paediatr. 2008;97:1529–1534. doi: 10.1111/j.1651-2227.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 76.Sorensen LC, Greisen G. The brains of very preterm newborns in clinically stable condition may be hyperoxygenated. Pediatrics. 2009;124:e958–963. doi: 10.1542/peds.2008-2394. [DOI] [PubMed] [Google Scholar]
- 77.Seidel HM, Ball JW, Dains JE, et al. Mosby’s guide to physical examination. 5. St. Louis: Mosby, Inc; 2003. Chest and lungs; pp. 356–413. [Google Scholar]
- 78.Seidel HM, Ball JW, Dains JE, et al. Mosby’s guide to physical examination. 5. St. Louis: Mosby, Inc; 2003. Blood vessels; pp. 462–495. [Google Scholar]
- 79.Blackburn S. Environmental impact of the NICU on developmental outcomes. J Pediatr Nurs. 1998;13:279–289. doi: 10.1016/S0882-5963(98)80013-4. [DOI] [PubMed] [Google Scholar]


