Abstract
Synapses are specialized junctions that mediate information flow between neurons and their targets. A striking feature of the nervous system is the specificity of its synaptic connections: an individual neuron will form synapses only with a small subset of available presynaptic and postsynaptic partners. Synaptic specificity has been classically thought to arise from homophilic or heterophilic interactions between adhesive molecules acting across the synaptic cleft. Over the past decade, many new mechanisms giving rise to synaptic specificity have been identified. Synapses can be specified by secreted molecules that promote or inhibit synaptogenesis, and their source can be a neighboring guidepost cell, not just presynaptic and postsynaptic neurons. Furthermore, lineage, fate, and timing of development can also play critical roles in shaping neural circuits. Future work utilizing large-scale screens will aim to elucidate the full scope of cellular mechanisms and molecular players that can give rise to synaptic specificity.
Introduction
The human brain is an immensely complicated organ, with an estimated hundred billion neurons forming trillions of synapses. A striking property of the nervous system is the precision of its vast numbers of synaptic connections, which are organized into specific neural circuits. Specificity of neural connections was first observed by the renowned neuroanatomist Santiago Ramón y Cajal, who successfully used the Golgi staining method to first demonstrate that the brain consists of many individual cells rather than being one large syncytium (Cajal, 1954). Cajal also observed that neurons have specific connectional relationships both in terms of choice of their synaptic partner and the subcellular location of these synapses. He noted, for instance, that cerebellar Purkinje cells receive inputs from different cell types in a highly stereotyped fashion, such that certain presynaptic neurons preferentially synapse onto Purkinje cell axon initial segments, while others selectively target Purkinje cell dendrites (Cajal, 1954). Thus, neurons can be selective both in terms of neuron subpopulations they form synapses with and the specific subcellular sites onto which these synapses are made; these two features are collectively referred to as “synaptic specificity.” Cajal’s early neuroanatomical explorations were extended by the advent of electron microscopy, which further provided evidence for synaptic specificity in the cerebral cortex (Somogyi et al., 1998; White, 2007).
How does connectional specificity of the nervous system arise? This question has been a matter of considerable debate, with evidence pointing both in the direction of experience-dependent plasticity (“nurture”) and genetic hardwiring (“nature”). For example, classical studies by Hubel and Weisel have demonstrated that blockade of visual experience during critical period of development can disrupt formation of ocular dominance columns in the visual cortex, leading to vision impairment (Wiesel and Hubel, 1963a, b). Subsequent studies have shown that neural circuitry can be shaped both by spontaneous neural activity and activity evoked by sensory experiences (Holtmaat and Svoboda, 2009; Katz and Shatz, 1996). On the other hand, a classical study by Roger Sperry demonstrated that regenerating frog retinal ganglion cells are predetermined to target specific postsynaptic targets in the tectum, even when experimental manipulation has rendered targeting of these neurons inappropriate, resulting in inverted vision (Sperry, 1963). Sperry proposed that there exist “highly specific cytochemical affinities” between axons and their targets that guide appropriate neuronal wiring, and suggested that orderly mapping that utilized multiple molecular gradients could lead to the development of topographic maps (Sperry, 1963). Subsequent work has identified a number of instances of target recognition that is precise from the earliest stages of innervation (Benson et al., 2001).
Synaptogenesis and circuit formation can be considered the culmination of a number of sequential developmental events (Benson et al., 2001). First, subsets of neurons are born and specified to a particular cell fate by combinatorial sets of transcription factors, which make different neuronal subtypes structurally and physiologically unique (Shirasaki and Pfaff, 2002). Next, developing axons need to be guided to the appropriate general target area through a combination of chemoattractive and chemorepulsive axon guidance cues (O’Donnell et al., 2009). Once they reach the appropriate area, axons need to recognize their specific synaptic targets out of the multitude of surrounding possible partners, as well as select which cellular subdomain of the target neuron they will synapse with (Benson et al., 2001). The neurotransmitter choice at a given synapse also needs to be coordinated, such that the neurotransmitter produced by the presynaptic neuron is matched by the neurotransmitter receptor expressed by the postsynaptic neuron (Spitzer et al., 2004). Finally, certain synaptic contacts will be stabilized, strengthened or eliminated, leading to the formation of mature neural circuits (Eaton and Davis, 2003).
Following synapse specification, synapses need to undergo assembly, whereby they physically accumulate synaptic machinery, and subsequent refinement and maturation, which involve attainment of appropriate synaptic density, morphology and functional maturation. Synapses are complex structures, requiring a diverse set of presynaptic proteins to support the release of neurotransmitter into the synaptic cleft following an action potential. The electrical depolarization of the presynaptic terminal leads to the opening of voltage-gated calcium channels and a local rise in calcium, which induces fusion of synaptic vesicles at the active zone and neurotransmitter release (Sudhof, 2004). Subsequently, synaptic vesicles undergo endocytosis, recycle and refill with neurotransmitters, and are ready for a new round of release. On the postsynaptic side, neurotransmitter receptors of the appropriate type are concentrated at the postsynaptic density, where they receive the signal from the presynaptic cell and propagate it by inducing changes in the postsynaptic potential. Modification of postsynaptic receptor levels or properties is thought to be the basis of plasticity and learning in the brain (Shepherd and Huganir, 2007).
The remainder of this review will be dedicated to the diverse molecular mechanisms that are involved in specifying synapses, focusing on adhesion and secreted molecules that can shape neural circuits, as well as intrinsic mechanisms that play a role in generating synaptic specificity.
Adhesion molecules and synaptic specificity
Synaptic specificity is classically thought to be mediated by adhesive molecules acting to establish connections across the synaptic cleft (Akins and Biederer, 2006). In this model, homophilic or heterophilic interactions between molecules expressed by the pre- and postsynaptic neurons would lead to the initial partner recognition and subsequent synapse formation and stabilization (Figure 1 A and B). Since many structures in both vertebrate and invertebrate brains are laminated, studying targeting to a layer has served as a convenient proxy for investigating synaptic specificity. For instance, in the vertebrate retina, immunoglobulin superfamily (IgSF) adhesion molecules Sidekick-1 and 2 and Dscam/DscamL were found to be important for correct targeting to different sublamina of inner plexiform layer (Yamagata and Sanes, 2008; Yamagata et al., 2002). These molecules are expressed in the presynaptic and postsynaptic cells that “meet” in the same retinal sublamina, are necessary and sufficient to direct neuronal projections to that retinal sublayer, and mediate homophilic adhesion in vitro (Yamagata and Sanes, 2008). Thus, an IgSF code seems to direct laminar specificity in the vertebrate retina.
Figure 1.
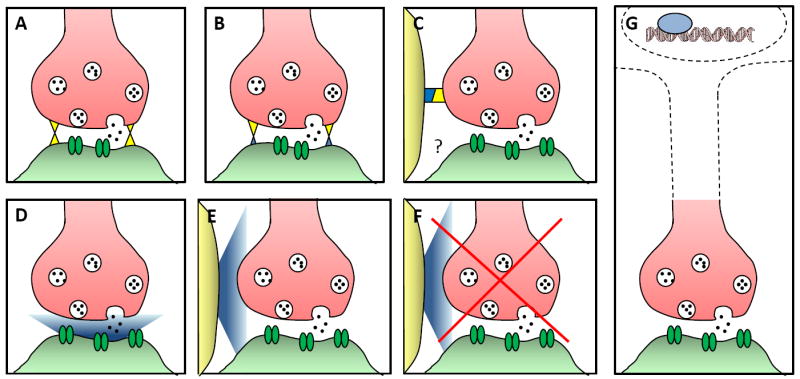
A schematic diagram of different molecular mechanisms that can give rise to synaptic specificity. A) Homophilic adhesion between pre- and postsynaptic partners (e.g. Sidekick, Dscam, N-cadherin, Capricious); B) Heterophilic adhesion between pre- and postsynaptic partners (e.g. neurexins and neuroligins, LAR and NGL-3); C) Heterophilic adhesion between the presynaptic neuron and a guidepost cell (e.g. SYG-1 and SYG-2); D) Secreted synaptogenic molecule produced by the presynaptic or the postsynaptic partner (e.g. Wnt7a, FGF22); E) Secreted synaptogenic molecule produced by a guidepost cell (e.g. Netrin, Thrombospondins); F) Secreted antisynaptogenic molecule produced by a guidepost cell (e.g. Netrin, Wnts); G) Lineage, fate and timing of development can lead to synaptic specificity via transcriptional mechanisms (e.g. UNC-4, Sequoia).
Screens for visual behavior defects in Drosophila have identified several adhesion molecules required for proper photoreceptor laminar targeting (Lee et al., 2001; Lee et al., 2003). In flies mutant for N-cadherin, R7 cells mistarget to the R8 laminar layer, while R1-R6 cells choose the correct layer but fail to extend out of the ommatidial bundle to the appropriate target cartridges (Lee et al., 2001). N-cadherin acts in both pre- and postsynaptic cells to mediate homophilic attractive interactions resulting in correct photoreceptor targeting (Prakash et al., 2005). Two other molecules identified in this screen, LAR receptor tyrosine phosphatase and α-liprin, have very similar targeting defects to N-cadherin, but appear to act exclusively presynaptically (Choe et al., 2006; Clandinin et al., 2001). The atypical cadherin Flamingo is also critical for R1-R6 target selection and acts non-cell autonomously in presynaptic cells as a short-range homophilic signal (Chen and Clandinin, 2008; Lee et al., 2003). Importantly, N-cadherin and Flamingo are broadly expressed in the eye and they direct targeting of multiple photoreceptor types, raising the possibility that they play a permissive rather than instructive role in synapse specification.
Another adhesion molecule that acts in the Drosophila eye is Capricious, a transmembrane protein with leucine-rich repeats. In contrast to N-cadherin and Flamingo, which are broadly expressed in the eye, Capricious is expressed only in R8 cells and their target layer (Shinza-Kameda et al., 2006). In capricious mutants, R8 cells display local targeting errors and layer change, while ectopic expression of capricious in R7 cells leads to their mistargeting to the R8 medullar layer (Shinza-Kameda et al., 2006). Interestingly, a recent study showed that normal targeting of R7 cells is accomplished by active repression of the R8 targeting program by a transcription factor NF-YC, which represses Senseless, a direct activator of Capricious transcription (Morey et al., 2008). Thus, Capricious is one of the main determinants of R8 targeting specificity. Capricious is also critical at the Drosophila neuromuscular junction for specifying connectivity between muscle 12 and the motoneurons that normally innervate it (Shishido et al., 1998).
A series of studies in C. elegans demonstrated that adhesive synaptogenic interactions may involve guidepost cells [Figure 1 C; (Shen and Bargmann, 2003; Shen et al., 2004)]. The authors focused on the motor neuron HSNL, which controls egg-laying in C. elegans by forming synapses onto the vulva muscles and the VC interneurons. HSNL achieves this specificity in target choice by positioning presynaptic sites at a specific location along the axon. This precise positioning of synapses is mediated by primary vulval epithelial cells that express SYG-2, an IgSF molecule. SYG-2 interacts with SYG-1, another transmembrane molecule that is expressed in HSNL, and thus recruits SYG-1 to the location where presynaptic sites are formed (Shen and Bargmann, 2003; Shen et al., 2004).
How does SYG-1 ensure formation of presynaptic sites at the appropriate location in HSNL? During development, transient presynaptic sites form at multiple locations along the HSNL axon (Ding et al., 2007). However, most of these presynaptic sites are eliminated by adulthood and only those where SYG-1 localizes remain. Ding and colleagues showed that an E3 ubiquitin ligase, a Skp1-Cullin-F-box (SCF) complex, acts in HSNL to eliminate unwanted presynaptic sites. Animals with loss-of-function mutations in components of this complex have delayed or incomplete elimination of additional presynaptic sites. SYG-1 binds to the Skp1 homolog, SKR-1, preventing it from interacting with the rest of the SCF complex. Thus, SYG-1 plays a protective role by locally inhibiting the SCF complex and preventing the degradation of presynaptic sites at the SYG-2-marked location (Ding et al., 2007).
In addition to laminar targeting and postsynaptic partner choice, transmembrane adhesion molecules can also direct synapse formation onto specific subcellular compartments. A recent study showed that cerebellar basket neurons synapse precisely onto the axon initial segment (AIS) of Purkinje cells in a manner dependent on another IgSF molecule, Neurofascin 186 (Ango et al., 2004). Neurofascin 186 is normally present in a subcellular gradient along the AIS-soma axis, and this localization is dependent on AIS-restricted cytoskeletal adaptor ankyrinG. When the Neurofascin gradient is disrupted, basket neuron axons follow Neurofascin to ectopic locations, and synapse formation at AIS is greatly reduced (Ango et al., 2004). Thus, Neurofascin 186 is critical for subcellular domain-restricted GABAergic innervation in the cerebellum.
Two other families of adhesion molecules, neurexins and neuroligins, were found to be sufficient to induce synapse formation in vitro. Neurexins are type I membrane proteins located on presynaptic terminals, originally identified as receptors for black widow spider toxin (Ushkaryov et al., 1992), while neuroligins are their postsynaptically localized receptors (Ichtchenko et al., 1995). Both sets of molecules have been implicated in autism spectrum disorders (Sudhof, 2008). Initial evidence that these molecules function at synapses came from studies showing that neuroligins expressed by non-neuronal cells can induce formation of presynaptic specializations in co-cultured neurons (Scheiffele et al., 2000), while non-neuronal expression of neurexins can induce postsynaptic formation (Graf et al., 2004). However, a mouse triple knockout for all three neuroligin isoforms showed no significant defect in number or ultrastructure of synapses, but had severely impaired synaptic transmission (Varoqueaux et al., 2006). Current models reconcile in vitro and in vivo findings by suggesting that increases in synapse numbers seen with neurexins and neuroligins in culture may represent stabilization of transient synaptic contacts. Thus, these molecules likely mediate synapse maturation and function but are not required for synapse formation per se (Sudhof, 2008). Interestingly, mutations in the single Drosophila neurexin gene result in defects in synaptic growth at glutamatergic neuromuscular junctions, defective synapse ultrastructure, and alterations in synaptic transmission, which is also consistent with an evolutionarily conserved role for these molecules in synaptic maturation but not specificity (Li et al., 2007).
The neuron-fibroblast coculture assays have demonstrated synaptogenic activity of additional cell adhesion molecules. SynCAM is a brain-specific, IgSF protein that is sufficient to induce formation of presynaptic terminals when expressed by non-neuronal cells in vitro (Biederer et al., 2002). Multiple isoforms of SynCAM engage in specific heterophilic complexes, increase the number of presynaptic terminals, and enhance excitatory neurotransmission (Fogel et al., 2007). Similarly, the netrin G ligand (NGL) family of adhesion molecules can also induce functional presynaptic differentiation when expressed heterologously (Kim et al., 2006). NGL-1 and NGL-2 bind the GPI-anchored netrin-G in an isoform-specific manner, while NGL-3 has recently been shown to bind LAR receptor protein tyrosine phosphatase (Woo et al., 2009). Heterologously expressed NGL-3 and LAR can induce pre- and postsynaptic differentiation in contacting axons and dendrites of cocultured neurons, respectively, suggesting that trans-synaptic adhesion between NGL-3 and LAR regulates excitatory synapse formation (Woo et al., 2009).
Recent studies have undertaken large-scale screens to identify novel proteins involved in synaptogenesis and target selection. One such study screened 410 genes encoding cell-surface and secreted proteins in Drosophila embryos and larvae, searching for those whose overexpression by all muscle fibers causes motor axons to make targeting errors (Kurusu et al., 2008). The authors found thirty such genes, sixteen of which contained an extracellular protein interaction module called a leucine-rich repeat (LRR). Interestingly, a LRR protein family was also identified in a recent unbiased expression screen for synaptogenic proteins using the vertebrate neuron-fibroblast coculture assay (Linhoff et al., 2009). LRRTM proteins were found to induce presynaptic differentiation in contacting axons and to localize to excitatory synapses; furthermore, a knockout mouse for one of the family members, LRRTM1, displayed a mild defect in distribution of VGLUT1, possibly indicating a local dispersal of synaptic vesicles. The results of these large-scale screens highlight the prevalence of LRR proteins as synaptic organizers, but also indicate that many other synaptogenic proteins remain to be identified (Linhoff et al., 2009).
It is worth noting that overexpression screens and coculture screens can identify new synaptogenic molecules in a high-throughput manner with relative speed and ease, but may be susceptible to artifacts. The artificial overexpression of a given protein may not capture its native location, timing, or level of expression, thus possibly resulting in de novo phenotypes that are not necessarily representative of the endogenous function of the protein. However, overexpression screens have a major advantage of being able to identify molecules that act in parallel and mutually redundant pathways; these molecules would be remarkably difficult to identify using traditional genetic screens. Thus, overexpression screens have an important role to play in the discovery of new synaptogenic molecules, but it remains critical to validate overexpression phenotypes with subsequent loss-of-function studies.
Secreted molecules, synaptogenesis and synaptic specificity
Secreted factors can also act to focally induce synapse formation and shape neural circuits (Figure 1 D-F). Classical studies of the vertebrate neuromuscular junction have identified Agrin, a molecule secreted by motor neurons that induces precise localization of acetylcholine receptor clusters on the target muscle membrane. Agrin acts through its receptor Musk as an “anti-declustering” factor to prevent acetylcholine-induced dispersion of AChRs (Kummer et al., 2006). In addition, studies at the Drosophila NMJ have implicated a number of morphogens in regulating synapse growth. Mutants for Bone Morphogenetic Protein (BMP) ortholog, Gbb, have reduced NMJ synapse size, impaired synaptic transmission, and aberrant synapse ultrastructure (McCabe et al., 2003). Further analysis revealed that expression of BMP in the postsynaptic muscles can largely rescue the mutant phenotype, suggesting that BMP signals retrogradely across the synapse to regulate synaptic growth. A similar NMJ phenotype was observed in mutants for a Drosophila Wnt, Wingless, which is secreted by the presynaptic boutons and endocytosed by the muscles (Packard et al., 2002). Thus, here a secreted factor acts either as an autocrine signal to induce presynaptic differentiation, or alternatively, it induces production of a retrograde signal [which is likely not BMP (McCabe et al., 2003)] in the muscle that then stimulates presynaptic growth. Interestingly, a recent study also implicated Wnts in prepatterning AChR clusters at the vertebrate NMJ (Jing et al., 2009).
Wnts were also identified as synaptogenic signals in the murine cerebellum. Mossy fibers undergo extensive remodeling upon contacting cerebellar granule cells, with which they form complex synaptic structures called rosettes. Both mossy fiber axon remodeling and synapse formation are blocked by a Wnt antagonist and stimulated by Wnt-7a, which is normally expressed by granule cells (Hall et al., 2000). Wnt-7a deficient mice show developmental delays in maturation of glomerular rosettes, indicating that this Wnt is important for cerebellar synapse formation but not irreplaceable, as other factors can compensate for its absence. Another granule cell-derived synaptogenic cue in the cerebellum is fibroblast growth factor (FGF) 22, which was biochemically purified based on its ability to cluster synaptic vesicles in cultured neurons (Umemori et al., 2004). FGF22 is expressed in granule cells, while its receptor FGFR2 is expressed in mossy fiber neurons. Through an elegant series of experiments, Umemori and colleagues demonstrated that neutralization/inactivation of either the FGF ligand or its receptor inhibits mossy fiber presynaptic differentiation both in vitro and in vivo. Thus, cerebellar granule cells utilize multiple secreted factors to promote synapse formation in their presynaptic partners.
Interestingly, Wnts can also act as antisynaptogenic factors to shape synaptic specificity. Using single-cell microarray analysis of two neighboring muscle cells in Drosophila, M12 and M13, Inaki and colleagues found that Wnt4 is preferentially expressed in M13 muscle (Inaki et al., 2007). In the absence of Wnt4, or its putative receptor (Frizzled), neurons that normally innervate M12, MN12s, form ectopic synapses with M13 and smaller synapses with M12. Conversely, ectopic expression of Wnt4 in M12 inhibits synapse formation by MN12s. Thus, Wnt4 is acting as an inhibitory cue to prevent synapse formation of MN12s with the incorrect muscle target.
An antisynaptogenic role for Wnts was also demonstrated in a recent study that examined synapse formation in the C. elegans tail motor neuron DA9 (Klassen and Shen, 2007). DA9 is a bipolar cholinergic neuron whose presynaptic terminals are restricted to a specific segment of its axon. Klassen and Shen found that the asynaptic domain of the DA9 axon is created by the local inhibitory action of the Wnt receptor, LIN-17/Frizzled. The Wnt ligand, LIN-44, mediates localization of LIN-17/Frizzled to this asynaptic domain and thus acts as a long-range inhibitory cue to pattern DA9 presynaptic terminals. Hence, inhibitory activity of Wnts in synaptogenesis is evolutionarily conserved.
Further studies of the C. elegans DA9 neuron have shown that the classical axon guidance molecule, Netrin, also has antisynaptogenic activity (Poon et al., 2008). In animals mutant for UNC-6/Netrin or one of its receptors, UNC-5, synaptic vesicle markers and active zone components are present in the DA9 dendrite. Through a series of experiments, Poon and colleagues have shown that the role of Netrin is to exclude presynaptic components from the DA9 dendrite, which lies just adjacent to the source of Netrin. Furthermore, the authors demonstrate that Netrin and Wnt are interchangeable in terms of antisynaptogenic activity, as ectopic expression of Netrin from Wnt-secreting cells can rescue Wnt mutant phenotype and vice versa. These results suggest that the final pattern of synaptic connectivity can arise not only from specifying synapse formation at the right location, but also from preventing synapses from being assembled at all the wrong places in the neuron.
Interestingly, UNC-6/Netrin can also promote synaptogenesis in C. elegans (Colon-Ramos et al., 2007). In the head of the worm UNC-6/Netrin is secreted by glia-like sheath cells to promote innervation of the nearby neurons AIY and RIA. Local secretion of Netrin localizes Netrin receptor UNC-40/DCC to the synapse-rich domain of presynaptic AIY neurons, where it promotes synapse assembly. In parallel, UNC-40/DCC plays a classical ventral axon guidance role in the postsynaptic RIA neurons, ensuring that the two neurons will meet and form synapses at a specific coordinate in the C. elegans nerve ring. Glia-like sheath cells appear to be instructive in this process, as perturbations of their morphology lead to concomitant changes in RIA axon guidance and AIY presynaptic terminal positioning (Colon-Ramos et al., 2007).
Glial cells have also been implicated in producing both synaptogenic and antisynaptogenic factors in vertebrates. Thrombospondins 1 and 2 are large extracellular matrix proteins secreted by immature astrocytes that promote CNS synaptogenesis in vitro (Christopherson et al., 2005). These synapses are ultrastructurally normal and presynaptically active but postsynaptically silent, due to the lack of AMPA receptors in the postsynaptic terminals. (There is evidence to suggest that other astrocyte-derived factors are required to make fully functional synapses.) Thrombospondin 1 and 2 double knockout mice have a 40% decrease in the number of synapses, consistent with the synaptogenic role of these molecules. Glia have also been implicated in synapse elimination, by stimulating postnatal neurons to express C1q, the initiating protein in the classical complement cascade (Stevens et al., 2007). Mice mutant for C1q and its main downstream effector C3 exhibit large defects in refinement of retinogeniculate projections. C1q and C3 localize to synapses, where they presumably play a local role in synapse elimination. Furthermore, C1q is upregulated and synaptically relocalized in a mouse model of glaucoma, suggesting that aberrant complement cascade-mediated synapse elimination may play a role in neurodegenerative disease.
Finally, a recent study implicated repulsive signaling by the secreted semaphorin Sema3E and its receptor Plexin-D1 in determining synaptic choice in the murine spinal cord (Pecho-Vrieseling et al., 2009). The authors focused on two nearby sensory-motor reflex arcs: the classic monosynaptic reflex arc innervating the triceps muscle, and the atypical reflex arc innervating the cutaneous maximus (Cm) muscle, which does not display monosynaptic connectivity between the Cm afferents and the Cm motoneurons. Cm motoneurons express Sema3E while triceps motoneurons do not, raising the possibility that semaphorin signaling may play a role in shaping connectivity of these two circuits. Indeed, in Sema3E mutants, almost half of Cm motoneurons now receive monosynaptic input from Cm afferents as assessed by electrophysiological recordings; this phenotype was phenocopied by a PlxnD1 conditional mutation in the proprioceptive afferents. Importantly, Cm motoneurons still lack monosynaptic input from triceps proprioceptive neurons in both of these mutants, indicating that motoneuron pool specificity was not altered in the absence of Sema3E and PlxnD1 (Pecho-Vrieseling et al., 2009). Thus, matching expression of a semaphorin ligand and its plexin receptor prevents synapse formation and hence regulates specificity of a sensory-motor reflex arc. However, a different matching mechanism must exist that ensures motor pool specificity, possibly involving a more global combinatorial code of repulsive and attractive signals that pattern the connectivity of the mammalian spinal cord.
Intrinsic determinants of synaptic specificity: lineage, fate and time
In addition to inductive models of synaptic specificity, in which synaptic partners or guidepost cells determine the location of nascent synapses, several studies have shown that neurons can also be prepatterned by fate and lineage to form specific connections (Figure 1G). One such study investigated second order neurons in the Drosophila olfactory system called projection neurons (PNs), which extend dendrites to specific glomeruli in the antennal lobe to receive converging input from olfactory receptor neurons (Jefferis et al., 2001). By using the MARCM method to perform systematic clonal analysis of projection neurons, Jefferis and colleagues found that PNs are prespecified by lineage and birth order to project dendrites to specific glomeruli. Thus, PNs are cell-autonomously patterned to form synapses with specific ORN axons independently of the presence of the ORNs themselves.
The importance of birth order in specifying PN dendrite projections to particular glomeruli was further demonstrated through the analysis of chinmo mutants in Drosophila. Chinmo is a BTB-zinc finger protein that governs neuronal temporal identity during postembryonic development of the Drosophila brain; high Chinmo levels specify early born neurons, while low Chinmo levels are characteristic of late born neurons in a given lineage (Zhu et al., 2006). In the PN lineage, loss of Chinmo causes early born PNs to adopt glomerular projection patterns of late born PNs, confirming that PN birth order specifies their connectivity. Additional studies have shown that two POU transcription factors, Acj6 and Drifter, are necessary and sufficient for dendritic targeting of two different PN sublineages (Komiyama et al., 2003), and that targeting of PN dendrites is mediated by the classical axon guidance molecule Semaphorin-1a (Komiyama et al., 2007). Sema-1a acts cell-autonomously as a receptor in the PNs, where it is expressed in a graded manner along the dorsolateral to ventromedial axis of the antennal lobe. Thus, a continuous gradient of a molecule can give rise not only to continuous topographic maps, as is the case for Ephrins and Eph receptors in the retinotectal system (Flanagan and Vanderhaeghen, 1998), but it can also help form a discrete neural map, as is the case here.
The role of fate and lineage in determining synaptic specificity was also demonstrated in the studies of the locomotion circuit in C. elegans. VA and VB motor neurons arise as lineal sisters but receive synaptic input from different interneurons. Past studies have shown that the specificity of the VA motor neuron synapses can be transcriptionally regulated by the homeodomain protein UNC-4 (Miller et al., 1992; White et al., 1992). In unc-4 mutants, the VA motor neurons display a pattern of synaptic inputs characteristic of the VB neurons while retaining VA morphology and axon trajectory. UNC-4 acts in VA neurons together with its transcriptional corepressor, UNC-37/Groucho, to repress the VB synaptic fate (Pflugrad et al., 1997). A recent study used a neuron-specific microarray strategy to identify genes that act downstream of unc-4 to regulate VA synaptic choice (Von Stetina et al., 2007). The authors show that a homeodomain protein ceh-12, which is normally expressed in VBs, is both necessary and sufficient to impose VB-like inputs onto VAs. Interestingly, the finding that the VA pattern of specific synaptic connections is formed by actively repressing VB fate is reminiscent of the recent study in Drosophila, where normal targeting of R7 cells is accomplished by active transcriptional repression of the R8 targeting program (Morey et al., 2008).
Photoreceptor connectivity in Drosophila is further determined by temporal expression dynamics of another transcription factor, Sequoia (Petrovic and Hummel, 2008). R7 and R8 cells have non-overlapping peaks of expression of Sequoia, which correspond to their sequential target innervation. In the absence of Sequoia, R7 cells misproject to the R8 target layer, while extending the window of expression of Sequoia leads to R8 cells terminating in the R7 layer. These findings led the authors to propose that Sequoia regulates axon competence to respond to an adhesion molecule that directly mediates targeting, which turns out to be N-cadherin (Lee et al., 2001). Thus, the role of Sequoia is to enable R8 and R7 cells to sequentially reuse the same broadly expressed adhesion molecule to find their correct target layer. This model is appealing as it allows for “recycling” of a small set of adhesion molecules to mediate specificity, and indicates that temporal identity can be used to generate connection diversity.
Finally, a recent study investigated the relationship between cell lineage and connectivity in the mammalian neocortex (Yu et al., 2009). Neocortical neurons are organized into functional columns, within which neurons are connected into precisely organized microcircuits. Yu and colleagues examined the patterns of connectivity between neurons arising from the same mother cell by labeling radial clones in utero using GFP-expressing retroviruses, and assessing their connectivity with multiple electrode recordings. Interestingly, sister clone neurons, which are closely related by lineage, have a six-fold higher probability of being connected by chemical synapses than the neighboring, more distantly related neurons do. These synaptic connections tend to be unidirectional, with the same synaptic directionality that is seen among mature cortical layers. Thus, sister excitatory neurons preferentially form synapses with each other and contribute to the formation of mature columnar circuits. The mechanisms for this lineage-dependent synaptic affinity are presently not known.
Conclusions and Future Directions
A number of recent studies have shown that a variety of different mechanisms can lead to the development of synaptic specificity. Cell-adhesion and secreted molecules can match synaptic partners by acting either directly, when they are produced by pre- or postsynaptic cells, or indirectly, when made by guidepost cells. In addition, intrinsic cell mechanisms, like lineage, fate, and timing of development, can also play critical roles in shaping neural circuits.
Despite the diversity of mechanisms and molecules that can give rise to synaptic specificity, some important themes are beginning to emerge. Synaptic specification can operate both at the level of partner choice and at the level of synapse formation onto a specific subcellular compartment. Targeting to a specific compartment can occur by active specification, as is seen in Neurofascin 186-mediated AIS targeting in the cerebellum (Ango et al., 2004), or by active exclusion, as is the case in the DA9 neuron in C. elegans (Klassen and Shen, 2007; Poon et al., 2008). One cell type can produce multiple synaptogenic cues, possibly acting at different steps during synapse formation and maturation; for example, cerebellar granule cells utilize both Wnt-7a and FGF22 to form synapses with their presynaptic partners, the mossy fiber cells (Hall et al., 2000; Umemori et al., 2004). Finally, the same molecule can play either a synaptogenic or antisynaptogenic role, depending on the developmental context. For instance, Netrin was recently shown to be able to promote or inhibit synapse formation in C. elegans, depending on which Netrin receptor is being utilized (Colon-Ramos et al., 2007; Poon et al., 2008). Netrin classically acts as an axon guidance molecule that can trigger both axon attraction via UNC-40/DCC receptor and axon repulsion via UNC-5 receptor (Round and Stein, 2007). Thus, there exists a striking parallel between synaptogenic and guidance effects of these receptors: UNC-40 promotes axon attraction and synapse formation, while UNC-5 leads to axon repulsion and inhibition of synaptogenesis. Whether these similarities are due to the use of some of the same downstream signaling machinery remains to be determined.
Recent studies also highlight the importance of neighboring cells in neural circuit formation. Glial cells can secrete synaptogenic factors like Thrombospondins (Christopherson et al., 2005) or induce production of molecules that mediate synapse elimination (Stevens et al., 2007). Neighboring cells can also play a guidepost role in circuit formation by initially determining spatial placement of the presynaptic sites along the axon, to which the postsynaptic partner gets subsequently guided by the same guidepost, or by some other mechanism. This phenomenon has been documented both in C. elegans (Colon-Ramos et al., 2007; Shen and Bargmann, 2003; Shen et al., 2004) and in vertebrates. For instance, subplate neurons act as transient guidepost cells in the maturation of the visual cortical circuit (Ghosh et al., 1990), while in the developing hippocampus, Cajal-Retzius cells and a set of GABAergic neurons ensure that two different populations of afferents synapse onto distinct subcellular domains of pyramidal dendrites (Del Rio et al., 1997). In both cases, guidepost cells transiently receive afferent synaptic input and therefore serve as “placeholders” for the presynaptic terminals until the dendritic processes of postsynaptic neurons grow in. Thus, guidepost cells seem to be particularly important when there is a temporal discrepancy between axonal and dendritic development.
Temporal control of expression of a broadly expressed synaptogenic molecule has also emerged as an important mechanism for achieving specificity. For instance, sequential peaks of expression of a transcription factor, Sequoia, enable R8 and R7 photoreceptors to target different postsynaptic layers while relying on the same adhesion molecule, N-cadherin, to execute the targeting (Petrovic and Hummel, 2008). This is an appealing model as it allows for recycling of a limited set of molecules to achieve complex patterns of neural connectivity. Other studies have emphasized the importance of neural fate determination in synapse specification, as the loss of active fate repression can result in a neuron population adopting synaptic fate of their sister cells (Miller et al., 1992; Morey et al., 2008). Finally, lineage itself can be instructive in synapse formation, as sister excitatory neurons preferentially form synapses with one another (Yu et al., 2009).
Although discoveries over the past decade have greatly contributed to our understanding of mechanisms of synaptic specificity, much remains to still be elucidated. Recent large-scale screens indicate that many synaptogenic molecules remain to be identified (Linhoff et al., 2009); characterization of these molecules, their patterns of expression and mechanisms of action will be critical for understanding how specific neural circuits in the brain form. Furthermore, cellular mechanisms that give rise to specificity are clearly varied; the question remains whether the nervous system utilizes all of them equally, or whether there is a preferred mode of synaptic matching in certain developmental contexts or regions in the brain. What are the downstream mechanisms that link synapse specification with synaptic component transport and synapse assembly? And conversely, how is the “upstream” information about neural lineage and identity subsequently translated into the specific pattern of connections that a neuron makes? The answers to these questions will lead to a better understanding of brain development and function, and its dysfunction in disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akins MR, Biederer T. Cell-cell interactions in synaptogenesis. Curr Opin Neurobiol. 2006;16:83–89. doi: 10.1016/j.conb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. Objective evidence of anatomical unity of nerve cells. Consejo Superior de Investigaciones Cientificas; Madrid: 1954. Neuron Theory or Reticular Theory? [Google Scholar]
- Chen PL, Clandinin TR. The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron. 2008;58:26–33. doi: 10.1016/j.neuron.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Prakash S, Bright A, Clandinin TR. Liprin-alpha is required for photoreceptor target selection in Drosophila. Proc Natl Acad Sci U S A. 2006;103:11601–11606. doi: 10.1073/pnas.0601185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL. Drosophila LAR regulates R1-R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- Ding M, Chao D, Wang G, Shen K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 2007;317:947–951. doi: 10.1126/science.1145727. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Davis GW. Synapse disassembly. Genes Dev. 2003;17:2075–2082. doi: 10.1101/gad.1113703. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr Biol. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Jing L, Lefebvre JL, Gordon LR, Granato M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron. 2009;61:721–733. doi: 10.1016/j.neuron.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Johnson WA, Luo L, Jefferis GS. From lineage to wiring specificity. POU domain transcription factors control precise connections of Drosophila olfactory projection neurons. Cell. 2003;112:157–167. doi: 10.1016/s0092-8674(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;59:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Lee RC, Clandinin TR, Lee CH, Chen PL, Meinertzhagen IA, Zipursky SL. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. 2003;6:557–563. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Miller DM, Shen MM, Shamu CE, Burglin TR, Ruvkun G, Dubois ML, Ghee M, Wilson L. C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature. 1992;355:841–845. doi: 10.1038/355841a0. [DOI] [PubMed] [Google Scholar]
- Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic M, Hummel T. Temporal identity in axonal target layer recognition. Nature. 2008;456:800–803. doi: 10.1038/nature07407. [DOI] [PubMed] [Google Scholar]
- Pflugrad A, Meir JY, Barnes TM, Miller DM., 3rd The Groucho-like transcription factor UNC-37 functions with the neural specificity gene unc-4 to govern motor neuron identity in C. elegans. Development. 1997;124:1699–1709. doi: 10.1242/dev.124.9.1699. [DOI] [PubMed] [Google Scholar]
- Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J, Stein E. Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron. 2006;49:205–213. doi: 10.1016/j.neuron.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shishido E, Takeichi M, Nose A. Drosophila synapse formation: regulation by transmembrane protein with Leu-rich repeats, CAPRICIOUS. Science. 1998;280:2118–2121. doi: 10.1126/science.280.5372.2118. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the Orderly Growth of Nerve Fiber Patterns and Connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Von Stetina SE, Fox RM, Watkins KL, Starich TA, Shaw JE, Miller DM., 3rd UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neurons in C. elegans. Genes Dev. 2007;21:332–346. doi: 10.1101/gad.1502107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL. Reflections on the specificity of synaptic connections. Brain Res Rev. 2007;55:422–429. doi: 10.1016/j.brainresrev.2006.12.004. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN. Mutations in the Caenorhabditis elegans unc-4 gene alter the synaptic input to ventral cord motor neurons. Nature. 1992;355:838–841. doi: 10.1038/355838a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J Neurophysiol. 1963a;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963b;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009 doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]


