Abstract
Objectives
Octreotide long acting repeatable (LAR) is commonly used to control the symptoms of patients with functional neuroendocrine tumors. Unfortunately, most patients escape control over time and require higher LAR doses or more frequent rescue therapy to remain asymptomatic. Previous work has shown that body weight and monthly LAR dose will significantly affect circulating plasma octreotide levels in patients undergoing therapy.
Methods
To determine if other parameters change circulating plasma octreotide levels, we prospectively studied 82 patients undergoing long-term LAR therapy.
Results
Multivariate analysis demonstrated that the plasma octreotide levels decrease by approximately 3.4% for each unit of body mass index (BMI) increase (P = 0.03), adjusting for sex and monthly LAR dose. Plasma octreotide levels for females were approximately 47.6% higher than those for males (P = 0.045), adjusting for BMI and monthly LAR dose. Initial and subsequent octreotide LAR doses should take into consideration sex and BMI. Males are estimated to require 14.1-mg (SD, 7.25) higher monthly LAR doses than females with the same BMI.
Conclusions
We have shown that plasma octreotide levels are affected by not only monthly LAR dose but also BMI and sex. We hope these observations will make choosing initial and subsequent octreotide LAR doses easier for physicians.
Keywords: octreotide, plasma, sex, body mass index, drug levels, height, weight
Octreotide and lanreotide are somatostatin analogs commonly used to control the symptoms of patients with neuroendocrine tumors (NETs). In the US, octreotide is approved for the treatment of carcinoid syndrome and VIPoma, as well as acromegaly.1 Lanreotide, which is commonly used out of indication to control NETs symptoms, is currently approved only for the treatment of acromegaly. Previous work from our group has shown that circulation of octreotide levels are dependent on monthly long acting repeatable (LAR) dose (milligram per month) and body weight (kilogram).2 In a retrospective trial, we demonstrated that plasma octreotide levels for a given monthly octreotide LAR dose had decreased by approximately 50% from mid 2005 until the current time. The reasons for this decrease still remain unknown. To determine what other variables will affect the circulating plasma octreotide levels in patients undergoing long-term octreotide LAR therapy for the control of carcinoid syndrome, we undertook a prospective study of 82 patients. The variables observed were monthly LAR dose expressed as milligrams of octreotide LAR per month, height, weight, body mass index (BMI), and sex.
MATERIALS AND METHODS
Patients undergoing long-term octreotide LAR (Novartis Pharmaceuticals Inc, Englewood, NJ) therapy from May 2007 through October 2008 had their plasma octreotide levels determined by a commercial octreotide assay at Inter Science Institute in Inglewood, Calif. All patients included in this study were on octreotide LAR therapy for at least 3 months to allow their octreotide drug level to reach a steady state. Eighty-two patients were enrolled in this prospective study approved by the Louisiana State University's institutional review board. Forty-three females and 39 males were studied. Weight was expressed in kilograms and height was expressed in meters, and from this data, BMI was calculated. The average female in this study weighed 70 ± 16 kg, whereas the average male weighed 88 ± 16 kg. All patients were on LAR therapy for at least 4 months, and individual patients were asked to have their octreotide levels measured on the day before their next dose of octreotide LAR (trough octreotide levels). Three females and 2 males received LAR at a dose of 20 mg/mo. Eighteen females and 14 males received LAR at a dose of 30 mg/mo. Seven females and 4 males received LAR at a dose of 40 mg/mo. Twelve females and 11 males received LAR at a dose of 60 mg/mo, and finally 2 female and 5 males received LAR at a monthly dose of 120 mg. The remainder of the patients received other doses (50 or 80 mg/mo) of LAR.
Statistical Analyses
Descriptive statistics such as mean and SD were reported for patient characteristics and outcome variables. Natural logarithm transformation was applied to plasma octreotide levels to improve normality. Scatter plot and bar plot were used to illustrate patterns of plasma octreotide levels with respect to BMI, LAR dose, and sex. The Pearson product moment correlation coefficient between BMI and the natural log-transformed plasma octreotide levels was reported. A multiple regression analysis was performed to study the effects of BMI, sex, and LAR dose level on plasma octreotide level.
RESULTS
Table 1 provides the summary statistics for patient characteristics used in this analysis. A negative association was observed between the natural log-transformed plasma octreotide level and BMI (Pearson R = −0.21). The log-transformed plasma octreotide level appears to decline linearly; thus, the original plasma octreotide level decreases exponentially as the BMI increases (Fig. 1). Females generally had higher plasma octreotide levels than males (Fig. 2). The most significant difference between females and males was observed for LAR dose of 30 mg/mo.
TABLE 1.
Summary of Patient Characteristics
| Female (n = 43) |
Male (n = 39) |
|||||
|---|---|---|---|---|---|---|
| Variables | Mean (SD) | Min | Max | Mean (SD) | Min | Max |
| Weight, kg | 69.7(15.7) | 48.53 | 120.20 | 87.8 (16.4) | 60.78 | 128.37 |
| Height, m | 1.6(0.1) | 1.45 | 1.80 | 1.8 (0.1) | 1.55 | 1.88 |
| BMI, kg/m2 | 26.1 (5.1) | 17.79 | 39.13 | 28.6 (6.5) | 18.69 | 49.5 |
| LAR dose, mg/mo | 44.0(21.8) | 20.0 | 120.0 | 53.6 (30.2) | 20.0 | 120.0 |
| Plasma octreotide, pg/mL | 4943.7 (4100.3) | 569.0 | 18,495.0 | 4201.0 (4033.3) | 100.0 | 19,042.0 |
| In (Plasma octreotide)* | 8.2 (0.8) | 6.34 | 9.83 | 7.9 (1.0) | 4.61 | 9.85 |
Natuxal log-transformed plasma octreotide.
Max indicates maximum; min, minimum.
FIGURE 1.
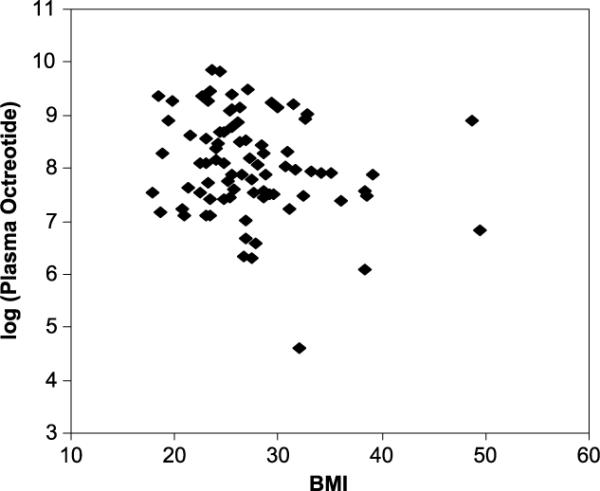
Natural log-transformed plasma octreotide level versus BMI (kilogram per square meter).
FIGURE 2.
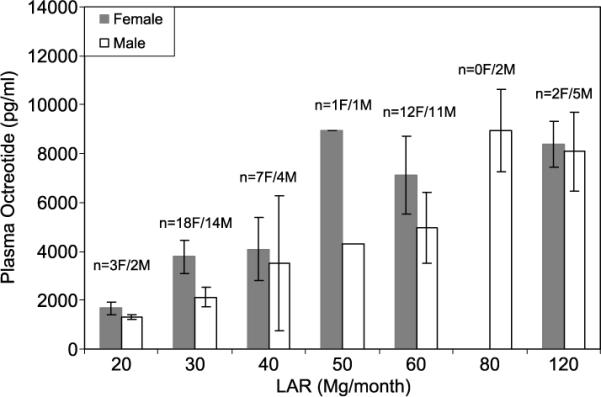
Summary (mean and SD) of plasma octreotide level (picogram per milliliter) by LAR dose (milligram per month) and sex.
Because most patients in a clinical practice do not receive LAR at a dose of 120 mg/mo or higher, we excluded the 7 subjects with LAR dose of 120 mg/mo and performed a multiple regression analysis (Table 2) to investigate the effects of BMI, sex, and monthly LAR dose on plasma octreotide levels. We also examined the effects of interaction between BMI, sex, and LAR dose; no significant effect was found. We noted that the plasma octreotide levels decreased exponentially as the BMI increased. The plasma octreotide level decreased by approximately 3.4% per unit of BMI increase (P = 0.03), adjusting for sex and monthly LAR dose. Similarly, plasma octreotide levels for females were approximately 47.6% higher than those of males (P = 0.045), adjusting for BMI and monthly LAR dose.
TABLE 2.
Multiple Regression Analysis for the Effects of BMI, Sex, and LAR Dose Level (Excluding 7 Subjects on 1 20-mg/mo LAR Dose) on the Natural Log-Transformed Plasma Octreotide Level
| Parameter | Parameter Estimate | P |
|---|---|---|
| BMI | −0.03 | 0.0299 |
| Sex | 0.39 | 0.0451 |
| Monthly LAR dose | 0.03 | <0.0001 |
The parameter for a continuous variable (such as BMI or monthly LAR dose) represents the mean change in the natural log-transformed plasma octreotide level per unit increase of the variable. The parameter for sex is the mean difference between females (sex = 1) andmales (sex = 0) in the natural log-transformed plasma octreotide level.
Our results suggest that physicians can make predictable LAR dose adjustments to achieve a desired plasma octreotide level. For instance, average males are estimated to require a 14.1-mg (SD, 7.25) higher monthly LAR dose than females to achieve the same plasma octreotide level, when they have identical BMI. Similarly, the monthly LAR dose should be increased by 1.3 mg (SD, 0.62) for each unit of BMI increase to achieve the same plasma octreotide level, adjusted for sex. The relatively large SDs for the estimated LAR dose adjustments indicate that in addition to sex and BMI, there are potentially other confounding factors that need to be accounted for. Future investigations should consider the effects of liver and renal function on circulating plasma octreotide levels.
DISCUSSION
Octreotide LAR is widely used to control diarrhea, flushing, and wheezing associated with carcinoid syndrome. Though not approved in the US, clinicians also commonly use octreotide or octreotide LAR for the control of the symptoms of other NETs such as gastrinomas.3 When octreotide LAR is used for the long term for the control of carcinoid syndrome, it is not uncommon for patients to require increases in their monthly LAR doses or more frequent use of rescue (aqueous octreotide) over time.4–6
Currently, the manufacturer's recommendation is that all patients start at a 20-mg/mo LAR dose with an assessment of symptom control after 3 or 4 months. This often leads to slow increases in dose for most patients.4,7 Very few patients in this study were on the manufacturer's recommended starting dose, most patients being on either 30 or 60 mg/mo. This highlights the significant differences in octreotide LAR dosing that patients require for adequate symptom control.
By prospectively studying this unique patient population, we have been able to identify specific patient characteristics that affect octreotide plasma values. We believe this study demonstrates that individualized dosing regimens are needed for men and women. Furthermore, we have demonstrated that BMI affects octreotide dosing among a spectrum of patients.
Additional data are needed to develop an algorithm for the adequate dosing of individual patients with octreotide LAR. This will require a large number of subjects and will need to also evaluate other predictors of plasma level that have not been investigated to date. Further analysis will also be needed to determine the precise mechanism as to why women have an elevated octreotide level as compared with men with the same BMI. However, for most clinicians dealing with NET patients, this study should help in determining the octreotide LAR dosing adjustments required to maintain optimal symptom control.
REFERENCES
- 1.Harris AG, O'Dorisio TM, Woltering EA, et al. Consensus statement: octreotide dose titration in secretory diarrhea. Diarrhea Management Consensus Development Panel. Dig Dis Sci. 1995;40:1464–1473. doi: 10.1007/BF02285194. [DOI] [PubMed] [Google Scholar]
- 2.Woltering EA, Mamikunian PM, Zietz S, et al. Effect of octreotide LAR dose and weight on octreotide blood levels in patients with neuroendocrine tumors. Pancreas. 2005;31:392–400. doi: 10.1097/01.mpa.0000186249.89081.0d. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, O'Dorisio MS, Hermann G, et al. Mechanisms of action of long acting analogs of somatostatin. Regul Pept. 1993;44:285–295. doi: 10.1016/0167-0115(93)90138-x. [DOI] [PubMed] [Google Scholar]
- 4.Anthony LB, Stafford S, Cronin M, et al. Octreotide LAR doses used in clinical practice: results from an internet survey and a clinical practice. Proc Am Soc Clin Oncol. 2004;23:4274. [Google Scholar]
- 5.Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 6.Woltering EA, Salvo VA, O'Dorisio TM, et al. Clinical value of monitoring plasma octreotide levels during chronic octreotide long-acting repeatable therapy in carcinoid patients. Pancreas. 2008;37(1):94–100. doi: 10.1097/MPA.0b013e31816907ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Öberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]


