Abstract
A general method for a highly regioselective, copper-catalyzed cross-coupling of two aromatic compounds by using iodine oxidant has been developed. The reactions involve an initial iodination of one arene followed by arylation of the most acidic C-H bond of the other coupling component. Cross-coupling of electron-rich arenes, electron-poor arenes, five- and six-membered heterocycles is possible in many combinations. Typically, 1/1.5 to 1/3 ratio of coupling components is used in contrast to existing methodology that often employs a large excess of one of the arenes. Common functionalities such as ester, ketone, aldehyde, ether, nitrile, nitro, and amine are well-tolerated.
1. Introduction
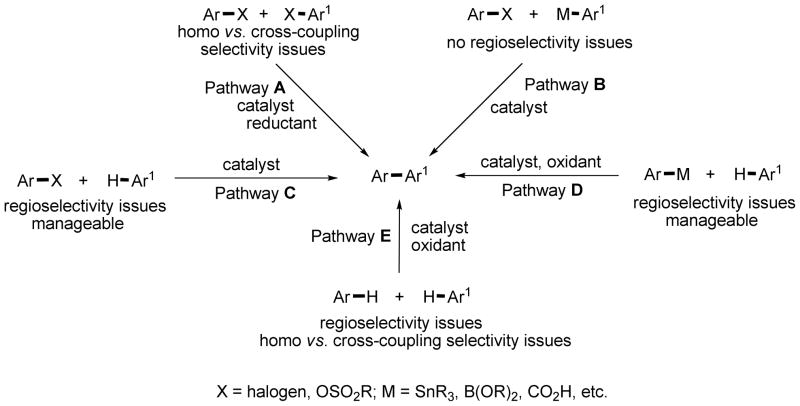
Biaryl moiety is a ubiquitous structural motif in natural products, pharmaceuticals, and functional materials.1 As a consequence, formation of aryl-aryl bonds has interested chemists since Ullmann reported a copper-catalyzed method for biaryl synthesis more than a century ago.2a The following methods can be used for creating an aryl-aryl bond (Scheme 1). Ullmann coupling is an example of coupling of two aryl halides affording a biaryl (Pathway A). A stoichiometric reducing agent is necessary and achieving selective cross-coupling is difficult for most intermolecular cases.2 Presently, the most widely used method for aryl-aryl bond formation is the coupling of an aryl metal with an aryl halide (Pathway B). Reactions such as Stille, Suzuki, Kumada, Negishi, and others allow for a highly controlled synthesis of biaryls possessing virtually any structural motifs.3 However, this methodology requires functionalized starting materials that may not be readily available resulting in longer synthetic sequences. Coupling of an aryl halide with arene C-H bond is also possible (Pathway C). Reoxidation is not required. Excellent regioselectivity with respect to arene C-H bond coupling component has been achieved for arylation of heterocycles,4,5 directing-group-containing arenes,4,6 and deprotonative arylation that includes functionalization of polyfluorobenzenes.4,7 However, for many simple substrates the arylation regioselectivity is still problematic. Often only symmetric arenes such as benzene or p-xylene are employed as C-H coupling components due to formation of regioisomer mixtures.4 Pathway D involves the coupling of an aryl metal or carboxylic acid with arene C-H bond.4,8 Regioselectivity issues are similar to the ones observed for Pathway C. Additionally, a stoichiometric oxidant, typically a transition metal salt, is required. Direct dehydrogenative arene coupling is perhaps the most efficient method for biaryl synthesis (Pathway E).4,9 Functionalized starting materials are not required and a stoichiometric oxidant is needed. However, this pathway presents the most formidable difficulties with respect to regio- and cross- vs. homocoupling selectivity. Often up to 100 equivalents of one of the coupling components are required to achieve good yields of cross-coupling product. A few recent reports show that in special cases minimal excess of one of the coupling components can be used.9d,m,n Achieving good coupling regioselectivity is also problematic unless five-membered ring heterocycles, directing-group-containing arenes, or polyfluorobenzenes are employed. Besides the above-mentioned issues, in most of the published cases palladium catalysts are used in conjunction with several equivalents of heavy metal (silver or copper) reoxidants. However, oxygen can sometimes be used as the stoichiometric oxidant avoiding generation of heavy metal waste.10
Scheme 1.
Methods for Aryl-Aryl Bond Formation
The generality of direct dehydrogenative arene coupling is relatively low. Success has been achieved either in the arylation of electron-rich heterocycles and directing-group-containing arenes with simple benzenes or cross-coupling of five-membered ring heterocycles. Therefore, the development of a new, general catalytic system for dehydrogenative cross-coupling is quite appealing. Considering the existing limitations, the new reaction system should deliver highly efficient cross-coupling with good regioselectivity while involving a readily available copper catalyst and a transition-metal-free oxidant. Additionally, the method should be highly general allowing for cross-coupling of many arene types. We report here a method for a highly regioselective, copper-catalyzed cross-coupling of arene C-H bonds by employing iodine as the terminal oxidant.
2. Method Development Considerations
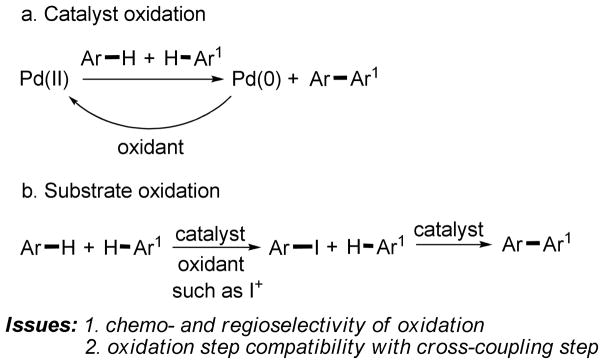
As mentioned above, most of the existing dehydrogenative arene cross-coupling methods are palladium-catalyzed and the scope of the published reactions is rather limited. The explanation for the lack of generality can be inferred from the proposed mechanisms where palladium catalyst governs both arylation selectivity and available substrate scope. The oxidant re-oxidizes Pd(0) to Pd(II) and has to be inert with respect to the substrates and reaction products. Alternatively, one can envision reaction conditions where, instead of the catalyst, one of the coupling partners would be oxidized to afford a functionalized intermediate that can participate in further reaction forming a cross-coupling product (Scheme 2).
Scheme 2.
Reaction Development Considerations
The oxidative direct arylation is now dissected into two in situ consecutive processes: oxidation and direct arylation. This approach may result in several advantages if the substrate oxidation step is designed correctly. A fast and selective oxidation of only one of the coupling components should result in minimization of the amount of the homodimerization byproduct. Additionally, a large excess of one of the coupling components should not be necessary. Regioselective oxidation would result in selective activation of only one of the many C-H bonds in substrate. Consequently, diverse and non-symmetric coupling partners could be employed. The oxidant must be able to regioselectively functionalize a broad range of aromatic compounds to afford intermediates that are reactive for subsequent copper-catalyzed direct arylation.7b–i Iodination usually occurs with high regioselectivity.11,12 Both electron-rich and electron-poor arenes can be iodinated under electrophilic or deprotonative reaction conditions, respectively.11,12 Aryl iodides can be employed as substrates in highly regioselective copper-catalyzed arylation of C-H bonds that is successful for arylation of a variety of heterocycles and electron-deficient arenes.7b–i The combination of copper-catalyzed direct arylation reaction with an in situ iodination should allow to create a general method for oxidative cross-coupling of two arenes. The iodinating reagent must be compatible with copper(I) catalyst. Strong oxidants must be avoided since they efficiently convert catalytically active Cu(I) to inactive Cu(II). We have reported a sequential iodination-cross coupling method that employs ICl oxidant.7i However, due to incompatibility of ICl with the Cu(I) catalyst the reaction has to be carried out in two steps. With those considerations in mind, we chose iodine since it is weaker oxidant than ICl and delivers an sp2 C-I functionality that is reactive under copper catalysis.
3. Results
3.1. Optimization of the iodination step
The method for oxidative arene cross-coupling consists of iodination followed by direct arylation that should be mutually compatible. The conditions for direct arylation employing copper catalysis have been extensively investigated before.7 The iodination method and its compatibility with the arylation step need to be investigated taking into account that iodination may proceed by different mechanisms for various arene types. Iodination of electron-rich arenes, electron-deficient arenes, five-member-ring heterocycles, and six-member-ring heterocycles will be discussed according to the mechanism by which the halogenation occurs.
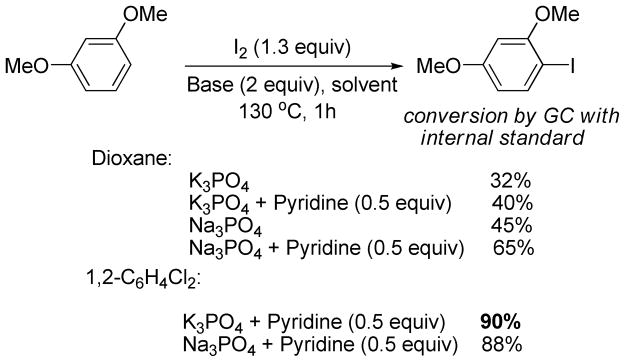
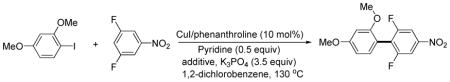
The iodination of electron-rich arenes proceeds through electrophilic aromatic substitution.11 Our initial attempts were directed towards developing optimized conditions for the iodination of electron-rich arenes by iodine that would be compatible with the subsequent cross-coupling step. 1,3-Dimethoxybenzene was used as the model substrate for iodination. A number of bases and additives were screened in dioxane and 1,2-dichlorobenzene solvent (Scheme 3).
Scheme 3.
Optimization of Iodination Conditions for Electron-Rich Arenes
The best conversion in dioxane was obtained when Na3PO4 base was used with the presence of 0.5 equiv of pyridine additive. However, excess of pyridine resulted in lower conversions. Unfortunately, Na3PO4 is not an efficient base for the subsequent cross-coupling step and thus the reaction was examined in 1,2-dichlorobenzene. To our delight, the conversion was improved to 90% by employing the K3PO4 base in the presence of pyridine additive. Thus, the conditions for iodination of electron-rich aromatic compounds involve K3PO4 base, I2, dichlorobenzene solvent, and pyridine additive. For more reactive substrates, iodination can be performed in dioxane. These conditions can be used for iodination of electron-rich arenes such as methoxy- or aminobenzenes as well as five-membered ring heterocycles such as thiophenes, indoles, pyrazoles, pyrroles, furans, and indolizines.
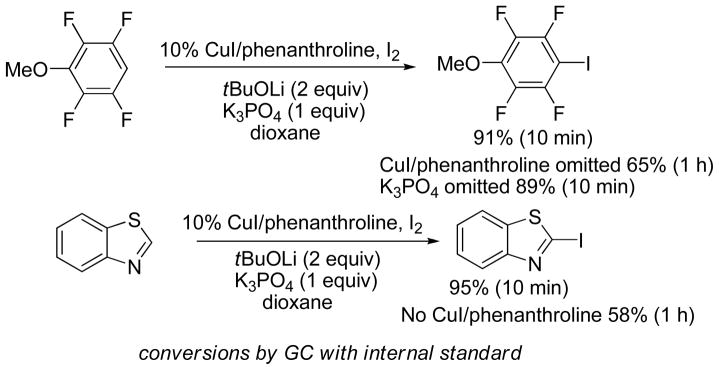
In contrast, electron-deficient arene iodination by electrophilic pathways requires harsh reaction conditions incompatible with the subsequent cross-coupling step.13 Consequently, deprotonative halogenation pathway should be employed.12 As reported earlier, dioxane solvent in combination with an alkoxide base and iodine proved to be efficient for regioselective iodination of the most acidic arene C-H bond (Scheme 4).14 The reaction conditions are applicable to the iodination of electron-poor arenes that contain several electron-withdrawing groups as well as the most acidic heterocycles such as oxazoles and thiazoles. Interestingly, the best results were obtained in the presence of copper catalyst that is required in the cross-coupling step, and by employing mixed tBuOLi/K3PO4 base. Huang and coworkers have reported that in non-polar solvents, five-membered ring heterocycle acidity is increased by copper complexation thus facilitating the deprotonation step.15
Scheme 4.
Influence of Copper Catalyst on Iodination of Electron-Poor Arenes and Acidic Heterocycles
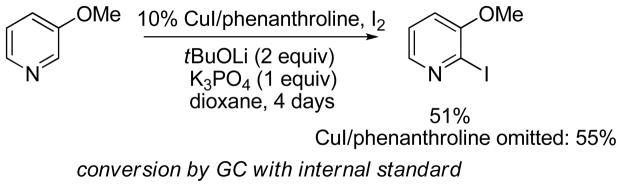
Finally, a method was developed for pyridine iodination at 2-position. Conditions similar to the ones used for the iodination of electron-poor arenes afforded reasonable conversions to 2-iodopyridines. Only pyridines possessing electron-donor substituents or halogens are reactive (Scheme 5). Interestingly, in this case CuI/phenanthroline additive slightly decreases conversion, in contrast to the iodination of electron-poor arenes.
Scheme 5.
Pyridine Iodination
3.2. Oxidative Arene Cross-Dimerization
The wide scope of iodination combined with generality of copper-catalyzed C-H bond arylation should allow for a very general method that allows to cross-couple many arene types. For example, by employing this methodology, one should be able to cross-couple electron-rich arenes with electron-poor arenes as well as five- and six-membered ring heterocycles. Any combinations of cross-coupling of electron-poor arenes, five-membered ring heterocycles, and six-membered ring heterocycles should also be possible. The types of cross-coupling reactions that could not be accomplished by employing this methodology include cross-coupling of electron-rich arenes. Additionally, electron-neutral arenes such as benzene could not be used as any of the coupling components. due to the requirement of a relatively acidic C-H bond with a DMSO pKa of 35–37 or below16 for the copper-catalyzed arylation step and their lack of reactivity in iodination step. For convenience, the cross-coupling examples below are classified according to arene type and not by the iodination mechanism.
3.3.1 Coupling of Electron-Rich Arenes with Heterocycles and Electron-Poor Arenes
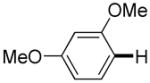
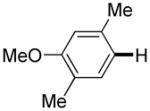
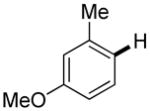
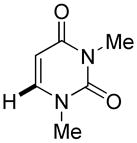
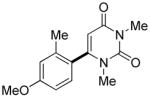
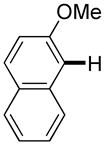
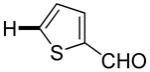
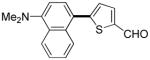
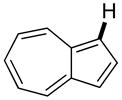
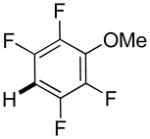
The reaction between 1,3-dimethoxybenzene and 3,5-difluoronitrobenzene was performed under the conditions developed for iodination of electron-rich arenes. By using 10 mol % CuI/phenanthroline catalyst, K3PO4 base, iodine oxidant, and pyridine additive in 1,2-dichlorobenzene the arenes were cross-coupled and an excellent yield of product was obtained (Table 1, Entry 1). If CuI/phenanthroline catalyst was omitted, the cross-coupling product was not formed. A number of electron-rich arenes were then cross-coupled with five- and six-membered ring heterocycles as well as electron-poor arenes. Typically, 1/1.5 to 1/3 arene ratio was used and no homocoupling products were observed. A single product regioisomer was obtained in all cases. Electron-rich arenes possessing either one dialkylamino, or an alkoxy substituent with an additional electron-donating group are reactive. 2,5-Dimethylanisole can be coupled with 2-cyanothiophene affording the coupling product in a good yield (entry 2). The coupling of 3-methylanisole with an uracil derivative gave the product in 61% yield (entry 3). Dimethylaniline derivatives can be arylated by a phenyloxazole (entry 4), 3,5-difluoropyridine (entry 5), and thiophene-2-carbaldehyde (entry 8). Coupling of 1- and 2-methoxynaphthalene with a polyfluorinated arene (entry 6) and 2,3,5,6-tetrafluoropyridine (entry 7) was also successful. Some polycyclic hydrocarbons such as azulene and pyrene can be arylated by polyfluorinated arenes (entries 9 and 10). The method appears to have a good functional group tolerance, with nitro, cyano, amide, chloro, and aldehyde substituents compatible with the reaction conditions. In all examples, the reactions proceed by initial iodination of the electron-rich arene followed by a copper-catalyzed cross-coupling. Only in one case formation of minor amounts of homocoupling byproduct was observed. Thus, 1-methoxynaphthalene arylation by heptafluorotoluene (entry 9) afforded <3% of perfluoro-4,4′-bitolyl in addition to the cross-coupling product.
Table 1.
Arylation of Electron-Rich Arenesa
 | |||||
|---|---|---|---|---|---|
| Entry | Electron-rich arene | Coupling partner | Product | Time | Yield |
| 1 |
|
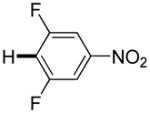
|
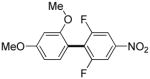
|
5 days | 82% |
| 2 |
|
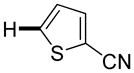
|
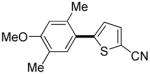
|
5 days | 72% |
| 3b |
|
|
|
2 days | 61% |
| 4c |
|
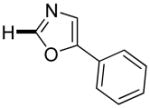
|
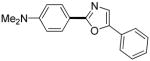
|
4 days | 57% |
| 5 |
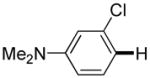
|
|
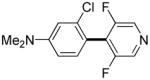
|
4 days | 80% |
| 6d |
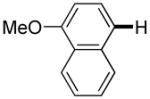
|
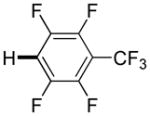
|
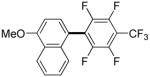
|
2 days | 85% |
| 7 |
|
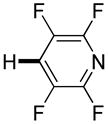
|
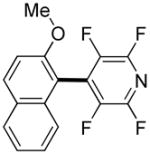
|
4 days 3 days |
55% 75%b |
| 8 |
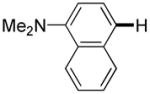
|
|
|
6 days | 64% |
| 9c |
|
|
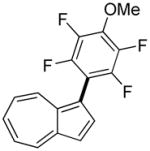
|
1 day | 49% |
| 10 |
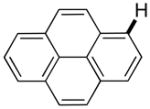
|
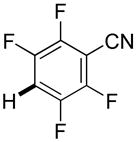
|
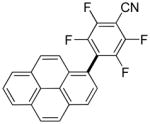
|
7 days | 53% |
Copper (I) iodide (0.1 mmol), phenanthroline (0.1 mmol), electron-rich arene (1–3 mmol), coupling partner (1–2 mmol), iodine (1.2–1.3 mmol), 1,2-dichlorobenzene solvent (0.7–1.0 mL), pyridine additive (0.5–1.0 mmol), 1–7 days. Yields are isolated yields. See the Supporting Information for details.
Sequential iodination/cross-coupling.
Dioxane solvent.
Less than 3% of perfluoro-4,4′-bitolyl formed.
3.3.2 Electron-Deficient Arene Arylation
A wide range of aromatic compounds bearing at least two electron-withdrawing groups such as chloro, fluoro, nitro, cyano, and ester can be selectively arylated at the most acidic C-H bond (Table 2). The entries are arranged with respect to the mechanism of iodination pathway involved in the coupling. The iodination occurs by an electrophilic aromatic substitution (entries 1–2), deprotonation-iodination (entries 3–8), and iodination of 6-membered ring heterocycles (entries 9–10). In entries 5–8 the electron-deficient arene is iodinated. For all other cases, the coupling partner is iodinated. Only one regioisomer of the coupling product is observed.
Table 2.
Arylation of Electron-Deficient Arenesa
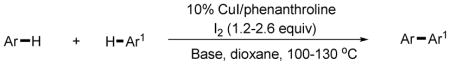 | |||||
|---|---|---|---|---|---|
| Entry | Electron-deficient arene | Coupling partner | Product | Time | Yield |
| 1b |
|
|
|
3 days | 52% |
| 2c |
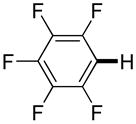
|
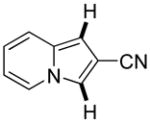
|
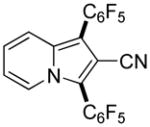
|
3 days | 78% |
| 3d |
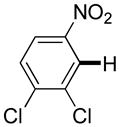
|
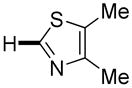
|
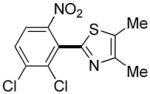
|
12 hours | 55% |
| 4e |
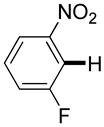
|
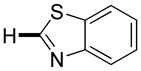
|
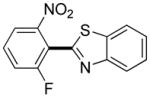
|
5 hours | 65% |
| 5f |
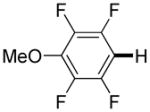
|
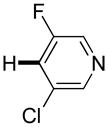
|
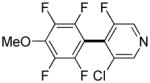
|
6 hours | 69% |
| 6g |
|
|
|
12 hours | 60% |
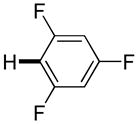
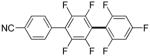
| 7h |
|
|
|
12 hours | 68% |
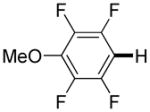
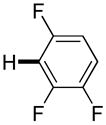
| 8i |
|
|
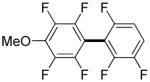
|
12 hours | 48% |
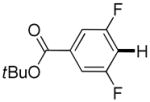
| 9 |
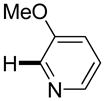
|
|
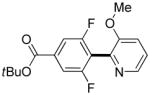
|
4 days | 50% |
| 10 |
|
|
|
5 days | 56% |
Copper (I) iodide (0.1 mmol), phenanthroline (0.1 mmol), electron-poor arene (1–4 mmol), coupling partner (1–3 mmol), iodine (1.2–2.6 mmol), dioxane solvent (0.8–2.0 mL), K3PO4 or tBuOLi/K3PO4 base, 5 hours–5 days. Yields are isolated yields. See the Supporting Information for details.
Pyridine additive (0.5 mmol), 1,2-dichlorobenzene solvent.
Pyridine additive (1.0 mmol), 20 mol % CuI/phenanthroline.
4,4′,5,5′-Tetramethyl-2,2′-bithiazole byproduct formed (13 %).
2,2′-Bibenzothiazole byproduct formed (15 %).
2,2′,3,3′,5,5′,6,6′-Octafluoro-4,4′-dimethoxybiphenyl byproduct formed (12%).
2′,2”,3′,3”,5′,5”,6′,6”-Octafluoro-p-quaterphenyl byproduct formed (8 %).
2,3,5,6-Tetrafluoro-4′-cyanobiphenyl dimer byproduct formed (12%).
2,2′,3,3′,5,5′,6,6′-Octafluoro-4,4′-dimethoxybiphenyl byproduct formed (18 %).
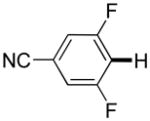
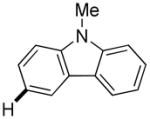
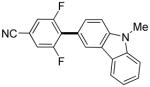
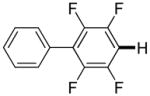
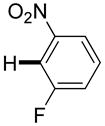
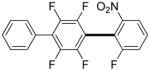
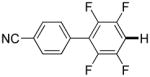
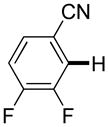
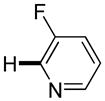
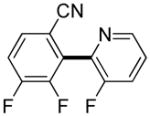
3,5-Difluorobenzonitrile can be coupled with N-methylcarbazole in an acceptable yield (entry 1). Coupling of pentafluorobenzene and several halogenated nitrobenzenes with five-membered ring heterocycles such as cyanoindoline (entry 2), dimethylthiazole (entry 3), and benzothiazole (entry 4) are successful. Interestingly, selective cross-coupling between two electron-deficient arenes is possible if there is a sufficient C-H bond acidity difference between the coupling partners. For example, tetrafluorobiphenyl can be coupled with 1-fluoro-3-nitrobenzene (entry 6). Coupling of a cyanated tetrafluorobiphenyl with 1,3,5-trifluorobenzene delivers the cross-coupling product selectively (entry 7). Finally, coupling of an arene possessing four fluorine substituents with an arene possessing three fluorines occurs in a moderate yield (entry 8). The cross-coupling product can be obtained because the iodination step requires electron-deficient arenes possessing at least four fluorine substituents. The more acidic arene is converted to aryl iodide which then couples with the less acidic arene affording the cross-coupling product. Arylation of pyridine derivatives by electron-deficient arenes affords 2-arylpyridines in moderate yields (entries 9 and 10). In the cases where deprotonation-iodination pathway operates, homocoupling byproduct formation is observed (entries 3–8).
3.3.3 Arylation of 5-Member-Ring Heterocycles
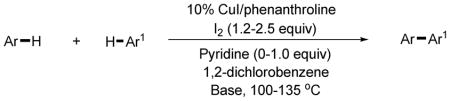
The reaction scope for the arylation of five-membered ring heterocycles is summarized in Table 3. The entries are arranged with respect to the mechanism of iodination pathway involved in the coupling. The iodination occurs by an electrophilic aromatic substitution (entries 1–8) or deprotonation-iodination (entries 9–10). In all cases except entry 9 the five-membered ring heterocycle is iodinated.
Table 3.
Arylation of Five-Membered Ring Heterocyclesa
 | |||||
|---|---|---|---|---|---|
| Entry | Five-membered ring heterocycle | Coupling partner | Product | Time | Yield |
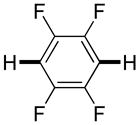
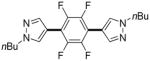
| 1b |
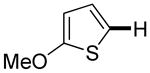
|
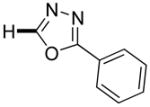
|
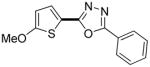
|
2 days | 60% |
| 2c |
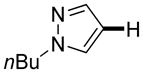
|
|
|
9 days | 80% |
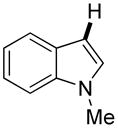
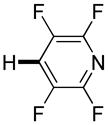
| 3d,e |
|
|
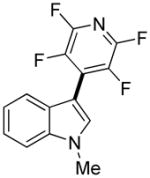
|
1 day | 82% |
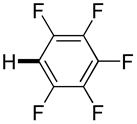
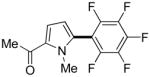
| 4f |
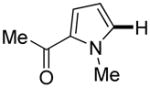
|
|
|
2.5 days | 55% |
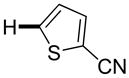
| 5 |
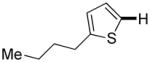
|
|
|
2 days | 61% |
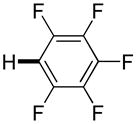
| 6g |
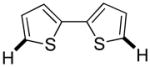
|
|
|
5 days | 62% |
| 7 |
|
|
|
2 days | 50% |
| 8 |
|
|
|
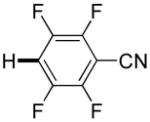
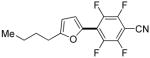
2 days | 61% |
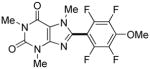
| 9h |
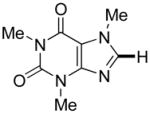
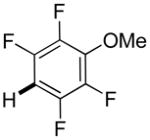
|
|
|
7 hours | 73% |
| 10i |
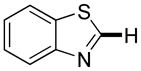
|
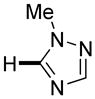
|
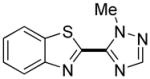
|
2 hours | 51% |
Copper (I) iodide (0.1 mmol), phenanthroline (0.1 mmol), heterocycle (1–3 mmol), coupling partner (1–4 mmol), iodine (1.2–2.5 mmol), 1,2-dichlorobenzene solvent (0.7–1.0 mL), K3PO4 or tBuOLi/K3PO4base, 2 hours–9 days. Yields are isolated yields. See the Supporting Information for details.
Dioxane solvent, <5% of 2-phenyloxazole dimer formed.
20 mol% CuCl/phenanthroline used.
Dioxane/DMF mixed solvent (9/1).
Sequential iodination/cross-coupling.
Minor amount (<5%) of regioisomer formed.
Decafluorobiphenyl byproduct formed (<3 %), 20 mol% CuCl/phenanthroline catalyst used.
Dioxane solvent, 2,2′,3,3′,5,5′,6,6′-octafluoro-4,4′-dimethoxybiphenyl byproduct formed (16 %).
Dioxane solvent, 2,2′-bibenzothiazole byproduct formed (15 %).
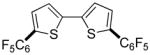
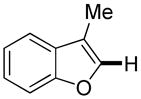
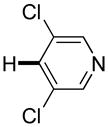
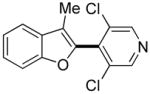
Thiophenes can be arylated by other five-membered ring heterocycles such as 2-phenyloxadiazole (entry 1), 2-cyanothiophene (entry 5), and electron-deficient arenes (entry 6). Especially interesting is the cross-coupling of two electronically different thiophenes (entry 5). This reaction proceeds with an excellent cross-coupling selectivity and no homocoupling products were observed in crude reaction mixture. Double arylation of 1,2,4,5-tetrafluorobenzene with N-butylpyrazole affords the coupling product in an excellent yield (entry 2). Arylation of N-methylindole and 1-methyl-2-acetylpyrrole by electron-deficient arenes is successful (entries 3 and 4). Furan derivatives can be arylated by electron-deficient arenes (entries 7 and 8). Caffeine is functionalized in a good yield (entry 9). Benzothiazole can be coupled with N-methyl-1,2,4-triazole and product is obtained in a moderate yield. Two examples in Table 3 show a four-fold C-H bond functionalization where the substrate is diarylated (entries 2 and 6). In some cases minor amounts of arene homodimerization products are observed (entries 1, 6, 9, and 10).
3.3.4 Pyridine Arylation by Heterocycles and Electron-Deficient Arenes
Table 4 summarizes the scope for arylation of pyridines derivatives. The data are arranged according to the mechanism of iodination pathway involved in the coupling. The iodination occurs by an electrophilic aromatic substitution (entries 1–3), deprotonation-iodination (entry 4), and pyridine iodination (entries 6–9). In entries 1–4, the coupling partner is iodinated. In all other cases, pyridine derivative is iodinated. Only one product isomer is observed in all cases.
Table 4.
Pyridine Arylationa
 | |||||
|---|---|---|---|---|---|
| Entry | Pyridine | Coupling partner | Product | Time | Yield |
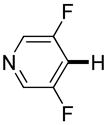
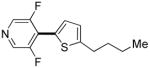
| 1b |
|
|
|
7 days | 79% |
| 2c |
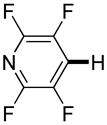
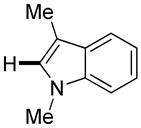
|
|
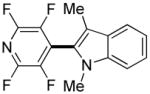
|
1 day | 71% |
| 3d |
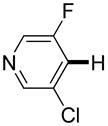
|
|
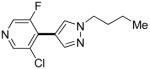
|
2 days | 79% |
| 4 |
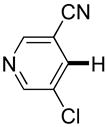
|
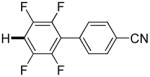
|
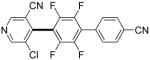
|
5 hours | 67% |
| 5 |
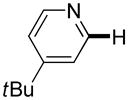
|
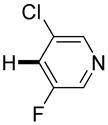
|
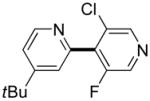
|
3 days | 42% |
| 6 |
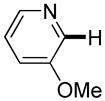
|
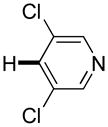
|
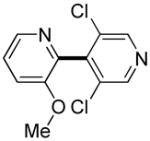
|
3 days | 47% |
| 7 |
|
|
|
3 days | 51% |
| 8 |
|
|
|
5 days | 53% |
| 9e |
|
|
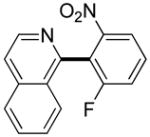
|
3 days | 32% |
Copper (I) iodide (0.1 mmol), phenanthroline (0.1 mmol), pyridine substrate (1.5–3.0 mmol), coupling partner (1.0 mmol), iodine (1.3–1.8 mmol), dioxane solvent (0.9–1.6 mL), K3PO4 or tBuOLi/K3PO4 base, 5 hours-7 days. Yields are isolated yields. See the Supporting Information for details.
1,2-Dichlorobenzene solvent.
Mixed 1,2-dichlorobenzene/DMF solvent (9.5/0.5).
1,2-Dichlorobenzene solvent, pyridine additive (0.5 mmol).
2,2′-Difluoro-6,6′-dinitrobiphenyl byproduct formed (16%).
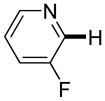
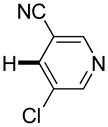
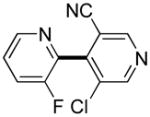
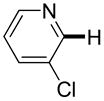
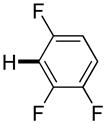
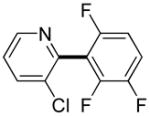
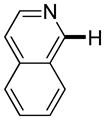
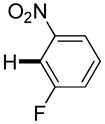
Polyfluoropyridines can be arylated by 2-butylthiophene (entry 1), 1,2-dimethylindole (entry 2), and N-butylpyrazole (entry 3). 3-Chloro-5-cyanopyridine is also reactive and can be coupled with tetrafluorocyanobiphenyl (entry 4). Pyridine itself is not reactive toward iodination/cross-coupling. However, pyridine derivatives possessing a halogen-, methoxy-, or alkyl substituent at 3- or 4-positions can be iodinated/cross-coupled under the general conditions with moderate yields and high selectivities (entries 5–8). Only monoarylation was observed even in the presence of excess iodine. Interestingly, isoquinoline can be regioselectively arylated with 3-fluoronitrobenzene, albeit in a moderate yield (entry 9). Low efficiency of isoquinoline iodination is responsible for the diminished yield in the latter case. 2,2′-Difluoro-6,6′-dinitrobiphenyl byproduct is formed in entry 9.
3.3.5. General Considerations
Copper-catalyzed arene cross-dimerization requires reaction times anywhere from few hours to nine days. Both the iodination and subsequent copper-catalyzed cross-coupling7d proceed on timescale of hours. For the methodology to be useful and predictable, factors that cause long reaction times need to be understood. Several control experiments were run to determine which components of the reaction mixture inhibit the reaction (Table 5).
Table 5.
Influence of Additivesa
 | |||
|---|---|---|---|
| Entry | Additive | Time | Conversion |
| 1 | None | 5 hours | 68% |
| 2 | 12 hours | > 98% | |
| 3 | LiI (1 equiv) | 12 hours | 88% |
| 4 | I2 (0.5 equiv) | 5 hours | <1% |
| 5 | 24 hours | <1% | |
| 6 | I2 (0.5 equiv) | 5 hours | <1% |
| 7 | + LiI (1 equiv) | 24 hours | 12% |
Conversions by GC with internal standard.
The cross-coupling of 1,3-dimethoxybenzene with 3,5-difluoronitrobenzene requires 5 days to achieve 82% yield (Table 1, entry 1). In contrast, 3,5-difluoronitrobenzene arylation by 2,4-dimethoxyiodobenzene proceeds to complete conversion in 12 hours (Table 5, entry 1). Iodination of 1,3-dimethoxybenzene to produce intermediate 2,4-dimethoxyiodobenzene requires an hour to achieve 90% conversion (Scheme 3). Clearly, one of the reaction components inhibits the copper-catalyzed cross-coupling of 1,3-dimethoxybenzene with 3,5-difluoronitrobenzene. Addition of LiI somewhat slows the reaction (entry 3). However, addition of 0.5 equiv of I2 shuts the reaction down completely (entries 3 and 4). Interestingly, if LiI and I2 are both added to the reaction mixture, some activity is restored (entries 6 and 7). Mechanism of inhibition most likely involves oxidation of catalytically active Cu(I) to inactive Cu(II).7i, 17 Analysis of the data from Tables 1–4 allows to conclude that reaction times depend on the efficiency of iodination process. If iodination is either slow or inefficient resulting in residual iodine presence in reaction mixture, the reactions are slow. Diarylation examples such as shown in Table 3, entries 2 and 6 are among the slowest reactions. The shortest reaction times are generally observed for examples where acidic heterocycle or polyfluoroarene coupling component is iodinated.
4. Summary
An operationally simple and general method for a copper-catalyzed cross-coupling of two aromatic compounds by employing iodine oxidant has been developed. The reactions proceed by iodination of one of the coupling components followed by arylation of the most acidic C-H bond of the other coupling component. Electron-rich arenes can be coupled with electron-poor arenes as well as five- and six-membered ring heterocycles. Additionally, any combinations of five- and six-membered ring heterocycles and electron-poor arenes can also be cross-coupled. In many cases, formation of homocoupling products is not observed. Coupling components are used in 1/1.5 to 1/3 ratio in contrast to existing methodology that often employs one of the arenes as the solvent. Most common functionalities such as ester, ketone, aldehyde, ether, nitrile, nitro, and amine are well-tolerated. For arenes containing multiple active C-H bonds, polyfunctionalization is possible.
4. Experimental Section
General procedure for coupling reactions
Outside the glovebox a 1-dram vial equipped with a magnetic stir bar was charged with 1,10-phenanthroline (10 mol%), CuI (10 mol %), iodine (1.2–2.6 equiv), the arene substrates in indicated ratio, pyridine (0–1.0 equiv), and 1,4-dioxane or 1,2-dichlorobenzene solvent. The vial was flushed with nitrogen, capped and placed inside glovebox. To this mixture was added base (K3PO4 or tBuOLi/K3PO4 mixture). The sealed vial was then taken out of the glovebox and stirred at appropriate temperature. After the completion of the reaction, the mixture was cooled to room temperature, diluted with CH2Cl2 (1.0 mL), and subjected to column chromatography on silica gel in hexanes followed by appropriate solvent to elute the products. After concentrating the fractions containing the product, the residue was dried under reduced pressure to yield pure product.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Sciences (Grant No. R01GM077635), A. P. Sloan Foundation, Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Footnotes
Supporting Information Available: Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem Rev. 2002;102:1359. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ullmann F, Bielecki J. Chem Ber. 1901;34:2174. [Google Scholar]; (b) Evano G, Blanchard N, Toumi M. Chem Rev. 2008;108:3054. doi: 10.1021/cr8002505. [DOI] [PubMed] [Google Scholar]
- 3.Reviews: Suzuki A. Chem Commun. 2005:4759. doi: 10.1039/b507375h.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem Int Ed. 2005;44:4442. doi: 10.1002/anie.200500368.Miura M. Angew Chem Int Ed. 2004;43:2201. doi: 10.1002/anie.200301753.Magano J, Dunetz JR. Chem Rev. 2011;111:2177. doi: 10.1021/cr100346g.
- 4.Reviews: Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n.Ackermann L, Vicente R, Kapdi AR. Angew Chem, Int Ed. 2009;48:9792. doi: 10.1002/anie.200902996.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem, Int Ed. 2009;48:5094. doi: 10.1002/anie.200806273.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173. doi: 10.1039/b606984n.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174. doi: 10.1021/cr0509760.Messaoudi S, Brion JD, Alami M. Eur J Org Chem. 2010:6495.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.Sun CL, Li BJ, Shi ZJ. Chem Commun. 2010:677. doi: 10.1039/b908581e.Livendahl M, Echavarren AM. Isr J Chem. 2010;50:630.Li CJ. Acc Chem Res. 2009;42:335. doi: 10.1021/ar800164n.
- 5.Selected examples: Akita Y, Inoue A, Yamamoto K, Ohta A, Kurihara T, Shimizu M. Heterocycles. 1985;23:2327.Catellani M, Chiusoli GP, Ricotti S. J Organomet Chem. 1985;296:C11.Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Bull Chem Soc Jpn. 1998;71:467.Rieth RD, Mankad NP, Calimano E, Sadighi JP. Org Lett. 2004;6:3981. doi: 10.1021/ol048367m.Chiong HA, Daugulis O. Org Lett. 2007;9:1449. doi: 10.1021/ol0702324.Lewis JC, Berman AM, Bergman RG, Ellman JA. J Am Chem Soc. 2008;130:2493. doi: 10.1021/ja0748985.Bellina F, Cauteruccio S, Rossi R. Eur J Org Chem. 2006:1379. doi: 10.1021/jo701496p.Lane BS, Sames D. Org Lett. 2004;6:2897. doi: 10.1021/ol0490072.Huestis MP, Fagnou K. Org Lett. 2009;11:1357. doi: 10.1021/ol900150u.Gracia S, Cazorla C, Métay E, Pellet-Rostaing S, Lemaire M. J Org Chem. 2009;74:3160. doi: 10.1021/jo802768n.Ackermann L, Vicente R, Born R. Adv Synth Catal. 2008;350:741.Park CH, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org Lett. 2004;6:1159. doi: 10.1021/ol049866q.Ohnmacht SA, Culshaw AJ, Greaney MF. Org Lett. 2010;12:224. doi: 10.1021/ol902537d.
- 6.Selected examples: Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. Angew Chem, Int Ed. 2003;42:112. doi: 10.1002/anie.200390037.Caron L, Campeau LC, Fagnou K. Org Lett. 2008;10:4533. doi: 10.1021/ol801839w.Chiong HA, Pham QN, Daugulis O. J Am Chem Soc. 2007;129:9879. doi: 10.1021/ja071845e.Deprez NR, Sanford MS. J Am Chem Soc. 2009;131:11234. doi: 10.1021/ja904116k.Kwak J, Kim M, Chang S. J Am Chem Soc. 2011;133:3780. doi: 10.1021/ja111670s.Xiao B, Fu Y, Xu J, Gong TJ, Dai JJ, Yi J, Liu L. J Am Chem Soc. 2010;132:468. doi: 10.1021/ja909818n.Scarborough CC, McDonald RI, Hartmann C, Sazama GT, Bergant A, Stahl SS. J Org Chem. 2009;74:2613. doi: 10.1021/jo802632v.Shabashov D, Daugulis O. J Org Chem. 2007;72:7720. doi: 10.1021/jo701387m.Kametani Y, Satoh T, Miura M, Nomura M. Tetrahedron Lett. 2000;41:2655.Ackermann L, Vicente R, Potukuchi HK, Pirovano V. Org Lett. 2010;12:5032. doi: 10.1021/ol102187e.
- 7.Palladium catalysis: Lafrance M, Rowley CN, Woo TK, Fagnou K. J Am Chem Soc. 2006;128:8754. doi: 10.1021/ja062509l.Copper catalysis: Do HQ, Daugulis O. J Am Chem Soc. 2007;129:12404. doi: 10.1021/ja075802+.Do HQ, Daugulis O. J Am Chem Soc. 2008;130:1128. doi: 10.1021/ja077862l.Do HQ, Khan RMK, Daugulis O. J Am Chem Soc. 2008;130:15185. doi: 10.1021/ja805688p.Ackermann L, Potukuchi HK, Landsberg D, Vicente R. Org Lett. 2008;10:3081. doi: 10.1021/ol801078r.Yotphan S, Bergman RG, Ellman JA. Org Lett. 2009;11:1511. doi: 10.1021/ol900103a.Yoshizumi T, Tsurugi H, Satoh T, Miura M. Tetrahedron Lett. 2008;49:1598.Besselièvre F, Piguel S, Mahuteau-Betzer F, Grierson DS. Org Lett. 2008;10:4029. doi: 10.1021/ol801512q.Do HQ, Daugulis O. Chem Commun. 2009:6433. doi: 10.1039/b912890e.Chen X, Dobereiner G, Hao XS, Giri R, Maugel N, Yu JQ. Tetrahedron. 2009;65:3085.Nickel catalysis: Canivet J, Yamaguchi J, Ban I, Itami K. Org Lett. 2009;11:1733. doi: 10.1021/ol9001587.Hachiya H, Hirano K, Satoh T, Miura M. Org Lett. 2009;11:1737. doi: 10.1021/ol900159a.
- 8.Selected examples: Oi S, Fukita S, Inoue Y. Chem Commun. 1998:2439.Chen X, Li JJ, Hao XS, Goodhue CE, Yu JQ. J Am Chem Soc. 2006;128:78. doi: 10.1021/ja0570943.Giri R, Maugel NL, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J Am Chem Soc. 2007;129:3510. doi: 10.1021/ja0701614.Kirchberg S, Tani S, Ueda K, Yamaguchi J, Studer A, Itami K. Angew Chem, Int Ed. 2011;50:2387. doi: 10.1002/anie.201007060.Zhao S, Wei Y, Xu J, Kan J, Su W, Hong M. J Org Chem. 2011;76:882. doi: 10.1021/jo102175f.Tredwell MJ, Gulias M, Bremeyer NG, Johansson CCC, Collins BSL, Gaunt MJ. Angew Chem, Int Ed. 2011;50:1076. doi: 10.1002/anie.201005990.Chu JH, Lin PS, Wu MJ. Organometallics. 2010;29:4058.Yang SD, Sun CL, Fang Z, Li BJ, Li YZ, Shi ZJ. Angew Chem, Int Ed. 2008;47:1473. doi: 10.1002/anie.200704619.Cornella J, Lu P, Larrosa I. Org Lett. 2009;11:5506. doi: 10.1021/ol902304n.Vogler T, Studer A. Org Lett. 2008;10:129. doi: 10.1021/ol702659a.Zhang F, Greaney MF. Angew Chem, Int Ed. 2010;49:2768. doi: 10.1002/anie.200906921.Wang DH, Mei TS, Yu JQ. J Am Chem Soc. 2008;130:17676. doi: 10.1021/ja806681z.Nishikata T, Abela AR, Huang S, Lipshutz BH. J Am Chem Soc. 2010;132:4978. doi: 10.1021/ja910973a.Wang C, Piel I, Glorius F. J Am Chem Soc. 2009;131:4194. doi: 10.1021/ja8100598.Shuai Q, Yang L, Guo X, Basle O, Li CJ. J Am Chem Soc. 2010;132:12212. doi: 10.1021/ja105396b.
- 9.(a) Cho SH, Hwang SJ, Chang S. J Am Chem Soc. 2008;130:9254. doi: 10.1021/ja8026295. [DOI] [PubMed] [Google Scholar]; (b) Itahara T. J Chem Soc, Chem Commun. 1981:254. [Google Scholar]; (c) Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]; (d) Xi P, Yang F, Qin S, Zhao D, Lan J, Gao G, Hu C, You J. J Am Chem Soc. 2010;132:1822. doi: 10.1021/ja909807f. [DOI] [PubMed] [Google Scholar]; (e) Kita Y, Morimoto K, Ito M, Ogawa C, Goto A, Dohi T. J Am Chem Soc. 2009;131:1668. doi: 10.1021/ja808940n. [DOI] [PubMed] [Google Scholar]; (f) Hull KL, Sanford MS. J Am Chem Soc. 2009;131:9651. doi: 10.1021/ja901952h. [DOI] [PubMed] [Google Scholar]; (g) Li R, Jiang L, Lu W. Organometallics. 2006;25:5973. [Google Scholar]; (h) He CY, Fan S, Zhang X. J Am Chem Soc. 2010;132:12850. doi: 10.1021/ja106046p. [DOI] [PubMed] [Google Scholar]; (i) Izawa Y, Stahl SS. Adv Synth Cat. 2010;352:3223. doi: 10.1002/adsc.201000771. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Wei Y, Su W. J Am Chem Soc. 2010;132:16377. doi: 10.1021/ja109383e. [DOI] [PubMed] [Google Scholar]; (k) Malakar CC, Schmidt D, Conrad J, Beifuss U. Org Lett. 2011;13:1378. doi: 10.1021/ol200065s. [DOI] [PubMed] [Google Scholar]; (l) Kitahara M, Umeda N, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2011;133:2160. doi: 10.1021/ja111401h. [DOI] [PubMed] [Google Scholar]; (m) Han W, Mayer P, Ofial AR. Angew Chem, Int Ed. 2011;50:178. doi: 10.1002/anie.201006208. [DOI] [PubMed] [Google Scholar]; (n) Brasse M, Ellman JA, Bergman RG. Chem Commun. 2011:5019. doi: 10.1039/c1cc10507h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Takahashi M, Masui K, Sekiguchi H, Kobayashi N, Mori A, Funahashi M, Tamaoki N. J Am Chem Soc. 2006;128:10930. doi: 10.1021/ja060749v. [DOI] [PubMed] [Google Scholar]; (p) Gong X, Song G, Zhang H, Li X. Org Lett. 2011;13:1766. doi: 10.1021/ol200306y. [DOI] [PubMed] [Google Scholar]; (q) Wang Z, Li K, Zhao D, Lan J, You J. Angew Chem, Int ed. 2011;50:5365. doi: 10.1002/anie.201101416. [DOI] [PubMed] [Google Scholar]; (r) Yamaguchi AD, Mandal D, Yamaguchi J, Itami K. Chem Lett. 2011;40:555. doi: 10.1021/ja209945x. [DOI] [PubMed] [Google Scholar]
- 10.(a) Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org Lett. 2007;9:3137. doi: 10.1021/ol071308z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yokota T, Sakaguchi S, Ishii Y. Adv Synth Catal. 2002;344:849. [Google Scholar]; (c) Brasche G, Garcia-Fortanet J, Buchwald SL. Org Lett. 2008;10:2207. doi: 10.1021/ol800619c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liégault B, Lee D, Huestis MP, Stuart DR, Fagnou K. J Org Chem. 2008;73:5022. doi: 10.1021/jo800596m. [DOI] [PubMed] [Google Scholar]; (e) Basle O, Li CJ. Chem Commun. 2009:4124. doi: 10.1039/b905275e. [DOI] [PubMed] [Google Scholar]; (f) Hagelin H, Oslob JD, Åkermark B. Chem-Eur J. 1999;5:2413. [Google Scholar]; (g) Do HQ, Daugulis O. J Am Chem Soc. 2009;131:17052. doi: 10.1021/ja907479j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavber S, Jereb M, Zupan M. Synthesis. 2008:1487. [Google Scholar]
- 12.(a) Do HQ, Daugulis O. Org Lett. 2009;11:421. doi: 10.1021/ol802411f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Popov I, Do HQ, Daugulis O. J Org Chem. 2009;74:8309. doi: 10.1021/jo9015369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA. J Am Chem Soc. 2004;126:15770. doi: 10.1021/ja0465247. [DOI] [PubMed] [Google Scholar]; (b) Deacon GB, Smith RNM. Aust J Chem. 1982;35:1587. [Google Scholar]
- 14.Do HQ, Daugulis O. Org Lett. 2010;12:2517. doi: 10.1021/ol100772u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Chan J, Chen Y, Borths CJ, Baucom KD, Larsen RD, Faul MM. J Am Chem Soc. 2010;132:3674. doi: 10.1021/ja100354j. [DOI] [PubMed] [Google Scholar]
- 16.(a) Shen K, Fu Y, Li JN, Liu L, Guo QX. Tetrahedron. 2007;63:1568. [Google Scholar]; (b) Bordwell FG. Acc Chem Res. 1988;21:456. [Google Scholar]
- 17.Examples of copper (II) iodide-phenathroline complexes: Ainscough EW, Plowman RA. Aust J Chem. 1970;23:691.Percy GC, Thornton DA. J Mol Struct. 1972;14:313.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.