Abstract
Purpose
To develop new therapies for children with solid tumors, we tested the cytotoxicity of natural killer (NK) cells expanded by coculture with K562-mb15-41BBL cells. We sought to identify the most sensitive tumor subtypes, clarify the molecular interactions regulating cytotoxicity, and determine NK anti-tumor potential in vivo.
Experimental Design
We tested in vitro cytotoxicity of expanded NK cells against cell lines representative of Ewing sarcoma (EWS) (n=5), rhabdomyosarcoma (n=4), neuroblastoma (n=3) and osteosarcoma (n=3), and correlated the results with expression of inhibitory and activating NK receptor ligands. We also compared expanded and primary NK cells, determined the effects of activating-receptor ligation and of chemotherapeutic drugs, and assessed the therapeutic effect of NK cell infusions in xenografts.
Results
In 45 experiments, EWS and rhabdomyosarcoma cell lines were remarkably sensitive to expanded NK cells, with median cytotoxicities at 1:1 effector:target ratio of 87.2% and 79.1%, respectively. Cytotoxicity was not related to levels of expression of NK receptor ligands, nor was it affected by pretreatment of target cells with daunorubicin or vincristine, but was markedly inhibited by preincubation of NK cells with a combination of antibodies against the NK activating receptors NKGD2 and DNAM-1. Expanded NK cells were considerably more cytototoxic than unstimulated NK cells, and eradicated EWS cells engrafted in NOD/scid IL2RGnull mice.
Conclusions
Among pediatric solid tumors, EWS and rhabdomyosarcoma are exquisitely sensitive to expanded NK cells. The NK expansion method described here has been adapted to large-scale conditions, and supports a Phase I clinical study including patients with these malignancies.
INTRODUCTION
Cure rates for children with acute leukemia have steadily increased, but despite aggressive treatment, nearly half of the patients with solid tumors such as Ewing sarcoma (EWS), rhabdomyosarcoma, osteosarcoma and neuroblastoma, have progressive disease [1-6]. Prognosis is particularly poor for those patients with metastatic disease, at least two-thirds of whom have disease progression [1-6]. Outcome after recurrence is generally dismal. For patients with recurrent EWS, for example, the likelihood of long-term survival is currently <20%, <10% if relapse occurs within 2 years [1, 7-12]. Therefore, new therapeutic approaches that bypass cellular mechanisms of drug resistance are urgently needed, particularly for patients with high-risk features, such as metastatic or recurrent disease.
The known potential of immune cells to recognize and kill tumor cells suggests their potential role in anti-cancer treatment. Natural killer (NK) cells can kill allogeneic hematopoietic cells, and have been administered either in the setting of hematopoietic stem cell transplantation or after non-myeloablative immunosuppressive therapy to enhance the effect of chemotherapy in patients with acute leukemia, yielding encouraging results [13-17]. NK cells can also lyse malignant non-hematopoietic cells as shown by reports indicating NK cells cytotoxicity against EWS and osteosarcoma cell lines in vitro [18-22], as well as neuroblastoma cell lines in vitro[19, 23-25] and in vivo [26]. Preliminary clinical data suggest that donor NK cells may exert anti-tumor activity in children with solid tumors undergoing allogeneic hematopoietic stem cell transplant [27].
NK cell cytotoxicity relies on the balance between activating stimuli and suppressive signals, including those delivered by killer immunoglobulin-like receptors (KIRs) that recognize specific major histocompatibility complex HLA class I alleles [28-30]. An increase in the NK cell activation status, a decrease in suppressive signals (such in the case of altered or mismatched HLA class I), or both should result in heightened NK cell cytotoxicity. Moreover, the magnitude of NK cell cytotoxicity is directly proportional to the ratio between the number of NK cells and target cells (i.e., effector: target ratio, or E : T). Thus, in the context of NK cell therapy, the infusion of large numbers of pre-activated, allogeneic NK cells is predicted to achieve maximum anti-cancer effect. To this end, we developed a method that allows specific activation and expansion of human NK cells from peripheral blood [31-33]. In the present study, we determined the cytotoxicity of NK cells generated by this method against pediatric solid tumors.
MATERIAL AND METHODS
Tumor cell lines
The EWS cell lines TC71, SK-N-MC, EW8 and A673 express the EWS-FLI1 fusion protein [34-36]; ES8 was derived at St. Jude Children’s Research Hospital and confirmed to contain the (11;22) translocation and express the EWS-FLI1 fusion (S. Ragsdale, St Jude Children’s Research Hospital, personal communication). The rhabdomyosarcoma cell line RH30 was derived at St. Jude Children’s Research Hospital from the bone marrow of a 16.5 year old male patient with alveolar rhabdomyosarcoma; RH36 (embryonal) and RH41 (alveolar) are extensively characterized rhabdomyosarcoma cell lines incorporated into the NCI-supported Pediatric Preclinical Testing Program (PPTP)[37]; TE-32 is also an established model of rhabdomyosarcoma [38, 39]. The neuroblastoma cell line JF was developed at St Jude Children’s Research Hospital from a 1 year old female patient with stage III-C neuroblastoma (S. Ragsdale, personal communication); SK-N-SH and NB1691 are established neuroblastoma cell lines [37, 40]. Likewise, the cell lines U-2 OS, HOS and MG-63 are well known models of osteosarcoma [41-43].
The cell lines U-2 OS, HOS, MG-63 were obtained from the American Type Culture Collection (ATCC; Rockville, MD). EW8, RH36, RH41, A673, SK-N-MC, SK-N-SH and TE-32 were provided by Dr. Peter Houghton, TC71 by Dr. Stephen Skapek, and NB-1691 by Dr. Andrew Davidoff (St. Jude Children’s Research Hospital). ES8, RH30 and JF were available from the St. Jude Children’s Research Hospital tissue repository.
The osteosarcoma cell lines U-2 OS, HOS and MG-63 were maintained in DMEM (Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and antibiotics. All other cells were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA), also supplemented with 10% FBS and antibiotics. The expression of ligands for NK activating, inhibitory and adhesion molecules was analyzed by staining with monoclonal antibodies and flow cytometry. Antibodies to human HLA-ABC (G46-2.6), MIC A/B (6D4), CD112 (R2.525), CD54 (LB-2), CD58 (IC3) were obtained from BD Biosciences (San Jose, CA); CD155 (300907), ULBP-1 (170818), ULBP-2 (165903), ULBP-3 (166510), CD48 (394307) from R&D Systems (Minneapolis, MN); and HLA-E (3D12HLA-E) from eBiosciences (San Diego, CA).
NK cell activation and expansion
The K562-mb15-41BBL cell line was made as previously described [31]. Briefly, K562 cells were first transduced with a construct encoding the “membrane-bound” form of IL-15 [IL-15 plus CD8α and green fluorescent protein (GFP)]. Cells with high expression of GFP and of surface IL-15 (“K562-mb15”) were then transduced with human 4-1BB ligand. We transduced the ES8, TC71, and EW8 cell lines with a murine stem cell virus (MSCV)- internal ribosome entry site (IRES)-GFP retroviral vector (from the St. Jude Vector Development and Production Shared Resource) containing the firefly luciferase gene (gift of Dr. K. Mihara, University of Hiroshima, Japan). Transduced cells were selected for their expression of GFP with a MoFlo Cell Sorter (Cytomation, Fort Collins, CO).
Peripheral blood samples were obtained from healthy adult donors. Mononuclear cells collected from the samples by centrifugation on a Lymphoprep density step (Nycomed, Oslo, Norway) were washed twice in RPMI-1640. To obtain primary NK cells, we used peripheral blood mononuclear cells and the Miltenyi NK Cell Isolation Kit (Miltenyi Biotec, Auburn, CA). Peripheral blood mononuclear cells (1.5 × 106) were incubated in a 24-well tissue culture plate with 1 × 106 K562-mb15-41BBL cells in the presence of 10 IU/mL human IL-2 (National Cancer Institute BRB Preclinical Repository, Rockville, MD) in RPMI-1640 and 10% FBS. Medium was exchanged every 2 days with fresh medium and IL-2. After 7 days of co-culture, residual T cells were removed with Dynabeads CD3 (Invitrogen, Carlsbad, CA).
Cytotoxicity assays
Target cells were suspended in RPMI-1640 tissue culture medium with 10% FBS, labeled with calcein AM, and plated in triplicate onto 96-well flat bottom plates (Costar, Corning, NY). The plates were placed in an incubator set at 37°C and 5% CO2 for 4 hours to allow for cell attachment. Expanded NK cells suspended in RPMI-1640 with 10% FBS and 100 IU/mL IL-2 were then added at various E : T ratios as indicated in Results. The plates were then centrifuged and incubated as above for various time periods as indicated in Results. Cells were then detached using trypsin plus EDTA, and stained with propridium iodide. Cytotoxicity was measured with a flow cytometry–based method, enumerating the number of viable target cells (calcein AM-positive, propidium-iodide negative, and with light scattering properties of viable cells) in cultures with and without NK cells. [32, 44]
In some experiments, we tested cytotoxicity using luciferase-labeled target cells. These were plated in 96-well, flat-bottom white Viewplates (Perkin-Elmer, Waltham, MA) and exposed to NK cells as described above. At the end of the cultures, an equal volume of Promega Bright-Glo luciferase reagent (Promega, Madison, WI) was then added to each test well and after 5 minutes luminescence was measured using a plate reader (Perkin-Elmer Waltham, MA) and analyzed with the Packard Fusion software (Packard Bioscience, Meriden, CT). Viability was calculated by comparing relative luminescent signal from control wells on each plate. All experiments were performed in triplicate. Anti-NKG2D (149810; R&D Systems) and anti-DNAM-1 (DX11; BD Biosciences) antibodies were used to tests the effect of these molecules on NK cell cytotoxicity.
Murine models
ES8 cells expressing luciferase were injected i.p. in 8 to 14-week-old NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NOD/scid IL2RGnull) mice (Jackson Laboratory, Bar Harbor, ME; 2 × 105 to 1 × 106cells per mouse). NK cells from healthy donors expanded for 7 days were resuspended in RPMI-1640 plus 10% FBS (1-3 × 107 cells per mouse) and then injected i.p. at the schedules indicated in the Results section. In some experiments, multiple injections of NK cells were given together with i.p. injections of IL-2 (20000 IU each). Mice receiving tissue culture medium with/or without IL-2 instead of NK cells served as controls. EWS engraftment and progression was evaluated using a Xenogen IVIS-200 system (Caliper Life Sciences, Hopkinton, MA), with imaging beginning 5 minutes after i.p. injection of an aqueous solution of D-luciferin potassium salt (3 mg/mouse). Photons emitted from luciferase-expression cells were quantified using the Living Image 3.0 software program. Some mice were treated with a single dose of 3.25 Gy whole-body irradiation 7 days after tumor cell injection. Irradiation was delivered by a cesium irradiator with attenuator shields to reduce the by approximately 10-fold from the source. The dose of 3.25 Gy was chosen based on previously published data regarding radiation sensitivity of NOD/scid IL2RGnull mice [45]. If NK cells were administered, the first infusion was performed within one hour after the irradiation.
RESULTS
Relative sensitivity of sarcoma and neuroblastoma cells to the cytotoxicity of expanded NK cells
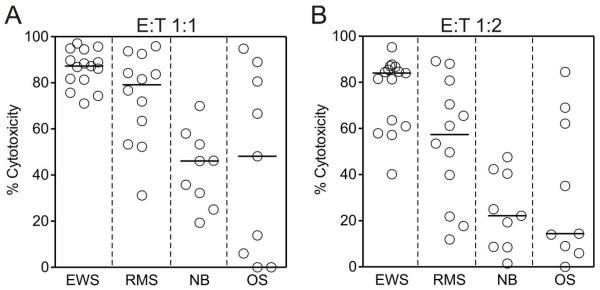
To determine the relative sensitivity of cells derived from pediatric solid tumors to the cytotoxicity of expanded NK cells, we obtained NK cells from 5 healthy donors and stimulated them for 7 days by coculture with irradiated K562-mb15-41BBL cells. After depletion of residual T cells, we tested the cytotoxicity of the expanded NK cells against EWS (TC71, SK-N-MC, ES8, EW8 and A673), rhabdomyosarcoma (RH30, RH36, RH41 and TE32), neuroblastoma (NB1691, JF and SK-N-SH) and osteosarcoma (U-2 OS, HOS and MG-63) cell lines, for a total of 45 experiments. After 4 hour of culture, there was considerable heterogeneity in the degree of sensitivity to NK cell cytotoxicity among the disease-defined groups. As shown in Fig. 1A, there was a median cytotoxicity of 87.2% at a 1 : 1 E : T with EWS cell lines, of 79.1% for rhabdomyosarcoma cells lines but only 46.1% and 48.1% for neuroblastoma and osteosarcoma cell lines, respectively (P = 0.0003). At 1 : 2, median cytotoxicity against EWS cells continue to exceed 80% and was considerably higher than that observed in the other groups (P = 0.0002; Fig. 1B). Thus, EWS cells are particularly sensitive to cytotoxicity of expanded NK cells. Among the other groups, susceptibility to NK cells was particularly heterogenous. For example, median cytotoxicity at 1:1 was 89.0% for the osteosarcoma cell line HOS but 6.0% with the MG-63 cell line.
Figure 1.
Susceptibility of cell lines derived from pediatric solid tumors to cytotoxicity by expanded NK cells. Shown are results of 4-hour cytotoxicity assays performed at 1:1 E:T ratio (A) and at 1:2 E:T ratio (B). Including in the tests were cell lines derived from Ewing sarcoma family of tumors (EWS) (TC71, SK-N-MC, ES8, EW8 and A673), rhabdomyosarcoma (RMS) (RH30, RH36, RH41 and TE32), neuroblastoma (NB) (NB1691, JF and SK-N-SH) and osteosarcoma (OS) U-2 OS, HOS and MG-63. Each symbol represents the mean of triplicate measurements relative to control cultures with no NK cells. Horizontal bars correspond to median values in each group.
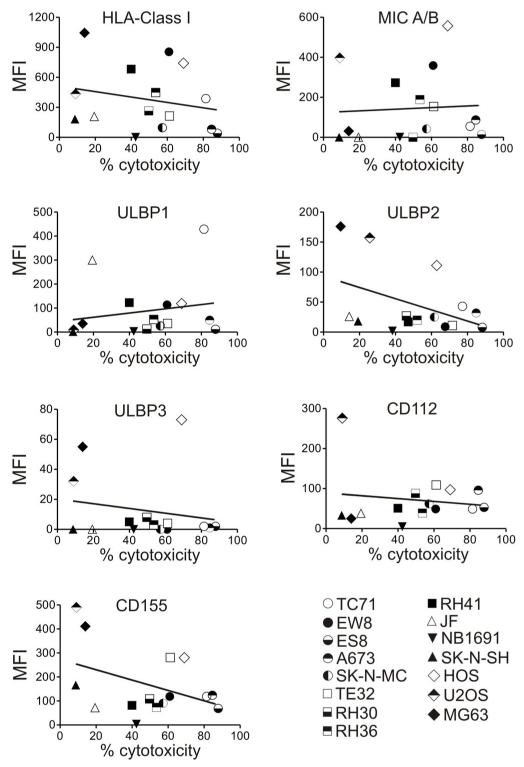
We examined whether the variable susceptibility to NK cell cytotoxicity was significantly related to their level of HLA Class I expression, but it was not (Fig. 2). We also examined expression of ligands known to trigger NK cell activation, including MIC A/B, ULBP1, ULBP2 and ULBP3 (NKG2D ligands), CD112 and CD155 (DNAM-1) and there was no apparent relation between the level of expression of these molecules and susceptibility to NK cell cytotoxicity (Fig. 2). Expression of other molecules such as CD54 and CD58 (adhesion molecules) and HLA-E (NKG2A/C ligand) was also not significantly related to cytotoxicity; CD48 (2B4 ligand), was not expressed in any of the cell lines (data not shown).
Figure 2.
Relation between susceptibility to expanded NK cell cytotoxicity and expression of ligands for NK cell inhibitory or activating receptors in pediatric solid tumor cell lines. For each surface molecule, the mean fluorescence intensity (MFI) (mean of triplicate measurements) measured in each cell line is plotted on the Y axis, with the percent cytotoxicity measured in 4-hour assays at 1:1 E:T ratio plotted on the × axis. Lines in each plot correspond to linear regression analyses. R2 values were 0.05 for HLA-Class I, 0.004 for MIC A/B, 0.19 for ULBP1, 0.19 for ULBP2, 0.03 for ULBP3, 0.06 for CD112, and 0.18for CD155.
Expanded NK cells are highly and specifically cytotoxic against EWS cells
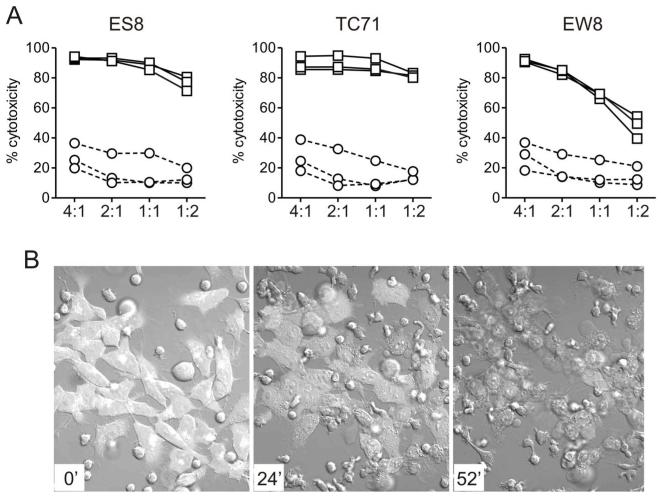
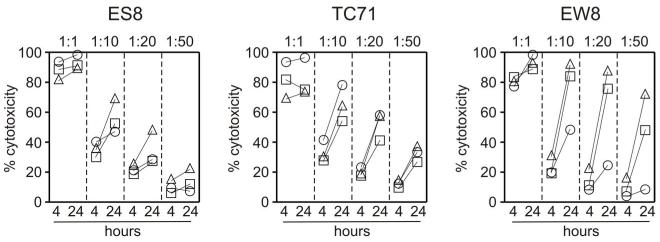
A previous study reported that EWS cells were sensitive to primary NK cells [20] but the cytotoxicities observed appeared to be much lower than those that we obtained with our expanded NK cells, suggesting that expanded NK cells are more effective. To formally test this possibility, we compared the cytotoxicity of primary and expanded NK cells. As shown in Fig. 3A, expanded NK cells were far more powerful than primary NK cells. For example, mean (± SD) cytotoxicity against the TC71 cell line at 1 : 1 was 87.8% ± 4.5% (n = 3) with expanded NK cells compared with 14.0% ± 9.3% for primary NK cells (P <0.001). Indeed, most EWS cells appeared to be lysed after only 1 hour of coculture (Fig. 3B). To define the limits of NK cell cytotoxicity against EWS cells, we performed tests at lower E : T ratios in 4 hour and 24 hour cultures. In 4 hour cultures, cytotoxicities dropped to less than 50% at 1: 10 and less than 25% at 1 : 20 (Fig. 4). However, when cultures were prolonged for 24 hours, cytotoxicities increased considerably; in the case of EW8, more than 70% cytotoxicities could be achieved with 2 of the 3 donors at 1 : 20 (Fig. 4).
Figure 3.
Cytotoxicity of expanded NK cells against EWS cells. (A) Cytotoxicity of expanded NK cells (squares) compared to that of primary NK cells (circles) against the EWS cell lines ES8 TC71 and EW8. Results obtained from 3 different donors at the E:T ratios indicated are shown. Each symbol is the mean of triplicate measurements relative to control cultures with no NK cells. (B) Live cell confocal photography of the EWS cell line (labeled with green fluorescent protein) cultured with expanded NK cells at 2:1 E:T ratio. The time of culture is shown in the lower left corner of each photograph. Microscopy was performed with a Nikon TE2000E2 microscope equipped with a Nikon C1Si confocal using 488nm and 561nm DPSS lasers for excitation. Temperature was maintained at ~37°C and 5% CO2 using an environmental control chamber. Images were acquired with a Nikon 40× 1.3 NA DIC objective every 20s for 2hr using Nikon EZC1 software. Full movie is in Supplementary Material.
Figure 4.
Cytotoxicity of expanded NK cells against EWS cell lines at low E:T ratios. Shown are percentage of cytotoxicity (relative to cultures with no NK cells) in co-cultures lasting 4 hours and 24 hours at different E:T ratios. Symbols are mean of triplicate measurements; each NK donor is indicated by a different symbol. In the case of the 4-hour measurements, standard deviations for all cell lines and all donors were <4% at 1:1, <5% at 1:10, <7% at 1:20 and at 1:50; for 24-hour measurements, they were <1% at 1:1, <5% at 1:10, <6% at 1:20 and <10% at 1:50.
Molecular mechanisms involved in the interaction between expanded NK cells and EWS cells
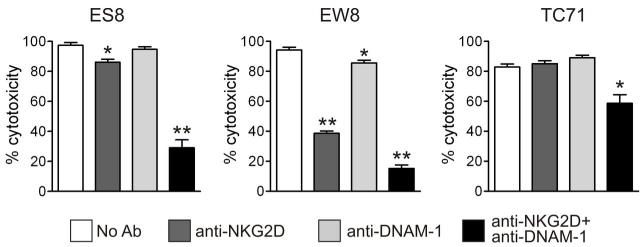
A previous study showed the ligation of NKGD2 and DNAM-1 in primary NK cells could decrease their cytotoxicity against EWS cells [20]. Because expanded NK cells express substantially higher levels of NKG2D than primary or IL-2 activated NK cells [32], we determined whether its ligation would affect cytotoxicity. Preincubation of expanded NK cells with the anti-NKG2D antibody 149810 inhibited their cytotoxicity against the Ewing sarcoma cell lines ES8 (P = 0.011, n = 10) and EW8 (P = 0.0004; n = 10) at E:T ratios of 1:1-1:4, but did not significantly affect cytotoxicity against TC71 (P = 0.061; n = 10). An anti-DNAM-1 antibody (DX11) had no significant effect on the cytotoxicity against ES8 and TC71; it had a significant but overall modest protective effect on EW8 (P = 0.008; n = 10). However, both antibodies combined markedly reduced cytotoxicity against all 3 cell lines (P = 0.0002 for ES8; P = 0.004 for TC71, and P = 0.0003 for EW8), whereas an anti-CD56 antibody, used as a control, had no discernible effect. These results (representative experiments shown in Fig. 5) point to cooperative role for NKG2D and DNAM-1 in the cytotoxicity of expanded NK cells against Ewing sarcoma cells.
Figure 5.
Effect of ligating NK-activating receptors on the cytotoxicity of expanded NK cells against EWS cell lines. Co-cultures of EWS with NK cells were performed after incubating NK cells for 20 minutes with anti-NKG2D and anti-DNAM-1 antibodies, alone or in combination. Each bar corresponds to mean percent cytotoxicity (± SD) of triplicate measurements (relative to control cultures with no NK cells). * = P <0.01; **= P<0.0001 by t test.
Finally, we determined whether pretreatment of cells with chemotherapy drugs commonly used to treat EWS would have any effect on the cells expression of activating ligands and/or the susceptibility to NK cells. Expression of HLA Class I, MIC A/B, ULBP1, ULBP2, CD112 and CD155 on ES8 and TC71 cells was not affected by exposure to LC50 concentrations of doxorubicin (10 nM) for 72 hours, and the specific cytotoxicity of NK cells expanded from 2 donors against the 2 cell lines remained unchanged. Similar results were obtained with vincristine (1 nM). Therefore, prior exposure to these cytotoxic drugs does not diminish their susceptibility to NK cell killing.
Cytotoxicity of expanded NK cells against EWS in vivo
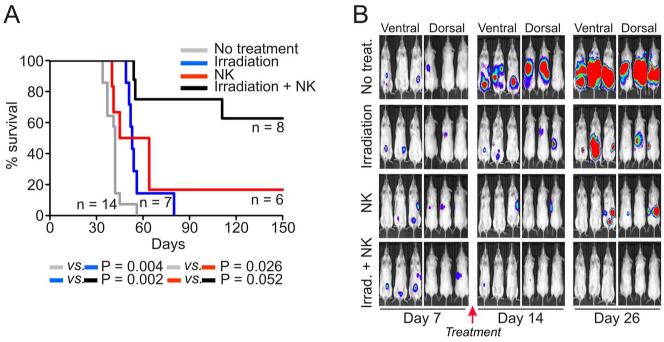
We generated a xenograft model of EFST by injecting luciferase-labeled ES8 cells in immunodeficient (NOD/scid IL2RGnull) mice (Fig. 6). ES8 cells (2 × 105) were injected i.p. in 14 mice; in all 14, ES8 cells formed tumors that progressively expanded. All 14 mice died or were euthanized because of signs of terminal illness and/or large tumor burden; median survival in this group was 42 days. Another group of 7 were injected with 2 × 105 ES8 cells i.p. and then irradiated 7 days later with 3.25 Gy. Irradiation prolonged survival (median, 53 days; P = 0.0043) but eventually all mice died. A third group of 6 mice was injected with an identical number of ES8 cells i.p. followed by 5 daily i.p. injections of expanded NK cells and IL-2 i.p. starting 7 days after tumor injection, a protocol previously shown to effectively eradicate leukemia in mice [32]. This group also showed a significant improvement in survival (median, 54.5; P = 0.0263) and 1 mouse survived disease-free for more than 100 days. Finally, we determined whether combining irradiation with NK cell therapy would further improved outcome: mice were irradiated on day 7 post-tumor engraftement with 3.25 Gy and then infused with NK cells (5 daily i.p. injections of expanded NK cells and IL-2 i.p starting approximately 1 hour after irradiation). Overall survival for the 8 mice treated with this approach was remarkably improved (P <0.0001), with 5 of the 8 mice surviving disease-free for more than 100 days.
Figure 6.
Anti-tumor capacity of expanded NK cells in vivo. (A) NOD/scid IL2RGnull were injected with 2 × 105 ES8 i.p. Seven days later mice were either irradiated with 3.25 Gy, treated with 1 × 107 expanded NK cells and 20000 IU IL-2 i.p. daily injections for 5 days with or without prior irradiation, or left untreated. Kaplan-Meier curves indicate the survival of each group of mice; P values in comparisons between groups by log rank test are shown. (B) Xenogen imaging of ES8-luciferase tumors in 4 groups of 3 mice each. Mice received the treatment described in A or no treatment.
DISCUSSION
The results of this study indicate that, among pediatric solid tumors, EWS cells are exquisitely sensitive to the cytotoxicity exerted by expanded, activated NK cells. Expanded NK cells could kill nearly all EWS cells within 4 hours at a 1 : 1 ratio, and still be considerably cytotoxic at a 1 : 10 or lower ratios if cultures were prolonged for 24 hours. Expanded NK cells had much more powerful anti-EWS capacity than primary, non-expanded, NK cells. The anti-EWS effect of NK cells was also seen in experiments with immunodeficient mice bearing EWS tumors, where NK cell infusions produced durable remissions. The interaction between NKG2D and DNAM-1 on the surface of NK cells with their ligands on tumors cells appears to be critical for NK cell cytotoxicity against EWS cells, as interference with these molecules considerably reduced cell killing. However, the key ligands remain to be identified.
The results with EWS cells recall those that we previously obtained with expanded NK cells against acute myeloid leukemia (AML) cells [32], suggesting that EWS and AML are the main targets for NK cell therapy of cancer in children. Most cell lines derived from other cancer cell types were also sensitive to expanded NK cell cytotoxicity. For example, rhabdomyosarcoma cells were as sensitive as EWS at 1 : 1 E :T ratio but less sensitive when the proportion of NK cells was reduced. The sensitivity of osteosarcoma cells varied widely. The molecular mechanisms underlying these differences remain to be elucidated but cannot be attributed to the levels of HLA Class I expression in the tumor cells alone. Neuroblastoma cell lines were significantly less sensitive than EWS cells to expanded NK cells, suggesting that neuroblastoma should have a lower priority among pediatric solid tumors eligible for NK cell therapy. NK cell cytotoxicity against neuroblastoma cells might be considerably enhanced by directing NK cells with antibodies reacting with neuroblastoma cell markers [46]. Moreover, Altvater et al. [47] using the method described here to expand NK cells demonstrated that cytotoxicity could be considerably enhanced by transducing NK cells with a chimeric signaling receptor recognizing the neuroblastoma cell marker GD2.
Expanded NK cells resulted in a substantial anti-tumor effect in mice engrafted with EWS cells, apparently achieving disease eradication in some animals. In our experiments, we also administered IL-2, as we previously observed that this cytokine significantly prolongs the survival of NK cells in immunodeficient mice [32]. Moreover, administration of IL-2 is often included in clinical NK cell infusions [17, 48]. We also administered multiple infusions of NK cells, as in preliminary experiments, single infusions of NK cells had anti-tumor activity but did not eradicate the disease. As a caveat, the xenografts that we included in our experiments are not orthotopic models of EWS, and NK cells might not migrate effectively to tissues were EWS tumor cells typically reside, resulting in suboptimal E : T ratios at the tumor site. However, we suggest that NK cell infusions might be more effective against tumor cells metastasizing to the bone marrow and lymphoid organs (tissues where NK cells thrive), rather than against primary tumor masses. Thus, infusions of allogeneic expanded NK cells should enhance the effects of chemotherapy followed by autologous hematopoietic cell transplantation, while reducing the risk of disease recurrence from carry over tumor cells infused with the autograft. Alternatively, NK cells could be directly injected into the tumor site after surgical removal, in efforts to deplete the margins of residual tumor cells.
To be effective, NK cell infusions must achieve E : T ratios estimated to exert substantial tumor cytoreduction. In this regard, the capacity of our method to specifically expand NK cells many fold, in addition to activate them, is particularly useful. Seven-day cultures, such as those used in this study, should yield sufficient NK cells for single infusions. Additional stimulation with K562-mb15-41BBL cells and extended cultures can dramatically increase the number of NK cells obtained, which should be adequate for multiple infusions. The NK expansion method described here has now been adapted to large scale, clinical grade, conditions, and a clinical protocol testing the feasibility and toxicity of this approach is now open. Because of the results of this study, eligibility includes patients with EWS and rhabdomyosarcoma.
Supplementary Material
Acknowledgments
This work was supported by grants CA113482, CA70089 and CA21765 from the National Cancer Institute, a grant from the Fondation des Gouverneurs de l’espoir, and by the American Lebanese Syrian Associated Charities (ALSAC)
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE
Infusions of NK cells are being increasingly considered as a means to improve cancer therapy. In efforts to develop new therapies for children with solid tumors, we sought to identify the tumor subtypes that were most sensitive to the cytotoxicity of natural killer (NK) cells expanded with a method developed in our laboratory. We found that Ewing sarcoma and rhabdomyosarcoma cells were exquisitely sensitive to these NK cells, which were considerably more cytototoxic than unstimulated NK cells and eradicated sarcoma cells engrafted in immunodeficient mice. Because of the results of this study, patients with Ewing sarcoma and rhabdomyosarcoma are now eligible for enrollment in a recently initiated clinical study testing infusions of allogeneic expanded NK cells.
REFERENCES
- [1].Rodriguez-Galindo C, Liu T, Krasin MJ, et al. Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children’s Research Hospital studies. Cancer. 2007;110:375–84. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- [2].Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–9. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Heare T, Hensley MA, Dell’Orfano S. Bone tumors: osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr. 2009;21:365–72. doi: 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- [4].Rodeberg DA, Stoner JA, Hayes-Jordan A, et al. Prognostic significance of tumor response at the end of therapy in group III rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol. 2009;27:3705–11. doi: 10.1200/JCO.2008.19.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–58. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- [6].Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–13. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108–14. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- [8].Miser JS, Krailo MD, Tarbell NJ, et al. Treatment of metastatic Ewing’s sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide--a Children’s Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22:2873–6. doi: 10.1200/JCO.2004.01.041. [DOI] [PubMed] [Google Scholar]
- [9].Miser JS, Goldsby RE, Chen Z, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy-a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49:894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- [10].Kolb EA, Kushner BH, Gorlick R, et al. Long-term event-free survival after intensive chemotherapy for Ewing’s family of tumors in children and young adults. J Clin Oncol. 2003;21:3423–30. doi: 10.1200/JCO.2003.10.033. [DOI] [PubMed] [Google Scholar]
- [11].Bacci G, Briccoli A, Longhi A, et al. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. 2005;44:748–55. doi: 10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- [12].Leavey PJ, Mascarenhas L, Marina N, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:334–8. doi: 10.1002/pbc.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- [14].Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–9. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- [15].Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–50. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- [17].Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in cancer patients. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- [18].Atzpodien J, Gulati SC, Shimazaki C, et al. Ewing’s sarcoma: ex vivo sensitivity towards natural and lymphokine-activated killing. Oncology. 1988;45:437–43. doi: 10.1159/000226661. [DOI] [PubMed] [Google Scholar]
- [19].Chin T, Toy C, Vandeven C, Cairo MS. Lymphokine-activated killer cytotoxicity in neonatal mononuclear cells: in vitro responses to tumor cell lines from pediatric solid tumors. Pediatr Res. 1989;25:156–60. doi: 10.1203/00006450-198902000-00016. [DOI] [PubMed] [Google Scholar]
- [20].Verhoeven DH, de Hooge AS, Mooiman EC, et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol. 2008;45:3917–25. doi: 10.1016/j.molimm.2008.06.016. [DOI] [PubMed] [Google Scholar]
- [21].Kubista B, Trieb K, Blahovec H, Kotz R, Micksche M. Hyperthermia increases the susceptibility of chondro- and osteosarcoma cells to natural killer cell-mediated lysis. Anticancer Res. 2002;22:789–92. [PubMed] [Google Scholar]
- [22].Honorati MC, Neri S, Cattini L, Facchini A. IL-17 enhances the susceptibility of U-2 OS osteosarcoma cells to NK cell lysis. Clin Exp Immunol. 2003;133:344–9. doi: 10.1046/j.1365-2249.2003.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alvarado CS, Findley HW, Chan WC, et al. Natural killer cells in children with malignant solid tumors. Effect of recombinant interferon-alpha and interleukin-2 on natural killer cell function against tumor cell lines. Cancer. 1989;63:83–9. doi: 10.1002/1097-0142(19890101)63:1<83::aid-cncr2820630114>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [24].Rossi AR, Pericle F, Rashleigh S, Janiec J, Djeu JY. Lysis of neuroblastoma cell lines by human natural killer cells activated by interleukin-2 and interleukin-12. Blood. 1994;83:1323–8. [PubMed] [Google Scholar]
- [25].Castriconi R, Dondero A, Corrias MV, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–4. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- [26].Castriconi R, Dondero A, Cilli M, et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother. 2007;56:1733–42. doi: 10.1007/s00262-007-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Perez-Martinez A, Leung W, Munoz E, et al. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr Blood Cancer. 2009;53:120–4. doi: 10.1002/pbc.21955. [DOI] [PubMed] [Google Scholar]
- [28].Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. %20-26. [DOI] [PubMed] [Google Scholar]
- [29].Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. 225-74. [DOI] [PubMed] [Google Scholar]
- [31].Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. Br J Haematol. 2009;145:606–13. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith MA, Morton CL, Phelps D, Girtman K, Neale G, Houghton PJ. SK-NEP-1 and Rh1 are Ewing family tumor lines. Pediatr Blood Cancer. 2008;50:703–6. doi: 10.1002/pbc.21099. [DOI] [PubMed] [Google Scholar]
- [35].Dunn T, Praissman L, Hagag N, Viola MV. ERG gene is translocated in an Ewing’s sarcoma cell line. Cancer Genet Cytogenet. 1994;76:19–22. doi: 10.1016/0165-4608(94)90063-9. [DOI] [PubMed] [Google Scholar]
- [36].Martinez-Ramirez A, Rodriguez-Perales S, Melendez B, et al. Characterization of the A673 cell line (Ewing tumor) by molecular cytogenetic techniques. Cancer Genet Cytogenet. 2003;141:138–42. doi: 10.1016/s0165-4608(02)00670-2. [DOI] [PubMed] [Google Scholar]
- [37].Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–40. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- [38].McAllister RM, Melnyk J, Finkelstein JZ, Adams EC, Jr., Gardner MB. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969;24:520–6. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [39].Tobey RA, Tesmer JG. Differential response of cultured human normal and tumor cells to trace element-induced resistance to the alkylating agent melphalan. Cancer Res. 1985;45:2567–71. [PubMed] [Google Scholar]
- [40].Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–52. [PubMed] [Google Scholar]
- [41].Ponten J, Saksela E. Two established in vitro cell lines from human mesenchymal tumours. Int J Cancer. 1967;2:434–47. doi: 10.1002/ijc.2910020505. [DOI] [PubMed] [Google Scholar]
- [42].McAllister RM, Gardner MB, Greene AE, Bradt C, Nichols WW, Landing BH. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971;27:397–402. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [43].Billiau A, Edy VG, Heremans H, et al. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977;12:11–5. doi: 10.1128/aac.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li L, Liu LN, Feller S, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147–54. doi: 10.1038/cgt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rgammanull mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- [46].Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12:1750–9. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–66. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.