Abstract
Background
Medical settings such as emergency departments (EDs) present an opportunity to identify and provide services for individuals with substance use problems who might otherwise never receive any form of assessment, referral, or intervention. Although Screening, Brief Intervention, and Referral to Treatment (SBIRT) models have been extensively studied and are considered effective for individuals with alcohol problems presenting in emergency departments and other medical settings, the efficacy of such interventions has not been established for drug users presenting in EDs.
Objectives
This paper describes the design of a NIDA Clinical Trials Network protocol testing the efficacy of an SBIRT model in medical EDs, highlighting considerations that that are pertinent to the design of other studies targeting substance use behaviors in medical treatment settings.
Methods
The protocol is described, and critical design decisions are discussed.
Results
Design challenges included defining treatment conditions, study population, and site characteristics; developing the screening process; choosing the primary outcome; balancing brevity and comprehensiveness of assessment; and selecting the strategy for statistical analysis.
Conclusion
Many of the issues arising in the design of this study will be relevant to future studies of interventions for addictions in medical settings.
Scientific Significance
Optimal trial design is critical to determining how best to integrate substance abuse interventions into medical care.
BACKGROUND
Harmful or hazardous use of drugs and alcohol has a tremendous impact on individual health status, contributing to a variety of medical conditions having high levels of associated mortality and morbidity (1), and increasing healthcare costs. For these reasons, there has been an increased focus on developing, implementing, and evaluating methods to identify and provide appropriate services to non-treatment-seeking individuals with substance use problems who are seen in health care settings. The Emergency Department (ED) appears to be a particularly promising setting in which to identify and engage problematic drug users. Relatively high rates of psychoactive substance use disorders have been found in EDs (2–3), exceeding that found in primary care settings (4–5). This has led clinicians and researchers to argue for the development of more effective methods of screening and case finding, brief interventions and referral to appropriate specialty treatment, as well as linkage between substance abuse services and general medical care, for individuals using illicit drugs who are seen in EDs and trauma centers (2, 6–7).
Compared to interventions dealing with hazardous or harmful drinking in ED settings, the evidence of the effectiveness of brief interventions has been considered only suggestive for drug use disorders (8). However, recent data suggest that that the effectiveness of such interventions may extend to drugs as well (9–13). The present study builds on the extensive experience in developing and implementing screening and brief interventions for harmful and hazardous alcohol use delivered in EDs and trauma centers, transferring and evaluating these procedures when applied to drug use in a multi-site study conducted by the NIDA Clinical Trials Network (NIDA CTN). This study breaks new ground for the CTN in that it is the first full scale multisite trial to be conducted by the CTN in a medical setting. Design of the study required consideration of a number of issues that may be important in the design of other studies investigating substance use interventions in medical settings. As such, a description of the study design and considerations that went into key design decisions is likely to be useful to others interested in the testing of substance use interventions throughout the health care system.
STUDY DESIGN OVERVIEW
Specific Aims
The primary aim of the study is to contrast substance use and substance-related outcomes among patients who endorse problematic substance use during an ED visit and are randomly assigned to one of three treatment conditions: 1) minimal screening only (MSO); 2) screening, assessment, and referral to treatment (if indicated or requested) (SAR); and 3) screening, assessment, and referral (if indicated or requested) plus a brief intervention (BI) with two telephone follow-up booster sessions (BI-B). We expect to observe significant differences among the three groups with respect to the primary outcome variable, days of use of the patient-defined primary problem substance during the 30 days prior to the 3-month assessment, as measured by the Time-Line Follow-Back (14). Secondary aims are to contrast the three groups with respect to treatment engagement and healthcare utilization, test the effect of several hypothesized moderators of outcome, and evaluate the dose-response effect of the number of booster sessions received among participants in the BI-B group.
Study Design
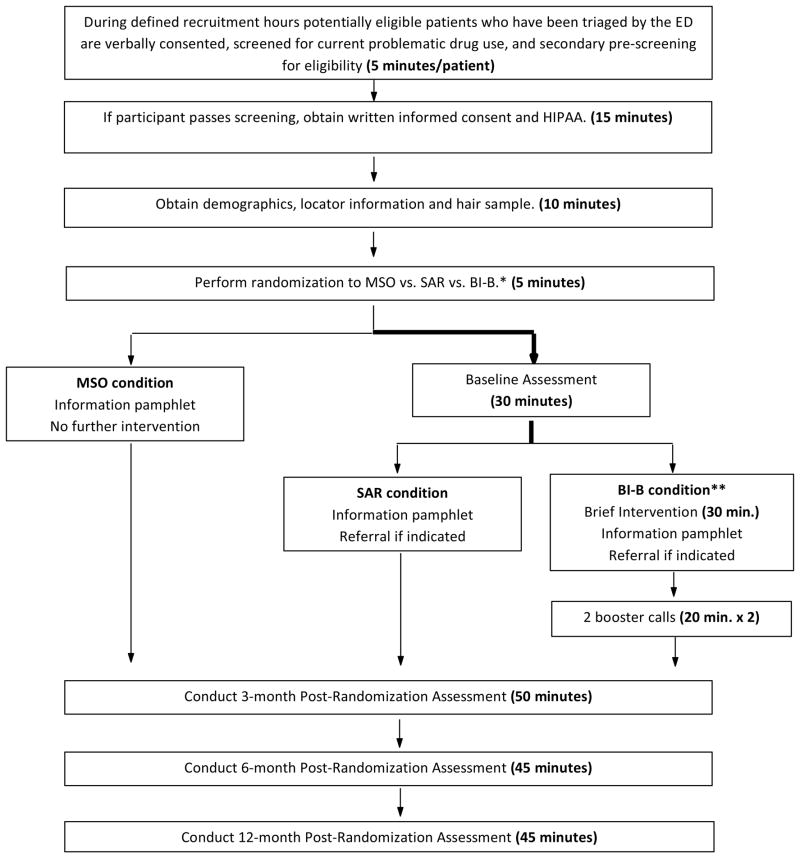
The project is a 3-group randomized, prospective trial with blinded assessments (See Figure 1). Individuals presenting to an ED who endorse problematic substance use on screening will be randomized in 1:1:1 ratio to MSO vs. SAR vs. BI-B. Randomization will occur after screening, and those randomized to MSO will receive an informational pamphlet and will not receive further assessment until follow-up at 3 months. Participants in the other two groups will receive a more extensive baseline assessment. To preserve the blind during the baseline assessment, assignment to the remaining two groups (SAR vs. BI-B) will not be revealed to either the participant or the research assistant until after the baseline assessment is complete. Those in the SAR group will be provided with an informational pamphlet and receive referral to addiction treatment if indicated (based on an ASSIST score of ≥ 27 for any drug or alcohol), while those assigned to the BI-B group will receive a brief intervention consisting of motivational enhancement therapy (MET) adapted for use in the ED, followed by the informational pamphlet and referral if indicated or requested. The BI-B group will also receive two booster telephone calls which will occur within 1 week of enrollment if possible, and in no cases later than 1 month following enrollment. Follow-up assessments will be conducted face-to-face at 3 months, 6 months, and 12 months post-enrollment.
Figure 1. Study Design.
*Allocation to SAR vs. BI-B is concealed until after completion of assessment.
**BI-B participants receive informational pamphlet and referral if indicated as part of the Brief Intervention.
CRITICAL DESIGN DECISIONS
Three-arm design
The issue of assessment reactivity has plagued brief intervention research. The impact of a comprehensive assessment on substance abuse history, treatment engagement and outcome can exceed that of the therapeutic intervention under investigation (15–17). This is particularly problematic when the intervention is so brief that the intensity of assessment (in terms of time spent by the participant with research staff) is comparable to that of the intervention. Without a “no assessment” or “minimal assessment” control, it is not possible to determine how much the assessment contributed to outcome, or, in the case of a null finding, whether it masked the effects of the therapeutic intervention.
A three-arm design was adopted to assess the independent contributions of assessment and brief intervention to outcomes in the study population. The contrast between the BI-B and the SAR groups can illuminate the effect of adding the brief intervention plus booster calls to assessment and referral. However, it tells us nothing about the effect of the assessment process on subsequent substance use outcomes. The inclusion of the MSO group, which receives as little interaction as possible at baseline, provides a control by which to measure the assessment effect. The comparison of BI-B to MSO provides a measure of the total effect of the intervention (including any therapeutic effects of assessment and referral). The MSO condition offers a better approximation of the true “treatment as usual” than SAR. Since the SAR condition represents a significantly greater intensity of intervention than MSO, the potential difference in outcome between SAR and MSO is of considerable interest. SAR may be considered a minimal intensity active intervention (in relation to MSO) as well as a control (in relation to BI-B). Thus each significant difference between groups, if found, would provide meaningful evidence of treatment effect, but with different implications for practice. An obvious limitation of this procedure is that the effects of the minimal screening, which cannot be assumed to be negligible, cannot be assessed. However, in this context there is no way to get around the necessity of asking participants some questions about substance use to assess eligibility for the trial.
Defining the population
Inclusion and exclusion criteria were chosen to achieve an acceptable balance between maximizing generalizability and minimizing heterogeneity, and to enroll a population that had a good chance of successfully completing study procedures and follow-up assessments. We did not find evidence to predict that the magnitude of the treatment effects would differ by primary drug of abuse or by severity of substance use problems (11, 18–19). We therefore chose to allow participants to qualify on the basis of use of any drug other than alcohol or nicotine, and included participants with a fairly broad range of current problematic use (described below). Other inclusion criteria include the following: registration as patient in the ED during study screening hours (Participants must be screened and randomized during their ED treatment, but will be retained in the study if subsequently admitted to the hospital); age 18 years or older (Including the adolescent population would introduce too much heterogeneity); English proficiency and literacy (Participants will be required to complete self-report questionnaires); ability to provide informed consent; and access to a working telephone (for booster sessions). Exclusion criteria are driven by ethical issues and the desire to maximize treatment effect and retention: inability to participate due to current ED treatment; significant impairment of cognition or judgment compromising capacity for informed consent; status as a prisoner at the time of treatment; current engagement in addiction treatment; residence more than 50 miles from the location of follow-up visits; inability to provide sufficient contact information (at least 2 reliable locators); and prior participation in the current study. Alcohol or nicotine use at any level are not exclusionary, nor is use of any secondary drug.
Choosing the sites
Because the efficacy of brief interventions for drug use disorders in the ED is not yet established, site selection placed a greater emphasis on internal validity than has been the case for most prior CTN studies. The study is being conducted in only 6 sites because using a smaller number of the most highly qualified sites is likely to result in more uniform and higher quality implementation of study procedures. Desirable sites are EDs that see a large volume of drug using patients, have prior research experience, are not currently using an SBIRT model for patients who use drugs, and have adequate staff and space to conduct the study. Most importantly, we required the demonstration of strong leadership for the protocol within the ED, in addition to the clinical trials support provided by the CTN Node. Finally, it is essential that the sites in aggregate provide a population broadly representative of the US population (ethnic and socioeconomic variability, urban and rural populations, etc.).
Screening
The initial screening in this study must define a population of patients who are likely to have problematic drug use, abuse, or dependence for inclusion in the study. In other words, the procedure needs to have a high positive predictive value (probability of the disorder given a positive test), whereas clinical screening instruments are designed to have high sensitivity (probability of a positive test given the disorder). The screening process must be relatively brief due to the pace and acuity of ED practice. Further, in order to minimize assessment reactivity in the MSO group, the substance-related screening questions must be kept to an absolute minimum.
In addition to assessing degree of drug use, the pre-screening questionnaire must 1) assess the degree of alcohol and drug problem severity, which will be used as stratification variables and thus needs to be determined prior to randomization; 2) determine the primary drug of abuse, the number of days it was used in the past 30 days, and the substance-relatedness of the ED visit; and 3) minimize potential participant discomfort with answering sensitive questions about drug use.
Screening instrument
No existing screening instrument was ideal for our purposes. Some instruments commonly used to screen for substance use disorders in medical settings are too long, such as the World Health Organization Alcohol Smoking, Substance Involvement Screening Test (ASSIST) (20), used as part of the assessment battery in this trial. A two-item screen for both alcohol and drug use disorders (21) was found to have a positive predictive value of 51.8% in a primary care population, but this instrument does not distinguish between alcohol and drugs. Other short instruments, such as the Case finding and Help Assessment Tool (CHAT) (22) have only partial validation, and it was not possible to say where its cut-off point would lie in relation to well-known instruments such as the Drug Abuse Screening Test (DAST) (23).
To satisfy the requirements of the study, we constructed a 20-item composite screening instrument of four sections, three of which comprise validated scales. The instrument begins with the 4-item Heavy Smoking Index (24–25) to avoid initiating the questionnaire with drug-related questions. Alcohol involvement is assessed using the 3-question Alcohol Use Disorders Identification Test (26–27), which performs about as well as the full AUDIT in identifying alcohol use disorders (27). Drug use is assessed using the 10-item DAST (23), which has moderate to high validity, sensitivity, and specificity (28). Finally, 3 questions determine primary substance of abuse, days of use of the primary substance, and substance-relatedness of the ED visit. The screen will be considered positive only if the participant has a score ≥ 3 on the DAST-10 and reports past 30-day use of the primary substance. The requirement for past 30-day use increases the stringency of the screen above that of the DAST drug use questions alone.
Procedure
To the extent possible, study staff will attempt to screen all potentially eligible patients (or a representative sample, e.g. every other patient) who register at the ED during defined recruitment hours. Specific details of the screening process will be different across sites due to the variability in patient flow and clinical routine among EDs. The research assistant (RA) will approach triaged ED patients and ask if they are willing to participate in an anonymous screening. Using a brief IRB-approved script patients will be asked to provide verbal consent for the anonymous collection of screening data. The screening data will be collected by the RA and by participant self-report using direct entry into tablet computers to facilitate rapid screening and mobility within the ED setting.
Designing the interventions
Brief intervention
Because the efficacy of brief interventions for drug use disorders in the ED is not well established, the intervention for this study is intended to be as robust as is practical for use in the ED. The duration of up to 30 minutes is significantly longer than that used in most prior ED SBIRT studies. While in the ED either waiting to be seen or in a room, participants randomized to the BI-B group will receive an in-person manual-guided brief intervention based on motivational interviewing principles, including feedback based on screening information, the FRAMES heuristic, and development of a change plan, as delivered in previous trauma center trials and the WASBIRT projects (29–33). BI-B participants will receive the informational pamphlet and referral for treatment (if indicated) from the BI interventionist. Brief interventions will be done by members of the study staff cross trained as research assistants conducting screening and assessments for the study as well as providing the intervention. The brief intervention will be performed by these study interventionists/RAs who have received training and will receive regular fidelity monitoring and ongoing supervision. For a given study participant, the brief intervention will be performed by an RA who has not conducted assessments with that individual.
Telephone booster sessions
Brief motivational interventions may have somewhat more limited effectiveness with drug abusers than with alcohol-involved individuals with respect to engaging them into treatment (34–35) or modifying their drug use (36–38); what positive effects are obtained deteriorate over time (39). Thus the “teachable moment” associated with care in an ED may fade rapidly as the time increases between the intervention and identification of a substance use disorder, leading to the recommendation that “booster sessions” be provided, either in person or via phone (9, 40–41).
Therefore, in addition to the initial brief intervention, all BI-B participants who can be reached will receive 2 telephone “booster” sessions in which the interventionist will check to see whether they have engaged in treatment, review and reinforce change plans, and seek a commitment from them (42). Each of these booster calls will be approximately 20 minutes long. The content of these boosters is patterned after sessions in MET (40, 43) and is similar to those previously used in EDs (42, 44), and primary care (45). In order to capitalize on the motivational effects of both the ED visit and the brief intervention, the target window for the initial phone booster call will be within 3 days of discharge from the ED. The second call ideally will be made within 7 days of discharge from the ED. If initial attempts to complete the booster sessions are unsuccessful, further attempts to engage participants will be made for up to one month post-discharge from the ED. Booster calls will be made from a centralized, study-wide intervention booster call center by interventionists who receive standardized training and ongoing supervision similar to that of those providing the initial brief intervention in the ED. Centralization of the booster calls is intended to standardize the process and also to ensure that sufficient attention is given to completing these calls.
Choosing the primary outcome variable and time point
It was necessary to choose a primary outcome variable that is valid across users of a variety of drugs used alone or in varying combinations. Because the focus of the brief intervention is on the patient-identified primary drug, use of that drug is a logical outcome measure. We considered including all drugs by using abstinent days as the primary outcome. However, this measure could be insensitive to improvement in patients who are using multiple drugs, and would not distinguish between substances that are more or less problematic. For example, a participant using marijuana for 30/30 days but no other drug would be rated the same as a patient using marijuana, heroin, and crack cocaine on each day. We also considered incorporating intensity of drug use in to the primary outcome, e.g., number of times used or dollar amount. However, these measures do not behave similarly for different substances of abuse. We therefore chose days of use of the primary problem substance as the primary outcome.
We chose the 3-month time point for the primary outcome because treatment effects for motivational interventions tend to attenuate with time (18, 39) (although there are examples of sleeper effects in brief intervention studies conducted in trauma centers (46–47). Follow-up rates are also expected to be higher at earlier time points, providing an advantage in statistical power and representativeness of the sample. On the other hand, a follow-up of less than three months would not provide adequate evidence of lasting behavior changes.
Keeping it lean
The selected assessment battery attempts to balance the value of comprehensive data against the costs of data collection in terms of staff time, feasibility of completion in the ED, financial cost, and assessment reactivity (discussed above). Lengthy assessments may be difficult to implement consistently in ED settings due to the pace of emergency care and the variable, often short time period during which the assessment can be completed. Given that the study is testing the efficacy of a relatively low-intensity intervention that could have a fairly small effect, a relatively large sample size is required, and the size of the assessment battery has a large impact on the cost of the study. Therefore, several domains traditionally assessed in addiction treatment trials, such as psychiatric and substance use diagnoses, quality of life, and potential mediators such as motivation and self-efficacy, were intentionally omitted. Included assessments are limited to those that contribute directly to the objectives of the study or are necessary for safety or regulatory compliance.
Table 1 illustrates the assessment battery used in the study. The Time-Line Follow-Back (TLFB; Sobell & Sobell, 1992, 1995) is the primary measure of substance use. This procedure quantifies use of each class of substance for each day in the assessment period. The NIDA-modified version (NM-ASSIST, http://www.nida.nih.gov/nidamed/) of the WHO ASSIST (20) is a secondary measure of substance use and consequences. Hair testing is the objective measure of substance use. Hair testing lacks temporal resolution, does not allow quantification of substance use, and is relatively expensive. However, it validly measures within-subject change and covers a 3-month period with a single sample, a great advantage for the current study as frequent urine drug testing introduces serious of assessment reactivity issues. In order to assess addiction treatment engagement and other health care utilization, an abbreviated version of the Treatment Services Review (TSR) (48) will be administered. A short non-study treatment form is used to capture non-study treatment received related to the index ED visit. Participants receiving a brief intervention will complete the 12-item version of the Working Alliance Inventory (WAI-SR) (49), based Bordin’s (50) formulation of working alliance. Readiness rulers will measure motivation during the brief intervention session. Finally, all participants will complete the 25-item Barriers to Treatment Inventory (51) at 3-month follow-up.
Table 1.
Assessment and Intervention Schedule
| CRF | Est. Time (Minutes) | Done By | Screen MSO | Screen SAR | Screen BI-B | Booster 1 BI-B | Booster 2 BI-B | 3 Month | 6 Month | 12 Month |
|---|---|---|---|---|---|---|---|---|---|---|
| Tobacco, Alcohol, & Drug Questionnaire | 5 | PT | X | X | X | |||||
| Secondary Pre-Screening Form | 2 | RA/PT | X | X | X | |||||
| Informed Consent | 10 | RA/PT | X | X | X | |||||
| Locator Information Form | 5 | RA/PT | X | X | X | |||||
| Update Baseline Locator Information Form | 5 | RA | X | X | X | |||||
| Hair Analysis Form | 2 | RA | X | X | X | X | X | X | ||
| Pamphlet | 1 | RA | X | X | X | |||||
| NIDA-Modified ASSIST V1.0 | 10 | RA/PT | X | X | X | X | X | |||
| Time-Line Follow-Back Assessment Period Form | 3 | RA | X | X | X | X | X | |||
| Time-Line Follow-Back | 10 | RA/PT | X | X | X | X | X | |||
| Working Alliance Inventory - Short Form - Revised | 5 | PT | X | |||||||
| Referral Form | 2 | RA | X | X | X | |||||
| Referral List | 1 | RA | * | * | ||||||
| Brief Intervention | 30 | INT | X | |||||||
| Booster By Phone | 20 | TBSI | X | X | ||||||
| Treatment Services Review | 10 | RA/PT | X | X | X | |||||
| Barriers to Treatment | 5 | PT | X |
Administer when indicated
Completed by RA based on review of medical record approximately 1 month following the index ED visit
MSO = Minimal Screening Only
SAR = Screening, Assessment, and Referral
BI-B = Brief Intervention plus telephone Booster sessions
RA=Research Assistant
INT=Interventionist
TBSI=Telephone Booster Session Interventionists
PT=Participant
All outcome measures for the planned analyses are derived from the TLFB, the NM ASSIST, and the TSR. The primary outcome, discussed above, is captured by the TLFB. Secondary outcomes from the TLFB data at each available time point are days of primary substance use, days of abstinence from all drugs, days of heavy drinking, quantity of primary drug use, and change in primary drug use relative to baseline (SAR and BI-B groups only). Additional secondary outcomes at each follow-up time point include relative change in hair sample results for the primary substance and each individual drug of abuse; Consequences of drug use (based on the NM ASSIST), participation in addiction treatment (from the TSR), and health care utilization (from the TSR).
Data analysis
It was not immediately clear whether it would be more effective to base the primary analysis on the value of the outcome variable or its change from baseline. Simulations were used to compare the observed statistical power using the following outcomes as a function of the within-group pre-post correlation in the outcome variable.
A - analyze 3-month outcomes only
B - analyze change only
C - Use baseline as a fixed-effect covariate to analyze 3-month outcomes
D – At trial’s end, calculate the average pre-post correlation within the two treatment groups. If this exceeds 0.5, analyze changes. If it is less than 0.5, analyze 3-mo outcome.
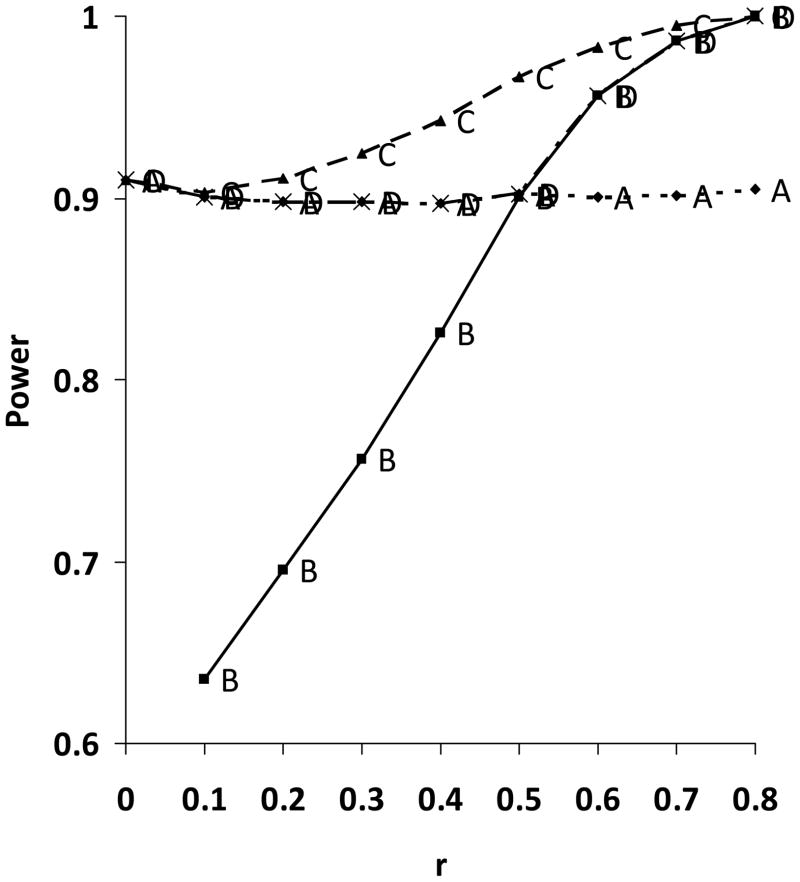
As seen in Figure 2, for the approaches incorporating the baseline value, the power decreases markedly as the pre-post correlation decreases. However, at all values of the pre-post correlation, the greatest power was observed for the method based on 3-month outcome including the baseline value as a covariate. Based on the WASBIRT study data we expect a pre-post correlation of approximately 0.31. In this case using an approach based on change scores would have been detrimental to power.
Figure 2. Power calculations based on simulations using 4 different analysis strategies.
- A - Analyze 3-month outcomes only.
- B - Analyze change only.
- C - Use baseline as a fixed-effect covariate to analyze 3-month outcomes.
- D - At trial’s end, calculate the average pre-post correlation within the two treatment groups. If this exceeds 0.5, analyze changes. If it is less than 0.5, analyze 3-mo outcome.
The primary analyses will make all three pair-wise comparisons between MSO, SAR and BI-B with respect to the primary outcome variable using a simple closed testing procedure to control family-wide type I error at no more than 0.05. The primary outcome will be analyzed according to the intent-to-treat principle in the sense that patients will be considered to belong to the randomized group even though they may not be fully adherent to the prescribed treatment or follow-up. Patients who refuse treatment will still be followed for outcome in their assigned group, but no attempt will be made to impute outcomes for patients who refuse follow-up.
The primary analysis will compare the primary outcome between each pair of treatments using a linear mixed model taking into account possible variability in the overall level of drug use between the sites, possible site-by-treatment interaction, and the level of a baseline covariate (number of days use of the primary substance of abuse in the 30 days prior to baseline assessment). Site and site-by-treatment interaction will be included as random effects.
Sample Size and Statistical Power
Based on data from the WASBIRT study we estimate the mean number of use days within prior 30 days (as evaluated at 3 months post intervention) in the SAR group to be ms(SAR) = 14 days with standard deviation (SD) equal to 11 days. We considered 3 days to be a clinically significant difference in days of use. Considering type I error α=0.05/3 (two-tailed) and 15% attrition, one needs 1285 subjects (429 in each arm) to have 90% power.
DISCUSSION
The evidence for SBIRT targetting drug use in ED settings is unusual in that effectiveness studies were conducted prior to definitive efficacy studies. Although the results from the CSAT-funded project are quite promising with respect to the effects of SBIRT interventions on drug use, controlled trials are required to support causal inference. To justify large-scale change in practice, clear demonstration of benefit associated with the higher level of assessment and intervention is required. Because of these considerations, the design of the study represents a hybrid between efficacy and effectiveness models (52), and is closer to the efficacy end of this spectrum than most of the trials that have been conducted in the NIDA CTN. Design consequences include use of a relatively intensive brief intervention; tight control over intervention and assessments; booster sessions conducted through a call center rather than the ED; relatively few, experienced sites; and relatively narrow, proximal outcome measures. On the other hand, the patient population remains broadly representative of the ED population, consistent with the effectiveness model, because there was no clear scientific advantage to limiting the study to a narrower group.
Another set of considerations has to do with the concern about possible assessment reactivity. This issue is relevant to all clinical trials, but is of greater concern for relatively brief psychosocial interventions where the effect size is expected to be relatively small. These considerations implied the need for brevity of screening and assessment procedures, which in turn required hard choices about what instruments to include and what to omit. While the selected assessment battery is sufficient to address the aims of the study, its limits the ability to examine a number of potentially meaningful mediators of change.
The issue of assessment reactivity in studies of SBIRT models can be reframed as a question concerning the efficacy of specific components of the intervention, i.e. the screening and assessment that precede the brief intervention. The addition of a minimal screening arm to this study provides a means to quantify the effects of the more extensive assessment, coupled with referral if indicated, relative to this minimal screening condition. This dismantling of the SBIRT model provides information on the value of the screening and referral process as a stand-alone intervention in the ED.
An interesting and generalizable finding in the development of the statistical analysis plan was the clear superiority, given the expected low within-group pre-post correlations, of the approach based on the value of the primary outcome variable at the primary endpoint with the inclusion of the baseline value as a covariate, rather than the approach based on change in the primary outcome variable. This approach is superior over the full range of pre-post correlations tested, but the difference is pronounced at lower pre-post correlations.
Brief interventions are an attractive method for engaging patients which substance use disorders who present for treatment across a wide range of medical settings. However, they are far from the only possible form of addiction intervention to be used in medical settings. Brief treatments involving multiple sessions provided by an addiction treatment specialist are possible in some settings (12). Indeed, our own brief intervention pushes the SBIRT model in this direction by providing up to 70 minutes of treatment over three sessions (one on-site intervention plus two telephone boosters). More active referral procedures may be a useful feature in some settings. Medical settings also present an opportunity for rapid induction of pharmacotherapy once a substance use disorder has been identified. Clearly we are just beginning to scratch the surface of what can be done to identify and address substance use disorders in the context of medical treatment. Optimal trial design is critical to determining how best to integrate substance abuse interventions into medical care.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (U10DA020024, Adinoff, PI; U10DA015833, Bogenschutz, PI; U10DA013714, Donovan, PI). The following individuals made significant contributions to the development of this protocol: Collen Allen, Cindy Claassen, Debbie Drosdick, Andrzej Kosinski, Jeff Sharp, Rose Singh, and Paul Wakim.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA: Journal of the American Medical Association. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Rockett IRH, Putnam SL, Jia H, Chang CF, Smith GS. Unmet substance abuse treatment need, health services utilization, and cost: A population-based emergency department study. Annals of emergency medicine. 2005;45(2):118–27. doi: 10.1016/j.annemergmed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Rockett IRH, Putnam SL, Jia H, Smith GS. Declared and undeclared substance use among emergency department patients: a population-based study. Addiction. 2006;101(5):706–12. doi: 10.1111/j.1360-0443.2006.01397.x. [DOI] [PubMed] [Google Scholar]
- 4.Calle PA, Damen J, De Paepe P, Monsieurs KG, Buylaert WA, Belg AC. A survey on alcohol and illicit drug abuse among emergency department patients. Acta Clinica Belgica. 2006;61(4):188–95. doi: 10.1179/acb.2006.033. [DOI] [PubMed] [Google Scholar]
- 5.Cherpitel CJ, Ye Y. Drug use and problem drinking associated with primary care and emergency room utilization in the US general population: Data from the 2005 national alcohol survey. Drug and Alcohol Dependence. 2008;97(3):226–30. doi: 10.1016/j.drugalcdep.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn CW, Ries R. Linking substance abuse services with general medical care: Integrated, brief interventions with hospitalized patients. American Journal of Drug & Alcohol Abuse. 1997;23(1):1–13. doi: 10.3109/00952999709001684. [DOI] [PubMed] [Google Scholar]
- 7.Lucas CE. The impact of street drugs on trauma care. Journal of Trauma. 2005;59(3 Suppl):S57–60. doi: 10.1097/01.ta.0000176044.21200.b0. [DOI] [PubMed] [Google Scholar]
- 8.Babor TF, Kadden RM. Screening and interventions for alcohol and drug problems in medical settings: what works? Journal of Trauma. 2005;59(3 Suppl):S80–7. doi: 10.1097/01.ta.0000174664.88603.21. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein E, Edwards E, Dorfman D, Heeren T, Bliss C, Bernstein J. Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Academic Emergency Medicine. 2009;16(11):1174–85. doi: 10.1111/j.1553-2712.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estee S, He L. Use of alcohol and other drugs declined among emergency department patients who received brief interventions for substance use disorders through WASBIRT. Olympia, WA: Washington State Department of Social and Health Services, Analysis RaD; 2007. Contract No.: 4.60.WA.2007.1. [Google Scholar]
- 11.The InSight Project Research Group. SBIRT outcomes in Houston: Final report on InSight, a hospital district-based program for patient at risk for alcohol or drug use problems. Alcoholism: Clinical and Experimental Research. 2009;33(8):1374–81. doi: 10.1111/j.1530-0277.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- 12.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: Comparison at intake and 6 months later. Drug and alcohol dependence. 2009;99(1–3):280–95. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humeniuk R, Dennington V, Ali R. The effectiveness of a brief intervention for illicit drugs linked to the alcohol, smoking and substance involvement screening test (ASSIST) in primary health care settings: a technical report of phase III findings of the WHO ASSIST randomized controlled trial. 2008 [Google Scholar]
- 14.Sobell LC, Sobell MB. Timeline Follow Back: A calendar method for assessing alcohol and druq use (User’s Guide) Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- 15.Clifford PR, Maisto SA. Subject reactivity effects and alcohol treatment outcome research. J Stud Alcohol. 2000;61(6):787–93. doi: 10.15288/jsa.2000.61.787. [DOI] [PubMed] [Google Scholar]
- 16.Clifford PR, Maisto SA, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: part I. Alcohol use and related consequences. J Stud Alcohol Drugs. 2007;68(4):519–28. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- 17.Maisto SA, Clifford PR, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: part II. Treatment engagement and involvement. J Stud Alcohol Drugs. 2007;68(4):529–33. doi: 10.15288/jsad.2007.68.529. [DOI] [PubMed] [Google Scholar]
- 18.Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 19.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: Comparison at intake and 6 months later. Drug and alcohol dependence. 2008 doi: 10.1016/j.drugalcdep.2008.08.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction (Abingdon, England) 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown RL, Leonard T, Saunders LA, Papasouliotis O. A two-item conjoint screen for alcohol and other drug problems. The Journal of the American Board of Family Practice/American Board of Family Practice. 2001;14(2):95–106. [PubMed] [Google Scholar]
- 22.Goodyear-Smith F, Coupe NM, Arroll B, EElley CR, Sullivan S, McGill A. Case finding of lifestyle and mental health disorders in primary care: validation of the ‘CHAT’ tool. British Journal of General Practice. 2008;58:26–31. doi: 10.3399/bjgp08X263785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 24.de Leon J, Diaz FJ, Becona E, Gurpegui M, Jurado D, Gonzalez-Pinto A. Exploring brief measures of nicotine dependence for epidemiological surveys. Addict Behav. 2003;28(8):1481–6. doi: 10.1016/s0306-4603(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 25.Diaz FJ, Jané M, Saltó E, Pardell H, Salleras L, Pinet C, et al. A brief measure of high nicotine dependence for busy clinicians and large epidemiological surveys. Australian and New Zealand Journal of Psychiatry. 2005;39(3):161–8. doi: 10.1080/j.1440-1614.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 26.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism: Clinical and Experimental Research. 2007;31(7):1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 27.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 28.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of Substance Abuse Treatment. 2007;32(2):189–98. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Dunn C. Brief motivational interviewing interventions targeting substance abuse in the acute care medical setting. Seminars in Clinical Neuropsychiatry. 2003;8(3):188–96. doi: 10.1016/s1084-3612(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 30.Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction. 2001;96(12):1725–42. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 31.Dunn C, Ostafin B. Brief interventions for hospitalized trauma patients. Journal of Trauma. 2005;59(3 Suppl):S88–93. doi: 10.1097/01.ta.0000174682.13138.a3. [DOI] [PubMed] [Google Scholar]
- 32.Dunn CW, Donovan DM, Gentilello LM. Practical guidelines for performing alcohol interventions in trauma centers. Journal of Trauma. 1997;42:299–304. doi: 10.1097/00005373-199702000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Field C, Hungerford DW, Dunn C. Brief Motivational Interventions: An Introduction. Journal of Trauma. 2005;59(3 Suppl):S21–S6. doi: 10.1097/01.ta.0000179899.37332.8a. [DOI] [PubMed] [Google Scholar]
- 34.Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan KL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149–60. doi: 10.1046/j.1360-0443.2001.96811498.x. [DOI] [PubMed] [Google Scholar]
- 35.Tait RJ, Hulse GK, Robertson SI. Effectiveness of a brief-intervention and continuity of care in enhancing attendance for treatment by adolescent substance users. Drug and Alcohol Dependence. 2004;74(3):289–96. doi: 10.1016/j.drugalcdep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Marsden J, Stillwell G, Barlow H, Boys A, Taylor C, Hunt N, et al. An evaluation of a brief motivational intervention among young ecstasy and cocaine users: no effect on substance and alcohol use outcomes. Addiction. 2006;101(7):1014–26. doi: 10.1111/j.1360-0443.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 37.Miller WR, Yahne CE, Tonigan JS. Motivational interviewing in drug abuse services: a randomized trial. J Consult Clin Psychol. 2003;71(4):754–63. doi: 10.1037/0022-006x.71.4.754. [DOI] [PubMed] [Google Scholar]
- 38.Stein MD, Herman DS, Anderson BJ. A motivational intervention trial to reduce cocaine use. Journal of Substance Abuse Treatment. 2009;36(1):118–25. doi: 10.1016/j.jsat.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 39.McCambridge J, Strang J. Deterioration over time in effect of Motivational Interviewing in reducing drug consumption and related risk among young people. Addiction. 2005;100 (4):470–8. doi: 10.1111/j.1360-0443.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 40.Longabaugh R, Woolard RE, Nirenberg TD, Minugh AP, Becker B, Clifford PR, et al. Evaluating the effects of a brief motivational intervention for injured drinkers in the emergency department. Journal of Studies on Alcohol. 2001;62(6):806–16. doi: 10.15288/jsa.2001.62.806. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein E, Bernstein J. Effectiveness of alcohol screening and brief motivational intervention in the Emergency Department setting. Annals of Emergency Medicine. 2008;51 (6):751–4. doi: 10.1016/j.annemergmed.2008.01.325. [DOI] [PubMed] [Google Scholar]
- 42.Mello MJ, Longabaugh R, Baird J, Nirenberg T, Woolard R. DIAL: A telephone brief intervention for high-risk alcohol use with injured emergency department patients. Annals of Emergency Medicine. 2008;51(6):755–64. doi: 10.1016/j.annemergmed.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 43.Miller WR, Zweben A, Diclemente CC, Rychtarik RG. In: Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Mattson ME, editor. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. [Google Scholar]
- 44.Bischof G, Grothues JM, Reinhardt S, Meyer C, John U, Rumpf H-J. Evaluation of a telephone-based stepped care intervention for alcohol-related disorders: A randomized controlled trial. Drug and Alcohol Dependence. 2008;93(3):244–51. doi: 10.1016/j.drugalcdep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Curry SJ, Ludman EJ, Grothaus LC, Donovan D, Kim E. A randomized trial of a brief primary-care-based intervention for reducing at-risk drinking practices. Health Psychology. 2003;22(2):156–65. [PubMed] [Google Scholar]
- 46.Gentilello LM, Rivara FP, Donovan DM, Jurkovich GJ, Daranciang E, Dunn CW, et al. Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg. 1999;230(4):473–80. doi: 10.1097/00000658-199910000-00003. discussion 80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zatzick D, Roy-Byrne P, Russo J, Rivara F, Droesch R, Wagner A, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Archives of general psychiatry. 2004;61(5):498–506. doi: 10.1001/archpsyc.61.5.498. [DOI] [PubMed] [Google Scholar]
- 48.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180(2):101–10. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Hatcher RL, Gillaspy JA. Development and validation of a revised short version of the Working Alliance Inventory. Psychotherapy Research. 2006;16(1):12–25. [Google Scholar]
- 50.Bordin ES. Theory and research on the therapeutic working alliance: New directions. In: Horvath AO, Greenberg LS, editors. The working alliance: Theory, research, and practice. Oxford England: John Wiley & Sons; 1994. pp. 13–37. [Google Scholar]
- 51.Rapp RC, Xu J, Carr CA, Lane DT, Wang J, Carlson R. Treatment barriers identified by substance abusers assessed at a centralized intake unit. Journal of substance abuse treatment. 2006;30(3):227–35. doi: 10.1016/j.jsat.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carroll KM, Rounsaville BJ. Bridging the gap: a hybrid model to link efficacy and effectiveness research in substance abuse treatment. Psychiatric Services. 2003;54(3):333–9. doi: 10.1176/appi.ps.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]