Abstract
Purpose
To construct a humanized SLE mouse that resembles the human disease to define pathophysiology and targeted for treatments.
Methods
We infused peripheral blood mononuclear cells (PBMC) from SLE patients into BALB-Rag2−/−IL2Rgc−/−mice (DKO), which lack T, B and NK cells. PBMC from 5 SLE patients and 4 normal donors (ND) at 3–5×106/mouse were infused IV/IP to non-irradiated 4–5 weeks old mice. We evaluated the engraftment of human CD45+cells and monitored the plasma human IgG, anti-dsDNA, anti-cardiolipin (aCL) antibodies, proteinuria, and kidney histology.
Results
We found 100% successful engraftment of 40 DKO mice infused with human PBMC. In both SLE-DKO and ND-DKO mice, 50–80% human CD45+ cells were observed in PBMC fraction 4–6 weeks post engraftment, with 70–90% CD3+ cells. There were fewer CD3+4+cells (5.5±2.1%) and more CD3+8+cells (79.4±3.6%) in the SLE-DKO mice, as in the SLE patients. CD19+B cells and CD11c+Monocytic cells were found in the spleen, lung, liver and bone marrow. There was no significant difference in plasma human IgG levels and anti-dsDNA antibodies between SLE-DKO and ND-DKO mice. Levels of aCL antibody were significantly higher in all SLE-DKO mice infused with PBMC from a SLE patient with high titers of aCL antibodies. SLE-DKO mice had proteinuria, human IgG deposits in the kidneys and shorter life span. In SLE- DKO mice engrafted from the aCL-positive patient, we found micro-thrombi and infiltration of CD3+, CD8+ and CD19+ cells in the glomeruli, recapitulating APS in these mice.
Conclusion
A novel humanized SLE-DKO mouse is established, exhibiting many characteristics of immunologic and clinical features of SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a prototypic multisystem autoimmune disease characterized by autoantibodies and immune complex deposition. Anti-double stranded DNA antibodies (anti-dsDNA Ab) and antiphospholipid antibodies (APL Ab) are present in 70% and 30–40% of patients respectively (1–3). Although it is presumed that autoantibodies contribute to disease pathogenesis, the cause of SLE remains unclear and therapies are limited. While studies of spontaneous or induced of mouse models have advanced our understanding of SLE, each strain has unique advantages and disadvantages, no strain is a perfect phenocopy of human SLE, and effective treatments in SLE mouse models often do not translate to human disease (3, 4).
To overcome these limitations and attempt to mimic the pathophysiology of human SLE, we infused peripheral blood mononuclear cells (PBMC) of SLE patients into a newly generated inbred severe-combined-immunodeficient (SCID) mouse, the BALB-Rag2−/− IL2Rgc−/− (double knock-out, DKO) mouse, which lacks T, B and NK cells. (5, 6) Both the Rag2, recombination activation gene 2, responsible for rearrangement of T and B cell receptors and the IL2R gamma chain (receptor to IL2, 4, 7, 9, 15 and 21) required by T/NK cells, are deleted in DKO mice. As such, there is no detectable murine IgG or IgM, (5, 7) A similar IL2Rgc–KO mouse was constructed on the NOD/SCID background resulting in NSG/NOG mice (7, 8). The SCID genotype of NSG/NOG mice was derived from a spontaneous point mutation of DNA-dependent protein kinase in CB17(BALB/c)/SCID mouse (9, 10). Both DKO and NSG/NOG mice have been successfully used to engraft human CD34+ stem cells, reconstituting the full components of human hematopoietic system (7, 8, 11). The NSG/NOG mice have been used extensively for tumor xenografts (7, 8). The CB17/SCID, DKO and NSG/NOG mice have also been used to engraft human PBMC and cord blood mononuclear cells to study HIV and vaccines. (11, 12) We chose to study DKO mice rather than NSG/NOG mice, because the latter are deficient in lytic complement activity ((13), Jackson Laboratory web-site), a key pathway implicated in SLE.
Previous studies support the rationale and feasibility of our approach. Two groups of investigators infused 15–30 million PBMC from SLE patients into CB17/SCID mice and detected anti-dsDNA antibodies in the circulation and mouse C3 deposition in the kidneys after 8–12 weeks (14, 15). Their work was limited because only 65% of mice were successfully engrafted with human cells and they required large numbers of PBMC which is challenging in SLE patients who are often lymphopenic. (16, 17). We hypothesized that the engraftment of SLE PBMC into DKO mice would consistently generate SLE disease and provide a unique and improved humanized mouse model of SLE to facilitate translational studies with human cells as targets for therapy. We report our observations on DKO mice, engrafted with PBMC from 5 SLE patients, including 2 with APS and 4 normal donors (ND).
MATERIALS AND METHODS
Subjects
Approximately 10–30 ml of heparinized peripheral blood was obtained from 4 disease-free consented ND volunteers and 5 patients who fulfilled ACR criteria for SLE, 2 of which had APS. The exclusion criteria were pregnancy and acute infection. Clinical characteristics of the patients are detailed in Table 1. All patients gave informed consent for this study, and the study was approved by the Institutional Review Board at Hospital for Special Surgery.
Table 1.
Description of SLE patients and SLE-DKO mice studied in this report.
| P | Age Gender | Disease Manifestation | Antibodies | SLE-DKO | |||||
|---|---|---|---|---|---|---|---|---|---|
| SLEDAI | anti-dsDNA (IU/ml) | aCL IgG (GPL U) | Medications | anti-dsD (IU/ml) | aCL IgG | Proteinuria | |||
| 1 | 44 M | GN (Class 3/5) | 4 | 147 | neg | MMF 3.0 g HCQ 400mg |
+ | neg | + |
| 2 | 61 F | GN (Class 3/5) | 4 | 41 | neg | MMF 2.5 g, MP 7.5 mg HCQ 400mg |
+ | neg | + |
| 3 | 24 M | GN (Class 4) | 2 | 98 | neg | MMF 2 g, MP 2 mg, HCQ 400mg |
+ | neg | + |
| 4 | 67 F | Malar rash; arthritis; pericarditis; arterial and venous thrombosis thrombocytopenia | 3 | 59 | 19 | Pred 2.5 mg HXQ 200mg Warfarin 3mg |
neg | neg | neg |
| 5 | 45 F | Photosensitivity pleuritis; arterial thrombosis, thrombocytopenia | 1 | 11 | >80 | Warfarin 10mg |
+ | + | + |
SLEDAI = Systemic Lupus Erythematosus Disease Activity Index; anti-ds-DNA = anti- double strand DNA antibodies (abnormal is >10 IU/ml); (IU) = international units; aCL = anticardiolipin antibodies (abnormal is >15 GPL U); MMF = mycophenolate mophetil; HXQ = hydroxychloroquine; MP = methylprednisolone
Engraftment of SLE-DKO mice: P1: n=2; P2: n=3; P3: n=3; P4: n=2; P5: n=7.
Mice and engraftment of human PBMC
Adult male and female DKO (BALB-Rag2−/− IL2Rgc−/−) breeding pairs were kindly provided to us by Dr. M. Kondo from Duke University, NC, through a Material Transfer Agreement from Dr. M. Ito, Central Institute for Experimental Animals, Kawasaki, Japan (5). They are maintained on Sulfatrim diet with autoclaved bedding and cages in a barrier facility. Four to five weeks old female and male mice were used for engraftment of human PBMC. PBMC were obtained by standard ficoll separation, washed in Hank’s solution, and suspended in RPMI, supplemented with 10% fetal calf serum, and injected half IV and half IP into the DKO mice at total of 3–5×106/mouse.
At 1–2 weeks post inoculation of PBMC and then at 7–10 days intervals, 100–150 ul blood was collected into EDTA tubes (BD, NJ). Red blood cells were lysed with ACK solution (Invitrogen, CA) and the PBMC were stained with various anti-human cell markers: CD45, CD3, CD4, CD8, CD19, CD11c (e-Bioscience, CA). After staining, the cells were fixed by Lyse-fix solution (BD, CA) and analyzed by FACS Calibur (BD Bioscience, CA) and FlowJo software (Tree Star, Inc. Ashland, OR).
In some experiments, spleen, lung, liver, kidney cell suspensions were prepared from the mice to assess the engraftment of human cells. Samples were also collected for histology and quantitative mRNA analyses. RNA was obtained by using the RNeasy Mini Kit (Qiagen, CA), followed by reverse transcription, using SuperScript II Rnase H Reverse Transcriptase (Invitrogen). Real time quantitative PCR was performed by using the iCycler iQ thermal cycler and detection system (Bio-Rad, CA). The primers for quantitative PCR are:
Human GAPDH: Forward-5′-GGTGGTCTCCTCTGACTTCAACA-3′;
Reverse- 5′-GTGGTCGTTGAGGGCAATG-3′;
Mouse GAPDH: Forward 5′-ACCCAGAAGACTGTGGATGG-3′;
Reverse ‘5-GGATGCAGGGATGATGTTCT-3′. Relative expression of human GAPDH in the kidneys of SLE-DKO and ND-DKO mice was normalized to mouse GAPDH, and then to DKO (set as 1).
All the procedures performed on these mice were approved by the Institute of Animal Care and Usage Committee at Hospital for Special Surgery.
ELISA and Anti-nuclear assays for human antibodies (Ab) and proteinuria
Plasma and urine from humanized DKO mice were collected weekly after engraftment of human PBMC. ELISA kits were used to assess: anti-dsDNA Ab, (detecting both human IgG and IgM antibodies, Bio-Rad Labs, CA), anti-cardiolipin antibodies (aCL-IgG, GCL units, Louisville APL Diagnostics, TX), and proteinuria (Albuwell-M and Creatinine-Companion-Elisa, Exocell, PA.). Human IgG and IgM levels were assessed by coating plates with goat anti- human IgG or IgM specific antibodies and using horse radish peroxidase labeled anti-human antibody for detection. (Invitrogen, CA). Anti-nuclear antibody (ANA) assay was performed with ANA/Hep-2 Cell culture IFA Test System (Zeus Scientific, NJ) according to manufacturer’s instructions, except that mouse plasma was diluted to 1:4 rather than 1:40 recommended for clinical assays. ANA’s were scored as 0 to 3+.
Pathophysiology and Immunohistochemistry (IHC)
Tissues from humanized and control DKO mice were fixed in 10% buffered formalin for standard paraffin sections. Hematoxylin, Periodic acid–Schiff (PAS) or immunohistochemical stains were performed on paraffin sections to assess pathophysiology and cellular infiltrates in the tissues. Antibody to: CD3 (rabbit monoclonal antibody, Vector Lab, CA), CD8 (mouse monoclonal antibody, Dako, CA) and CD19 (mouse monoclonal antibody, Serotec, UK), and appropriate HRP conjugated secondary antibodies were used to determine the subset of cells in the kidney section. In some experiments, fresh kidney tissues were embedded in OCT (Sigma), frozen and sectioned for IHC staining to examine human IgG deposits in the kidney, with HRP conjugated goat anti-human IgG (Invitrogen, CA).
Statistical analyses
Mean ± SEM were presented for data and Student’s T tests were used for statistical analyses. P<0.05 was considered significant.
RESULTS
Successful Engraftment of PBMC from SLE and normal donors in non irradiated DKO mice
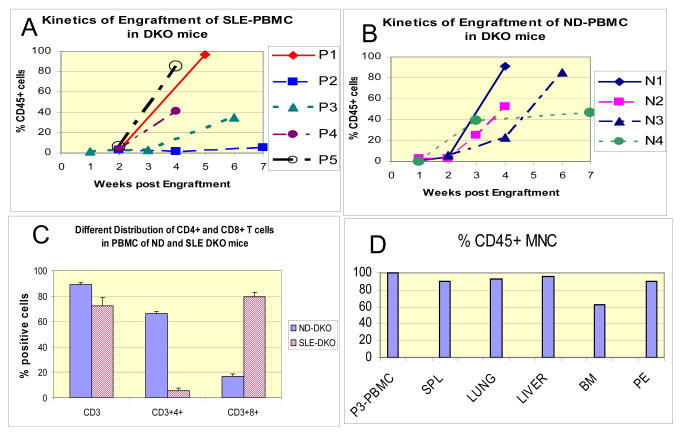
We engrafted 1–5 mice from each of 5 SLE patients (designated P1–5) depending on the yield of PBMC. The clinical and laboratory characteristics of SLE patients are presented on Table 1. Of note, P4 and P5 were SLE patients who also had antiphospholipid syndrome (APS). We detected 5–10% human CD45+ cells in the peripheral blood of DKO mice 2 weeks after inoculation with human PBMC. Figure 1A shows the kinetics of engraftment of 5 SLE donors. PBMC from 4 normal donors (ND) were engrafted in a similar manner and ND-DKO mice showed similar patterns of engraftment (Figure 1B). At 4–5 weeks, P1, P3, P4 and P5 SLE-DKO mice showed 20–80 % human CD45+ cells in the PBMC (Figure 1A). Engraftment was delayed in mice inoculated with PBMC from P2 and her SLE-DKO mice achieved 20% CD45+ cells after 8–9 weeks.
Figure 1.
Kinetics of engraftment of human peripheral blood mononuclear cells (PBMC) from (A) SLE or (B) Normal donors (ND) in DKO mice. Data represent percent human CD45+ cells among PBMC. Average engraftment of human CD45+ cells was 41% in SLE DKO and 53% in ND DKO mice at 4 weeks post engraftment, and they were not significantly different. n= 1–3 mice per donor group; variability or SEM was <10% for each data point. (C) Differential distribution of CD3+CD4+ and CD3+CD8+ cells in CD45+ PBMC of DKO mice engrafted with 4 normal donors (ND-DKO) and 5 SLE donors (SLE-DKO), n=12 mice/group. There were less CD3+CD4+ cells and more CD3+CD8+ cells in SLE-DKO, whereas ND-DKO mice had normal distribution of CD3+CD4+ and CD3+CD8+ (~2–3:1 ratio) cells in the PBMC (SLE-DKO vs. ND-DKO: P<1.3X10−8 for CD4+; P< 2.1×10−7 for CD8+ populations). (D) Distribution of CD45+ human hematopoietic cells in mononuclear cells in various tissues of a representative humanized SLE-DKO mouse engrafted with P3 PBMC. The tissues studied were spleen, lung, liver, bone marrow (BM) and peritoneum exudates (PE), compared to P3 PBMC.
Within 3–4 weeks, CD3+ cells were predominantly detected in the CD45+ populations in PBMC from both SLE- and ND- DKO mice (Figure 1C). In SLE-DKO mice, there were significantly fewer CD3+CD4+cells (5.5±2.1 %) and more CD3+CD8+ cells (79.4±3.6%); this contrasted with a more typical distribution of CD3+CD4+ (66.2±2.5%) and CD3+8+ (16.5±2.1%) cells in the ND-DKO mice (SLE-DKO vs. ND-DKO: P<10−8 for CD4+, P<10−7 for CD8+ populations, n=12 mice/group, representing all P1-5 SLE-DKO mice, and N1-4 ND-DKO mice) (Figure 1C). SLE patients were reported to have a skewed ratio of CD4/CD8 distribution, with less CD4+ and more CD8+ cells in the PBMC (18). That this abnormal distribution was evident in the SLE–DKO mice supports the concept that SLE-DKO mice mimic features of human SLE.
Figure 1D shows 85–95% of CD45+cells in the mononuclear cells in the spleen, lung, liver, and peritoneal lavage and 62% in bone marrow of a representative SLE-DKO mouse engrafted with patient P3. Percent of CD45+CD3+ cells ranged from 65–90% in these tissues, where CD8+ cells predominated, similar to PBMC. B cells were detectable at 5–10% in the tissues, with 20% in the peritoneum. Similarly, CD11c cells were not detectable in the blood, but were found in the spleen (18%) and other tissues (5–10%). CD56+ NK cells were not detectable in the tissues examined. Such distribution of subset of mononuclear cells was representative of 3 other SLE-DKO mice studied, generated from P3 and P5.
Plasma IgG and IgM levels and pathogenic antibodies in SLE-DKO mice
Given that the human PBMC were well engrafted in SLE- DKO mice, we examined other parameters of SLE disease, such as anti-dsDNA, ANA antibodies, aCL IgG and proteinuria in both SLE-DKO and ND-DKO mice at 4–8 weeks post engraftment, when they were at their highest engraftment of CD45+ cells (Figure 1). Figure 2A shows the plasma levels of human IgG in SLE-DKO and ND-DKO mice (n=7 mice each, representing mice derived from all the donors). There was no significant difference (P<0.25) in plasma human IgG levels between SLE-DKO and ND-DKO mice (32.3±13 and 26.4±7 ug/ml, respectively). Levels of IgG were 0.1–1% of that in normal human individuals (~10 mg/ml IgG), as previously reported in humanized SCID mice. (12, 19) Similarly, IgM levels were not significantly different (P<0.9) between the SLE-DKO and ND-DKO mice; SLE-DKO, 2.86±0.84 ug/ml; ND-DKO, 2.98±0.6 ug/ml; and un-engrafted DKO, 0.02 ug/ml.
Figure 2.
Plasma immunoglobulins and antibody levels detected in SLE-DKO and ND-DKO mice at 4–8 weeks post engraftment. A. Plasma IgG levels, representing all SLE-DKO and ND-DKO derived from P1-5 and N1-4 donors. B. Anti-dsDNA antibody, in humanized SLE and ND DKO mice. There were 1–7 mice/group tested from SLE-DKO and ND-DKO derived from P1-5 and N1-4 donors. There was no significant difference (P< 0.26) between the 2 groups. The 2 highest values were from SLE-DKO mice engrafted with P1 and P5 donors. C. Anti-CL (GCL) in SLE-DKO, and ND-DKO mice. SLE-DKO mice from donor P5 (APS) and P1, 2, and 4 were presented. aCL levels of P1, 2, and 4 SLE-DKO mice were similar to ND-DKO mice. P5-DKO vs. ND-DKO: P< 0.017; P5-DKO vs. SLE (P1, 2, 4)–DKO: P<0.01; SLE (P1, 2, 4) -DKO vs. DKO (no engraftment): P< 0.22; ND-DKO vs. DKO: P<0.9. Each point represents 1 mouse in all the graphs. Elevated ACL levels were demonstrated in all 7 P5-DKO mice at 2–3 different time points within 2–4 weeks post engraftment.
Three out of the five SLE-DKO groups (SLE-DKO of P1, P2 and P5 mice) had detectable anti-dsDNA antibodies within 5–7 weeks post engraftment as did two out of the 12 ND-DKO mice. Fig 2B shows the distribution of anti-dsDNA antibodies in SLE-DKO and ND-DKO mice (n=12 each) representing all the donors in each group (SLE-DKO mice, 7.6 ±2.64 IU/ml; ND-DKO mice, 4.4 ±1.37 IU/ml;, un-engrafted DKO, 1± 0.3). Although there were some slightly elevated levels, there were no significant differences between the SLE-DKO and ND-DKO mice (P<0.26) which may be due to variability among SLE-DKO mice.
We tested SLE-DKO, ND-DKO (4–8 weeks after engraftment) and unengrafted DKO mice for the presence of ANA using plasma at 1:4 dilution. Four of seven SLE-DKO mice had ANA scores of 2+ (ANA score range 0 – 2+) whereas none of five ND-DKO mice had 2+ ANA (ANA score range 0–1+) and none of three unengrafted DKO mice were positive for ANA (all ANA score 0).
One of the SLE patients, P5, had high titer of antiphospholipid antibodies (>80 GCL, Table 1). We detected high aCL levels in all 7 P5-DKO mice engrafted with her cells, as shown in Figure 2C, and all 7 mice had detectable aCL at 2–3 different times within 2–4 weeks post engraftment. In P5-DKO mice, the aCL levels were 15.9±2.7 GCL units (n=7), compared to 6.08±0.5 units (n=5) in the other SLE-DKO from P1–P4 mice. ND-DKO (n=6) had aCL levels of 7.2±0.3 GCL units (P5-DKO vs SLE-DKO, P<0.01; P5-DKO vs ND-DKO, P<0.018); un-engrafted DKO had 4.9±1.5 GCL-U (n=4, P< 0.22, SLE-DKO vs DKO). Our finding that the presence of aCL IgG was specific to P5-DKO mice bearing cells from the SLE patient with APS patient (P5) who had persistently high levels of aCL, indicates that the engrafted mice reflect an accurate phenocopy of certain autoantibodies.
Lupus nephritis in SLE-DKO mice
SLE-DKO mice had evidence of mild proteinuria, measured by urinary albumin/creatinine (A/C) ratio, after 4–6 weeks of engraftment (SLE-DKO, 93.5±25, n=15 mice from P1–5 SLE donors; ND-DKO: 22±3.3 ug/mg A/C ratio; P<0.01, n=9 mice from 1–4 ND; un-engrafted DKO: 17.8 ug/mg, n=5 mice, Fig 3A). The proteinuria phenotype was associated with nephritis in donor SLE patients (P1, P2 and P3; Table 1). The SLE–DKO mouse with severe proteinuria (3556 A/C ratio ug/mg) received PBMC from P1, a patient with active nephritis at the time when cells were obtained for engrafting (Table 1 and Fig 3A).
Figure 3.
A. Assessment of proteinuria in humanized SLE DKO and ND DKO mice at 4–8 weeks post engraftment. Proteinuria was determined by the urine albumin and creatinine levels, and the ratio of albumin/creatinine ug/mg was assessed to normalize the values (see Materials and Methods). Each point represents 1 mouse. 2–7 mice/group were tested from all SLE-DKO and ND-DKO derived from P1-5 and N1-4 donors. The highest value of 3556 ug/mg A/C was from SLE-DKO derived from P1 from the first engraftment and 12.5 ug/mg was from the second engraftment (see Results for details). SLE-DKO (n=15 mice, excluding P1), ND-DKO (n=9 mice), DKO (n=5 mice); SLE-DKO vs. ND-DKO: P< 0.01; SLE-DKO vs. un-engrafted DKO: P< 0.007. B. Median survival of humanized SLE DKO (n=18) mice was 6 weeks, where as ND DKO (n=14) mice was 7 weeks and beyond, significantly different between the 2 groups. (P<0.02)
We engrafted PBMC from P1 on 2 separate occasions. Previous to his first engraftment, P1 had active nephritis and his SLE-DKO mouse exhibited severe proteinuria in 4 weeks post engraftment (Fig 3A; proteinuria, A/C = 3556 ug/mg), and died soon after. Three months later, P1 had little proteinuria and his anti-dsDNA decreased by 30%, and we obtained PBMC for a second engraftment. The SLE-DKO mouse engrafted with these PBMC survived longer (euthanized at 7 weeks) and did not show proteinuria (A/C = 12.5 ug/mg, Figure 3A). Importantly, the differences in disease phenotype occurred in mice with similar engraftment of cells from P1 (CD45+ cells 80–90%. In this case, the humanized SLE-DKO mice appeared to simulate the disease state of the SLE donor.
Consistent with the proteinuria were the deposits of human IgG in the glomeruli of SLE-DKO mice, as shown by representative kidney sections of P1, P3 and P5 DKO mice (Figure 4A). No human IgG deposit was detected in kidney sections of ND-DKO and un-engrafted DKO mice. Detailed histological analyses were conducted in the P5-DKO, ND-DKO, and control un-engrafted DKO mice (Figures 4B and C). Both P5-DKO and ND-DKO mice had high engraftment of 70–80% CD45+ cells at the time of euthanasia. In P5-DKO kidney, the infiltrates of human cells were prominent in the perivascular regions and in small clusters or single cells in the renal interstitium. There was diffuse glomerular and tubular damage. (Supplementary Figure 1A). The glomeruli were enlarged, showing severe capillary thrombosis and endothelial cell necrosis. Multifocal acute tubular necrosis with hyaline casts was also evident. The overall appearance of the kidney was similar to a human lupus class IV-G proliferative nephritis (20). In the ND-DKO kidney, the infiltrates of human cells were minimal in perivascular regions with occasional interstitial elements. The glomeruli appeared normal as did tubules.
Figure 4.
Immunohistochemistry and histology of the Kidneys of SLE–DKO, ND-DKO, and un-engrafted DKO mice. A. Human IgG deposits were present in the glomeruli of P1, P3 and P5 SLE-DKO kidneys, and absent in the ND-DKO and un-engrafted DKO kidneys. B. H & E and PAS stains of kidneys of P5-DKO, ND-DKO, and DKO mice. There was abundant perivascular infiltrate of human cells in SLE-DKO kidney, compared to the less prominent infiltration in ND-DKO kidney. The DKO kidney displayed no infiltrate. In the kidney of P5-DKO mouse, the glomeruli were swollen with cellular necrosis. The hyaline thrombi present in the glomerular capillary loops and capillaries (white arrows) were evident on PAS stain. Glomeruli of both the ND-DKO and DKO mice appeared normal C. CD3+, CD8+ and CD19+ human cells were present in the glomeruli of P5-DKO, scanty in ND-DKO, and absent in DKO kidney.
Immunohistochemical stains showed infiltrates of human cells strongly positive for CD3 and with less intensity for CD19 in the P5-DKO and the ND-DKO kidney. (Supplementary Figure 1B). Figure 4C showed CD3, CD8 and CD19 positive cells in the glomeruli of the P5-DKO kidney but none in the ND-DKO or un-engrafted DKO kidney. By flow cytometric analyses we also observed human CD3+, CD8+ and CD19+ cells in kidneys of other SLE-DKO mice (data not shown), similar to that represented in Figure 1D, and discussed above. In the P5-DKO liver, there was a prominent periportal infiltrate of human cells with diffuse interstitial streaming and extensive coagulative hepatocellular necrosis, without involvement of the biliary ductules. In the ND-DKO liver there was a less prominent periportal infiltrate of human cells without evidence of hepatocellular or bile duct necrosis (supplementary Figure 1C).
To have a better quantification of the cellular infiltrates in the SLE-DKO kidneys, we assessed the presence of human GAPDH mRNA, as a surrogate for human cells. We performed quantitative PCR of human GAPDH in kidney tissues of P5-DKO and ND-DKO mice to compare with the histological analyses. Consistent with the histology and immunohistochemistry findings, we detected an average of 7.8 times more human GAPDH expression in kidneys of 3 different P5-DKO mice (average relative expression=168±39) compared to ND-DKO (average relative expression=22±15, n=3 mice from 2 different ND donors, P<0.049).
A correlation of SLE disease phenotype was observed in the SLE-DKO mice engrafted with PBMC from APS patient (P5) whose cells were engrafted from samples obtained 2 different times using 3–4 mice at each time point. P5 had high aCL antibody titers (>80 GPL units, Table 1) at both time points. All P5 SLE-DKO mice tested had detectable aCL antibodies (7/7 mice, Figure 2C), proteinuria (6/7 mice, Figure 3A), abnormal kidney pathology with microthrombi in kidneys and liver (3/3 mice examined, Fig 4, data not shown for liver), and died within 4–5 weeks of engraftment (7/7 mice). Although P5 donor did not have clinical evidence of proliferative nephritis (the lesion identified in SLE-DKO mice engrafted with her cells), she did not have a kidney biopsy, and the absence of renal dysfunction does not exclude histological disease (21). In contrast to P5-DKO mice, those engrafted with PBMC from P4, another SLE patient with APS (Table 1), did not express aCL antibodies, microthrombi, and no proteinuria (Figure 2C and 3A). Of note, P4 had low levels of aCL antibodies at the time her cells were engrafted, and P4-DKO had an average of 40% engraftment of CD45+ cells compared with over 80% in P5-DKO mice (Figure 1A).
Premature death of SLE- DKO mice compared to ND-DKO mice
ND-DKO mice had significantly better survival than SLE-DKO mice (ND-DKO- median survival = 7 weeks n=14 mice vs. SLE-DKO median survival = 6 weeks; n=18 mice; P<0.02) (Figure 3B). Assessment of seven week survival is presented, because we euthanized mice at this time point to assay for clinical parameters described above when engraftment of CD45+ cells reached 70–90% in the PBMC. Notably, approximately 50% of the SLE-DKO mice were dead in 4–5 weeks. The cause of death is not clear, but could have been due to multiple organ failure in some mice; P5-DKO showed hepatic necrosis associated with elevated levels of serum transaminases (data not shown) and as well as renal damage involving glomeruli and tubules. Engraftment of the ND-DK was equally high (70–90%), but they appeared normal and healthy, and, more importantly, all survived beyond 4–5 weeks. ND-DKO mice appeared to die from weight loss and anemia, but no sign of hair loss, skin lesions, typical of allogeneic/xenogeneic graft versus host disease (GVHD) (13, 22). Interestingly, one ND-DKO as well as one P2-SLE-DKO mouse lived up to 4.5– 5 months, with 20–23% human CD45+ cells. Un-engrafted DKO had normal life span in our animal facility, where we have kept them for over 8 months.
DISCUSSION
We have developed a humanized SLE-DKO mouse that exhibits many clinical and immunological characteristics of SLE, and mice infused with PBMC from a SLE patient with APS and high levels of aCL antibodies manifested features of APS. As DKO mice are more permissive hosts than CB17/scid mice, we observed 100% successful engraftment of 40 DKO mice infused with human PBMC, without conditioning regimens, such as irradiation (11, 23). High rate of successful engraftment with only 3–5×106 PBMC provides the opportunity to construct many humanized SLE-DKO mice derived from the same donor, even among lymphopenic SLE patients (16). Phenotypically, the engrafted mice appeared healthy for 3–4 weeks post engraftment of SLE PBMC, even with 50–70% engraftment of CD45+ cells. However, within the next 2 weeks the SLE-DKO mice, engrafted with PBMC from P1 and P5 experienced >10% weight loss and proteinuria, resulting in moribund conditions, distinctly different from ND-DKO mice. SLE-DKO mice demonstrated some pathological features of genetic or spontaneous SLE mouse models (4), but the onset of disease was without manipulation, sooner than 20–30 weeks in SLE mice, and driven by human cells. Within 5–6 weeks, SLE-DKO mice developed some key features of SLE disease, including autoantibodies like their donors, and nephritis, although they do not show renal failure. Human SLE disease is heterogeneous, but SLE-DKO mice generated from well characterized donors may provide a predictive pre-clinical model for evaluation of therapeutics as well as personalized therapies tested on personalized mice.
To our knowledge this is the first report on a humanized APS mouse model, as currently there is lack of suitable animal model to characterize the mechanism of APS, and many investigators rely on passive transfer of aCL antibodies into normal mice (24, 25). The presence of persistent stable levels of aCL antibodies in SLE/APS-DKO mice could truly mimic the human APS conditions (Figure 2C). The demonstration of thrombi in the kidneys and liver of SLE/APS-DKO mice enables detailed elucidation of the interaction of inflammatory cells and antibody in thrombotic events in APS (Figure 4). Sensitized and damaging T cells may preferentially home to the kidneys of SLE-DKO mice, contributing to the tissue damage observed. It is noteworthy that SLE-DKO mice from P4 with a history of APS (Table 1), but low aCL levels at the time when PBMC were obtained showed neither pathogenic aCL antibodies nor proteinurea (Figure 3A). It is possible that prolonged treatment with prednisone had eliminated the damaging effector T or B cells or that the aCL antibodies were of different specificities. Nonetheless, the humanized SLE-DKO appeared to phenocopy the disease state of the respective patients.
In this study we observed similar high levels of engraftment of PBMC from SLE or normal donors into DKO mice within 6–7 weeks, and similar kinetics of engraftment (Figure 1A&B). However, T cell populations engrafted differed between the groups with the ratio of CD8 T cells predominating in SLE-DKO mice (Figure 1C). The maintenance of normal ratio of CD4 and CD8 cells in ND-DKO mice was consistent with our earlier findings in humanized non-irradiated NSG mice, and more recently also reported in xenogeneic GVHD model of irradiated ND-NSG/NOG mice (11, 13, 26). In contrast, the lower CD4+ cells in SLE-DKO mice could reflect lower CD4+ CD25+ T regulatory cells reported in SLE patients (27, 28). Furthermore, ND-DKO mice did not show evidence for autoimmunity; they did not have ANA and aCL antibodies, lupus nephritis, T and B cell infiltrates in kidneys or accelerated death (Figure 2–4). Supporting our observations, Ito et al also observed that ND-DKO mice survived beyond 2 months when they were not irradiated (13). Although we cannot rule out that infusion of SLE PBMC into non-irradiated DKO mice induced some elements of xenogeneic GVHD, the contrast between the characteristics of the humanized SLE-DKO and ND-DKO argue against GVHD as a general phenomenon to explain autoimmunity and lupus nephritis in our model. Furthermore, the distinguishing features of the human SLE-DKO mice could facilitate studies of pathophysiology of SLE.
The infiltration of inflammatory cells in the kidneys SLE-DKO mice is similar to those reported in the kidneys of murine and human SLE (20, 29). Triantafyllopoulou et al noted similar proliferative nephritis in SLE mice treated with polyinosinic:polycytidylic acid, inducing interferon–alpha production to accelerate the nephritis process (30). Although CD4+ cells were reported in the kidney of interferon-alpha induced murine SLE (31), we did not detect human CD4 cells in the SLE-DKO kidneys by Flow cytometry. In human SLE, however, CD8+ T cells as well as B cells were found in involved kidneys, similar to the phenotype of the SLE-DKO mice reported here (29, 32, 33). Accordingly, to further determine the cellular components that initiate the SLE/APS disease, human T, B, NK and monocytic cells could be transferred singly or in combination into DKO mice. With species specific reagents for human and mice, SLE/APS-DKO mice provide the unique experimental system to distinguish and analyze the contribution or products of human or mouse hematopoietic cells, or mouse endothelial cells in SLE/APS disease processes.
In conclusion, we were successful in creating a novel and reproducible humanized SLE DKO mouse with PBMC from SLE patients. This translational mouse model can provide a platform to dissect the cellular and inflammatory components longitudinally in the SLE/APS conditions and test personalized targets of treatment in mice generated from precisely phenotyped SLE donors.
Supplementary Material
Supplementary Figure 1A shows damaged kidney tubules in SLE-DKO mouse
Supplementary Figure 1B shows abundant CD3 and CD19 staining in the interstitium of P5-DKO kidney, but less staining in the ND-DKO kidney and scanty to none in the DKO kidneys
Supplementary figure 1C: In P5-DKO mouse there were significant hepatic cellular infiltrates and necrosis, which were absent in ND-DKO or un-engrafted DKO mice
Acknowledgments
This work was supported in part by NIH RO1AR038889 (JES), Mary Kirkland Center for Lupus Research, and National Council of Technological and Scientific Development Grant of Brazil, 200591/2008 (DA)
We thank: our patients and normal donors; Dr. Diana Goldenberg and Emily Miller for recruiting the patients, Dr. Victor Andrade at Memorial Sloan Kettering Cancer Center for advice on the pathology of SLE-DKO and ND-DKO mice, Dr. Mamoru Ito from Central Institute for Experimental Animals, Kawasaki, Japan and Dr. Motonari Kondo from Duke University for generous provision of the DKO mice, Irina Shuleshko and Maria Jiao for assistance with histology and immunohistochemistry, and Dr. Philip Rusli for help with the micrographs. We are grateful to Drs. Theresa Lu, Alessandra Pernis and Francis Dumont for critical review of the manuscript.
Footnotes
Please send reprint requests to Gloria C. Koo or Jane E Salmon; salmonj@hss.edu
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Salmon JE, Girardi G, Theodore E. Woodward Award: antiphospholipid syndrome revisited: a disorder initiated by inflammation. Trans Am Clin Climatol Assoc. 2007;118:99–114. [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg R. Why can’t we find a new treatment for SLE? J Autoimmun. 2009;32(3–4):223–30. doi: 10.1016/j.jaut.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan MJ. Young Investigator Award Lecture of the APS Water and Electrolyte Homeostasis Section, 2008: The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1258–67. doi: 10.1152/ajpregu.90864.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 6.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68(5):855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 7.Ito M, Kobayashi K, Nakahata T. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol. 2008;324:53–76. doi: 10.1007/978-3-540-75647-7_3. [DOI] [PubMed] [Google Scholar]
- 8.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 9.Miller RD, Hogg J, Ozaki JH, Gell D, Jackson SP, Riblet R. Gene for the catalytic subunit of mouse DNA-dependent protein kinase maps to the scid locus. Proc Natl Acad Sci U S A. 1995;92:10792–105. doi: 10.1073/pnas.92.23.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki R, Fujimori A, Hamatani K, Mita K, Saito T, Mori M, Fukumura R, Morimyo M, Muto M, Itoh M, Tatsumi K, Abe M. Nonsense mutation at Tyr-4046 in the DNA-dependent protein kinase catalytic subunit of severe combined immune deficiency mice. Proc Natl Acad Sci U S A. 1997;94:2438–43. doi: 10.1073/pnas.94.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo GC, Hasan A, O’Reilly RJ. Use of humanized severe combined immunodeficient mice for human vaccine development. Expert Rev Vaccines. 2009;8(1):113–20. doi: 10.1586/14760584.8.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosier DE, Gulizia RJ, Baird SM, Wilson DB, Spector DH, Spector SA. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991;251(4995):791–4. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 13.Ito R, Katano I, Kawai K, Hirata H, Ogura T, Kamisako T, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87(11):1654–8. doi: 10.1097/TP.0b013e3181a5cb07. [DOI] [PubMed] [Google Scholar]
- 14.Duchosal MA, McConahey PJ, Robinson CA, Dixon FJ. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990;172(3):985–8. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauermann N, Sthoeger Z, Zinger H, Mozes E. Amelioration of lupus manifestations by a peptide based on the complementarity determining region 1 of an autoantibody in severe combined immunodeficient (SCID) mice engrafted with peripheral blood lymphocytes of systemic lupus erythematosus (SLE) patients. Clin Exp Immunol. 2004;137(3):513–20. doi: 10.1111/j.1365-2249.2004.02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohm I. Apoptosis: the link between autoantibodies and leuko-/lymphocytopenia in patients with lupus erythematosus. Scand J Rheumatol. 2004;33(6):409–16. doi: 10.1080/03009740410006907. [DOI] [PubMed] [Google Scholar]
- 17.Vila LM, Alarcon GS, McGwin G, Jr, Bastian HM, Fessler BJ, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum. 2006;55(5):799–806. doi: 10.1002/art.22224. [DOI] [PubMed] [Google Scholar]
- 18.Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52(1):201–11. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- 19.Camacho RE, Wnek R, Fischer P, Shah K, Zaller DM, Woods A, et al. Characterization of the NOD/scid-[Tg]DR1 mouse expressing HLA-DRB1*01 transgene: a model of SCID-hu mouse for vaccine development. Exp Hematol. 2007;35(8):1219–30. doi: 10.1016/j.exphem.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan SK, Ordonez NG, Feitelson PJ, Lim VS, Spargo BH, Katz AI. Lupus nephropathy without clinical renal involvement. Medicine (Baltimore) 1977;56(6):493–501. doi: 10.1097/00005792-197711000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Lu SX, Holland AM, Na IK, Terwey TH, Alpdogan O, Bautista JL, et al. Absence of P-selectin in recipients of allogeneic bone marrow transplantation ameliorates experimental graft-versus-host disease. J Immunol. 185(3):1912–9. doi: 10.4049/jimmunol.0903148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126(3):303–14. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(Suppl 2):ii46–50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshan SV, Franzke CW, Redecha P, Monestier M, Mackman N, Girardi G. Role of tissue factor in a mouse model of thrombotic microangiopathy induced by antiphospholipid antibodies. Blood. 2009;114(8):1675–83. doi: 10.1182/blood-2009-01-199117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157(1):104–18. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183(10):6346–58. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz DA. Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther. 2008;10(6):227. doi: 10.1186/ar2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao W, Thacker SG, Hodgin JB, Zhang H, Wang JH, Park JL, et al. The peroxisome proliferator-activated receptor gamma agonist pioglitazone improves cardiometabolic risk and renal inflammation in murine lupus. J Immunol. 2009;183(4):2729–40. doi: 10.4049/jimmunol.0804341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A. 107(7):3012–7. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, et al. Interferon alpha accelerates murine SLE in a T cell dependent manner. Arthritis Rheum. doi: 10.1002/art.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 2008;74(4):448–57. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- 33.Dolff S, Abdulahad WH, van Dijk MC, Limburg PC, Kallenberg CG, Bijl M. Urinary T cells in active lupus nephritis show an effector memory phenotype. Ann Rheum Dis. 69(11):2034–41. doi: 10.1136/ard.2009.124636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1A shows damaged kidney tubules in SLE-DKO mouse
Supplementary Figure 1B shows abundant CD3 and CD19 staining in the interstitium of P5-DKO kidney, but less staining in the ND-DKO kidney and scanty to none in the DKO kidneys
Supplementary figure 1C: In P5-DKO mouse there were significant hepatic cellular infiltrates and necrosis, which were absent in ND-DKO or un-engrafted DKO mice