Abstract
While previous research has linked executive attention to emotion regulation, the current study investigated the role of attentional alerting (i.e., efficient use of external warning cues) on younger (N = 39) and older (N = 44) adults’ use of gaze to regulate their mood in real time. Participants viewed highly arousing unpleasant images while reporting their mood and were instructed to deliberately manage how they felt and to minimize the effect of those stimuli on their mood. Fixations toward the most negative areas of the images were recorded with eye tracking. We examined whether looking less at the most negative regions, compared to each individual’s own tendency, was a beneficial mood regulatory strategy and how it interacted with age and alerting ability. High alerting older adults, who rely more on external cues to guide their attention, experienced a smaller decline in mood over time by activating a less-negative-looking approach (compared to their own average tendency), effectively looking away from the most negative areas of the images. More negative gaze patterns predicted better mood for younger adults, though this effect decreased over time. Alerting did not moderate gaze-mood links in younger adults. Successful mood regulation may thus depend on particular combinations of age, fixation, and attention.
Keywords: aging, gaze, mood, attentional functioning
Contrary to the negative stereotypes related to aging, theoretical and empirical literatures suggest that emotion regulation skills are maintained or may even be enhanced throughout adulthood. Older adults report experiencing fewer negative emotions and more positive emotions than their younger counterparts (e.g., Gross et al., 1997; Lawton, et al., 2001). They consider themselves to have effective regulatory strategies with which to control emotional experience and expression (Gross et al., 1997; Lawton, Kleban, Rajagopal, & Dean, 1992; Phillips, Henry, Hosie, & Milne, 2008; Shiota & Levenson, 2009). Socioemotional selectivity theory (SST; Carstensen, Isaacowitz, & Charles, 1999) provides one explanation for why older adults appear to be better emotion regulators. According to SST, emotional goals become more salient as people age and they perceive their time to be more limited. As a result, older adults are motivated to pursue emotional satisfaction and maintain emotional balance, which benefits them in the present, while younger adults perceive their time to be expansive and are motivated to seek knowledge, which may be useful for them at a later time.
Mood as a Function of Age and Gaze
One way older adults may be able to maintain better mood is by focusing on more positive information and avoiding negative information; this pattern has been termed an “age-related positivity effect” (Carstensen & Mikels, 2005; Carstensen, Mikels, & Mather, 2006; Mather & Carstensen, 2005). Although there are many similarities between young and older adults in how they process emotional stimuli (Murphy & Isaacowitz, 2008), age-related positivity effects have been consistently found for visual attention (fixation or gaze) in full attention conditions (Isaacowitz, Wadlinger, Goren, & Wilson, 2006a, 2006b; Mather & Carstensen, 2003; Knight et al., 2007; Allard & Isaacowitz, 2008). SST provides a motivational explanation for these gaze preferences, suggesting that they act as a regulatory tool that older adults use to optimize their affective experience (Carstensen et al., 1999, 2005).
A small, but growing, body of research has investigated whether positive gaze preferences are shown by older adults in order to promote, maintain, or enhance positive emotional states (e.g., Isaacowitz, Toner, Goren, & Wilson, 2008; Johnson, 2009). In Isaacowitz et al. (2008), age-related gaze preferences were directly related to individuals’ actual mood. Younger and older adults were induced into either a positive, negative, or neutral mood, via a mood induction procedure (Eich & Metcalfe, 1989). This induction of mood was then followed by an eye-tracking session in which synthetic emotional faces paired with neutral faces were viewed by the participants. The results revealed an age-related dissociation between fixation patterns and mood at the start of the eye-tracking. Younger adults showed mood-congruent gaze preferences such that they looked more toward happy faces when in a good mood, and more toward angry and afraid faces when in a bad mood. Older adults did not exhibit such mood-congruent gaze patterns; instead, they displayed mood-incongruent positive gaze preferences showing that they looked more toward happy faces and looked away from faces expressing negative emotion when in a bad mood. The finding that older adults display positive gaze preferences when in a negative mood suggests that older adults actually use gaze not to reflect mood, but that their gaze may actually serve a regulatory function.
The Role of Individual Differences in Age-Related Gaze-Mood Links
While trying to understand the conditions under which gaze preferences help individuals to improve their mood, it is important to also consider individual characteristics which might moderate the gaze-mood relationship, especially given recent findings suggesting a large degree of variability in mood among older adults (Stanley & Isaacowitz, in press). Furthermore, Mather and colleagues have argued that adequate cognitive control is necessary for older adults to display positivity effects in attention and memory (Knight et al., 2007; Mather & Knight, 2005). This assertion is supported by the finding that older adults with better cognitive control (as measured by executive attention) show the largest positivity effects in their gaze and memory (Knight et al., 2007; see also, Petrican, Moscovitch, & Schimmack, 2008, for a link between cognitive control and the retrieval of emotional memories). On the other hand, Allard and Isaacowitz (2008) found that older adults display positive gaze preferences even in the context of dual-task performance, and a more recent study (Allard, Wadlinger, & Isaacowitz, 2010) further demonstrated that activating positive gaze preferences does not seem to demand high cognitive effort, as measured by pupil dilation.
While these studies above have not directly addressed the relationships between positive preferences, mood-regulation, and cognitive control, one analysis has linked gaze patterns to mood change, as a function of cognitive control (Isaacowitz, Toner, & Neupert, 2009). In that study (2009), older adults with better executive attention best resisted mood decline (comparing mood states at the start and end of the eye-tracking session) by displaying a more positive looking pattern (looking toward happy and away from angry faces). In contrast, younger adults with better executive attention were able to resist mood decline by displaying a negative looking pattern (looking away from happy and toward angry faces). These findings further extend Mather and colleagues’ (e.g., Knight et al., 2007) assertion of the cognitively controlled nature of positivity effects in old age and suggest that the extent to which positive preferences enhance older adults’ mood regulation is dependent on adequate cognitive control. Yet, this raises the question of why younger adults with better executive attention feel best when they look at negative materials? Isaacowitz et al. (2009) argued that older adults rely on an attention deployment strategy involving disengaging from negative material, whereas younger adults may prefer to use regulation strategies other than attention deployment. For example, reappraisal involves reinterpreting the meaning of the negative stimuli (Gross, 1998): by visually attending to the negative stimuli, young adults may gather useful information to use for reappraisal.
Such findings are also consistent with research on contra-hedonic motivations, suggesting there are age-differences in how people attempt to influence their feelings (e.g., Riediger, Schmiedek, Wagner, & Lindenberger, 2009; Tamir, 2009). Motivations to enhance or maintain negative affect are most common among adolescents and less prevalent in older adults (Riediger et al., 2009). Older adults are found to be more motivated to maintain positive affect and decrease negative affect (Riediger et al., 2009). These differential motivations may reveal themselves in different patterns of gaze-mood links.
Research on the importance of attentional resources in emotion regulation has mostly focused on executive control (see Zelazo & Cunningham, 2007, for a review). What other attentional substrates may moderate gaze-mood links beyond executive attention? Posner and Petersen (1990) identified two more types of attention abilities: alerting and orienting. The alerting ability helps achieve and maintain a state of high sensitivity to incoming stimuli. The orienting ability involves selection of information from sensory input (Fan, McCandliss, Sommer, Raz, & Posner, 2002). Alerting and orienting may also play roles in attempts at regulating emotion. For example, individuals might need to maintain their state of alertness to detect potentially highly arousing negative stimuli in their environment and to selectively attend to particular (less disturbing) regions of visual stimuli. As the attentional abilities are thought to be independent of one another (Fan et al., 2002), each may have different roles in regulating emotions. Indeed, there is some evidence that alerting and orienting are differentially associated with various mood states (Compton, Wirtz, Pajoumand, Claus, & Heller, 2004): while negative affect was associated with alerting effects, positive affect was associated with orienting effects. This suggests a link between the alerting function of sensitivity to external cues and the modulation of negative affect in the context of emotion regulation. Therefore, we focused exclusively on the alerting ability in our investigation of the role of attention in response to a context in which negative affective response needs to be regulated. The alerting ability is assessed with the Attention Network Test (ANT; Fan et al., 2002).: the efficiency of alerting is determined by measuring the relative response times (RTs) to cue trials. High alerting scores reflect the benefits associated with an external warning cue that contains no spatial information regarding the upcoming targets.
There are other reasons to think that alerting may be an especially important attentional process in terms of the gaze-mood links across adulthood. First, it has been suggested that reliance on environmental (external) cues increases with age (Spieler, Mayr, & LaGrone, 2006). Use of external cues may be a compensatory mechanism supporting older adults’ information processing in the face of age-related general slowing (e.g., Salthouse, 1996) and noisy internal representation systems (Spieler et al., 2006). While some report that the alerting function decreases with normal aging (Festa-Martino, Ott, & Heindel, 2004; Jennings, Dagenbach, Engle, & Funke 2007), one study actually found larger alerting effects in older adults compared to their younger counterparts when the cues persisted over time (Fernandez-Duque & Black, 2006). Jennings et al. (2007) argued that age-related changes in the noradrenergic system, which modulates phasic arousal, make it difficult to maintain a vigilant state for older adults; however, longer cue presentation may provide enough environmental support to allow older adults to benefit from external cues to guide their attention, perhaps even more so than young adults (Jennings et al., 2007). If older adults show a general bias towards emotional information (Leclerc & Kensinger, 2008), it is possible that those older adults whose alerting system allows them to use external cues effectively can also respond to external emotional cues. This pattern of response might then allow older adults with good alerting ability to direct their attention in ways that will help them regulate their mood. Following this reasoning, the current study sought to understand what role alerting ability plays when older adults try to use their gaze to regulate themselves out of negative moods.
The Current Study: Mood as a Function of Age, Gaze and Alerting Ability
While previous studies have highlighted that positive gaze preferences may help older adults to alleviate bad mood (Isaacowitz et al., 2008, 2009), several questions remain. First, participants were instructed to look “naturally” at the emotional stimuli in past studies. It is unclear whether both younger and older participants were actually motivated to make attempts to improve their mood. Second, the previous studies did not directly assess how gaze-mood links change over time. Change over time is important because successful mood regulation is a temporally dynamic process (Larcom & Isaacowitz, 2009), and gaze-based regulation may take some time to come into “effect.” As older adults activate positive gaze preferences especially when in a negative mood (Isaacowitz et al., 2008), it is possible that gaze–mood links may emerge only when their “current” mood state is threatened after prolonged exposure to unpleasant stimuli.
Previous work has only linked overall mood change and between-group differences in overall fixation patterns for younger and older participants. However, fixation patterns may change over time within individuals as people attempt to regulate their mood; therefore, it is critical to examine how changes in fixation patterns within individuals are associated with changes in mood over time. This approach will provide a better understanding of gaze-mood links. Finally, participants in previous research were exposed to a mix of positive and negative stimuli, which makes it difficult to determine the impact of particular looking patterns on real-time mood change. Any, or all, of these limitations may have complicated attempts to understand the use of gaze for real-time mood regulation, and may have hindered the ability to understand how the gaze-mood link is affected by age and attentional ability.
Therefore, our goal in the current study was to investigate age differences in deliberate mood regulation by providing participants with explicit instructions to actively manage mood. To ensure that we exposed our participants to an emotionally demanding situation so that they could deliberately manage their mood, we presented a series of highly arousing negative images while recording participants’ eye movements to assess gaze patterns. Consistent with previous findings (e.g., Isaacowitz et al., 2006a, 2006b; Isaacowitz & Choi, in press), we expected that older adults, relative to younger adults, would show less negative preferences in gaze by looking away from the most negative areas of the stimuli.
We aimed to extend previous research by investigating another attentional substrate, the alerting network, to address to what extent the efficiency of alertness related to links between age-related use of gaze and mood change. The alerting ability was measured using the ANT (Fan et al., 2002), a performance-based measure of individual differences in the efficacy of attentional networks. Gaze preferences were measured objectively via eye-tracking. In contrast to our previous work (Isaacowitz et al., 2009) focusing on the differences in persons in the association between mood and fixation patterns at the aggregated level, the current study examined the within-person moment-to-moment association between mood and fixation patterns. In order to assess the within-person changes in fixation patterns during each short-time interval, we calculated the deviation in fixation duration that took place from each participant’s own average fixation tendency, and linked it to the mood-change in that interval. In this way, we were able to conduct more fine-tuned analysis of real-time mood-change in relation to changes in fixation patterns.
We hypothesized that both younger and older adults’ moods would decline over time as they were being exposed to highly arousing negative stimuli; however, the rate of mood decline would depend on fixation patterns and alerting ability. Following previous eye-tracking work (e.g., Isaacowitz et al., 2008, 2009), we hypothesized that older adults would respond to this situation by looking less at the most negative areas of the stimuli to regulate their mood, whereas younger adults would engage visually with the most negative areas of the stimuli. However, we expected that age differences in within-person gaze-mood links in this negative affect-laden context would be more pronounced for those with high alerting ability given the suggested link between alertness and negative affect (Compton, 2004). Critically, high alerting ability would enable individuals to be more sensitive to the most negative regions of the stimuli and use those parts as cues for their mood regulation. This would be reflected by within-person changes in fixation patterns to help individuals regulate their mood, albeit in age-specific ways. Specifically, we hypothesized that high alerting older adults would benefit most in terms of their mood over time from activating a less-negative-looking approach (compared to their own average tendency), effectively looking away from the most negative areas of the images. On the other hand, high alerting younger adults would feel best when they activated an engagement strategy (Isaacowitz et al., 2009), looking more at the most negative areas compared to their own average tendency. We expected these effects to strengthen over time as the potential effects of the arousing images on mood accumulated.
Method
Participants
Forty-one young (Mage = 18.51, SD = .78, 68% females) and 53 older adults (Mage = 70.85, SD = 7.07, 74% females) participated in the current study. Younger participants were college undergraduates. Older adults were recruited from our previous database and from the local Boston area. All participants received either course credit or a monetary stipend for their participation. Two young and 11 older participants from the sample were not trackable because of obfuscation of the pupil or reflective glasses. This left 95% of (N = 39) young and 79% of the older sample (N = 44) available for data analysis. Participants were highly educated and had either attended some college or were college graduates. Approximately eighty-four percent of the sample was Caucasian, 4.8% Asian-American, 3.6% African American, 2.4% Hispanic, 3.6% East Asian, and 1.2% provided no response. None of the participants showed symptoms of dementia and their mean score on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975) were in the normal range (> 25, maximum score 30). All participants were tested on the Snellen (Hetherington, 1954) chart for distance vision and the Rosenbaum (Rosenbaum, 1984) chart for near vision. All had acceptable visual acuity, which is 20/40 or better (20/20 is designated as normal vision), where the numerator represents the distance in feet between a person and the chart at a distance of 20 feet and the denominator is the distance from which the person with normal vision can read a line of letters. Older adults (M = 1.68, SD = .24) exhibited a decline in their contrast sensitivity compared with young adults (M = 1.91, SD = .09), t(81) = 6.08, p <.001, in the Pelli-Robson Contrast Sensitivity Test (Pelli, Robson, & Wilkins, 1988). On a 5-point scale (1 = poor; 5 = excellent), participants rated their overall health. The groups did not differ in terms of their ratings (M = 3.89, SD = .83 for younger adults; M = 3.77, SD = .80 for older adults), t < 1.
Materials and Apparatus
Attention Network Test (ANT)
In the ANT, participants were asked to press the designated key indicating the direction of the centrally presented target arrow (left or right) that appears above or below a fixation cross, shown in the center of the screen. Each arrow trial is preceded by one of four cue conditions: no cue, center cue (a cue at the fixation cross), double cue (simultaneous cues at possible target arrow positions above and below the cross), and spatial cue (a cue above or below the cross predicting the arrow position). Both center and double cues provide temporal information about the target’s appearance (alerting). Following Fan and colleagues (2002), an index of the alerting effect is calculated by subtracting the mean RT of the double cue condition from that of the no cue condition. A higher RT means that the individual’s RT is faster in the presence of alerting cues. No age differences were found in alerting, t(78) = 1.46, p = .15.
Analog slider
A potentiometer slider (Empirosoft Corporation, New York, NY) was used to record participants’ self-reported current mood throughout the experiment. The left end point on the slider was rated as 0 (extremely negative) and the right end point was rated as 100 (extremely positive).
Eye tracker
Each participant’s gaze at emotional stimuli was measured with an Applied Science Laboratories eye tracker (Model 504). The tracker records the movements and position of the participant’s left eye sixty times per second with a camera and a non-invasive beam of infrared light. The visual fixations were defined as those series of gazes in which an individual stays within a 1º visual angle for 100 ms or longer (Manor & Gordon, 2003). Gaze patterns and visual fixations to each image were recorded throughout the presentation of emotional pictures on a Dell Dimension 8400, 17” desktop using GazeTracker software (Eye Response Technologies, Inc., Charlottesville, VA).
Emotional stimuli
Emotional stimuli were 117 highly arousing and negatively-valenced images selected from the International Affective Picture System images (IAPS; Lang, Bradley, & Cuthbert, 1999). Arousing and valence ratings of the IAPS images were taken from the IAPS manual and the selected images for the current study were highly arousing (M = 5.57, SD = .76) and negatively valenced (M = 2.71, SD = .73) rated on a 9-point scale (1 = extremely negative or low arousal and 9 = extremely positive or high arousal). Each IAPS image contains both highly negative parts as well as less negative, or even neutral, parts. In order to assess fixation patterns to the most negative areas of the stimuli, areas of interest (AOIs) were created to include the most negative area within each IAPS image (see also, Isaacowitz & Choi, in press; Wadlinger & Isaacowitz, 2008). First, each of the images was initially evaluated by a member of the research team and preliminary AOIs were set for the most negative area. Eight raters who were unaware of the goal of the current study then rated these AOIs and the remaining parts of each image on their valence and arousal levels using a 7-point scale: the valence (1- extremely unpleasant, 7 -extremely pleasant) and arousal (1- extremely calm, 7 - extremely arousing). Paired t-tests comparing the valence of these AOIs to the rest of the images revealed that the AOIs were rated as significantly more negative (M = 2.59, SD = .40) than the remaining areas (M = 3.59, SD = .23), t(7) = 9.57, p < .001. These AOIs were also rated as significantly more arousing (M = 5.38, SD = .51) than the remaining areas (M = 4.34, SD = .30), t(7) = 7.73, p < .001. Using these AOIs, we calculated the percentage of fixations to the most negative areas of each image relative to the amount of fixation on the entire image. We examined the average of these fixation percentages to the most negative AOIs between each two consecutive mood ratings (approximately 1 min worth of fixations between two ratings), in order to assess links between fixation and simultaneous mood change.
Procedure
After providing informed consent, participants completed self-reported measures of demographic, followed by vision tests. Next, participants’ left-eye was calibrated using the eye-tracker. For calibration, participants were asked to adjust their gaze to 17 points presented on the screen in order to ensure that the tracker recorded within 1° visual angle of each point (Isaacowitz et al., 2006a, 2006b). After calibration, participants were presented with affective images while their gaze was recorded. Before beginning the experimental trials, participants were asked to deliberately manage their mood, using the following instructions: “You will be seeing some real-world images which may evoke some unpleasant emotions in you. Your goal is to try to manage the emotions you feel while looking at the images. In other words, try to minimize the effect of these pictures on your mood.” Previous studies have used similar instructions to motivate participants to focus on emotion regulation (e.g., Xing & Isaacowitz, 2006). No specific instructions about the specific strategies that the participants should employ to regulate their mood were given so that participants could utilize the regulatory strategies they use naturally in their everyday life.
Prior to the presentation of the affective pictures, participants reported their “current” mood by using the mood slider. This was the first mood rating. Once the participant had made their first mood rating, they saw a presentation of 117 trials. In each trial, a negative emotional picture was displayed for 5 seconds followed by a 0.5-s fixation cross slide to realign gaze to the center of the screen. After each set of 13 negative images (approximately every minute), a slide was displayed instructing the participants to report their “current” mood at that moment. This interval was chosen to be short enough to capture mood changes that take place after looking at a reasonable number of slides. Once participants reported their mood, the next set of 13 trials was presented. This continued through the roughly 10 minutes of the image presentation. After the eye-tracking session, participants performed the ANT task.
Results
Age Differences in Overall Fixation
Collapsed across all time intervals, the average percent fixation to the most negative AOIs was higher for younger adults ((M = 51.78, SD = 11.00) compared to older adults (M = 36.44, SD = 17.17), t(81) = 4.90, p < .001. Replicating previous findings, younger adults generally fixated more on the most negative of areas of the IAPS images than did older adults (e.g., Isaacowitz & Choi, in press).
Predicting Age Differences in Mood Over Time as a Function of Gaze and Alerting Ability
Multilevel modeling (MLM) was utilized to test hypotheses concerning gaze-mood links over time. MLM accounts for the hierarchical data structure, containing repeated measurements of time and fixation (Level 1) nested within participants (Level 2) with mood as the dependent variable (Raudenbush & Bryk, 2002; Singer & Willet, 2003). The MLM analysis provides fixed effects describing the average mean level (intercept) and within-person trajectories (slope) as well as random effects describing interindividual differences in these parameters (i.e., individuals’ deviation from the sample mean trajectory) and it does not hold an assumption of balanced data (Raudenbush & Bryk, 2002; Singer & Willet, 2003), which is important for the current study given the unequal number of younger and older adults. All MLMs were implemented using SAS PROC MIXED (Littell, Milliken, Stroup, & Wolfinger, 2006). Time was centered at the first occasion so the initial time of mood report served as the reference point. Another Level 1 predictor, within-person effect of fixation (WPfixation), was centered at each participant’s own average fixation duration across all time intervals (i.e., person-mean centering) and represents increases or decreases from his or her own mean fixation level. At Level 2, between-person effect of fixation (BPfixation) was also included as a covariate given the age differences in the overall fixation level. This reflects the individual’s mean level of fixation centered at 50 (i.e., where 0 represents that participants fixated equally to the most negative area of the images and to less negative areas). The Level 2 predictor, age group variable, was dummy-coded with young adults as the reference group (younger adults = 0, older adults = 1). Finally, another Level 2 predictor, alerting, was grand-mean centered by subtracting the respective sample mean (M = 34.98).
The results are presented in two parts. First, the findings from the preliminary analysis regarding the initial inspection of the amount of within-person variability in the mood data, together with results on time-related trends in mood, are reported. Second, findings from the final model testing age differences in within-person gaze-mood links and the moderating role of alerting ability are reported.
Preliminary Analysis
Results of the unconditional means model (i.e., without predictors) for mood revealed that 25% of total variance in mood scores was attributable to within-person variability. Next, we fitted a set of unconditional growth models to examine time-related trends in mood states. A visual inspection of mood data indicated that each participant’s mood declined over time, but the rate of decline decelerated, suggesting the need for a quadratic effect of time. Therefore, three unconditional growth models were inspected: a fixed linear and random linear time model; a fixed quadratic time and random linear time model; and a random quadratic time model. Models with differing fixed effects were estimated using maximum likelihood and models differing in random effects only were estimated using restricted maximum likelihood. Nested models were compared using the difference in the model -2 Log Likelihood (LL) values (i.e., χ2 values) as a function of degrees of freedom, which were equal to the difference in the number of model parameters (see Snijders & Bosker, 1999); smaller -2LL values indicate better fit of the model. The fixed quadratic and random linear time model showed improved fit over the fixed linear and random linear time model, LL ratio χ2(1) = 43.2, p < .001. The random quadratic time model had a better fit than the fixed quadratic and random linear time model, LL ratio χ2(3) = 361.0, p < .001, indicating that the random quadratic time model provided the best fit; therefore, it was used as the baseline model for hypothesis tests.
Is There Evidence that Alerting Ability Plays a Role in Age-Related Gaze-Mood Links?
To test whether there were age-related gaze-mood links over time, and whether the alerting function served as a moderator, the following model was specified:
Moodit represents mood for person i at time t. In the Level 1 equation, β0t represents the within-person level of mood at the initial mood assessment, β1t represents the linear time-related slope (i.e., change in mood over time), and β2it represents the quadratic time- related slope, and rit denotes the residual within-person variance. β3t is the expected change in mood associated with deviations from within-person mean fixation and β4t is the expected change in mood associated with the within-person relationship between the linear effect of time and deviations from the within-person mean fixation. In the Level 2 equation, γ00 represents the initial mood for younger adults with average alerting. The slopes denote the expected changes in mood for a one-unit increase in a given predictor variable when controlling for the effects of the other predictors (i.e., when all other predictors are zero).
Age group differences in the initial mood are represented by γ01, while average percent fixation (BPfixation) and alerting differences in initial mood are represented by γ02 and γ03, respectively. Whether age group differences in the initial mood depend on alerting is represented by γ04. The linear effect of time on mood for younger adults is reflected in γ10 and cross-level interactions examining age group and alerting functioning differences in the linear effect of time on mood are reflected in γ11 and γ12, respectively. Whether or not age group differences in the linear effect of time depend on alerting functioning is represented by γ13. γ20 represents the quadratic effect of time in mood for younger adults. The within-person relationship between deviations from within-person mean fixation and mood for younger adults is represented by γ30 and cross-level interactions testing for age group and alerting functioning differences in the within-person relationship between deviations from within-person mean fixation and mood are specified in γ31 and γ32, respectively.γ33 tests for the simultaneous interaction between age group and alerting functioning on the within-person relationship between fixation and mood (i.e., Time X Age group X WPfixation). γ40 tests for the within-person interaction between the linear effect of time and deviations from within-person mean fixation for younger adults. γ41 tests for age group differences in the within-person interaction between the linear effect of time and deviations from within-person mean fixation and γ42 tests for alerting functioning differences. γ43 tests for the simultaneous interaction between age group and alerting functioning on the within-person interaction between the linear effect of time and deviations from within-person mean fixation (i.e., Time X Age group X WPfixation X Alert interaction). Finally, u0i, u1i, and u2i represent person-specific deviations from intercept, linear slope and quadratic slope, respectively.
The results of the model are summarized in Table 1. The model for the fixed effects accounted for 10% of the variance in mood. 1 Compared to the baseline model, Level 1 residual and Level 2 intercept variances decreased by 4.42 and 1.72 %, respectively. The significant linear and quadratic effects of time indicated that younger adults’ mood linearly declined over time; however, the rate of decline decelerated over time. A significant Time x Age Group interaction suggests that age groups differ in the linear rate of change, such that older adults’ mood deteriorated to a greater degree than younger adults.2 The effect of deviations from within-person mean fixation (γ30) was significant for younger adults (the reference group), indicating that they felt better when they looked more at the most negative areas of the stimuli compared to their own average. Neither the two-way WPfixation X Age group (γ31) and WPfixation X Alerting (γ32) interactions, nor the three-way WPfixation X Age group X Alerting (γ33) interaction, were significant. A significant interaction of Time X WPfixation (γ40), however, indicates that the effect of the deviations from within-person mean fixation depended on the linear effect of time, as the positive effect on mood of a more-negative-looking approach for younger adults became less positive during the task.
Table 1.
Multilevel Modeling Results Predicting Mood From Time, Fixation, Age group, and Alerting Ability.
| Mood | ||
|---|---|---|
| Parameters | Estimate | SE |
| Fixed effects | ||
| Intercept | 53.99*** | 4.33 |
| Time | −4.09*** | 0.06 |
| Time2 | 0.24*** | 0.06 |
| WPfixation | 0.09* | 0.05 |
| Age group | 9.54 | 5.81 |
| BPfixation | 0.02 | 0.16 |
| Alert | −0.04 | 0.13 |
| Age group X Alert | 0.15 | 0.19 |
| Age group X WPfixation | −0.11 | 0.08 |
| Time X WPfixation | −0.02** | <0.01 |
| Time X Age group | −1.46*** | 0.58 |
| Time X Alert | <0.01 | 0.01 |
| Time X Age group X Alert | −0.02 | 0.02 |
| WPfixation X Alert | <0.01 | <0.01 |
| WPfixation X Age group X Alert | −0.01 | <0.01 |
| Time X WPfixation X Age group | 0.03 | 0.02 |
| Time X WPfixation X Alert | <−0.01 | <0.01 |
| Time X WPfixation X Age group X Alert | <0.01* | <0.01 |
| Variance Components | ||
| Intercept | 592.91*** | 101.62 |
| Linear slope | 38.16*** | 7.57 |
| Quadratic slope | 0.20*** | 0.05 |
| Residual | 25.88*** | 1.68 |
| Modeled Variancea | ||
| Between persons (pseudo R2Intercept) | 4.42% | |
| Within persons (pseudo R2Residual) | 1.72% | |
Note. SE = standard errors; WPfixation = deviations from within-person mean fixation; BPfixation = between-person effect of fixation.
Proportional reductions in the variance component intercept and residual in comparison to baseline model (i.e., random quadratic model).
p<.05,
p<.01,
p<.00
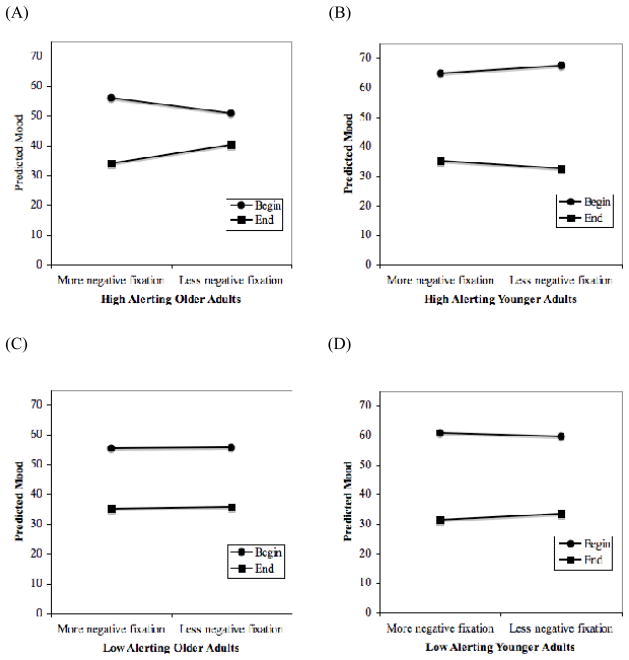
It is important to note, however, that there was a significant four-way Time X WPfixation X Age Group X Alert (γ43) interaction. To examine the nature of the four-way interaction, separate models were first run for young and old groups and then the three-way Time X WPfixation X Alert interaction within age group was plotted. Following the procedure outlined by Dawson and Richter (2006), simple slopes of mood regressed on time were tested for significant differences between pairs of slopes at + / − 1SD for both alerting and WPfixation. This was run using Dawson’s (nd) on-line resource for probing three-way interactions. Figure 1(A) and 1(B) depict these interactions. To show the pattern of results most clearly, only the beginning and end time points were plotted in the graphs. The planned tests revealed that the difference in slopes between the low vs. high level of fixation among high alerting older adults was significant (t = −2.25, p < .02). High alerting older adults experienced a lesser decline in mood from the beginning of the session to the end when they looked less at the most negative areas of the stimuli than their average tendency, as compared to when they looked more at the most negative areas of the stimuli than their average tendency. This is shown in Figure 1(A) by the smaller distance from the beginning to the end for less negative fixation than for more negative fixation. No difference was found for high alerting younger adults (t < 1), indicating that the relationship between the level of fixation and mood change over time did not depend on better alerting functioning (see Figure 1(B)).
Figure 1.
Predicting mood over time as a function of deviations from within-person mean fixation for older and younger participants with high and low alerting ability. Figure is adjusted for quadratic time effect and between-person effect of fixation
Next, we explored additional slope differences through post-hoc comparisons, using a Bonferroni adjustment alpha level of 0.1 per test (Dawson & Richter, 2006). None of the comparisons reached significance after adjustment. Thus, in partial support of our predictions, older adults with high alerting ability who used a less-negative-looking approach compared to their average tendency showed a less negative mood trajectory over time. In contrast, we did not find evidence that alerting ability moderated the gaze-mood change relationship in low alerting older adults or in younger adults.
Discussion
In this study we investigated the moderating role of alerting ability on the relationship between age-related gaze preferences and mood regulation. Consistent with previous findings, overall fixation patterns indicate that older adults showed a less-negative-looking approach toward the negative images than did younger adults (e.g., Isaacowitz & Choi, in press). To extend previous findings, the current study tested a direct link between age-related gaze preferences and mood change in the context of deliberate mood regulation by providing participants explicit instructions to actively manage mood while viewing highly arousing negative stimuli. In order to connect the gaze preferences to mood, we used a person-centered approach which examined how within-person changes in fixation might covary with mood change over time; this expanded our previous findings regarding age-related gaze-mood links (e.g., Isaacowitz et al., 2008, 2009). Results from the baseline time-trend model indicated that individuals’ moods declined over time as they were continually exposed to highly arousing unpleasant images; however, the rate of decline tended to decelerate over time. Yet, the results from the follow-up model suggest that age only predicted a linear change in mood, showing that older adults’ mood deteriorated to a greater degree than younger adults, contrary to previous findings on self-report in mood and laboratory mood-regulation tasks (e.g., Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Larcom & Isaacowitz, 2009). This may suggest that it is more difficult for older adults to regulate their mood in a situation with continual exposure to highly arousing negative stimuli (e.g., Charles, 2005; Charles & Carstensen, 2008). Older adults have been found to perceive IAPS pictures as more negative and arousing than younger adults (Gruhn & Scheibe, 2008). Nevertheless, these age differences in the rate of mood change depended on within-person variability in fixation and individual differences in alerting ability.
The Role of Alerting Ability in Age-Related Gaze-Mood Change Links
We found evidence that individual differences in alerting ability moderated age-related gaze-mood change links. Among older adults, the level of fixation when alerting ability was high was shown to be more important than when alerting ability is low. That is, older adults with high alerting ability experienced a lesser decline of mood at the end of the session when they showed less-negative-looking patterns (looking less at the most negative areas of stimuli) than their average tendency compared to when they showed more-negative-looking patterns. Older adults with worse alerting ability did not show a significant pairing between a less-negative-looking approach (compared with their own average tendency) and mood change. Therefore, activating a less-negative-looking approach was a more beneficial mood regulatory strategy for those older adults with better alerting ability. This finding indicates that older adults who are more responsive to external warning cues may also be better at utilizing their gaze toward emotional stimuli to effectively manage how they feel. These results support a direct link between a less-negative-looking approach and better mood among older adults, but only for those with good alerting ability.
In an extension of previous findings (Isaacowitz et al., 2008, 2009), younger adults experienced better mood when they looked more at the most negative areas of the stimuli compared to their own average. This suggests that younger adults experience better moods when they engage visually with the most negative aspects of the stimuli. It is possible that young adults rely more on emotion regulation strategies other than attention deployment: perhaps they attempt to gather useful information for reappraisal of the negative stimuli by visually engaging with them (see also Isaacowitz, under review). However, the magnitude of the effect reduced over time. In other words, younger adults experienced less benefit from using negative gaze to regulate mood as time progressed. The nature of these effects requires further investigation. Unlike for older adults, though, there was no clear pattern linking gaze and mood to alerting ability in young adults.
The current findings suggest that it is important to examine not only the difference between persons in the association between gaze preferences and mood, but also the within-person, moment-to-moment association. While previous studies focused on how individual differences in mean fixation level to emotional stimuli predicted mean change in mood (e.g., Isaacowitz et al., 2009), the results from current study demonstrated that an individual’s momentary deviations around his or her own fixation average influences change in mood over time. Interestingly, the link between within-person changes in fixation and changes in mood over time emerged as most relevant for older adults with high alerting ability. For these older adults to regulate their mood successfully, they had to “turn on” a less-negative-looking pattern in real-time.
Why, though, does good alerting ability facilitate the successful use of within-person gaze shifts to regulate mood? As mentioned earlier, high alerting scores refer to increased response preparation in reaction to external warning cues (Fan et al., 2002; Posner, 2008), whereas low alerting scores indicate less dependence on alerting cues to achieve a vigilant state. Thus, older individuals who are especially reliant on cues in order to be alerted may be more sensitive to detecting emotionally relevant stimuli in their environment and may be more adept at using those cues as cause to activate preferential gaze to help them regulate their mood. Given that alerting cues persisting over time enhance older adults’ alertness to use external cues effectively (Fernandez-Duque & Black, 2006; Jennings et al., 2007) and that negative affect enhances the level of alertness (Compton et al., 2004), high alerting older adults may be better able to use the most negative areas as cues during prolonged exposure to negative stimuli. These higher alerting older adults likely direct their attention away from the most negative areas by activating a less-negative-looking approach as a mood regulatory tool.
On the other hand, because older adults with low alerting ability are less able to use external cues to direct their attention, they may be less adept at using less negative looking preferences for successful mood regulation. Why is alerting ability not associated with younger adults’ gaze-mood links? One possibility is that younger adults may not rely on emotional cues to activate preferential gaze as a regulatory tool to the same extent as older adults, making attentional processes other than alerting more relevant to their regulatory efforts.
A few limitations should be considered when interpreting the findings reported above. One of the limitations of the current study is that, while participants were instructed to regulate their mood, we did not have any measure beyond eye-tracking of what they actually did in response to the instructions. Future research should ask participants to report what they actually did in order to follow the instructions and whether they were motivated to improve their mood. Another potential limitation is that the deliberate mood regulation instruction may have introduced a demand effect on the mood ratings. However, this does not seem to be the case, as participants clearly indicated that their mood declined over time. Thus, it does not appear that they adjusted how they were feeling to meet the expectations of the researchers. Finally, participants’ mood change patterns may actually reflect simple habituation effects. This is certainly possible, but the higher-order interactions predicting mood change suggest that the direction of mood change over time depend on age, use of gaze, and attention ability.
Conclusion
What can such findings tell us about the effects of age on links between emotion and cognition? Extending the previous findings on the role of executive control in age-related gaze-mood links (Isaacowitz et al., 2009), the current study suggests that alerting ability is also an important attentional substrate in the context of older adults’ use of gaze patterns for deliberate mood regulation. Older adults who have good alerting abilities are able to effectively use their gaze by visually disengaging more from the most unpleasant parts of images to facilitate their mood regulation over time. In contrast, younger adults better regulate their mood using a more negative gaze pattern than their usual tendency. However, gaze-mood links in younger adults do not depend on alerting ability, suggesting the possibility that younger adults may rely more on other attentional substrates to utilize a preferential gaze for regulatory purposes. The results highlight that the use of gaze for mood regulation happens in real-time, but the interplay between the two over time may vary not only by age but by other individual difference factors as well.
Acknowledgments
This research was supported by National Institute on Aging Grant R01 AG026323 to Derek M. Isaacowitz. We are grateful to Xiaodong Liu, Shevaun Neupert, and Michaela Riediger for their helpful suggestions concerning this study.
Footnotes
Two categories of effects sizes were utilized, global and local effect sizes (Peugh, 2010; Singer & Willet, 2003). Global effect size R2 quantifies the proportion of the variance in the dependent variable explained by all predictors in the model. Local effect sizes R2, on the other hand, quantify the proportion reduction in Level-1 (R2Residual) and Level-2 (R2Intercept) variance from adding predictors to the subsequent model.
We repeated this model by also including an age group by quadratic effect of time term; however, the interaction was not significant and was excluded from the model.
A separate model was also run to test whether between-person fixation differences predicted mood change over time. Although the mean level fixation did not predict a time-related trend in mood, there was evidence that, on average, older adults with high alerting ability who had the average tendency to fixate on less negative aspects of the stimuli, were able to maintain a higher level of overall mood than those who used a negative looking approach (i.e., BPfixation X Age Group X Alerting interaction, t = 2.23, p < .05); whereas younger adults with high alerting ability showed a similar level of overall mood regardless of fixation patterns. Thus, at the mean level, we found a similar effect of alerting on age-related gaze-mood links.
References
- Allard ES, Isaacowitz DM. Are preferences in emotional processing affected by distraction? Examining the age-related positivity effect in visual fixation within a dual-task paradigm. Aging, Neuropsychology, and Cognition. 2008;15(6):725–743. doi: 10.1080/13825580802348562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard ES, Wadlinger HA, Isaacowitz DM. Positive gaze preferences in older adults: Assessing the role of cognitive effort with pupil dilation. Aging, Neuropsychology, and Cognition. 2010;17:296–311. doi: 10.1080/13825580903265681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the Intersection of Cognition, Motivation, and Emotion. In: Birren JE, Schaire KW, editors. Handbook of the Psychology of Aging. 6. Amsterdam: Elsevier; 2006. pp. 343–362. [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade J. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Charles ST. Viewing injustice: Greater emotion heterogeneity with age. Psychology and Aging. 2005;20:159–165. doi: 10.1037/0882-7974.20.1.159. [DOI] [PubMed] [Google Scholar]
- Charles ST, Carstensen LL. Unpleasant situations elicit different emotional responses in younger and older adults. Psychology and Aging. 2008;23:495–504. doi: 10.1037/a0013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Piazza JR. Age differences in affective well-being: Context matters. Social and Personality Psychology compass. 2009;3:1–14. [Google Scholar]
- Compton RJ, Wirtz D, Pajoumand G, Claus E, Heller W. Association between positive affect and attentional shifting. Cognitive Therapy and Research. 2004;28:733–744. [Google Scholar]
- Dawson J. Interpreting interaction effects. n.d Retrieved from http://www.jeremydawson.co.uk/slopes.htm.
- Dawson JF, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference test. Journal of Applied Psychology. 2006;91:917–926. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- Eich E, Metcalfe J. Mood dependent memory for internal versus external events. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15(3):443–455. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Festa-Martino E, Ott BR, Heindel WC. Interactions between phasic alerting and spatial orienting: Effects of normal aging and Alzheimer’s disease. Neuropsychology. 2004;18:258–268. doi: 10.1037/0894-4105.18.2.258. [DOI] [PubMed] [Google Scholar]
- Folsetin MF, Folstein SE, McHugh PR. “Mini-mental”: A practical method for grading the cognitive state of patients for clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer’s Disease. Neuropsychology. 2006;20:133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Gotestam Skorpen C, Hsu AYC. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12:590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Grühn D, Scheibe S. Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): Do ratings become more extreme with age? Behavior Research Methods. 2008;40:512–521. doi: 10.3758/brm.40.2.512. [DOI] [PubMed] [Google Scholar]
- Hetherington R. The Snellen Chart as a test of visual acuity. Psychologische Forschung. 1954;24:349–357. doi: 10.1007/BF00422033. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM. Does looking at the positive mean feeling good? Age makes a difference. (under review) Manuscript submitted for publication. [Google Scholar]
- Isaacowitz DM, Choi Y. Malleability of age-related positive gaze preferences: Training to change gaze and mood. Emotion. doi: 10.1037/a0021551. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006a;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An Eye-tracking study. Psychology and Aging. 2006b;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson HR. Looking while unhappy: Mood congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19:848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Neupert SD. Use of gaze for real-time mood regulation: Effects of age and attentional functioning. Psychology and Aging. 2009;24:989–994. doi: 10.1037/a0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, Dagenbach DD, Engle CM, Funke LJ. Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Aging, Neuropsychology, and Cognition. 2007;14:353–369. doi: 10.1080/13825580600788837. [DOI] [PubMed] [Google Scholar]
- Johnson DR. Goal-directed attentional deployment to emotional faces and individual differences in emotional regulation. Journal of Research in Personality. 2009;43:8–13. [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (lAPS): Instruction manual and affective ratings. Gainsville, FL: Center for Research in Psychophysiology. University of Florida; 1999. [Google Scholar]
- Larcom MJ, Isaacowitz DM. Rapid Emotion Regulation After Mood Induction: Age and Individual Differences. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2009;64B:733–741. doi: 10.1093/geronb/gbp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Moss M, Hoffman C, Kleban MH, Ruckdeschel K, Winter L. Valuation of life: A concept and a scale. Journal of Aging and Health. 2001;13:3–31. doi: 10.1177/089826430101300101. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS for mixed models. 2. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free- viewing visuocognitive tasks. Journal of Neuroscience Methods. 2003;128:85–93. doi: 10.1016/s0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-Directed Memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: A meta-analysis of memory and attention tasks. Psychology and Aging. 2008;23:263–286. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychology and Aging. 2008;23:585–594. doi: 10.1037/a0013176. [DOI] [PubMed] [Google Scholar]
- Peugh JL. A practical guide to multilevel modeling. Journal of School Psychology. 2010;48:85–112. doi: 10.1016/j.jsp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Henry JD, Hosie JA, Milne AB. Effective Regulation of the Experience and Expression of Negative Affect in Old Age. Journals of Gerontology Series B: Psychological Sciences & Social Sciences. 2008;36B:138–145. doi: 10.1093/geronb/63.3.p138. [DOI] [PubMed] [Google Scholar]
- Posner MI. Measuring alertness. Annals of the New York Academy of Sciences. 2008;1129:193–199. doi: 10.1196/annals.1417.011. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Riediger M, Schmiedek F, Wagner GG, Lindenberger U. Seeking pleasure and seeking pain: Differences in prohedonic and contra-hedonic motivation from adolescence to old age. Psychological Science. 2009;20:1529–1535. doi: 10.1111/j.1467-9280.2009.02473.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JG. The biggest reward for my invention isn't money. Medical Economics. 1984;61:152–163. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Catran E, Meiran N. Reappraisal (but not distraction) is going to make you sweet: Physiological evidence for self-control effort. International Journal of Psychophysiology. 2009;71:91–96. doi: 10.1016/j.ijpsycho.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Methods for Studying Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Spieler DH, Mayr U, LaGrone S. Outsourcing cognitive control to the environment: Adult age differences in the use of task cues. Psychonomic Bulletin & Review. 2006;13:787–793. doi: 10.3758/bf03193998. [DOI] [PubMed] [Google Scholar]
- Stanley JT, Isaacowitz DM. Age-related differences in profiles of mood-change trajectories. Developmental Psychology. doi: 10.1037/a0021023. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir M. What do people want to feel and why?: Pleasure and utility in emotion regulation. Current Directions in Psychological Science. 2009;18:101–105. [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Looking happy: The experimental manipulation of a positive visual attention bias. Emotion. 2008;8:121–126. doi: 10.1037/1528-3542.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Isaacowitz DM. Aiming at happiness: How motivation affects attention to and memory for emotional images. Motivation and Emotion. 2006;30:249–256. [Google Scholar]
- Zelazo PD, Cunningham W. Executive function: Mechanisms underlying emotion regulation. In: Gross J, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 135–158. [Google Scholar]