Abstract
Copy number variants (CNVs) are known to be associated with complex neuropsychiatric disorders (e.g., schizophrenia and autism) but have not been explored in the isolated features of aggressive behaviors such as intermittent explosive disorder (IED). IED is characterized by recurrent episodes of aggression in which individuals act impulsively and grossly out of proportion from the involved stressors. Previous studies have identified genetic variants in the serotonergic pathway that play a role in susceptibility to this behavior, but additional contributors have not been identified. Therefore, to further delineate possible genetic influences, we investigated CNVs in individuals diagnosed with IED and/or personality disorder (PD). We carried out array comparative genomic hybridization on 113 samples of individuals with isolated features of IED (n = 90) or PD (n = 23). We detected a recurrent 1.35-Mbp deletion on chromosome 1q21.1 in one IED subject and a novel ∼350-kbp deletion on chromosome 16q22.3q23.1 in another IED subject. While five recent reports have suggested the involvement of an ∼1.6-Mbp 15q13.3 deletion in individuals with behavioral problems, particularly aggression, we report an absence of such events in our study of individuals specifically selected for aggression. We did, however, detect a smaller ∼430-kbp 15q13.3 duplication containing CHRNA7 in one individual with PD. While these results suggest a possible role for rare CNVs in identifying genes underlying IED or PD, further studies on a large number of well-characterized individuals are necessary. © 2011 Wiley-Liss, Inc.
Keywords: aggression, array CGH, genomic disorders, segmental duplication, 15q13.3
INTRODUCTION
Recent analyses of copy number variants (CNVs) have revealed associations of genomic changes with a variety of human diseases and disorders. A majority of rare (frequency of <1%) CNVs arise within genomic “hotspots” due to unequal crossover between segmental duplications of high sequence identity [Lupski, 1998; Bailey et al., 2002]. Genome-wide surveys of these hotspots initially detected an enrichment of rare CNVs among individuals with intellectual disability [Sharp et al., 2006; Stankiewicz and Lupski, 2010] and were subsequently identified in individuals with schizophrenia [Walsh et al., 2008], autism [Sebat et al., 2007], obesity [Bochukova et al., 2010; Walters et al., 2010], and epilepsy [Helbig et al., 2009; Mefford and Mulley, 2010].
Aggression is a complex behavior regulated and influenced by an individual's environment, neural brain circuitry, and genetic makeup [Brunner et al., 1993; Manuck et al., 1999; Seroczynski et al., 1999; Volavka, 1999; Davidson et al., 2000; Bevilacqua et al., 2010]. Impulsive aggression as opposed to premeditated aggression is believed to arise from an inability to fully regulate negative emotion and often leads to acts of spontaneous violence [Davidson et al., 2000; Siever, 2008]. This behavior is highly prevalent among violent offenders and has led investigators to explore the possible biological and neurological contributions to the aggression phenotype [Seroczynski et al., 1999; Rajender et al., 2008; Siever, 2008; Craig and Halton, 2009]. Twin and family studies suggest that impulsive acts of aggression are influenced by genetics, with heritability estimates of 44–72% [Miles and Carey, 1997; Seroczynski et al., 1999; Slutske, 2001; Rhee and Waldman, 2002; Yeh et al., 2010]. More than a decade of genetic research using linkage and association studies has identified several candidate genes as contributors to impulsive aggression [Brunner et al., 1993; Manuck et al., 1999; Caspi et al., 2002; Craig and Halton, 2009; Bevilacqua et al., 2010].
Interestingly, aggression, impulsivity, and mood disorders have been reported in many individuals with large genomic rearrangements, particularly those carrying an approximately 1.6-Mbp recurrent deletion on chromosome 15q13.3 spanning segmental duplication breakpoints 4 and 5 (BP4 and BP5). In fact, five separate studies on this particular deletion reported aggressive behaviors in individuals with developmental delay or autism [Ben-Shachar et al., 2009; Miller et al., 2009; Shinawi et al., 2009; van Bon et al., 2009; Cubells et al., 2011]. For example, Ben-Shachar et al. [2009] reported that 9 out of 14 children with the deletion also exhibited some form of abnormal behavior including rage, repeated head banging, and/or attention deficit hyperactivity disorder (ADHD). Behavioral disorders have also been reported in other patients with large CNVs including 1q21.1 cases with intellectual disability [Brunetti-Pierri et al., 2008] and 16p13.11 cases with autism [Ullmann et al., 2007], intellectual disability [Hannes et al., 2009], or schizophrenia [Ingason et al., 2009]. These findings suggest that haploinsufficiency of certain genomic regions affects specific neurobiological pathways predisposing individuals to aggressive and impulsive behavior. However, to date, no study has specifically examined the role of CNVs in isolated aggressive behaviors, such as intermittent explosive disorder (IED).
IED is characterized by recurrent episodes of aggressive behavior and, according to the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV) [Association, 1994], cannot be explained by other mental disorders (e.g., borderline personality disorder), the effects of substance use (e.g., medication or drug abuse), or a medical condition (e.g., head trauma). The disorder is estimated to affect approximately 3–4% of the general population, with an early onset age around 14 years [Coccaro et al., 2005; Kessler et al., 2006]. Here we undertook a systematic analysis to assess the contribution of CNVs in 113 individuals diagnosed with IED (n = 90) and/or personality disorder (PD, n = 23) without cognitive deficits. We performed comparative genomic hybridization (CGH), using a custom whole-genome microarray targeted to genomic hotspots, to identify rare, potentially pathogenic CNVs contributing to IED not present in 306 control individuals. For further comparison, we also assessed CNV data from a large set of 5,570 normal adult individuals matched for ethnicity [Consortium, 2008; Itsara et al., 2009].
MATERIALS AND METHODS
Subjects
All subjects were physically healthy Caucasian individuals who were systematically evaluated as part of a larger program designed to study the biological correlates of personality traits in human subjects. Study subjects (85 males, 62 females) were recruited by newspaper and public service announcements seeking subjects with and without histories of anger and aggression to take part in medically related studies. Written informed consent, using an IRB-approved consent form, was obtained from all subjects after all procedures were fully explained. The medical health of all subjects was documented by medical history, physical examination, and a variety of clinical laboratory studies, including a urine screen for illicit drugs.
Diagnostic Assessment
All diagnoses of IED were made as previously described [Coccaro et al., 2009]. A diagnosis of IED was made using the Integrated Research Criteria for IED [IED-IR; Coccaro, 2011], which combines the critical aspects of the DSM-IV and earlier Research Criteria for IED [Coccaro et al., 1998]. Subjects with a life history of bipolar disorder, schizophrenia (or other psychotic disorder), or intellectual disability were excluded from this study. All of the IED-IR subjects (n = 117) met the DSM-IV criteria for at least one lifetime Axis I disorder, and nearly all (n = 107) met DSM-IV criteria for a PD (Table I). Most of the PD subjects (n = 21) met DSM-IV criteria for at least one lifetime Axis I disorder and, by definition, all PD subjects (n = 30) met DSM-IV criteria for a PD (Table I). Subjects from both groups had clear evidence of impaired psychosocial functioning (IED, mean GAF score = 60.5 ± 7.3; PD, mean GAF 65.2 ± 5.3). Aggression was assessed dimensionally (in most, though not all, subjects) using the aggression scales of the Life History of Aggression assessment [Coccaro et al., 1997] and the Buss–Perry Aggression Questionnaire [Buss and Perry, 1992]. Impulsivity was evaluated using the Life History of Impulsive Behavior Questionnaire [Schmidt et al., 2004] and the impulsivity score from the Eysenck Personality Questionnaire-II [Eysenck and Eysenck, 1975].
TABLE I.
Summary of Lifetime Axis I and Personality Disorder Diagnoses
| IED-IR (n = 117) | PD (n = 30) | |
|---|---|---|
| Mean GAF score | 60.5 ± 7.3 | 65.2 ± 5.3 |
| Lifetime Axis I disorders | 117 (100%) | 21 (70%) |
| Any mood | 74 (63.2%) | 14 (46.7%) |
| Major depression | 63 (53.8%) | 13 (43.3%) |
| Dysthymia | 12 (10.3%) | 1 (3.3%) |
| Depressive NOSa | 8 (6.8%) | 1 (3.3%) |
| Any anxiety | 46 (39.3%) | 8 (26.7%) |
| Phobic | 23 (19.7%) | 6 (20%) |
| Non-phobic | 46 (39.3%) | 7 (23.3%) |
| Substance use | 52 (44.4%) | 3 (10%) |
| Alcoholism | 41 (35%) | 3 (10%) |
| Drug dependence | 28 (23.9%) | 2 (6.7%) |
| Non-IED impulse control | 8 (6.8%) | 0 (0%) |
| Eating | 19 (16.2%) | 3 (10%) |
| Adjustment | 8 (6.8%) | 3 (10%) |
| Somatoform | 5 (4.3%) | 0 (0%) |
| Personality disorders | 107 (91.5%) | 30 (100%) |
| Cluster A | 21 (17.9%) | 2 (6.7%) |
| Paranoid | 20 (17.1%) | 1 (3.3%) |
| Schizoid | 1 (0.9%) | 1 (3.3%) |
| Cluster B | 62 (53%) | 11 (36.7%) |
| Borderline | 46 (39.3%) | 7 (23.3%) |
| Antisocial | 21 (17.9%) | 0 (0%) |
| Narcissistic | 19 (16.2%) | 4 (13.3%) |
| Histrionic | 8 (6.8%) | 1 (3.3%) |
| Cluster C | 35 (29.9%) | 13 (43.3%) |
| Obsessive-compulsive | 23 (19.7%) | 7 (23.3%) |
| Avoidant | 13 (11.1%) | 7 (23.3%) |
| Dependent | 2 (1.7%) | 1 (3.3%) |
| PD-NOSa | 28 (23.9%) | 11 (36.7%) |
NOS = not otherwise specified; Note that 113/147 total samples were of good DNA quality and were evaluated for CNVs.
DNA Samples
DNA was extracted from whole blood by the University of Chicago's Clinical Research Center and the extracted DNA was frozen and stored at −70°C until genotyping. Of the 147 (IED, n = 117; PD, n = 30) individuals evaluated in our research program, 113 samples (IED, n = 90; PD, n = 23) passed DNA quality control and were hybridized using a custom targeted microarray. The control cohort consisted of 306 DNA samples obtained from the Rutgers University Cell and DNA Repository (http://www.rucdr.org). These individuals were ascertained by the National Institute of Mental Health (NIMH) Genetics Initiative [Moldin, 2003] through an online self-report based on the Composite International Diagnostic Instrument Short-Form (CIDI-SF) [Kessler and Ustun, 2004] and screened for major depression, bipolar disorder, and psychosis. Those who did not meet DSM-IV criteria for major depression, who denied a history of bipolar disorder or psychosis, and who reported exclusively European origins were included [Baum et al., 2008; Talati et al., 2008].
CNV Discovery and Analysis
We utilized the duplication architecture of the human genome to custom design a DNA oligo microarray targeted to the genomic hotspots [Bailey et al., 2002]. This hotspot-targeted microarray consists of 135,000 probes (by Roche NimbleGen) with a median spacing 2.6 kbp in 107 genomic hotspots and 36 kbp in the genomic backbone. Array CGH of patient samples was carried out as described [Selzer et al., 2005] using a single, unaffected male (GM15724, Coriell) as a reference. All arrays were then analyzed by mapping probe coordinates to the human genome assembly build 36 (hg18). Using chromosome-specific means and standard deviations, normalized log intensity ratios for each sample were transformed into z-scores. These z-scores were then classified as “increased,” “normal,” or “decreased” in copy number using a three-state Hidden Markov Model (HMM). For each sample, HMM state assignments of probes were merged into segments if consecutive probes of the same state were less than 50 kbp apart when merged. If two segments of the same state were separated by an intervening sequence of ≤5 probes and ≤10 kbp, both segments and the intervening sequence were called as a single variant. Subsequently, we automatically filtered and divided putative CNVs based on size, z-scores, and probe counts. With these filtering criteria, we were able to thoroughly scan HMM outputs for CNV events and manually check the validity of each call by examining the normalized log intensity ratios across a chromosome.
RESULTS
A total of 350 CNVs greater than 50 kbp were detected using array CGH among the 113 samples that passed DNA quality control measures (Supplementary Table I). At a similar rate of detection, 1,074 CNVs greater than 50 kbp were seen in our 306 NIMH controls (Supplementary Table II). Overall, there was no difference in the frequency of large CNVs (>500 kbp) between individuals with IED and controls (6/113 vs. 6/306, respectively; Fisher's exact test, P = 0.072) assayed on the same microarray platform. After filtering for known copy number polymorphisms, we identified three large rare CNVs: a recurrent 1q21.1 deletion containing GJA8 [Brunetti-Pierri et al., 2008; Consortium, 2008; Mefford et al., 2008; Stefansson et al., 2008], a novel 16q22.3q23.1 deletion in individuals with IED, and an approximately 430-kbp 15q13.3 duplication containing CHRNA7 in an individual with PD (Table II). None of these events were observed in our 306 NIMH controls.
TABLE II.
Clinical Diagnoses of Subjects Carrying Rare CNV Deletions
| Subject | Sex | Deletion | Frequency in controlsa | IED-IR | Other | Axis II | Suicide attempts |
|---|---|---|---|---|---|---|---|
| Axis I | |||||||
| 1 | Female | 1q21.1 (1.5 Mbp)b | 0/5,570; 0/306 | Current | MDD, recurrent; AD, partial remission; SD, full remission; PSD, current; ODD, current; ADHD, current | Borderline PD | 4 |
| 2 | Female | 16q22.2–q23.1 (350 kbp)b | 0/5,570; 0/306 | Current | CA, past; HD, full remission; GAD, past; ODD, past | Histrionic PD; narcissistic PD | 2 |
| 3 | Male | 15q13.3 (430 kbp)b | 35/5,570; 0/306 | N/A | MDD, recurrent; AD, partial remission; SD, full remission; SP, current; GAD, current, ODD, past | Avoidant PD, borderline PD, self-defeating PD | 0 |
MDD, major depressive disorder; AD, alcohol dependence; SD, poly-substance dependence; PSD, post-traumatic stress disorder; ODD, oppositional defiant disorder; ADHD, attention deficit hyperactivity disorder; CA, cannabis abuse; HD, hallucinogen dependence; GAD, generalized anxiety disorder; SP, social phobia; PD, personality disorder.
Counts from two control groups: 5,570 as previously described by schizophrenia Consortium [2008] and Itsara et al. [2009]; 306 NIMH controls were run using the same NimbleGen microarray design.
Approximate size of copy number variant; actual breakpoints unknown.
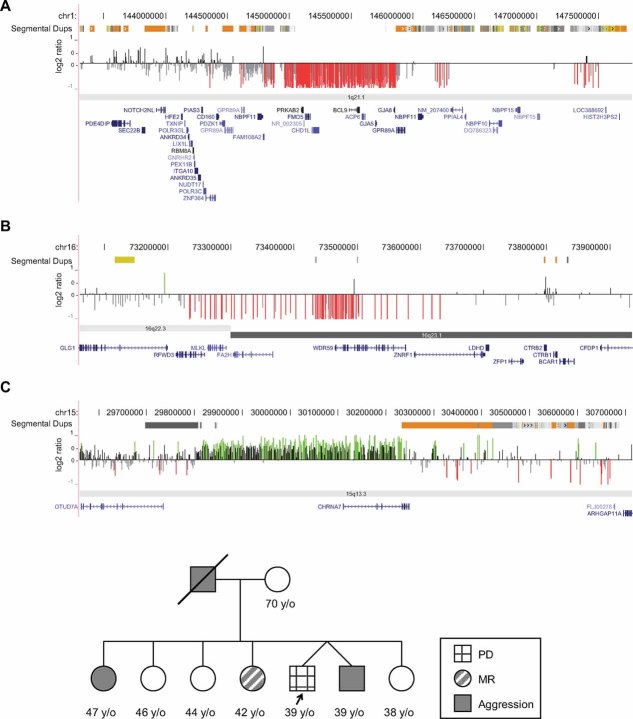
The ∼1.35-Mbp deletion (chr1: 144,979,471–145,863,720) on chromosome 1q21.1 (Fig. 1A) was detected in Subject 1, who had features of IED as well as ADHD, borderline personality disorder, and a history of major depressive disorder, alcohol dependence, and poly-substance dependence. Family history suggests problems with anger and aggression in the father but not in the mother. The subject's older brother has a history of anger and aggression, depression, and a possible diagnosis of schizophrenia while the older sister has had individual and family therapy for emotional problems and difficulties with anger. The subject's younger sister has a history of anorexia, substance abuse, and has also been in therapy. The subject also reported experiencing physical and sexual abuse for herself and her siblings from their father. Detailed evaluation showed that this individual has had at least three verbal arguments/temper tantrums per week over the past 23 years (since 18 years old). There is also a history of more than 20 juvenile arrests for physical fighting albeit without convictions. Outbursts were described to involve snapping, yelling, swearing, and physical fighting, sometimes escalating to property damage. The greatest period in which the subject reported property damage occurred at a frequency of two times per month between the ages of 18–26. For about a decade, between the ages of 19–30 years old, the subject reported involvement in physical assault at about two times per week at the highest frequency. One extreme episode resulted in a week spent in jail and a requirement to take anger management class. In addition to these outbursts, the subject reported multiple attempts at suicide (Supplementary Material).
FIG. 1.
Rare CNVs in IED and PD. A: A recurrent ∼1.5-Mbp deletion was detected in 1 of 90 individuals with IED (Subject 1). The undefined breakpoints lie within segmental duplications (green) flanking the deleted region. B: A large deletion on 16q22.3–q23.1. This rare deletion was detected in 1 out of 90 subjects diagnosed with IED (Subject 2), which is unreported and remains uncharacterized. The approximately 350-kbp region intersects a total of six genes, including two that are highly expressed in the human nervous system: fatty acid 2-hydroxylase (FA2H) and zinc finger and ring finger protein 1 (ZNRF1). C: A recurrent duplication on 15q13.3. This duplication was identified in 1 out of 23 subjects diagnosed with PD (Subject 3). Currently, it is unclear whether this duplication is pathogenic. The lower panel shows a family pedigree of Subject 3 (arrow) who was diagnosed with PD (checkered) and family members with a history of aggression (solid) and mental retardation (diagonal) are also depicted.
The ∼350-kbp deletion we detected on chromosome 16q22.3q23.1 (chr16: 73,290,945–73,626,825) in Subject 2, encompasses five RefSeq genes including FA2H and ZNRF1 (Fig. 1B). This event was not seen in any of the 5,876 controls analyzed in our study, suggesting that this event is novel. There is no history of anger and aggression or substance abuse in the parents. However, anger problems were reported in the subject's sister. There is no childhood history of physical or sexual abuse. Subject 2 typically engaged in two verbal arguments per week for the past 6 years (since 18 years old). These outbursts were described to include yelling, insulting, and swearing at others. This individual recalled one incident of property damage and one episode of physical aggression; however, both events were minor and had no legal consequences. Aggressive episodes occurred while the subject was sober and were spontaneous in nature. This subject was also diagnosed with histrionic and narcissistic PD along with a history of drug abuse, hallucinogen dependence, generalized anxiety disorder, and oppositional defiant disorder (Supplementary Material).
While we did not identify the larger 15q13.3 (BP4–BP5) deletion previously associated with aggressive behaviors, we detected an ∼430-kbp duplication nested within the BP4–BP5 locus and encompassing CHRNA7 in Subject 3 (Fig. 1C). Subject 3 does not have IED but was diagnosed with avoidant, borderline, and self-defeating PDs, social phobia, generalized anxiety disorder, and a history of depression, alcohol dependence, drug dependence, and oppositional defiant disorder. Further evaluation of family members indicated a history of aggression in a twin brother and a sister and intellectual disability in another sister (Fig. 1C). The father was described to have problems with aggression and alcohol. DNA samples from family members were not available for further testing so the carrier status of this 15q13.3 duplication in other family members is not known.
DISCUSSION
In this study we aimed to determine whether rare pathogenic CNVs are a predisposing factor for impulsive aggressive behavior, due to growing evidence that genomic architecture is highly significant to human biology and disease [Marques-Bonet et al., 2009; Mefford and Eichler, 2009]. We were particularly interested to see if 15q13.3 (BP4–BP5) deletions would be enriched in our subjects. Previous reports have emphasized the presence of neuropsychiatric disorders and neurobehavioral problems in patients with this particular alteration on chromosome 15q13.3 (Table II). We did not find any 15q13.3 (BP4–BP5) deletions in our cohort of 113 individuals; however, we recognize that the small sample size of our study cohort limits the power to detect this rare rearrangement. In a study of patients with schizophrenia, this deletion was found in 9/3,391 patients as opposed to 0/3,181 controls [Consortium, 2008]. Our results, therefore, do not completely rule out the possible association of this deletion with aggressive and impulsive behavior.
While the larger 15q13.3 deletion was not observed among IED subjects, we did find a smaller duplication nested within BP4 and BP5 in one subject with PD. This duplication was observed in 35/5,570 and 0/306 of our two control groups. While a heterozygous loss of the region is associated with variable neurodevelopmental phenotypes [Shinawi et al., 2009], the clinical significance of a gain is still undetermined. Recently, Szafranski et al. [2010] attempted to determine whether these duplications are benign or pathogenic by investigating this alteration in 59 patients with a range of phenotypes. After mapping the breakpoints for each event, they were able to determine two classes of large ∼1.5-Mbp (BP4–BP5) duplications (n = 4) and five classes of smaller duplications (n = 55), similar to that found in Subject 3. Six of eleven index patients with a smaller duplication, who were diagnosed with developmental delay or intellectual disability, also had some neuropsychiatric issues, including bipolar disorder, anxiety disorder, disruptive behavior disorder, and severe pica. While these observations suggest the possible association of the smaller 15q13.3 duplications with neurocognitive and neuropsychiatric disorders, the comparable detection rates of this CNV in controls argues otherwise. It is possible, however, that control cohorts were not screened for mild neuropsychiatric behaviors and confound current estimates. Subject 3 in our study is affected by a number of PDs and our findings support the need for more robust studies of 15q13.3 duplications as a susceptibility locus to milder neurobehavioral disorders (Supplementary Material).
The 1q21.1 deletion we identified in Subject 1 has been associated with variable neurodevelopmental phenotypes and is enriched in affected populations compared to controls. The recurrent 1.35-Mbp deletion has been detected in 25/5,218 unrelated patients with mental retardation, microcephaly, cardiac abnormalities, or cataracts [Mefford et al., 2008] and 10/3,391 patients with schizophrenia [Consortium, 2008]. In comparison, the event was only seen in 1/7,918 total controls. Behavioral abnormalities have also been observed in patients with this deletion. Brunetti-Pierri et al. [2008] describe anxiety/depression, antisocial behavior, and aggression in 3 out of 21 probands with dysmorphic features. A loss at this particular locus may be associated with a broader range of phenotypes than is currently described, possibly influencing the behaviors seen in our subject; however, a greater number of samples must be analyzed to draw a definitive conclusion (Table III).
TABLE III.
Summary of Studies Reporting Aggressive Behaviors in Individuals With Rare CNVs
| CNV | Ascertainment | Total cases | (+) Aggressive and/or impulsive behavior | Study |
|---|---|---|---|---|
| 15q13.3 (del) | Developmental delay, epilepsya | 10 | 1 | Shinawi et al. [2009] |
| 15q13.3 (del) | Developmental delay, epilepsy, autism spectrum disorder | 5 | 5 | Miller et al. [2009] |
| 15q13.3 (del) | Developmental delay, autism spectrum disorderb | 14 | 9 | van Bon et al. [2009] |
| 1q21.1 (del) | Congenital heart defects, developmental delay, schizophreniac | 21 | 1 | Brunetti-Pierri et al. [2008] |
| 16p13.1 (del/dup) | Intellectual disability, multiple congenital abnormalities | 13 | 4 | Hannes et al. [2009] |
| 16p13.1 (del/dup) | Intellectual disability, autism | 7 | 2 | Ullmann et al. [2007] |
Ten individuals from four unrelated families.
Fourteen children from 12 unrelated families.
Two probands also described to have behavioral problems.
The second rare CNV identified in this study, a deletion on chromosome 16q22.3q23.1, is currently uncharacterized and was not seen in 5,876 controls. Three RefSeq genes (MLKL, FA2H, and WDR59) are completely deleted by this event, and two (RFWD3 and ZNRF1) are partially deleted. Notably, fatty acid 2-hydroxylase (FA2H) and zinc finger and ring finger 1 (ZNRF1) are two genes involved in the formation and insulation of neuronal axons. Specifically, FA2H contributes to myelin formation and ZNRF1 plays a role in Schwann cell repair; both are highly expressed in nervous tissue [Araki et al., 2001; Alderson et al., 2004]. Reduced white matter integrity (myelinated axons in the brain) has been reported in schizophrenics with significant histories of aggression [Hoptman et al., 2002] and in subjects with IED-IR [Coccaro, unpublished data]. It is possible that the disruption of FA2H and ZNRF1 creates a deficiency in neural maintenance and repair, predisposing an individual to deleterious injuries that can result in neuropsychiatric disorders such as IED.
Our results highlight the possible role of genomic rearrangements in impulsive aggressive behavior and PDs. However, the complex association of these rearrangements with a variety of phenotypes suggests that other factors in addition to CNVs are influencing what ultimately manifests in individuals [Girirajan et al., 2010]. In IED, environmental effects and individual life experiences seem to play a vital role in whether individuals with predisposing genetic variants actually present with clinical features. It may be that impulsive aggression is a behavior that frequently co-occurs with genomic disorders as a result of underlying biological processes that are disrupted. In future work, systematic examination of psychosocial factors, sequencing top priority genes, or carrying out exome or whole-genome sequencing of affected individuals, in addition to CNV analysis, may provide insight into the genetic variability that contributes to IED.
Acknowledgments
We thank the participating subjects and staff at the recruitment centers. We thank Arthur Ko, Karyn Meltz Steinberg, Catarina D. Campbell, and Megan Y. Dennis for critical review of the manuscript. E.E.E. is an investigator of Howard Hughes Medical Institute.
Supplementary material
Additional supporting information may be found in the online version of this article.
REFERENCES
- Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279(47):48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- Araki T, Nagarajan R, Milbrandt J. Identification of genes induced in peripheral nerve after injury. Expression profiling and novel gene discovery. J Biol Chem. 2001;276(36):34131–34141. doi: 10.1074/jbc.M104271200. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Association Press; 1994. [Google Scholar]
- Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, Schwartz S, Adams MD, Myers EW, Li PW, Eichler EE. Recent segmental duplications in the human genome. Science. 2002;297(5583):1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar S, Lanpher B, German JR, Qasaymeh M, Potocki L, Nagamani SC, Franco LM, Malphrus A, Bottenfield GW, Spence JE, Amato S, Rousseau JA, Moghaddam B, Skinner C, Skinner SA, Bernes S, Armstrong N, Shinawi M, Stankiewicz P, Patel A, Cheung SW, Lupski JR, Beaudet AL, Sahoo T. Microdeletion 15q13.3: A locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet. 2009;46(6):382–388. doi: 10.1136/jmg.2008.064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S, Belmer A, Hodgkinson CA, Dell'osso L, Suvisaari J, Coccaro E, Rose RJ, Peltonen L, Virkkunen M, Goldman D. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468(7327):1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O'Rahilly S, Hurles ME, Farooqi IS. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Berg JS, Scaglia F, Belmont J, Bacino CA, Sahoo T, Lalani SR, Graham B, Lee B, Shinawi M, Shen J, Kang SH, Pursley A, Lotze T, Kennedy G, Lansky-Shafer S, Weaver C, Roeder ER, Grebe TA, Arnold GL, Hutchison T, Reimschisel T, Amato S, Geragthy MT, Innis JW, Obersztyn E, Nowakowska B, Rosengren SS, Bader PI, Grange DK, Naqvi S, Garnica AD, Bernes SM, Fong CT, Summers A, Walters WD, Lupski JR, Stankiewicz P, Cheung SW, Patel A. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40(12):1466–1471. doi: 10.1038/ng.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63(3):452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Intermittent explosive disorder: Development of integrated research criteria for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Compr Psychiatry. 2011;52(2):119–125. doi: 10.1016/j.comppsych.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: Development and psychometric characteristics. Psychiatry Res. 1997;73(3):147–157. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Berman ME, Lish JD. Intermittent explosive disorder-revised: Development, reliability, and validity of research criteria. Compr Psychiatry. 1998;39(6):368–376. doi: 10.1016/s0010-440x(98)90050-5. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Posternak MA, Zimmerman M. Prevalence and features of intermittent explosive disorder in a clinical setting. J Clin Psychiatry. 2005;66(10):1221–1227. doi: 10.4088/jcp.v66n1003. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: Serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2009;35(2):435–444. doi: 10.1038/npp.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig IW, Halton KE. Genetics of human aggressive behaviour. Hum Genet. 2009;126(1):101–113. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- Cubells JF, DeOreo EH, Harvey PD, Garlow SJ, Garber K, Adam MP, Martin CL. Pharmaco-genetically guided treatment of recurrent rage outbursts in an adult male with 15q13.3 deletion syndrome. Am J Med Genet Part A. 2011;155A(4):805–810. doi: 10.1002/ajmg.a.33917. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck personality questionnaire (junior and adult) London: Hodder and Stoughton; 1975. [Google Scholar]
- Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, Itsara A, Vives L, Walsh T, McCarthy SE, Baker C, Mefford HC, Kidd JM, Browning SR, Browning BL, Dickel DE, Levy DL, Ballif BC, Platky K, Farber DM, Gowans GC, Wetherbee JJ, Asamoah A, Weaver DD, Mark PR, Dickerson J, Garg BP, Ellingwood SA, Smith R, Banks VC, Smith W, McDonald MT, Hoo JJ, French BN, Hudson C, Johnson JP, Ozmore JR, Moeschler JB, Surti U, Escobar LF, El-Khechen D, Gorski JL, Kussmann J, Salbert B, Lacassie Y, Biser A, McDonald-McGinn DM, Zackai EH, Deardorff MA, Shaikh TH, Haan E, Friend KL, Fichera M, Romano C, Gecz J, DeLisi LE, Sebat J, King MC, Shaffer LG, Eichler EE. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42(3):203–209. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannes FD, Sharp AJ, Mefford HC, de Ravel T, Ruivenkamp CA, Breuning MH, Fryns JP, Devriendt K, Van Buggenhout G, Vogels A, Stewart H, Hennekam RC, Cooper GM, Regan R, Knight SJ, Eichler EE, Vermeesch JR. Recurrent reciprocal deletions and duplications of 16p13.11: The deletion is a risk factor for MR/MCA while the duplication may be a rare benign variant. J Med Genet. 2009;46(4):223–232. doi: 10.1136/jmg.2007.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A, Muhle H, de Kovel C, Baker C, von Spiczak S, Kron KL, Steinich I, Kleefuss-Lie AA, Leu C, Gaus V, Schmitz B, Klein KM, Reif PS, Rosenow F, Weber Y, Lerche H, Zimprich F, Urak L, Fuchs K, Feucht M, Genton P, Thomas P, Visscher F, de Haan GJ, Moller RS, Hjalgrim H, Luciano D, Wittig M, Nothnagel M, Elger CE, Nurnberg P, Romano C, Malafosse A, Koeleman BP, Lindhout D, Stephani U, Schreiber S, Eichler EE, Sander T. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41(2):160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: A preliminary study. Biol Psychiatry. 2002;52(1):9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Ingason A, Rujescu D, Cichon S, Sigurdsson E, Sigmundsson T, Pietilainen OP, Buizer-Voskamp JE, Strengman E, Francks C, Muglia P, Gylfason A, Gustafsson O, Olason PI, Steinberg S, Hansen T, Jakobsen KD, Rasmussen HB, Giegling I, Moller HJ, Hartmann A, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Bramon E, Kiemeney LA, Franke B, Murray R, Vassos E, Toulopoulou T, Muhleisen TW, Tosato S, Ruggeri M, Djurovic S, Andreassen OA, Zhang Z, Werge T, Ophoff RA, Rietschel M, Nothen MM, Petursson H, Stefansson H, Peltonen L, Collier D, Stefansson K, Clair DM. Copy number variations of chromosome 16p13.1 region associated with schizophrenia. Mol Psychiatry. 2009;16(1):17–25. doi: 10.1038/mp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, Krauss RM, Myers RM, Ridker PM, Chasman DI, Mefford H, Ying P, Nickerson DA, Eichler EE. Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet. 2009;84(2):148–161. doi: 10.1016/j.ajhg.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Coccaro EF, Fava M, Jaeger S, Jin R, Walters E. The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63(6):669–678. doi: 10.1001/archpsyc.63.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR. Genomic disorders: Structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. 1998;14(10):417–422. doi: 10.1016/s0168-9525(98)01555-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999;45(5):603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Marques-Bonet T, Girirajan S, Eichler EE. The origins and impact of primate segmental duplications. Trends Genet. 2009;25(10):443–454. doi: 10.1016/j.tig.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Eichler EE. Duplication hotspots, rare genomic disorders, and common disease. Curr Opin Genet Dev. 2009;19(3):196–204. doi: 10.1016/j.gde.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Mulley JC. Genetically complex epilepsies, copy number variants and syndrome constellations. Genome Med. 2010;2(10):71. doi: 10.1186/gm192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Sharp AJ, Baker C, Itsara A, Jiang Z, Buysse K, Huang S, Maloney VK, Crolla JA, Baralle D, Collins A, Mercer C, Norga K, de Ravel T, Devriendt K, Bongers EM, de Leeuw N, Reardon W, Gimelli S, Bena F, Hennekam RC, Male A, Gaunt L, Clayton-Smith J, Simonic I, Park SM, Mehta SG, Nik-Zainal S, Woods CG, Firth HV, Parkin G, Fichera M, Reitano S, Lo Giudice M, Li KE, Casuga I, Broomer A, Conrad B, Schwerzmann M, Raber L, Gallati S, Striano P, Coppola A, Tolmie JL, Tobias ES, Lilley C, Armengol L, Spysschaert Y, Verloo P, De Coene A, Goossens L, Mortier G, Speleman F, van Binsbergen E, Nelen MR, Hochstenbach R, Poot M, Gallagher L, Gill M, McClellan J, King MC, Regan R, Skinner C, Stevenson RE, Antonarakis SE, Chen C, Estivill X, Menten B, Gimelli G, Gribble S, Schwartz S, Sutcliffe JS, Walsh T, Knight SJ, Sebat J, Romano C, Schwartz CE, Veltman JA, de Vries BB, Vermeesch JR, Barber JC, Willatt L, Tassabehji M, Eichler EE. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med. 2008;359(16):1685–1699. doi: 10.1056/NEJMoa0805384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DR, Carey G. Genetic and environmental architecture of human aggression. J Pers Soc Psychol. 1997;72(1):207–217. doi: 10.1037//0022-3514.72.1.207. [DOI] [PubMed] [Google Scholar]
- Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, Cox GF, Dickinson H, Gentile J, Harris DJ, Hegde V, Hundley R, Khwaja O, Kothare S, Luedke C, Nasir R, Poduri A, Prasad K, Raffalli P, Reinhard A, Smith SE, Sobeih MM, Soul JS, Stoler J, Takeoka M, Tan WH, Thakuria J, Wolff R, Yusupov R, Gusella JF, Daly MJ, Wu BL. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet. 2009;46(4):242–248. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldin SO. NIMH human genetics initiative: 2003 update. Am J Psychiatry. 2003;160(4):621–622. doi: 10.1176/appi.ajp.160.4.621. [DOI] [PubMed] [Google Scholar]
- Rajender S, Pandu G, Sharma JD, Gandhi KP, Singh L, Thangaraj K. Reduced CAG repeats length in androgen receptor gene is associated with violent criminal behavior. Int J Legal Med. 2008;122(5):367–372. doi: 10.1007/s00414-008-0225-7. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychol Bull. 2002;128(3):490–529. [PubMed] [Google Scholar]
- Schmidt CA, Fallon AE, Coccaro EF. Assessment of behavioral and cognitive impulsivity: Development and validation of the Lifetime History of Impulsive Behaviors Interview. Psychiatry Res. 2004;126(2):107–121. doi: 10.1016/j.psychres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer RR, Richmond TA, Pofahl NJ, Green RD, Eis PS, Nair P, Brothman AR, Stallings RL. Analysis of chromosome breakpoints in neuroblastoma at sub-kilobase resolution using fine-tiling oligonucleotide array CGH. Genes Chromosomes Cancer. 2005;44(3):305–319. doi: 10.1002/gcc.20243. [DOI] [PubMed] [Google Scholar]
- Seroczynski AD, Bergeman CS, Coccaro EF. Etiology of the impulsivity/aggression relationship: Genes or environment? Psychiatry Res. 1999;86(1):41–57. doi: 10.1016/s0165-1781(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, Hurst JA, Stewart H, Price SM, Blair E, Hennekam RC, Fitzpatrick CA, Segraves R, Richmond TA, Guiver C, Albertson DG, Pinkel D, Eis PS, Schwartz S, Knight SJ, Eichler EE. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet. 2006;38(9):1038–1042. doi: 10.1038/ng1862. [DOI] [PubMed] [Google Scholar]
- Shinawi M, Schaaf CP, Bhatt SS, Xia Z, Patel A, Cheung SW, Lanpher B, Nagl S, Herding HS, Nevinny-Stickel C, Immken LL, Patel GS, German JR, Beaudet AL, Stankiewicz P. A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat Genet. 2009;41(12):1269–1271. doi: 10.1038/ng.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158–162. doi: 10.1007/s11920-001-0014-1. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Moller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Muhleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nothen MM, Peltonen L, Collier DA, St Clair D, Stefansson K. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455(7210):232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafranski P, Schaaf CP, Person RE, Gibson IB, Xia Z, Mahadevan S, Wiszniewska J, Bacino CA, Lalani S, Potocki L, Kang SH, Patel A, Cheung SW, Probst FJ, Graham BH, Shinawi M, Beaudet AL, Stankiewicz P. Structures and molecular mechanisms for common 15q13.3 microduplications involving CHRNA7: Benign or pathological? Hum Mutat. 2010;31(7):840–850. doi: 10.1002/humu.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati A, Fyer AJ, Weissman MM. A comparison between screened NIMH and clinically interviewed control samples on neuroticism and extraversion. Mol Psychiatry. 2008;13(2):122–130. doi: 10.1038/sj.mp.4002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann R, Turner G, Kirchhoff M, Chen W, Tonge B, Rosenberg C, Field M, Vianna-Morgante AM, Christie L, Krepischi-Santos AC, Banna L, Brereton AV, Hill A, Bisgaard AM, Muller I, Hultschig C, Erdogan F, Wieczorek G, Ropers HH. Array CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 2007;28(7):674–682. doi: 10.1002/humu.20546. [DOI] [PubMed] [Google Scholar]
- van Bon BW, Mefford HC, Menten B, Koolen DA, Sharp AJ, Nillesen WM, Innis JW, de Ravel TJ, Mercer CL, Fichera M, Stewart H, Connell LE, Ounap K, Lachlan K, Castle B, Van der Aa N, van Ravenswaaij C, Nobrega MA, Serra-Juhe C, Simonic I, de Leeuw N, Pfundt R, Bongers EM, Baker C, Finnemore P, Huang S, Maloney VK, Crolla JA, van Kalmthout M, Elia M, Vandeweyer G, Fryns JP, Janssens S, Foulds N, Reitano S, Smith K, Parkel S, Loeys B, Woods CG, Oostra A, Speleman F, Pereira AC, Kurg A, Willatt L, Knight SJ, Vermeesch JR, Romano C, Barber JC, Mortier G, Perez-Jurado LA, Kooy F, Brunner HG, Eichler EE, Kleefstra T, de Vries BB. Further delineation of the 15q13 microdeletion and duplication syndromes: A clinical spectrum varying from non-pathogenic to a severe outcome. J Med Genet. 2009;46(8):511–523. doi: 10.1136/jmg.2008.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J. The neurobiology of violence: An update. J Neuropsychiatry Clin Neurosci. 1999;11(3):307–314. doi: 10.1176/jnp.11.3.307. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, Falchi M, Chen F, Andrieux J, Lobbens S, Delobel B, Stutzmann F, El-Sayed Moustafa JS, Chevre JC, Lecoeur C, Vatin V, Bouquillon S, Buxton JL, Boute O, Holder-Espinasse M, Cuisset JM, Lemaitre MP, Ambresin AE, Brioschi A, Gaillard M, Giusti V, Fellmann F, Ferrarini A, Hadjikhani N, Campion D, Guilmatre A, Goldenberg A, Calmels N, Mandel JL, Le Caignec C, David A, Isidor B, Cordier MP, Dupuis-Girod S, Labalme A, Sanlaville D, Beri-Dexheimer M, Jonveaux P, Leheup B, Ounap K, Bochukova EG, Henning E, Keogh J, Ellis RJ, Macdermot KD, van Haelst MM, Vincent-Delorme C, Plessis G, Touraine R, Philippe A, Malan V, Mathieu-Dramard M, Chiesa J, Blaumeiser B, Kooy RF, Caiazzo R, Pigeyre M, Balkau B, Sladek R, Bergmann S, Mooser V, Waterworth D, Reymond A, Vollenweider P, Waeber G, Kurg A, Palta P, Esko T, Metspalu A, Nelis M, Elliott P, Hartikainen AL, McCarthy MI, Peltonen L, Carlsson L, Jacobson P, Sjostrom L, Huang N, Hurles ME, O'Rahilly S, Farooqi IS, Mannik K, Jarvelin MR, Pattou F, Meyre D, Walley AJ, Coin LJ, Blakemore AI, Froguel P, Beckmann JS. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463(7281):671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh MT, Coccaro EF, Jacobson KC. Multivariate behavior genetic analyses of aggressive behavior subtypes. Behav Genet. 2010;40(5):603–617. doi: 10.1007/s10519-010-9363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.