Abstract
Aging compromises restoration of the cardiac mechanical function during reperfusion. We hypothesized that this was due to an ampler release of mitochondrial reactive oxygen species (ROS). This study aimed at characterising ex vivo the mitochondrial ROS release during reperfusion in isolated perfused hearts of middle-aged rats. Causes and consequences on myocardial function of the observed changes were then evaluated. The hearts of rats aged 10- or 52-week old were subjected to global ischemia followed by reperfusion. Mechanical function was monitored throughout the entire procedure. Activities of the respiratory chain complexes and the ratio of aconitase to fumarase activities were determined before ischemia and at the end of reperfusion. H2O2 release was also evaluated in isolated mitochondria. During ischemia, middle-aged hearts displayed a delayed contracture, suggesting a maintained ATP production but also an increased metabolic proton production. Restoration of the mechanical function during reperfusion was however reduced in the middle-aged hearts, due to lower recovery of the coronary flow associated with higher mitochondrial oxidative stress indicated by the aconitase to fumarase ratio in the cardiac tissues. Surprisingly, activity of the respiratory chain complex II was better maintained in the hearts of middle-aged animals, probably because of an enhanced preservation of its membrane lipid environment. This can explain the higher mitochondrial oxidative stress observed in these conditions, since cardiac mitochondria produce much more H2O2 when they oxidize FADH2-linked substrates than when they use NADH-linked substrates. In conclusion, the lower restoration of the cardiac mechanical activity during reperfusion in the middle-aged hearts was due to an impaired recovery of the coronary flow and an insufficient oxygen supply. The deterioration of the coronary perfusion was explained by an increased mitochondrial ROS release related to the preservation of complex II activity during reperfusion.
Keywords: Myocardial aging, Ischemia, Oxidative stress, Respiratory chain complexes
Introduction
Amongst subjects admitted in care units for an acute myocardial infarction, in-hospital and long-term mortalities are significantly higher in patients over 65 years old when invasive strategies are not endeavored (Polewczyk et al. 2008). This is probably related to the observed increased incidence of diabetes, hypertension, and heart failure in this population and to the associated activation of the adrenergic drive (Hu et al. 2008). From data presented in the literature, the aged heart has a diminished functional and adaptive reserve capacity, an increased susceptibility to incur damage and a limited practical ability for repair/regeneration (Juhaszova et al. 2005). The reduced tolerance to ischemic insult with aging is also noticed in the laboratory animal with a reduced recovery of the coronary flow and contractility during reperfusion occurring in mice (Azhar et al. 1999; Willems et al. 2003; Willems et al. 2005) and rats (Leichtweis et al. 2001; Xia et al. 2003) as soon as they reach middle age.
Oxidative stress seems to play a central role in the age-associated vulnerability to ischemia/reperfusion. Indeed, administration of superoxide dismutase and catalase during the pathology affords protection to the senescent heart by preventing vasoconstriction and protein oxidation (Besse et al. 2006). Although some authors emphasize the involvement of the vascular NADPH oxidase in this pathology (Oudot et al. 2006), others point out the prominent responsibility of the mitochondrial reactive oxygen species (ROS) (Lesfnefsky and Hoppel 2003) through the well-known ROS-induced release process (Zorov et al. 2006). Due to cardiolipin oxidation by ROS attack, interfibrillar mitochondria of the senescent heart display lower oxidative capacities and increased ROS release (Fannin et al. 1999; Lesnefsky et al. 2001a; Hoppel et al. 2002). Ischemia further amplifies these abnormalities (Lesnefsky et al. 2001b; Lesnefsky and Hoppel 2008; Lesnefsky et al. 2009), but only in the aged myocardium.
The augmented vulnerability to calcium of senescent cardiomyocytes (Tsukube et al. 1996; Hansford et al. 1999; Lakatta et al. 2001; Jahangir et al. 2001) might be responsible for the amplified oxidative stress during ischemia/reperfusion. Indeed, rising levels of matrix calcium favors mitochondrial ROS release (Martin et al. 2007) through a mechanism that can imply succinate accumulation (Sentex et al. 1999). Succinate-ubiquinone reductase (complex II) re-oxidizes succinate, which can support important ROS production at the level of complex I via reverse electron flux (Capel et al. 2005; Lacraz et al. 2008). This mechanism is probably responsible for the huge ROS production that transitorily occurs at early reperfusion in the adult heart (Demaison et al. 2001), which is most likely amplified in the aging heart. Indeed, in ischemia/reperfusion, old hearts have more oxidized proteins than young hearts (Nagy et al. 1996).
Ischemia/reperfusion-induced oxidative stress has a noticeable influence on NADH-ubiquinone reductase (complex I) activity, since it transitorily diminishes it (Paradies et al. 2004). Complex II activity is also robustly reduced by reperfusion-induced oxidative stress (Pasdois et al. 2006; Chen et al. 2008) via deglutathionylation of its 70-kDa flavin protein and tyrosine nitration by peroxynitrite (Chen et al. 2007, 2008). The significance of the decrease in activities of the complexes I and II at resumption of the coronary flow is not known, but these complexes could be used as fuses to prevent perpetuation of the huge ROS release occurring during this period. Nevertheless, the early and important ROS release at the beginning of reperfusion could be responsible for the oxidative modifications of numerous proteins and the alterations of their functioning. It could explain several abnormalities of the senescent reperfused heart including impaired oxidative capacities and cardiac metabolic efficiency through changes in respiratory complex activities, but also the low restoration of the coronary flow via reduction of the NO bioavailability and/or endothelin-1 over-activity through oxidation of its receptors (Amrani et al. 1996; Goodwin et al. 1999; Besse et al. 2001; Nakamura et al. 2003).
This study was aimed at determining whether the impaired contractile recovery observed in the post-ischemic aged heart is related to an augmented mitochondrial ROS release at resumption of the coronary flow and at evaluating the causes and consequences of this phenomenon. The hearts of young adults (10 weeks) and middle-aged (52 weeks) animals were thus perfused and subjected to ischemia followed by reperfusion. To estimate the ex vivo mitochondrial ROS release during reperfusion, we evaluated the aconitase to fumarase ratios before ischemia and at the end of the reperfusion and we contrasted these values to the rate of H2O2 released by mitochondria isolated from the pre-ischemic myocardium. The causes and consequences of the observed changes were determined by measuring the activities of several respiratory complexes known to be reduced through ischemia/reperfusion-induced oxidative stress and by examining scrupulously several parameters of cardiac functioning including mechanical activity, oxidative capacities, metabolic efficiency, and coronary perfusion.
Materials and methods
Animals and treatments
The experiments followed the European recommendation guidelines for the use of laboratory animals and were approved by the local ethics review board (authorization number, 380537). Thirty-six male Wistar rats from an inbred colony were housed in individual cages in an animal facility with controlled temperature, dark/light cycle, and hygrometry. They were fed standard commercial pellets (A04, Safe, Gannat, France) ad libitum with free access to water. Animals were randomly divided into two groups according to their age. The young adult group was composed by 10-week-old rats while the middle-aged group by 52-week-old rats.
Isolated heart perfusion
The animals were anesthetized with sodium pentobarbital (50 mg/kg) and heparinized (1,000 I.U./kg) through the saphenous vein. After rapid excision of the heart, the aorta was cannulated in Langendorff mode of non-recirculating coronary perfusion at constant pressure (60 mmHg) at 37°C. Modified Krebs–Henseleit perfusion buffer contained (in mM): NaCl (119), MgSO4 (1.2), NaHCO3 (25), KCl (4.8), CaCl2 (2.5), and glucose (11) as sole energy source and was equilibrated with 95% O2–5% CO2. A latex balloon linked to a pressure probe was inserted into the left ventricle cavity to determine diastolic and systolic pressures. Baseline diastolic pressure was set at approximately 10 mmHg. The coronary flow was estimated by weight determination of 30-s collected samples of effluent. The pulmonary artery was cannulated for a direct coronary sinus sampling of the coronary effluent. Arterial and venous oxygen contents were measured by anaerobic collection of effluent in hermetically closed capillary tubing. The samples were then kept in ice until determination of oxygen concentration with a blood gas analyser (Radiometer™ABL 700, Brønshøj, Danemark). Developed pressure was calculated as the difference between the systolic and diastolic pressures. The rate × pressure product (RPP) was the product between the heart rate and developed pressure. Oxygen consumption was calculated as the product of the arterio-venous oxygen content difference and the coronary flow. Since the blood gas analyser gave results of the oxygen concentration in kPa, the arterio-venous oxygen content difference was calculated assuming that 1 kPa = 10 nmoles of oxygen × ml−1 (Gnaiger 2001). Cardiac metabolic efficiency was estimated as the total RPP (expressed in mHg/min) to total oxygen consumption ratio (expressed in μmoles/min) of each heart and was expressed in mHg/μmole.
Ischemia-reperfusion protocol and sampling
In a first set of experiments, isolated hearts issued from ten young adult and ten middle-aged rats were perfused for 30 min to establish stabilized baseline performances before inducing total global normothermic ischemia for 25 min and reperfusion for 45 min. During ischemia, rigorous conditions of temperature verification were applied by bathing the heart in Krebs–Heinseleit buffer maintained at 37°C. At the end of the reperfusion period, the hearts were freeze-clamped in liquid nitrogen and stored at −80°C until determination of cardiac dry weight, aconitase to fumarase ratio, and respiratory chain complex activities.
In a second set of experiments, hearts from young adult and middle-aged rats (eight per group) were perfused in baseline conditions for 30 min. At the end of the perfusion protocol, a piece of myocardium (about 200 mg) from the apex of the heart was immediately freeze-clamped and stored at −80°C in order to measure respiratory chain complex activities and aconitase to fumarase ratio. The other part of the myocardium was immediately used for isolated mitochondria preparation.
Mitochondria preparation
After the perfusion, atria and the remaining aorta were cut off from the heart. Myocardium was minced with scissors in a cold isolation buffer composed of (in mM) sucrose (150), KCl (75), Tris–HCl (50), KH2PO4 (1), MgCl2 (5), and EGTA (1), pH 7.4, fatty acid-free serum albumin 0.2%. The pieces of myocardium were rinsed several times on a filter and put in an Elvehjem potter containing 15 ml of isolation buffer. A protease (subtilisin 0.02%) was added for 1 min to digest myofibrils at ice temperature, and the totality was then homogenized with the potter (300 rpm, 3 to 4 transitions). Subtilisin action was stopped by addition of the isolation buffer (30 ml). The homogenate was then centrifuged (800 g, 10 min, 4°C), and the resulting supernatant was collected and filtered. Mitochondria were then washed through two series of centrifugation (8,000 × g, 10 min, 4°C). The last pellet of mitochondria was re-suspended in sucrose 250 mM, Tris–HCl 10 mM, EGTA 1 mM, pH 7.4 at a concentration of approximately 20 mg/ml.
Respiration measurements
The rate of mitochondrial oxygen consumption was measured at 30°C in an incubation chamber with a Clarke-type O2 electrode filled with 1 ml of incubation medium (KCl 125 mM, Tris–HCl 20 mM, KH2PO4 10 mM, EGTA 1 mM, pH 7.2, fatty acid-free bovine serum albumin 0.15%). All measurements were performed using mitochondria (0.25 mg mitochondrial protein/ml) incubated either with glutamate (5 mM)/malate (2.5 mM) or/and succinate (5 mM) as substrates, in the presence (state 3) and in the absence (state 4) of ADP 100 mM. The incubation medium was constantly stirred with a built-in electromagnetic stirrer and bar flea. Coupling of the mitochondrial oxidative phosphorylation was assessed by the state 3/state 4 ratio which measures the degree of control imposed on oxidation by phosphorylation (respiratory control ratio (RCR)).
Mitochondrial reactive oxygen species release
The rate of mitochondrial H2O2 production was measured at 30°C which followed linear increase in fluorescence (excitation at 560 nm and emission at 584 nm) due to enzymatic oxidation of amplex red by H2O2 in presence of horseradish peroxidase, modified to kinetically follow the rate of production of H2O2 by isolated mitochondria on a SFM25 computer-controlled Kontron fluorometer. Reaction conditions were 0.25 mg of mitochondrial protein/ml, 5 U/ml of horseradish peroxidase, 1 μM of amplex red, with glutamate/malate or/and succinate (in the same concentrations as in respiration measurements). They were added in order to start the reaction in the same incubation buffer with that used for measurements of mitochondrial oxygen consumption. Mitochondrial ROS was measured in the absence of ADP (state 2 respiration rate). Rotenone (1 μM) and antimycin A (0.5 μM) were sequentially added to determine, respectively, the maximum rate of H2O2 production of complexes I and I + III of the respiratory chain.
Measurement of respiratory chain complex activities
Activities of the NADH-ubiquinone oxydo-reductase (complex I), succinate-ubiquinone oxydo-reductase (complex II), ubiquinol cytochrome c reductase (complex III), cytochrome c oxidase (complex IV), NADH cytochrome c reductase (activity of complex I + III) and succinate cytochrome c reductase (activity of complex II+ III) were determined as commonly performed in this laboratory. Heart samples (100 mg) were homogenized at 4°C with 0.9 ml of a potassium phosphate buffer 100 mM, pH 7.4. The homogenates were centrifuged (1,500 × g, 5 min, 4°C), and the resulting supernatants were stored at −80°C until determination of the various enzymatic activities.
Complex I activity was determined spectrophotometrically by measuring the disappearance of NADH at 340 nm in the presence of decylubiquinone (Kramer et al. 2005). The reaction medium was a potassium phosphate buffer 47.5 mM, pH 7.4 containing bovine serum-albumin (3.75 mg/ml), NADH (0.1 mM) and decylubiquinone (0.1 mM). The reaction was initiated by adding the 200-times diluted sample and the change in absorbance at 37°C was measured for 2 min with and without rotenone (10 μM) in order to evaluate the specific and non-specific activities.
Complex II activity was also evaluated spectrophotometrically in the presence of succinate by measuring the reduction of dichloro-inophenol (DCIP) (Miro et al. 1999). In this reaction, succinate oxidation by complex II reduces decylubiquinone which spontaneously transfers its electrons to DCIP. The 200-times diluted sample with the reaction medium (potassium phosphate buffer 45.4 mM, pH 7.4, bovine serum-albumin 2.5 mg/ml, antimycin A 9.3 μM, rotenone 5 μM, DCIP 100 μM and succinate 30 mM) were stirred at 37°C for 10 min, and the reaction was initiated by addition of decylubiquinone (50 μM). The change in absorbance was then measured at 600 nM for 5 min.
Complex III activity was determined by measuring the reduction of cytochrome c when decylubiquinol was used as substrate and complexes I and IV were blocked by specific inhibitors (Krähenbühl et al. 1994). The change in absorbance at 550 nm was first evaluated at 30°C for 1 min in the presence of potassium phosphate buffer 90.7 mM, pH 7.4, EDTA 50 μM, bovine serum-albumin (1 mg/ml), KCN 1 mM, oxidized cytochrome c (100 μM), decylubiquinol 0.11 mM and the 200 times diluted sample in order to evaluate total activity. The non-specific activity was then measured with antimycin A (5 μg/ml) for 2 min and complex III activity was then calculated by subtraction.
Complex IV assay was performed by determining the oxidation of reduced cytochrome c in a medium containing potassium phosphate buffer 50 mM, pH 7.4 and cytochrome c 1 mM reduced at 90% with sodium dithionite (Veitch and Hue 1994). The reaction was initiated by addition of the 200-times diluted sample and it was followed at 37°C and 550 nm for 2 min. The non-specific activity was then measured by addition of KCN 1 mM.
Complexes I + III and II + III activities were evaluated by using NADH (0.25 mM) and succinate (25 mM) as substrates and measuring the reduction of cytochrome c at 550 nm (Veitch and Hue 1994). The reaction was initiated in a medium containing potassium phosphate buffer 90 mM, pH 7.4, EDTA 0.5 mM, sodium azide 2 mM and oxidized cytochrome c 50 μM by addition of the 200-times diluted sample and it was maintained at 30°C for 5 min. In the case of the complex II + III activity determination, rotenone (1 μM) was added to avoid reverse electron flux through complex I. The non-specific activities was evaluated by using specific inhibitors of complexes I and II (rotenone 1 μM and thenoytrifluoroacetone 10 pM, respectively).
Other enzymatic assays
Activity of the citrate synthase was determined according to Faloona and Srere (1969). Aconitase and fumarase activities were determined according to Gardner et al. (1994), with the modification that the extraction medium was supplemented with tri-sodium citrate to stabilize aconitase activity ex vivo. In order to evaluate myocardial mitochondrial density, activity of the citrate synthase was divided by the protein amount of the homogenate. The activities of respiratory chain complexes were expressed in units per mg of proteins. Proteins were measured using the bicinchoninic acid method with a commercially available kit (Thermo Scientific, Rockford, IL).
Statistical analysis
The results are presented as mean ± SEM. General data on animal morphology and baseline normoxic cardiac function were contrasted across the animal age by 1-way analysis of variance (ANOVA). Enzymatic measurements were analysed through a two-way ANOVA depicting the effects of aging, ischemia/reperfusion, and the cross-interaction between these two factors. Measurements made during ischemia-reperfusion (diastolic pressure, developed pressure, heart rate, RPP, oxygen consumption, metabolic efficiency) were treated with repeated-measures ANOVA to test the effect of animal age (external factor), perfusion time (internal factor), and their interaction. When required, group means were contrasted with a Fisher’s LSD test. A probability (p) less than 0.05 was considered significant. Statistical analyses were performed using the NCSS 2007 software.
Results
General data
Animal size was estimated by measuring the length from insertion of the collarbones on the sternum to the outer sexual organ (Table 1). Middle-aged rats displayed a higher value (+10%) than that of their young adult counterparts. Interestingly, the relative thorax length was increased (+23%) in the older animals, due to a rise in thorax length (+32%) without any change in abdomen length. Animal and heart weights were augmented by aging (+37 and +28%, respectively), but the ratio between heart and body weight was unaffected.
Table 1.
General morphological data
| Young adults | Middle aged | ANOVA | |
|---|---|---|---|
| Thorax length (cm) | 4.4 ± 0.1 | 5.8 ± 0.1 | p < 0.001 |
| Abdomen length (cm) | 9.8 ± 0.1 | 9.8 ± 0.4 | NS |
| Total length (cm) | 14.2 ± 0.1 | 15.6 ± 0.4 | p < 0.001 |
| Relative thorax length (%) | 31 ± 1 | 38 ± 1 | p < 0.001 |
| Relative abdomen length (%) | 69 ± 1 | 62 ± 1 | p < 0.001 |
| Animal weight (g) | 323 ± 5 | 444 ± 33 | p < 0.001 |
| Heart dry weight (mg) | 204 ± 6 | 262 ± 8 | p < 0.001 |
| Relative heart weight (mg/kg) | 634 ± 19 | 612 ± 5 | NS |
Thorax length was measured from the insertion of the collarbones on the sternum to the apex of the sternum. Abdomen length was determined by measuring the distance from the apex of the sternum to the penis. Total length was the sum of the thorax and abdominal lengths. Relative heart weight was the ratio between heart dry weight and animal weight. The number of experiments was ten per group
ANOVA analysis of variance, NS not significant
Basal cardiac functioning
Under pre-ischemic conditions, the systolic pressure, left ventricle developed pressure, heart rate, and rate × pressure product were high and not affected by the animal age (Table 2). Since we aimed at evaluating the global heart functioning, but not the myocardial efficiency to produce its mechanical activity, these parameters were not divided by the cardiac dry weight. Coronary flow and oxygen consumption were similar in young adult and middle-aged rats. However, the cardiac metabolic efficiency was significantly decreased (−17%) by aging.
Table 2.
Basal cardiac functioning
| Young adults | Middle aged | ANOVA | |
|---|---|---|---|
| Syst P (mmHg) | 139 ± 5 | 155 ± 8 | NS |
| Diast P (mmHg) | 12 ± 1 | 13 ± 1 | NS |
| LVDP (mmHg) | 127 ± 5 | 142 ± 8 | NS |
| Heart rate (beats/min) | 271 ± 8 | 257 ± 14 | NS |
| RPP (mHg/min) | 34.3 ± 1.2 | 36.3 ± 2.3 | NS |
| Coro flow (ml/min/g) | 51.8 ± 3.6 | 53.1 ± 4.6 | NS |
| Ox cons (μmoles/min/g) | 32.0 ± 2.2 | 31.9 ± 2.4 | NS |
| Met. Eff. (mHg/μmole) | 5.41 ± 0.24 | 4.48 ± 0.31 | p < 0.05 |
The number of experiments was ten per group.
Syst P systolic pressure, Diast P diastolic pressure, LVDP left ventricle developed pressure, RPP rate × pressure product, Coro flow coronary flow, Ox cons oxygen consumption, Met. Eff. metabolic efficiency, ANOVA analysis of variance, NS not significant
Cardiac functioning during ischemia
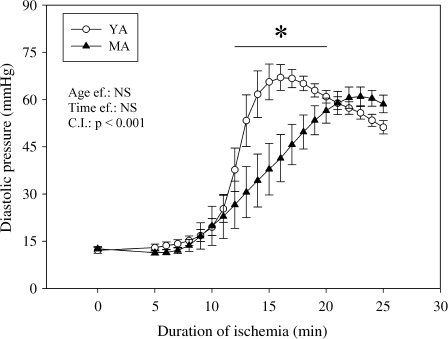
As soon as the coronary flow was stopped, the left ventricle developed pressure and rate × pressure product became null. The heart rate stopped soon after cessation of contraction and the diastolic pressure began to increase from the 5th min of ischemia (Fig. 1), indicating the occurrence of a progressive contracture. The rise in diastolic pressure was less important in the middle-aged group from the 12th to the 19th min of ischemia in comparison with the young group (−30, −43, −44, −42, −38, −31, −24, and −10% at the 12th, 13th, 14th, 15th 16th, 17th, 18th, and 19th min of ischemia, respectively). Thereafter, the diastolic pressure of the middle-aged animals equalized that of their young counterparts until the end of ischemia.
Fig. 1.
Effects of aging on myocardial rigor tension evaluated by the diastolic pressure during ischemia. The number of experiments was ten per group. YA young adult rats, MA middle-aged rats, Age ef. effect of the age, Time ef. effect of the duration of ischemia, C.I. cross-interaction, asterisk significantly different
Cardiac mechanical function during reperfusion
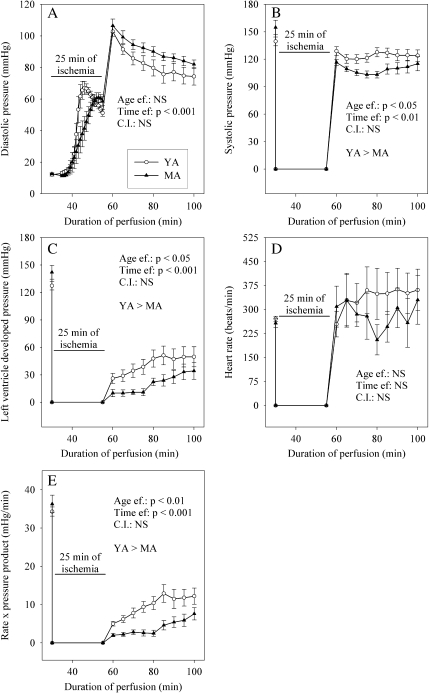
As soon as resumption of the coronary flow, the diastolic pressure increased from approximately 54 ± 2 mmHg at the end of ischemia to 104 ± 3 mmHg at the 5th min of reperfusion (Fig. 2). Thereafter, the diastolic pressure progressively decreased to a value close to 78 ± 3 mmHg at the 45th min of reperfusion. During the whole reperfusion, values of the diastolic pressure were not affected by aging. The systolic pressure at the 5th min of reperfusion was slightly lower compared with the pre-ischemic value (−8 and −25% in the young adult and middle-aged rats, respectively), and less restored in the older animals (−10%) than in the youngest rats. This statement was true for the whole duration of reperfusion. The left ventricle developed pressure and rate × pressure product evolved similarly during reperfusion. They gradually recovered from a null value at the end of ischemia to positive values whose magnitude depended on the age of the animals. The restoration of these two parameters was indeed reduced in the middle-aged rats (−73 and −76% for the left ventricle developed pressure and rate × pressure product in comparison with the young animals at the 25th min of reperfusion). However, although still significantly different, this huge difference tended to be lower at the 45th min of reperfusion (−33% and −38% for the left ventricle developed pressure and rate × pressure product). Recovery of the heart rate was never significantly affected by the age of the animals.
Fig. 2.
Effects of aging on cardiac mechanical function during post-ischemic reperfusion. a Diastolic pressure; b systolic pressure; c left ventricle developed pressure; d heart rate; e rate × pressure product. The number of experiments was ten per group. YA young adult rats, MA middle-aged rats, Age ef. effect of the age, Time ef. effect of the duration of reperfusion
Cardiac metabolic function during reperfusion
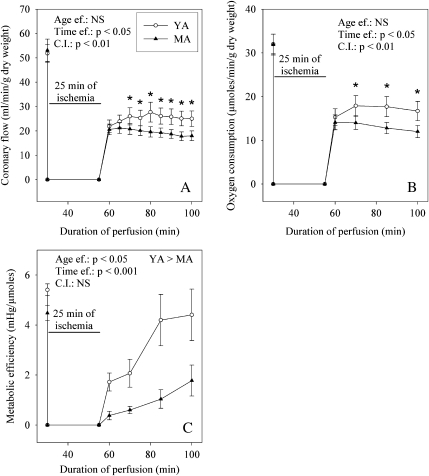
The coronary flow (Fig. 3) was similarly restored in the two age groups at the 5th of reperfusion (42 ± 4 and 42 ± 7% of the pre-ischemic value for the young and mature adults), but evolved differently thereafter. In the group of the young adult animals, the coronary flow continued to increase until the 45th min of reperfusion to a value equal to 25 ± 3 ml/min/g dry weight. Contrariwise, in the older animals, this parameter tended to decrease until the end of reperfusion to 18 ± 2 ml/min/g dry weight. Consequently, the rate of the coronary flow was significantly lower in the older animals (−28%) compared with that of the younger ones. The oxygen consumption progressed similarly during reperfusion. Although it was similar in the two groups at the 5th min of reperfusion, it was lower in the middle-aged rats (−28%) compared with their young counterparts at the 45th min of reperfusion. The metabolic efficiency progressively recovered to achieve the pre-ischemic value at the 45th min of reperfusion in the younger animals, whereas it remained constantly low in the older ones. At the end of reperfusion, the metabolic efficiency was reduced by 60% by aging.
Fig. 3.
Effects of aging on cardiac metabolism during post-ischemic reperfusion. a Coronary flow; b oxygen consumption; c metabolic efficiency. The number of experiments was ten per group. YA young adult rats, MA middle-aged rats, Age ef. effect of the age, Time ef. effect of the duration of reperfusion, C.I. cross-interaction; asterisk significantly different
Aconitase to fumarase ratio of myocardial biopsies
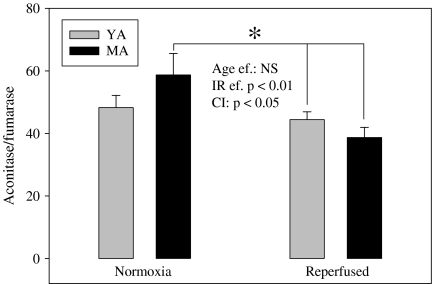
The aconitase to fumarase ratio (Fig. 4) inversely related to the mitochondrial oxidative stress was reduced by ischemia/reperfusion (−22%). Interestingly, the decrease was minimal in the heart of young adult animals (−8%) whereas it was significantly different in the myocardium of middle age rats (−34%, p < 0.05).
Fig. 4.
Effects of aging and of ischemia/reperfusion on the aconitase to fumarase ratio. The measurements have been performed on the freeze-clamped hearts at the end of the stabilization and reperfusion periods. The number of experiments was eight and ten per group for the normoxic and reperfused hearts, respectively. YA young adult rats, MA ten middle-aged rats; asterisk significantly different
Mitochondrial oxygen consumption and ROS release
Cardiac mitochondria were purified after a 30-min normoxic perfusion and their respiration properties were determined using several substrates (glutamate/malate as NADH-linked substrate, succinate/rotenone as FADH2-linked substrate and glutamate/malate/succinate as NADH- and FADH2-linked substrates). Whatever the substrate used, the parameters of mitochondrial respiration (Table 3) were similar between young adult and middle-aged animals. This was verified with only exception the RCR value with glutamate/malate as substrate. Indeed, this value was lower in the mitochondria of middle-aged rats in comparison with those of young adults (−33%).
Table 3.
Mitochondrial oxidative capacities after 30 min of normoxic perfusion
| Respiratory state | Young adults | Middle aged | ANOVA | |
|---|---|---|---|---|
| GM | Substrate | 23 ± 1 | 20 ± 1 | NS |
| +ADP | 153 ± 6 | 131 ± 15 | NS | |
| +Oligomicine | 26 ± 3 | 33 ± 6 | NS | |
| +DNP | 219 ± 14 | 183 ± 26 | NS | |
| RCR | 6.43 ± 0.68 | 4.28 ± 0.38 | p < 0.05 | |
| S | Substrate | 70 ± 4 | 78 ± 4 | NS |
| +ADP | 239 ± 16 | 276 ± 14 | NS | |
| +Oligomicine | 70 ± 4 | 81 ± 5 | NS | |
| +DNP | 214 ± 15 | 246 ± 11 | NS | |
| RCR | 3.46 ± 0.04 | 3.45 ± 0.19 | NS | |
| GMS | Substrate | 59 ±4 | 67 ± 3 | NS |
| +ADP | 263 ± 11 | 245 ± 31 | NS | |
| +Oligomicine | 74 ± 4 | 80 ± 7 | NS | |
| +DNP | 259 ± 21 | 279 ±13 | NS | |
| RCR | 3.33 ±0.13 | 3.57 ± 0.27 | NS |
Except the RCR which is the ratio between the state III and the state IV respiration rates, the results are expressed in ng atoms of oxygen/min/mg of proteins. The number of experiments was eight per group
GM glutamate (5 mM) + malate (2.5 mM), S succinate (5 mM) + rotenone (2.5 μM), GMS glutamate (5 mM) + malate (2.5 mM) + succinate (5 mM), substrate state II respiration rate, +ADP state III respiration rate, +oligomicine state IV respiration rate, +DNP state uncoupled by dinitrophenol, RCR respiratory complex ratio
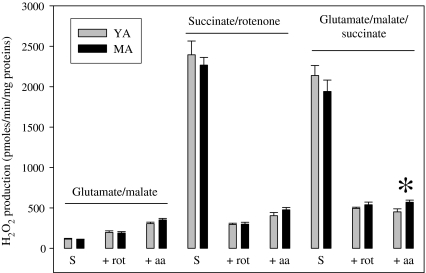
Mitochondrial H2O2 release was also evaluated (Fig. 5). It was determined during the state II respiration rate. Under these conditions, H2O2 release was low with glutamate/malate and high with succinate/rotenone or glutamate/succinate/malate. Inhibition of complex I by rotenone slightly augmented H2O2 release with glutamate/malate, but strongly reduced it with succinate/rotenone and glutamate/malate/succinate. Additional inhibition of complex III with antimycin A aggravated H2O2 release with glutamate/malate and succinate/rotenone, but not with glutamate/malate/succinate. Aging had no effect on H2O2 release with glutamate/malate and succinate/rotenone, but enlarged the one measured with glutamate/malate/succinate when complexes I and III were simultaneously blocked.
Fig. 5.
Effect of the transition from youth to middle age on H2O2 release by isolated mitochondria from normoxic myocardium. The number of experiments was eight per group. YA young adults, MA middle-aged animals, S substrate alone (state II respiration rate), +rot substrate + rotenone, +aa substrate + rotenone + antimycine A, asterisk significantly different
Respiratory complex activities in myocardial biopsies
Citrate synthase activity (4.37 ± 0.13 in the young adults vs. 3.87 ± 0.28 mU/mg of proteins in the middle-aged hearts) was not modified by ischemia/reperfusion and by aging. In pre-ischemic myocardium, aging did not modify activities of the different respiratory complexes (Table 4), except that of cytochrome c oxidase which was slightly increased (+29%). The effect of ischemia/reperfusion was different in the adult and middle-aged hearts. In the younger hearts, the pathological event reduced the succinate-ubiquinone oxido-reductase (−27%) and NADH cytochrome c reductase (−43%) activities without sparing the ratio between NADH cytochrome c reductase and succinate cytochrome c reductase (−50%). In the middle-aged hearts, ischemia/reperfusion did not trigger any decrease in these parameters. Ubiquinol cytochrome c reductase tended to be lower (−23%), but this reduction was not significant.
Table 4.
Complexes of the respiratory chain at the end of reperfusion
| Young adults | Middle aged | |||
|---|---|---|---|---|
| Pre-ischemica | Reperfusedb | Pre-ischemica | Reperfusedb | |
| CIc | 1.49 ± 0.17 | 1.65 ± 0.20 | 1.86 ± 0.23 | 1.50 ± 0.25 |
| CIId | 0.86 ± 0.04 a | 0.63 ± 0.05 b | 0.92 ± 0.05 a | 0.86 ± 0.10 a |
| CIIIe | 1.65 ± 0.32 | 1.76 ± 0.21 | 1.51 ± 0.21 | 1.16 ± 0.36 |
| CIVf | 0.21 ± 0.01 a, d | 0.26 ± 0.01 b, c | 0.27 ± 0.01 b, c | 0.24 ± 0.02 c, d |
| CI + IIIg | 0.054 ± 0.009 a | 0.031 ± 0.005 b | 0.045 ± 0.001 a, b | 0.044 ± 0.006 a, b |
| CII + IIIh | 0.031 ± 0.001 | 0.039 ± 0.004 | 0.042 ± 0.003 | 0.041 ± 0.005 |
| CI + III/CII + IIIi | 1.56 ± 0.20 a | 0.78 ± 0.10 b | 1.24 ± 0.13 a, c | 1.08 ± 0.07 b, c |
All the results are expressed in mU/mg of mitochondrial proteins. The number of experiments was eight and ten for the normoxic and reperfused groups. Lowercase letters (a, b, c, and d) are significantly different
aHearts collected at the end of the stabilisation period
bReperfused: hearts collected at the end of the reperfusion period
cNADH dehydrogenase
dSuccinate dehydrogenase
eUbiquinol cytochrome c reductase
fCytochrome c oxidase
gNADH cytochrome c reductase
hSuccinate cytochrome c reductase
iRatio between the activities of NADH cytochrome c reductase and succinate cytochrome c reductase
Discussion
In this study, we verified that the well-known increase in ischemia/reperfusion-induced functional abnormalities occurring with aging also arises in middle-aged animals and we tried to evaluate the possible biochemical mechanisms involved in these changes. Fifty-two-week-old Wistar rats, considered as middle-aged animals with a life period approximating half of the longevity of this animal strain, were thus compared with 10-week-old animals, considered as young adults. Their hearts were collected, perfused according to the constant-pressure Langendorff mode and subjected to global zero-flow ischemia followed by reperfusion. Ischemic contracture was delayed in the middle-aged rats, suggesting better maintenance of ATP concentration in the older animals. In the literature, there is a study in apparent contradiction with this statement. Indeed, Headrick (1998) indicated that aging tended to increase ischemic- and hypoxic-contracture. Contrariwise, Ramani et al. (1996) showed a decelerated ischemic contracture. The two studies contrasted young or adult vs. aged animals. Our work supports the fact that middle age is associated with a delayed ischemic-related contracture. Rigor tension is explained by cellular ATP depletion due to arrest of the anaerobic glycolysis which results from proton-induced inhibition of the phosphofructokinase (PFK) reaction. This occurs in the diverse cells composing the myocardium at different times, depending on their localization (epi- vs. endocardium) and finally the intensity of their metabolism. The gap junction connexin 43 content has been tightly involved in the development of rigor tension, since it facilitates cell-to-cell communication and generalization of the behavior of a cell minority to the total cellular population of the heart. It is known to be decreased with aging, starting precisely at middle duration of the lifespan (Boengler et al. 2007). Since middle age reduces the gap junction connexin 43 content, excess proton accumulated in the most metabolically active cells should not be easily diffused in the less active cells, which should delay PFK inhibition in these last cells and allow the upholding of their metabolic activity. This decline in the cell-to-cell communication should delay the development of the ischemic contracture in the middle-aged myocardium (Ruiz-Meana et al. 2008), but it should also augment the pH decrease in those cells mostly resistant to proton-induced metabolic inhibition.
In spite of this apparent beneficial effect on the ischemic contracture, recovery of the mechanical activity (left ventricular developed pressure and rate × pressure product) during post-ischemic reperfusion was hampered in the middle-aged animals. This is in agreement with numerous studies showing that middle-aged and senescent hearts (Azhar et al. 1999; Leichtweis et al. 2001; Xia et al. 2003; Willems et al. 2003; Willems et al. 2005) augment reperfusion-induced contraction disorders. Delayed ischemic contracture with preservation of residual metabolic activity might be responsible for these intensified abnormalities. Ischemia triggers acidosis that is likely redoubled by continuation of the metabolic activity. Intracellular protons are extruded from the cells by the Na+/H+ exchanger (NHE-1 in the heart) that favors firstly intracellular sodium accumulation and secondly cellular calcium entry through the Na+/Ca2+ exchanger (Sniecinski and Liu 2004) when deprivation of ATP does not allow functioning of the Na+/K+-ATPase. Middle-aged and senescent hearts have been characterized by increased cellular sodium accumulation at the end of ischemia (Tani et al. 1997 and 1999) compared with young hearts. They are protected against reperfusion-induced cellular damage by either the NHE-1 inhibitor cariporide (Nakai et al. 2002; Besse et al. 2004; Simm et al. 2008) or the Na+/Ca2+ exchanger (NCX) inhibitor KB-R7943 (Yamamura et al. 2001). This stresses the importance of ischemia-induced proton production and NHE-1/NCX axis in genesis of the ischemia/reperfusion-induced functional disturbances of the aged heart.
The lower salvage of the cardiac mechanical activity during reperfusion in the older animals was associated with a reduced recovery of the oxygen consumption (−28%). This reflects a depressed oxidative metabolism leading to reduced energy production. The impairment of this low oxygen consumption-related energy production was further amplified by a powerless restoration of the metabolic efficiency that never exceeded 37 ± 13% of the pre-ischemic value in the middle-aged group versus 80 ± 19% in the young adult group. Reduced cardiac metabolic efficiency during reperfusion is due to several processes involving decreased mitochondrial energy production (mitochondrial calcium loading, bulge of ROS production, opening of the permeability transition pore and mitochondrial uncoupling) and augmented energy wasting (elimination of excess sodium and calcium via the Na+/K+-ATPase and Ca2+-ATPase). If and when these abnormalities disappear, the cardiac metabolic efficiency is restored. In the middle-aged hearts, restoration of this parameter during reperfusion was strongly delayed, which could be explained by a higher proton production, increased sodium, and calcium loadings and resulting abnormalities of energy production and utilization. Contraction was thus more depressed in the older animals compared with the younger ones and this accounted for the lower recovery of the rate × pressure product in the middle-aged rats. The decreased energy availability of the reperfused aged hearts might be explained by a distortion of the cellular functioning as well as by an abnormality of the coronary perfusion leading to insufficient oxygen supply. We observed lower recovery of the coronary flow of the middle-aged hearts compared with that of the young adults. This has been already mentioned by Willems et al. (2005) who showed that impaired salvage of the ventricular contractility in reperfused hearts of senescent animals correlates with recovery of the coronary flow. Furthermore, Besse et al. (2006) have shown that the post-ischemic coronary flow of the aged heart can be improved by treatment with superoxide dismutase and catalase, highlighting the prominent role of superoxide anions. This enhancement of the coronary perfusion was accompanied with a considerable improvement of the contractile function. This strongly suggests that insufficient coronary perfusion is rate-limiting during reperfusion of aged hearts with a major role of the abnormal vascular tone and the vascular cell dysfunction.
Insufficient coronary perfusion is thus probably related to the oxidative stress (Besse et al. 2006). ROS production has been reported to be associated with reduced nitric oxide (NO) bioavailability through scavenging by superoxide anions (Wei et al. 2006) and/or endothelial NO synthase uncoupling (Crabtree et al. 2009). In the present study, we evaluated the instant mitochondrial ROS impregnation at two moments of the perfusion: just before ischemia and at the end of reperfusion. It was performed by measuring the aconitase to fumarase ratio in cardiac homogenates (Gardner et al. 1994). Transition from normoxia to end of reperfusion triggered a significant decrease in the aconitase to fumarase ratio in the middle-aged hearts, whereas it did not alter this parameter in the hearts of young adult animals. This strongly suggests an increased oxidative stress in the oldest animals. This was reinforced by the measurement of hydrogen peroxide (H2O2) release by mitochondria isolated from normoxic hearts. Indeed, this H2O2 release was either unaltered or amplified by aging, depending on the conditions of substrate supply and of respiratory chain complex inhibition. The ampler reduction of the aconitase to fumarase ratio in the older hearts reflected thus necessarily a higher ex vivo mitochondrial ROS release. Such an aging-related increase in the mitochondrial ROS release can partly explain the lower recovery of the coronary flow during reperfusion. However, other phenomena also probably intervene. Indeed, aging has been associated with augmented expression of NADPH oxidase in the vascular wall (Oudot et al. 2006). In any case, the low coronary perfusion reduced the oxygen supply and energy production, leading to delayed restoration of the mechanical function.
An argument issued from our results suggests the importance of the ROS release during reperfusion on the restoration of the coronary flow. Young adult myocardium, but not middle-aged hearts, had an ischemia/reperfusion-induced reduction of complex II activity. This decrease is known to be related to the oxidative stress (Chen et al. 2007). Indeed, at the beginning of reperfusion, calcium invades the mitochondrial matrix (Miyata et al. 1992) and opens the permeability transition pore, leading to release of NADH from the mitochondrial matrix (Batandier et al. 2004). Furthermore, it activates the α-ketoglutarate dehydrogenase, which triggers succinate accumulation in the mitochondrial environment (Sentex et al. 1999). Both phenomena favor oxidation of the FADH2-linked substrate succinate that is known to trigger ROS over generation. In the present study, we ascertained that succinate augments considerably H2O2 release by isolated cardiac mitochondria in comparison with the NADH-linked substrate glutamate plus malate. As indicated by the inhibitory effect of rotenone, this occurs at complex I level by reverse electron flux. The high-ROS release occurring during the first 3 min of reperfusion (Demaison et al. 2001) oxidizes the complex II portion of the respiratory chain (Chen et al. 2007). ROS-induced inhibition of complex II at restoration of the coronary flow has a huge effect on subsequent mitochondrial ROS production, since it provokes its inhibition. Utilization of complex II inhibitors such as 3-nitro-N-methyl-salicylamide, malonate, or 3-nitropropionic acid indeed protects the myocardium against reperfusion-induced contractile dysfunction by reducing the burst of ROS occurring ordinarily at the beginning of reperfusion (Zhang et al. 2006; Turan et al. 2006; Wojtovich and Brookes 2008). In our work, ischemia/reperfusion-induced inhibition of the complex II activity in young adults related to the burst of ROS occurring at early reperfusion tempered ROS release during the remaining duration of reperfusion as it was indicated by the unchanged aconitase to fumarase ratio before and after ischemia. This was associated with a better recovery of the coronary flow. On the contrary, complex II activity was maintained in the middle-aged heart and this was associated with a decreased aconitase to fumarase ratio. As a consequence of the higher ROS production, recovery of the coronary flow during reperfusion was worsened.
Complex II activity might be modulated by its lipid environment and its preservation after ischemia/reperfusion in middle-aged hearts suggests membrane protection against the huge oxidative stress occurring under these circumstances. In our study, such a protection was also observed for complex I + III whose activity was reduced by ischemia/reperfusion in the young adult hearts, but not in the middle-aged myocardium. This suggests that biochemical composition of the lipid environment surrounding the respiratory complexes was different in young adult and middle-aged hearts. According to Pepe et al. (1999), aged hearts displays less n-3 polyunsaturated fatty acids (PUFAs) than young ones. This ascertainment has also been verified in skeletal muscle (Martin et al. 2007). Yet, n-3 PUFAs are extremely sensitive to the oxidative stress, more than n-6 PUFAs. The lower content of n-3 PUFAs for the benefit of n-6 PUFAs in the aged myocardium could protect activity of the complexes II and I + III against ROS deleterious effect during ischemia/reperfusion. Such a membrane lipid-related protection has been already observed in the middle-aged rat myocardium (Kakarla et al. 2005) in which the antioxidant defense (SOD, catalase, glutathione reductase) and the lipid peroxidation are reduced, although the oxidative stress evaluated by the drop of the reduced glutathione content is increased.
A reduction of the activity of the complex I + III in young adult hearts was observed whereas activities of the complexes I and III were not altered, suggesting the involvement of the quinone pool. Coenzyme Q treatment has been shown to be cardioprotective during ischemia/reperfusion (Maulik et al. 2000; Crestanello et al. 2002; Lakomkin et al. 2002; Timoshin et al. 2003; Verma et al. 2007; Sahach et al. 2007). The protective effect is associated with a shift of the redox equilibrium between the semi-reduced forms of ubiquinone and flavine coenzymes to a higher output of ubisemiquinone (Timoshin et al. 2003). This contributes to lower succinate-related ROS release (Lakomkin et al. 2002), lipid peroxidation, and thiol oxidation (Maulik et al. 2000) during reperfusion. The mitochondrial function is hence preserved (Crestanello et al. 2002; Lakomkin et al. 2002) with a reduced capacity of opening the permeability transition pore (Sahach et al. 2007). The coronary perfusion is also protected (Lakomkin et al. 2002). Ischemia/reperfusion is thus accompanied with disturbances of the quinone metabolism that can be responsible for the associated perfusion and mechanical dysfunctions. In the aged rat heart, although complex I + III is not damaged in our study, cardioprotective effect of the CoQ is also observed (Timoshchuk et al. 2009) with better restorations of contractile dysfunction, coronary flow, and metabolic efficiency. This further confirms that ROS release is more important in the aged heart, although the membrane environment and respiratory complex activities are less damaged probably through reduction of the n-3 PUFAs to n-6 PUFAs ratio. Conversely, other important cellular targets of the oxidative stress such as proteins (thiol oxidation, tyrosine nitration, protein oxidation, ROS-induced uncoupling of eNOS, etc.) can be involved in the lower resistance of the aged myocardium to ischemia/reperfusion. As it was shown by Gao et al. (2000), the aged myocardium seems to have a reduced capacity of nitric oxide production.
In conclusion, middle age augmented reperfusion mechanical dysfunction via a lower recovery of the coronary flow and insufficient oxygen supply. Due to preservation of the respiratory complex II activity, reperfusion-induced mitochondrial ROS release was enlarged, which explained the lower restoration of coronary perfusion probably through decreased NO bioavailability.
Acknowledgments
This work was supported by the National Institute of Agronomical Research (INRA, France) and the Université Joseph Fourier.
References
- Amrani M, Chester AH, Jayakumar J, Yacoub MH. Aging reduces postischemic recovery of coronary endothelial function. J Thorac Cardiovasc Surg. 1996;111:238–245. doi: 10.1016/S0022-5223(96)70421-X. [DOI] [PubMed] [Google Scholar]
- Azhar G, Gao W, Liu L, Wei JY. Ischemia-reperfusion in the adult mouse heart: influence of age. Exp Gerontol. 1999;34:699–714. doi: 10.1016/S0531-5565(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- Besse S, Tanguy S, Riou B, Boucher F, Bulteau AL, Page C, Swynghedauw B, Liris J. Coronary and aortic vasoreactivity protection with endothelin receptor antagonist, bosentan, after ischemia and hypoxia in aged rats. Eur J Pharmacol. 2001;432:167–175. doi: 10.1016/S0014-2999(01)01417-0. [DOI] [PubMed] [Google Scholar]
- Besse S, Tanguy S, Boucher F, Page C, Rozenberg S, Riou B, Liris J, Swynghedaw B. Cardioprotection with cariporide, a sodium-proton exchanger inhibitor, after prolonged ischemia and reperfusion in senescent rats. Exp Gerontol. 2004;39:1307–1314. doi: 10.1016/j.exger.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Besse S, Bulteau AL, Boucher F, Riou B, Swynghedauw B, Liris J. Antioxidant treatment prevents cardiac protein oxidation after ischemia-reperfusion and improves myocardial function and coronary perfusion in senescent hearts. J Physiol Pharmacol. 2006;57:541–552. [PubMed] [Google Scholar]
- Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–H1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- Capel F, Rimbert V, Lioger D, Diot A, Rousset P, Mirand PP, Boirie Y, Morio B, Mosoni L. Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech Ageing Dev. 2005;126:505–511. doi: 10.1016/j.mad.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chen J, Rawale S, Varadharaj S, Kaumaya PP, Zweier JL, Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem. 2008;283:27991–28003. doi: 10.1074/jbc.M802691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- Crestanello JA, Doliba NM, Doliba NM, Babsky AM, Niborii K, Osbakken MD, Whitman GJ. Effect of coenzyme Q10 supplementation on mitochondrial function after myocardial ischemia reperfusion. J Surg Res. 2002;102:221–228. doi: 10.1006/jsre.2001.6324. [DOI] [PubMed] [Google Scholar]
- Demaison L, Moreau D, Vergely-Vandriesse C, Grégoire S, Degois M, Rochette L. Effects of dietary polyunsaturated fatty acids and hepatic steatosis on the functioning of isolated working rat heart under normoxic conditions and during post-ischemic reperfusion. Mol Cell Biochem. 2001;224:103–116. doi: 10.1023/A:1011934603667. [DOI] [PubMed] [Google Scholar]
- Faloona GR, Srere PA. Escherichia coli citrate synthase. Purification and the effect of potassium on some properties. Biochemistry. 1969;8:4497–4503. doi: 10.1021/bi00839a041. [DOI] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- Gao F, Christopher TA, Lopez BL, Friedman E, Cai G, Ma XL. Mechanism of decreased adenosine protection in reperfusion injury of aging rats. Am J Physiol Heart Circ Physiol. 2000;279:H329–H338. doi: 10.1152/ajpheart.2000.279.1.H329. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci USA. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E. Oxygen solubility in experimental media. Oroboros Bioenergetics Newsletter. 2001;6(3):1–6. [Google Scholar]
- Goodwin AT, Amrani M, Marchbank AJ, Gray CC, Jayakumar J, Yacoub MH. Coronary vasoconstriction to endothelin-1 increases with age before and after ischaemia and reperfusion. Cardiovasc Res. 1999;41:554–562. doi: 10.1016/S0008-6363(98)00253-3. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Tsuchiya N, Pepe S. Mitochondria in heart ischaemia and aging. Biochem Soc Symp. 1999;66:141–147. doi: 10.1042/bss0660141. [DOI] [PubMed] [Google Scholar]
- Headrick JP. Aging impairs functional, metabolic and ionic recovery from ischemia-reperfusion and hypoxia-reoxygenation. J Mol Cell Cardiol. 1998;30:1415–1430. doi: 10.1006/jmcc.1998.0710. [DOI] [PubMed] [Google Scholar]
- Hoppel CL, Moghaddas S, Lesnefsky EJ. Interfibrillar cardiac mitochondrial complex III defects in the aging rat heart. Biogerontology. 2002;3:41–44. doi: 10.1023/A:1015251212039. [DOI] [PubMed] [Google Scholar]
- Hu A, Jiao X, Gao E, Li Y, Sharifi-Azad S, Grunwald Z, Ma XL, Sun JZ. Tonic beta-adrenergic drive provokes proinflammatory and proapoptotic changes in aging mouse heart. Rejuvenation Res. 2008;11:215–226. doi: 10.1089/rej.2007.0609. [DOI] [PubMed] [Google Scholar]
- Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/S0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heat-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Kakarla P, Vadluri G, Reddy KS, Leeuwenburgh C. Vulnerability of the mid aged rat myocardium to the age-induced oxidative stress: influence of exercise training on antioxidant defense system. Free Radic Res. 2005;39:1211–1217. doi: 10.1080/10715760500315118. [DOI] [PubMed] [Google Scholar]
- Krähenbühl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts: application to mitochondrial encephalomyopathies. Clin Chim Acta. 1994;230:177–187. doi: 10.1016/0009-8981(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Kramer KA, Oglesbee D, Hartman SJ, Huey J, Anderson B, Magera MJ, Matern D, Rinaldo P, Robinson BH, Cameron JM, Hahn SH. Automated spectrophotometric analysis of mitochondrial respiratory chain complex enzyme activities in cultured skin fibroblasts. Clin Chem. 2005;51:2110–2116. doi: 10.1373/clinchem.2005.050146. [DOI] [PubMed] [Google Scholar]
- Lacraz G, Couturier K, Taleux N, Servais S, Sibille B, Letexier D, Guigas B, Dubouchaud H, Leverve X, Favier R. Liver mitochondrial properties from the obesity-resistant Lou/C rat. Int J Obes (Lond) 2008;32:629–638. doi: 10.1038/sj.ijo.0803779. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Sollott SJ, Pepe S. The old heart: operating on the edge. Novartis Found Symp. 2001;235:172–196. doi: 10.1002/0470868694.ch15. [DOI] [PubMed] [Google Scholar]
- Lakomkin VL, Korkina OV, Tsyplenkova VG, Timoshin AA, Ruuge EK, Kapel’ko VI. The protective action of ubiquinone at ischemia and reperfusion. Kardiologiia. 2002;42:51–55. [PubMed] [Google Scholar]
- Leichtweis S, Leeuwenburgh C, Bejma J, Ji LL. Aged rat hearts are not more susceptible to ischemia-reperfusion injury in vivo: role of glutathione. Mech Ageing Dev. 2001;122:503–518. doi: 10.1016/S0047-6374(00)00253-0. [DOI] [PubMed] [Google Scholar]
- Lesfnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged heart. Biochim Biophys Acta. 2008;1777:1020–1027. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–1015. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Martin C, Dubouchaud H, Mosoni L, Chardigny JM, Oudot A, Fontaine E, Vergely C, Keriel C, Rochette L, Leverve X, Demaison L. Abnormalities of mitochondrial functioning can partly explain the metabolic disorders encountered in sarcopenic gastrocnemius. Aging Cell. 2007;6:165–177. doi: 10.1111/j.1474-9726.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- Maulik N, Yoshida T, Engelman RM, Bagchi D, Otani H, Das DK. Dietary coenzyme Q(10) supplement renders swine hearts resistant to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;278:H1084–H1090. doi: 10.1152/ajpheart.2000.278.4.H1084. [DOI] [PubMed] [Google Scholar]
- Miro O, Barrientos A, Alonso JR, Casademont J, Jarreta D, Urbano-Marquez A, Cardellach F. Effects of general anaesthetic procedures on mitochondrial function of human skeletal muscle. Eur J Pharmacol. 1999;55:35–41. doi: 10.1007/s002280050589. [DOI] [PubMed] [Google Scholar]
- Miyata H, Lakatta EG, Stern MD, Silverman HS. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res. 1992;71:605–613. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- Nagy K, Takacs IE, Pankucsi C. Age-dependence of free radical-induced oxidative damage in ischemic-reperfused rat heart. Arch Gerontol Geriatr. 1996;22:297–309. doi: 10.1016/0167-4943(96)00700-5. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Horimoto H, Mieno S, Sasaki S. Na(+)/H(+) exchanger inhibitor HOE642 offers myoprotection in senescent myocardium independent of ischemic preconditioning mechanisms. Eur Surg Res. 2002;34:244–250. doi: 10.1159/000063396. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Al-Ruzzeh S, Chester AH, Dewar A, Rothery S, Severs NJ, Yacoub MH, Amrani M. Age-related changes in the protective effect of chronic administration of l-arginine on post-ischemic recovery of endothelial function. Eur J Cardiothorac Surg. 2003;23:626–632. doi: 10.1016/S1010-7940(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxydases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- Pasdois P, Beauvoit B, Tariosse L, Vinassa B, Bonoron-Adèle S, Santos PD. MitoK(ATP)-dependent changes in mitochondrial volume and in complex II activity during ischemic and pharmacological preconditioning of Langendorff-perfused rat heart. J Bioenerg Biomembr. 2006;38:101–112. doi: 10.1007/s10863-006-9016-3. [DOI] [PubMed] [Google Scholar]
- Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- Polewczyk A, Janion M, Gasior M, Gierlotka M. Myocardial infarction in the elderly. Clinical and therapeutic differences. Kardiol Pol. 2008;66:166–172. [PubMed] [Google Scholar]
- Ramani K, Lust WD, Wittingham TS, Lesnefsky EJ. ATP catabolism and adenosine generation during ischemia in the aging heart. Mech Ageing Dev. 1996;89:113–124. doi: 10.1016/0047-6374(96)01732-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Rodriguez-Sinovas A, Cabestrero A, Boengler K, Heusch G, Garcia-Dorado D. Mitochondrial connexin 43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2008;77:325–333. doi: 10.1093/cvr/cvm062. [DOI] [PubMed] [Google Scholar]
- Sahach VF, Vavilova HL, Rudyk OV, Dobrovol’s’kyĭ FV, Shymans’ka TV, Miedviediev OS. Inhibition of mitochondrial permeability transition pore is one of the mechanisms of cardioprotective effect of coenzyme Q10. Fiziol Zh. 2007;53:35–42. [PubMed] [Google Scholar]
- Sentex E, Laurent A, Martine L, Grégoire S, Rochette L, Demaison L. Calcium- and ADP-magnesium-induced respiratory uncoupling in isolated cardiac mitochondria: influence of cyclosporine A. Mol Cell Biochem. 1999;202:73–84. doi: 10.1023/A:1007074330569. [DOI] [PubMed] [Google Scholar]
- Simm A, Friedrich I, Scheubel RJ, Gursinsky T, Silber RE, Bartling B. Age dependency of the cariporide-mediated cardio-protection after simulated ischemia in isolated human atrial heart muscles. Exp Gerontol. 2008;43:691–699. doi: 10.1016/j.exger.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Sniecinski R, Liu H. Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium. Anesthesiology. 2004;100:589–597. doi: 10.1097/00000542-200403000-00019. [DOI] [PubMed] [Google Scholar]
- Tani M, Suganuma Y, Hasegawa H, Shinmura K, Ebihara Y, Hayashi Y, Guo X, Takayama M. Decrease in ischemic tolerance with aging in isolated perfused Fisher 344 rat hearts: relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol. 1997;29:3081–3089. doi: 10.1006/jmcc.1997.0533. [DOI] [PubMed] [Google Scholar]
- Tani M, Honma Y, Takayama M, Hasegawa H, Shinmura K, Ebihara Y, Tamaki K. Loss of protection by hypoxic preconditioning in aging Fisher 344 rat hearts related to myocardial glycogen content and Na+ imbalance. Cardiovasc Res. 1999;41:594–602. doi: 10.1016/S0008-6363(98)00256-9. [DOI] [PubMed] [Google Scholar]
- Timoshchuk SV, Vavilova HL, Strutyns’ka NA, Talanov SA, Petukhov DM, Kuchmenko OB, Donchenko HV, Sahach VF. Cardioprotective action of coenzyme Q in conditions of its endogenous synthesis activation in cardiac ischemia-reperfusion in old rats. Fiziol Zh. 2009;55:58–63. [PubMed] [Google Scholar]
- Timoshin AA, Lakomkin VL, Gubkin AA, Ruuge EK. Effect of coenzyme Q10 on free radical centers in isolated rat myocardium tissue. Biofizika. 2003;48:717–721. [PubMed] [Google Scholar]
- Tsukube T, McCully JD, Federman M, Krukenkamp IB, Levitsky S. Developmental differences in cytosolic calcium accumulation associated with surgically induced global ischemia: optimization of cardioplegic protection and mechanism of action. J Thorac Cardiovasc Surg. 1996;112:175–184. doi: 10.1016/S0022-5223(96)70194-0. [DOI] [PubMed] [Google Scholar]
- Turan N, Csonka C, Csont T, Giricz Z, Fodor G, Bencsik P, Gyongyosi M, Cakici I, Ferdinandy P. The role of peroxynitrite in chemical preconditioning with 3-nitropropionic acid in rat hearts. Cardiovasc Res. 2006;70:384–390. doi: 10.1016/j.cardiores.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Veitch K, Hue L. Flunarizine and cinnarizine inhibit complexes I and II: possible implication for Parkinsonism. Mol Pharmacol. 1994;45:158–163. [PubMed] [Google Scholar]
- Verma DD, Hartner WC, Thakkar V, Levchenko TS, Torchilin VP. Protective effect of coenzyme Q10-loaded liposomes on the myocardium in rabbits with an acute experimental myocardial infarction. Pharm Res. 2007;24:2131–2137. doi: 10.1007/s11095-007-9334-0. [DOI] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- Willems L, Garnham B, Headrick JP. Aging-related changes in myocardial purine metabolism and ischemic tolerance. Exp Gerontol. 2003;38:1169–1177. doi: 10.1016/j.exger.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–256. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim Biophys Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Godin DV, Ansley DM. Propofol enhances ischemic tolerance of middle-aged rat hearts: effects on 15-F(2t)-isoprostane formation and tissue antioxidant capacity. Cardiovasc Res. 2003;59:113–121. doi: 10.1016/S0008-6363(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Yamamura K, Tani M, Hasegawa H, Gen W. Very low dose of the Na(+)/Ca(2+) exchange inhibitor, KB-R7943, protects ischemic reperfused aged Fischer 344 rat hearts: considerable strain difference in the sensitivity to KB-R7943. Cardiovasc Res. 2001;52:397–406. doi: 10.1016/S0008-6363(01)00409-6. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang JH, Yu F, Zhao J, Jiang P, Chang L, Tang C, Xu J. Protective role of 3-nitro-N-methyl-salicylamide on isolated rat heart during 4 hours of cold storage and reperfusion. Transplant Proc. 2006;38:1247–1252. doi: 10.1016/j.transproceed.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]