Abstract
Phytoestrogens as selective estrogen receptor modulators like compounds may consider as a therapeutic option in osteoporosis. In this regard, the effect of phytoestrogens on bone biomarkers was examined in several trials which their results are controversial. We aimed this meta-analysis to evaluate the net effect of phytoestrogens on bone markers. A thorough search was conducted from 2000 to 2010 in English articles. All randomized clinical trials were reviewed, and finally, 11 eligible randomized clinical trials were selected for meta-analysis. Totally 1,252 postmenopausal women were enrolled in the study by considering the changes of pyridinoline (Pyd), desoxypyridinoline (Dpyd), bone alkaline phosphatase, and osteocalcin concentrations in urine and serum after phytoestrogens consumption. The urine Pyd and Dpyd levels decreased significantly in phytoestrogens consumers. Effect size and effect size for weighted mean difference of urine Pyd levels showed −1.229171 (95% confidence interval (CI) = −1.927639 to −0.530703) and −9.780623 (95% CI = −14.240401 to −5.320845), respectively, a significant results in comparison to control group and significant results for Dpyd −0.520132 (95% CI = −0.871988 to −0.168275) and −0.818582 (95% CI = −1.247758 to −0.389407), respectively. Meta-analysis indicates that phytoestrogens intake can prevent bone resorption, but its benefits on bone formation are not significant. This favorable effect was observed in low doses and in at least 3 weeks of phytoestrogens intake.
Keywords: Phytoestrogens, Soy isoflavone, Meta-analysis, Pyridinoline, Desoxypyridinoline, Bone-specific alkaline posphatase, Osteoporosis, Oxidative stress
Introduction
Osteoporosis, a chronic devastating disease, faced humanity with a serious challenge. As estimated in USA, one out of three women older than 50 years is at risk of osteoporosis and its complications which its annual direct cost is high. Regardless of many definitions for osteoporosis, World Health Organization explains it as a disease presented with low bone mass and enhanced fragility and consequently increased risk of fracture (Anonymous 2003). Osteoporosis is caused by an imbalance between bone formation and bone resorption which accelerates after menopause in women. Estrogen deficiency after menopause leads to brittle bone and weak muscles which increases the risk of fall and fracture. Therefore, there are many enthusiasms into its prevention and treatment. Although many prevention and treatment modalities are considered for osteoporosis, however, their side effects and low benefits limit their administration. Hormone replacement therapy as a substitute for estrogen deficiency increase bone mineral density (BMD) and decrease fracture risk, but their serious side effects lowlights their usage (Middleton and Steel 2007; Rossouw et al. 2002; Hulley et al. 1998). In the recent years, there are enthusiasms into application of natural products especially in prevention modalities. Considering the role of oxidative stress as well as inflammation in pathophysiology of osteoporosis (Abdollahi et al. 2005; Salari and Abdollahi 2009) and the relationship between cardiovascular disease and senile osteoporosis (Salari et al. 2008a, b), several natural products are under investigation for this purpose. For example, n-3 fatty acids as potent anti-inflammatory agents have been studied in different human and animal subjects, and it seems that they mostly inhibit bone resorption rather than affecting bone formation (Salari et al. 2008a, b, 2010). In this regard, nowadays herbal medicine as one branch of alternative medicine comes into attention.
Soy isoflavones as natural phytoestrogens have weak estrogen agonistic effect on estrogen receptor β, which make them similar to selective estrogen receptor modulators (SERMs). Soy isoflavones mostly consist of genistein, daidzein, and glycitein. While some studies talked about the possible preventive effect of soy isoflavones on bone loss, other studies indicated decreasing bone turnover (Picherit et al. 2001; Devareddy et al. 2006; Hidaka et al. 2003). In vitro studies stated a direct stimulatory effect of soy isoflavones on osteoblasts as well as its inhibitory effect on production of tumor necrosis factor-α, interleukin-6, and prostaglandin E2. Therefore, both its positive effects on bone formation and bone resorption were mentioned (Choi et al. 2001; Suh et al. 2003). Since 10 years ago, soy isoflavones have been taken into special attention in human food. Results from animal studies have been optimistic for continuing in human trials. Most of studies were conducted on postmenopausal women who are more vulnerable. The results of clinical trials are conflicting; therefore, there was a need for integrating all relevant clinical trials in order to analyze data powerfully and statistically. In the searched randomized clinical trials (RCTs), pyridinoline (Pyd), desoxypyridinoline (Dpyd), cross-link telopeptide (CTX), N-telopeptide of type I collagen (NTX) were measured as bone resorption markers, and osteocalcin (OC) and bone-specific alkaline posphatase (BALP) were measured as bone formation markers. In addition to the limitations of BMD as a diagnostic factor in osteoporosis, its less prognostic value has to be taken into account. Actually BMD predicts the risk of fracture while a recent study reported the underestimated value of BMD in risk evaluation (Siris et al. 2004). Therefore, the rate of bone remodeling can determine bone strength besides BMD, and its high rate is associated with severe forms of osteoporosis (Garnero et al. 1996; Riggs and Melton 2002). Not only BMD changes but also inhibition of bone remodeling reduces the risk of fracture. BMD changes need a long duration of time, and it is estimated that every 2 years, BMD changes 1% to 2%. Thus, in a short duration of time, only bone biomarkers are good predictors of bone remodeling and risk of fracture, which make them a useful prognostic tool in clinical trials. Bone resorption markers and bone formation markers change in parallel. In the selected clinical trials, OC and BALP as markers of bone formation and Pyd and Dpyd as markers of bone resorption were mostly measured. Pyd and Dpyd are produced during bone resorption and pass into urine (Srivastava et al. 2005) which can be measured by different methods such as radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA) with better sensitivity (Gunja-Smith and Boucek 1981). Both markers are reliable markers of bone resorption.
OC, a bone matrix protein, is synthesized by mature osteoblasts and is detected by RIA or ELISA. BALP is present in the surface of osteoblasts, and its detection by immunoassay provides a specific method for assessment of osteoblasts function (Farley et al. 1981).
Getting more powerful results of former studies and shedding a light on the way of future investigations, we analyzed all eligible reported studies considering the mentioned bone markers and BMD quantitatively.
Methods
Data sources
PubMed, Web of Sciences (ISI), and Scopus were searched from 2000 to 2010 by keywords such as osteoporosis, bone, BMD, soy, soy isoflavones, phytoestrogens, genistein, Pyd, Dpyd, OC, BALP, and BMD. We limited our search to the randomized clinical trials written in English. Studies were chosen for meta-analysis if they met the inclusion criteria including postmenopausal women as subjects, taking every herbal product as a phytoestrogen, and measurement of the bone markers (Pyd, Dpyd, OC, BALP, NTx, CTx) as the index of bone turnover. Three reviewers assessed each article independently to diminish the probability of duplication, analyzing reviews, case studies, and uncontrolled trials. Studies were precluded if they were uncontrolled or their results did not consider our goals.
Assessment of trial quality
Jadad score, which indicates the potentiality of the studies based on their description of randomization, blinding, and dropouts (withdrawals), was used to assess the methodological quality of trials (Uesugi et al. 2002, 2003; Yamori et al. 2002; Dalais et al. 2003; Morabito et al. 2002; Atkinson et al. 2004; Arjmandi et al. 2003; Ye et al. 2006; Zhang et al. 2007; Albertazzi et al. 2005; Kreijkamp-Kaspers et al. 2004). The quality scale ranges from 0 to 5 points with a low-quality report of score 2 or less and a high-quality report of score at least 3.
Statistical analysis
Data from selected studies were extracted in the form of 2 × 2 tables. Included studies were weighted and pooled. The data were analyzed using Statsdirect software version 2.7.7. Effect size and effect size for weighted mean difference and 95% confidence intervals (95% CI) were calculated using the Der Simonian–Laird method. The Cochran Q test was used to test heterogeneity. Funnel plot analysis was used as publication bias indicator.
Results
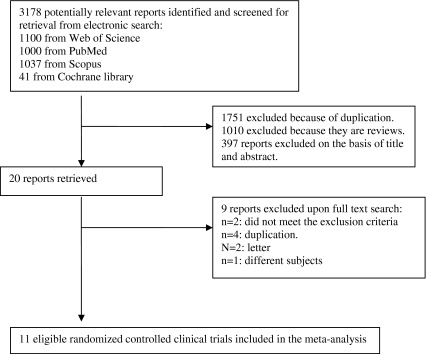
The electronic search provided 3,178 articles: 1,000 from PubMed, 1,100 from Web of Sciences, 1,037 from Scopus, and 41 from Cochrane library. Of those, 20 studies were scrutinized in full text, of which six trials were considered unsuitable while 11 trials were analyzed (Fig. 1).
Fig. 1.
Flow diagram for study selection
Concerning trial quality, all 11 trials received a Jadad score of 3 or more (Table 1). Totally 1,252 postmenopausal women were enrolled in the meta-analysis. A brief summary of trials are reported in Table 1. All incorporated trials in this meta-analysis were randomized and double-blinded.
Table 1.
Characteristics of 12 selected RCTs
| Study | Duration | No. of subjects | Phytoestrogens dose (mg) | Indices | Jadad score |
|---|---|---|---|---|---|
| Uesugi et al. (21) | 4 weeks | 23 | 61.8 | Pyd, Dpyd, OC | 3 |
| Yamori et al. (22) | 10 weeks | 40 | 37.3 | Pyd, Dpyd | 3 |
| Uesugi et al. (23) | 3 months | 22 | 61.8 | BMD, Pyd, BALP | 4 |
| Dalais et al. (24) | 3 months | 106 | 118 | Pyd, Dpyd | 4 |
| Morabito et al. (25) | 1 year | 90 | 54 | Pyd, Dpyd, BALP, OC | 3 |
| Atkinson et al. (26) | 1 year | 205 | 43.5 | Pyd, Dpyd, BALP, NTx | 5 |
| Arjmandi et al. (27) | 3 months | 71 | 88.4 | Dpyd, BALP | 4 |
| Ye et al. (28) | 24 weeks | 90 | 84, 126 | Dpyd, OC, BALP | 5 |
| Zhang et al. (29) | 2 years | 100 | 78 | Dpyd, OC, | 5 |
| Albertazzi et al. (30) | 6 weeks | 100 | 90 | CTx, OC | 5 |
| Kreijkamp-Kaspers et al. (31) | 1 year | 202 | 99 | BALP, BMD | 5 |
Pyd pyridinoline, Dpyd desoxypyridinoline, BALP bone-specific alkaline phosphatase, OC osteocalcin, CTX cross-link telopeptide, NTX N-telopeptide of type I collagen, BMD bone mineral density
Efficacy
Efficacy of phytoestrogens comparing to placebo in Pyd
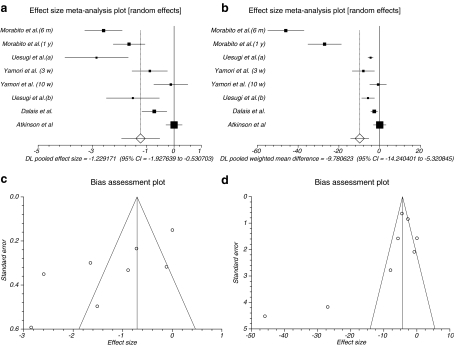
The summary effect size and effect size for weighted mean difference of urine Pyd levels for all reported data in different duration of time for follow-up in all doses in six trials (Uesugi et al. 2002, 2003; Yamori et al. 2002; Dalais et al. 2003; Morabito et al. 2002; Atkinson et al. 2004) were −1.229171 (95% CI = −1.927639 to −0.530703) and −9.780623 (95% CI = −14.240401 to −5.320845), respectively, a significant result (P = 0.0006, Fig. 2a and P < 0.0001, Fig. 2b). The Cochrane Q test for heterogeneity indicated that the studies are heterogeneous (P < 0.0001) and could not be combined; thus, the random effects for individual and summary of effect size and effect size for weighted mean difference were applied. Regression of normalized effect vs. precision for all included studies for Pyd among phytoestrogens vs. placebo therapy in different duration of time for follow-up and all doses for effect size was −6.321254 (95% CI = −11.339793 to −1.302715, P = 0.0216), and Kendall’s test on standardized effect vs. variance indicated tau = −0.571429, P = 0.0312 (Fig. 2c) and for effect size for weighted mean difference was −4.34888 (95% CI = −10.169707 to 1.471947, P = 0.1173) and Kendall’s test on standardized effect vs. variance indicated tau = −0.642857, P = 0.0141 (Fig. 2d).
Fig. 2.
a Individual and pooled effect size for the outcome of “Pyd” in the studies considering phytoestrogens comparing to placebo therapy. b Individual and pooled effect size for weighted mean difference for the outcome of “Pyd” in the studies considering phytoestrogens comparing to placebo therapy. c Publication bias indicators for the outcome of “Pyd” in the studies considering phytoestrogens comparing to placebo therapy (effect size). d Publication bias indicators for the outcome of “Pyd” in the studies considering phytoestrogens comparing to placebo therapy (effect size for weighted mean difference)
Efficacy of phytoestrogens comparing to placebo in Dpyd
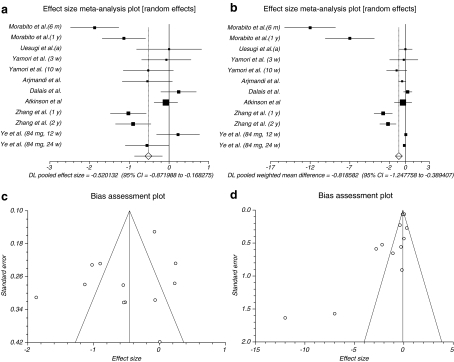
The summary effect size and effect size for weighted mean difference for Dpyd for all reported data in different duration of time for follow-up in all doses in postmenopausal women of eight trials (Uesugi et al. 2002; Yamori et al. 2002; Dalais et al. 2003; Morabito et al. 2002; Atkinson et al. 2004; Ye et al. 2006; Zhang et al. 2007) were −0.520132 (95% CI = −0.871988 to −0.168275) and −0.818582 (95% CI = −1.247758 to −0.389407), respectively, a significant result (P = 0.0038, Fig. 3a and P = 0.0002, Fig. 3b). The Cochrane Q test for heterogeneity indicated that the studies are heterogeneous (P < 0.0001) and could not be combined; thus, the random effects for individual and summary of effect size and effect size for weighted mean difference were applied. Regression of normalized effect vs. precision for all included studies for Dpyd among phytoestrogens vs. placebo therapy in different duration of time for follow-up and all doses for effect size was −2.296791 (95% CI = −8.139748 to 3.546166, P = 0.4017), and Kendall’s test on standardized effect vs. variance indicated tau = −0.121212, P = 0.5452 (Fig. 3c) and for effect size for weighted mean difference was −2.829871 (95% CI = −4.911233 to −0.74851, P = 0.0127) and Kendall’s test on standardized effect vs. variance indicated tau = −0.515152, P = 0.0138 (Fig. 3d).
Fig. 3.
a Individual and pooled effect size for the outcome of “Dpyd” in the studies considering phytoestrogens comparing to placebo therapy. b Individual and pooled effect size for weighted mean difference for the outcome of “Dpyd” in the studies considering phytoestrogens comparing to placebo therapy. c Publication bias indicators for the outcome of “Dpyd” in the studies considering phytoestrogens comparing to placebo therapy (effect size). d Publication bias indicators for the outcome of “Dpyd” in the studies considering phytoestrogens comparing to placebo therapy (effect size for weighted mean difference)
Efficacy of phytoestrogens comparing to placebo in BALP
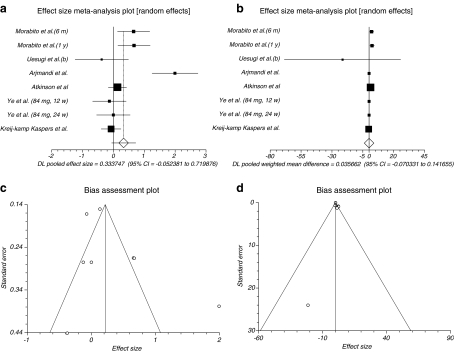
The summary effect size and effect size for weighted mean difference for BALP for all reported data in different duration of time for follow-up and all doses in six trials (Uesugi et al. 2003; Morabito et al. 2002; Atkinson et al. 2004; Arjmandi et al. 2003; Ye et al. 2006; Kreijkamp-Kaspers et al. 2004) were 0.333747 (95% CI = −0.052381 to 0.719876) and 0.035662 (95% CI = −0.070331 to 0.141655), respectively, a non-significant results (P = 0.0902, Fig. 4a and P = 0.5096, Fig. 4b). The Cochrane Q test for heterogeneity indicated that the studies are heterogeneous (P < 0.0001 and P = 0.0069) and could not be combined; thus, the random effects for individual and summary of effect size and effect size for weighted mean difference were applied. Regression of normalized effect vs. precision for all included studies for BALP among phytoestrogens vs. placebo therapy in different duration of time for follow-up and all doses for effect size was 2.60556 (95% CI = −3.065677 to 8.276798, P = 0.3039), and Kendall’s test on standardized effect vs. variance indicated tau = 0, P = 0.9049 (Fig. 4c) and for effect size for weighted mean difference was 0.345827 (95% CI = −1.40993 to 2.101584, P = 0.6469) and Kendall’s test on standardized effect vs. variance indicated tau = 0.214286, P = 0.5484 (Fig. 4d).
Fig. 4.
a Individual and pooled effect size for the outcome of “BALP” in the studies considering phytoestrogens comparing to placebo therapy. b Individual and pooled effect size for weighted mean difference for the outcome of “BALP” in the studies considering phytoestrogens comparing to placebo therapy. c Publication bias indicators for the outcome of “BALP” in the studies considering phytoestrogens comparing to placebo therapy (effect size). d Publication bias indicators for the outcome of “BALP” in the studies considering phytoestrogens comparing to placebo therapy (effect size for weighted mean difference)
Efficacy of phytoestrogens comparing to placebo in OC
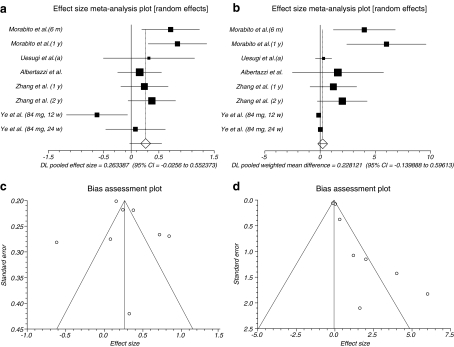
The summary effect size and effect size for weighted mean difference for OC for all reported data in different duration of time for follow-up and all doses in postmenopausal women of five trials (Uesugi et al. 2002; Morabito et al. 2002; Ye et al. 2006; Zhang et al. 2007; Albertazzi et al. 2005) were 0.263387 (95% CI = −0.0256 to 0.552373) and 0.263387 (95% CI = −0.0256 to 0.552373), respectively, a non-significant results (P = 0.074, Fig. 5a and P = 0.2244, Fig. 5b). The Cochrane Q test for heterogeneity indicated that the studies are heterogeneous (P = 0.0107 and P = 0.0002) and could not be combined; thus, the random effects for individual and summary of effect size and effect size for weighted mean difference were applied. Regression of normalized effect vs. precision for all included studies for OC among phytoestrogens vs. placebo therapy in different duration of time for follow-up and all doses for effect size was −0.065824 (95% CI = −7.956693 to 7.825044, P = 0.9844), and Kendall’s test on standardized effect vs. variance indicated tau = 0, P = 0.9049 (Fig. 5c) and for effect size for weighted mean difference was 2.018182 (95% CI = 0.859008 to 3.177355), P = 0.0053 and Kendall’s test on standardized effect vs. variance indicated tau = 0.428571, P = 0.1789 (Fig. 5d).
Fig. 5.
a Individual and pooled effect size for the outcome of “OC” in the studies considering phytoestrogens comparing to placebo therapy. b Individual and pooled effect size for weighted mean difference for the outcome of “OC” in the studies considering phytoestrogens comparing to placebo therapy. c Publication bias indicators for the outcome of “OC” in the studies considering phytoestrogens comparing to placebo therapy (effect size). d Publication bias indicators for the outcome of “OC” in the studies considering phytoestrogens comparing to placebo therapy (effect size for weighted mean difference)
Discussion
The similarities of phytoestrogens to SERMs and their weak estrogenic effects make them considerable functional therapeutic options in osteoporosis which may diminish the adverse effect of estrogen. Regarding the importance of BMD as well as bone biomarkers in bone health assessment, there are several different types of studies that assessed bone remodeling after phytoestrogens intake and their results are conflicting. Some studies in postmenopausal women reported significant decrease in bone resorption markers (Crisafulli et al. 2004; Evans et al. 2007) in contrast to the others who reported non-significant changes in bone resorption markers (Albertazzi et al. 2005; Wangen et al. 2000) and bone formation markers (Albertazzi et al. 2005; Weaver et al. 2009). In addition, the BMD changes after phytoestrogens intake were reported controversially (Cook and Pennington 2002; Kritz-Silverstein and Goodman-Gruen 2002; Harkness et al. 2004). Concerning the importance of bone biomarkers in clinical trials, we analyzed the effect of the phytoestrogens on bone markers. In the chosen 11 trials for meta-analysis, six trials assessed for changes in urinary Pyd levels. In four out of six trials, a significant decrease in urine Pyd level was seen (Uesugi et al. 2002, 2003; Yamori et al. 2002; Morabito et al. 2002), while the rest of the studies reported non-significant changes (Dalais et al. 2003; Atkinson et al. 2004) in the duration of 3 weeks–1 year. The meta-analysis shows a significant decrease in urinary Pyd level after phytoestrogens consumption.
Further analysis on the effect of phytoestrogens intake on Dpyd changes showed promising results. The results of eight studies were analyzed for this purpose; five out of eight trials reported significant decrease in Dpyd (Uesugi et al. 2002; Yamori et al. 2002; Morabito et al. 2002; Ye et al. 2006; Zhang et al. 2007), and the rest of them could not detect significant changes (Dalais et al. 2003; Atkinson et al. 2004; Arjmandi et al. 2003). Regardless of the study period as confirmed by our analysis (4 weeks–2 year), data show the significant decrease in Dpyd levels.
Previously in a literature review, the US Consensus Panel recommended daily intake of 90-mg isoflavones to get bone health advantage (Branca 2003), although in our study no change was found after exclusion of doses more than 90 mg. Yamori et al. observed significant decrease in Pyd and Dpyd levels by daily intake of 37.3 mg phytoestrogens while Dalais et al. could not show significant alteration in these bone markers after consumption of 118-mg phytoestrogens a day (Yamori et al. 2002; Dalais et al. 2003). Although some animal studies are confirming the most significant effect of low-dose phytoestrogens on bone retention rather than high doses (Anderson et al. 1998), however, Devareddy et al. (2006) indicated that higher doses of phytoestrogens are necessary for reversing bone loss. Also the results of a former meta-analysis confirm our results (Ma et al. 2008). Ma et al. (2008) reported significant bone resorption inhibition even with less than 90-mg isoflavone a day for less than 3 months. Interestingly, Uesugi et al. (2002) found significant reduction of Pyd and Dpyd in less than a month, but Atkinson et al. (2004) could not find any significant effect in 1 year. In our analysis, duration of the study had a wide range of 4 weeks to 1 year which could not affect the results significantly. We evaluated the effect of phytoestrogen intake on serum level of BALP in six RCTs. Our results show no significant changes in BALP. In the selected trials, only two trials indicated significant increase in BALP after phytoestrogens consumption (Morabito et al. 2002; Atkinson et al. 2004), and the remainder could not find any valuable alteration in serum concentration of BALP (Uesugi et al. 2003; Arjmandi et al. 2003; Ye et al. 2006; Kreijkamp-Kaspers et al. 2004). Morabito et al. (2002) and Atkinson et al. (2004) examined the dose as 54 mg and 43.5 mg of phytoestrogens, respectively, in 1 year. Morabito et al. (2002) could see the significant results as soon as 6 months. In contrast to our results, Ma et al. (2008) reported significant changes in BALP after phytoestrogens consumption. Our former study on the effect of n-3 fatty acids on bone biomarkers resulted significant change in urine Pyd levels as soon as after 2 month n-3 fatty acid consumption (Salari et al. 2010). In the chosen articles, five studies were analyzed for OC. In only one study, we saw the significant increase in OC level (Morabito et al. 2002) by the dose of 54-mg phytoestrogens and as early as 6 months, and the rest of the studies did not result in this way (Uesugi et al. 2002; Ye et al. 2006; Zhang et al. 2007; Albertazzi et al. 2005). Meta-analysis of these five studies resulted in no significant change in serum OC levels. The power of the present meta-analysis is high because only three trials got Jadad score of three and the rest of them got more scores. In addition, the relatively high number of pooled participants supports the statistical power for identifying a small effect. The large heterogeneity between studies in the effect of phytoestrogens on bone biomarkers are originated from different doses of phytoestrogens intake, different duration of studies, and the quality of studies that may cause difference between pooled results and the results of some individual studies. Considering our results that indicate phytoestrogens can decrease bone resorption without affecting bone formation, there is another meta-analysis concluding no significant benefit of soy isoflavone on BMD at lumbar spine or hip in postmenopausal women (Liu et al. 2009). Therefore, phytoestrogens seems beneficial in decreasing bone resorption in postmenopausal women, and to date, no advantage on bone formation has been determined. Thus, a meta-analysis on the effect of phytoestrogens on BMD and more RCTs is suggested to verify more possible beneficial effect of phytoestrogens on bone.
Acknowledgment
This paper is the outcome of an in-house study and was not supported financially by any source.
References
- Abdollahi M, Larijani B, Rahimi R, Salari P. Role of oxidative stress in osteoporosis. Therapy. 2005;2(5):787–796. doi: 10.2217/14750708.2.5.787. [DOI] [Google Scholar]
- Albertazzi P, Steel SA, Bottazzi M. Effect of pure genistein on bone markers and hot flashes. Climacteric. 2005;8:371–379. doi: 10.1080/13697130500345257. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Ambrose WW, Garner SC. Biphasic effects of genistein on bone tissue in the ovariectomized, lactating rat model. Proc Soc Exp Biol Med. 1998;217:345–350. doi: 10.3181/00379727-217-44243. [DOI] [PubMed] [Google Scholar]
- Anonymous. Prevention and management of osteoporosis. A report of a WHO scientific group 2003. http://whqlibdoc.who.int/trs/WHO_TRS_921.pdf
- Arjmandi BH, Khalil DA, Smith BJ, et al. Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J Clin Endocrinol Metab. 2003;88(3):1048–1054. doi: 10.1210/jc.2002-020849. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2004;79:326–333. doi: 10.1093/ajcn/79.2.326. [DOI] [PubMed] [Google Scholar]
- Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc. 2003;62:877–887. doi: 10.1079/PNS2003309. [DOI] [PubMed] [Google Scholar]
- Choi EM, Suh KS, Kim YS, Choue RW, Koo SJ. Soybean ethanol extract increases the function of osteoblastic MC3T3-E1. Phytochemistry. 2001;56:733–739. doi: 10.1016/S0031-9422(00)00484-2. [DOI] [PubMed] [Google Scholar]
- Cook A, Pennington G. Phytoestrogen and multiple vitamin/mineral effects on bone mineral density in early postmenopausal women: a pilot study. J Women’s Health Gend Based Med. 2002;11(1):53–60. doi: 10.1089/152460902753473462. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Altavilla D, Squadrito G, et al. Effects of the phytoestrogen genistein on the circulating soluble receptor activator of nuclear factor κB ligand-osteoprotegerin system in early postmenopausal women. J Clin Endocrinol Metab. 2004;89:188–192. doi: 10.1210/jc.2003-030891. [DOI] [PubMed] [Google Scholar]
- Dalais FS, Ebeling PR, Kotsopoulos D, McGrath BP, Teede HJ. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin Endocrinol. 2003;58(6):704–709. doi: 10.1046/j.1365-2265.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- Devareddy L, Khalil DA, Smith BJ, et al. Soy moderately improves microstructural properties without affecting bone mass in an ovariectomized rat model of osteoporosis. Bone. 2006;38:686–693. doi: 10.1016/j.bone.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Evans EM, Racette SB, Pelt RE, Peterson LR, Villareal DT. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause. 2007;14(3 Pt 1):481–488. doi: 10.1097/01.gme.0000243570.78570.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JR, Chesnut CH, 3rd, Baylink DJ. Improved method for quantitative determination in serum of alkaline phosphatase of skeletal origin. Clin Chem. 1981;27:2002–2007. [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z, Boucek RJ. Collagen cross-linking compounds in human urine. Biochem J. 1981;197:759–762. doi: 10.1042/bj1970759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness LS, Fiedler K, Sehgal AR, Oravec D, Lerner E. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J Womens Health. 2004;13(9):1000–1007. doi: 10.1089/jwh.2004.13.1000. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Okamoto Y, Miyazaki K, Uesugi T. Evaluation of a soybean product Fujiflavone P40 as an antiosteoporotic agent in rats. Phytother Res. 2003;17:112–119. doi: 10.1002/ptr.1047. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Goodman-Gruen DL. Usual dietary isoflavone intake, bone mineral density, and bone metabolism in postmenopausal women. J Women’s Health Gend Based Med. 2002;11(1):69–78. doi: 10.1089/152460902753473480. [DOI] [PubMed] [Google Scholar]
- Liu J, Ho SC, Su Y, Chen W, Zhang C, Chen Y. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone. 2009;44:948–953. doi: 10.1016/j.bone.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2008;62:155–161. doi: 10.1038/sj.ejcn.1602748. [DOI] [PubMed] [Google Scholar]
- Middleton ET, Steel SA. The effects of short-term hormone replacement therapy on long-term bone mineral density. Climacteric. 2007;10:257–263. doi: 10.1080/13697130701370435. [DOI] [PubMed] [Google Scholar]
- Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17(10):1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- Picherit C, Bennetau-Pelissero C, Chanteranne B, et al. Soybean isoflavones dose-dependently reduce bone turnover but do not reverse established osteopenia in adult ovariectomized rats. J Nutr. 2001;131:723–728. doi: 10.1093/jn/131.3.723. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., 3rd Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res. 2002;17:11–14. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, Writing Group for the Women’s Health Initiative Investigators et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Salari P, Abdollahi M. Controversial effects of non-steroidal anti-inflammatory drugs on bone: a review. Inflamm Allergy Drug Targets. 2009;8(3):169–175. doi: 10.2174/187152809788681065. [DOI] [PubMed] [Google Scholar]
- Salari P, Larijani B, Abdollahi M. Association of hyperhomocysteinemia with osteoporosis: a systematic review. Therapy. 2008;5(2):215–222. doi: 10.2217/14750708.5.2.215. [DOI] [Google Scholar]
- Salari P, Rezaie A, Larijani B, Abdollahi M. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med Sci Monit. 2008;14(3):RA37–RA44. [PubMed] [Google Scholar]
- Salari P, Asalforoush M, Ameri F, Larijani B, Abdollahi M. The effect of n-3 fatty acids on bone biomarkers in Iranian postmenopausal osteoporotic women: a randomized clinical trial. Age. 2010 doi: 10.1007/s11357-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Vliet EL, Lewiecki EM, et al. Clinical use of serum and urine bone markers in the management of osteoporosis. Curr Med Res Opin. 2005;21:1015–1026. doi: 10.1185/030079905X49635. [DOI] [PubMed] [Google Scholar]
- Suh KS, Koh G, Park CY, et al. Soybean isoflavones inhibit tumor necrosis factor-α-induced apoptosis and the production of interleukin-6 and prostaglandin E2 in osteoblastic cells. Phytochemistry. 2003;63:209–215. doi: 10.1016/S0031-9422(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: a four-week study. J Am Coll Nutr. 2002;21(2):97–102. doi: 10.1080/07315724.2002.10719200. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Toda T, Okuhira T, Chen JT. Evidence of estrogenic effect by the three-month-intervention of isoflavone on vaginal maturation and bone metabolism in early postmenopausal women. Endocr J. 2003;50(5):613–619. doi: 10.1507/endocrj.50.613. [DOI] [PubMed] [Google Scholar]
- Wangen KE, Duncan AM, Merz-Demlow BE, et al. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85(9):3043–3048. doi: 10.1210/jc.85.9.3043. [DOI] [PubMed] [Google Scholar]
- Weaver CM, Martin BR, Jackson GS, et al. Antiresorptive effects of phytoestrogen supplements compared with estradiol or residronate in postmenopausal women using 41Ca methodology. J Clin Endocrinol Metab. 2009;94:3798–3805. doi: 10.1210/jc.2009-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori Y, Moriguchi EH, Teramoto T, et al. Soybean isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: a ten-week study. J Am Coll Nutr. 2002;21(6):560–563. doi: 10.1080/07315724.2002.10719255. [DOI] [PubMed] [Google Scholar]
- Ye YB, Tang XY, Verbruggen MA, Su YX. Soy isoflavones attenuate bone loss in early postmenopausal Chinese women. A single-blind randomized. Placebo-controlled trial. Eur J Nutr. 2006;45:327–334. doi: 10.1007/s00394-006-0602-2. [DOI] [PubMed] [Google Scholar]
- Zhang G, Qin L, Shi Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J Bone Miner Res. 2007;22(7):1072–1079. doi: 10.1359/jbmr.070405. [DOI] [PubMed] [Google Scholar]