Abstract
Clinical data from 72 dog breeds of varying size and life expectancy were grouped according to breed body mass and tested for prevalence at ages 4 to 5, ages 7 to 10, and lifetime incidence of non-hereditary, age-related cataract (ARC). The incidence of ARC was found to be directly related to the relative life expectancies in the breed groups: The smallest dog breeds had a lower ARC prevalence between ages 4 and 5 than mid-size breeds and these, in turn, a lower prevalence than the giant breeds. A similar sequence was evident for ages 7 to 10 and for overall lifetime incidence of ARC. These differences became more significant when comparing small and giant breeds only. We could also confirm the inverse relationship between body size and life expectancy in these same sets of dog breeds. Our results show that body size, life expectancy, and ARC incidence are interrelated in dogs. Given that ARC has been shown to be at least partially caused by oxidative damage to lens epithelial cells and the internal lens, we suggest that it can be considered not only as a general biomarker for life expectancy in the canine and possibly other species, but also for the systemic damages produced by reactive oxygen species. This suggests new approaches to examine the gene expression pathways affecting the above-noted linkages.
Keywords: Dog, Age-related cataract, Breeds, Size, Life span
Introduction
One of the fascinating phenomena concerning life expectancy among several vertebrate and non-vertebrate species is that body size is positively correlated to longevity in comparisons made between species (Austad 2005; van Heemst et al. 2005; Galis et al. 2007), while this relationship seems to be inverse within individual species. The latter has been reported for mice (Miller et al. 2000; Bartke and Brown-Borg 2004; Austad 2005), horses (Brosnahan and Paradis 2003), humans (Samaras and Storms 1992; van Heemst et al. 2005), and dogs (Austad 2005; Galis et al. 2007; Greer et al. 2007).
It would be useful to identify non-invasive biomarkers of aging that relate to this phenomenon, but none have been reliably established thus far. In this context, age-related cataract (ARC) is a pathological lesion that occurs with aging in humans, other mammals, and birds (Slatter et al. 1983; Williams et al. 2004; Wolf et al. 2005; Zubenko et al. 2007) and is at least partly linked to lens oxidative damage (Davies 1995; Spector 1995; Reddy et al. 2001; Wolf et al. 2005; Pendergrass et al. 2006; Brennan and Kantorow 2009). We previously have shown a correlation between longevity in human nonagenarians and their delay in developing ARC (Zubenko et al. 2007), and also demonstrated that small GH-R, IGF-1 deficient mutant dwarf mice have both delayed ARC development and extended life spans (Wolf et al. 2005). This suggests that ARC has a connection to systemically acting factors controlling the rate of growth and scale of body size, which produce life-limiting events and also affect lens maintenance and lens health.
Given its wide variety in body size, mass, and life expectancy between different breeds, as well as the availability of large amounts of veterinary data, the domestic dog suggested itself as a useful model to test for correlations between body size, life expectancy, and the incidence and prevalence of ARC in order to examine whether ARC can be considered a biomarker of aging in this species, which could be useful for future studies of the underlying mechanisms.
Dogs, materials, and methods
Data on cataract status were based on veterinary eye exam results over a 10-year-period between 1996 and 2005 that were supplied by the Canine Eye Registry Foundation (CERF; Anonymous 1996–2005). The results had been established using ophthalmic examination including slit lamp examination of the lens by board-certified veterinary ophthalmologists, as required by the CERF database. Age groups included dogs aged 4 to 5, 5 to 6, 6 to 7, 7 to 8, 8 to 9, 9 to 10, and over 10 years. Cataracts that had been identified as hereditary at the time of examination were excluded from the data. We excluded breeds that did not have at least 100 individual exam entries for the lifetime studies and at least 25 entries for ages 4 to 5 in the database (n = 102) from the calculation in order to avoid skewing incidence percentages due to statistical fluctuation. Dogs younger than 4 years were also excluded to guard against the inclusion of congenital and juvenile hereditary cataracts (Ackermann 1999). Given that some sources only use dogs above the age of 7 to safeguard against hereditary cataracts (Gelatt and Mackay 2005), cataracts occurring between ages 7 and 10 were also incorporated into the analyses as a separate variable. There was no information about gender in the data. At the time of analysis, data consisted of n = 101,811 individual veterinary eye exams performed at various ages on dogs representing n = 72 breeds. Each examined dog was assigned a number. No individuals are entered into the database more than once.
Cataract incidence per breed was provided as the number of dogs diagnosed with non-hereditary cataracts in relation to the overall number of dogs that had been examined in each age group. Data on average breed mass (in pounds) were taken from The Encyclopedia of Dog Breeds (Coile 2005) entries for male dogs and merged with the cataract data provided by CERF described above.
Average life expectancies per breed were derived from previous studies (Coile 2005; Greer et al. 2007). Furthermore, we also considered the percentage of dogs examined over age 10 in relation to the total number of dogs examined per breed as a measure of life expectancy and used it in the analysis (see Tables 2, 4, 5 and 6).
Table 2.
Life expectancy and percentages of dogs examined over age 10 in relation to the total number of dogs examined per breed as a function of size groups
| Group | Life Expectancy ± SD | % Seen over 10 years ± SD |
|---|---|---|
| Small | 13.78 ± 1.44 | 8.36 ± 5.12 |
| 40 to 60 lb | 12.81 ± 0.83 | 5.04 ± 1.91 |
| 65 to 85 lb | 11.70 ± 1.10 | 4.03 ± 1.64 |
| Giant | 9.09 ± 1.74 | 3.13 ± 1.63 |
Table 4.
Generalized Linear Model (Type III Sum of Squares) showing the influence of the variables beneath on size class
| Variable | F value | P value |
|---|---|---|
| % Dogs seen >10 years | 22.75 | <0.0001 |
| Incidence 4 to 5 years | 3.83 | 0.0544 |
| Lifetime Incidence | 0.12 | 0.7326 |
There are three degrees of freedom. Coefficient of determination (R2) = 0.35, PModel < 0.0001
Table 5.
Four Generalized Linear Models (type III Sum of Squares) for variables explaining size class
| F | P | F | P | F | P | F | P | |
|---|---|---|---|---|---|---|---|---|
| LE | – | – | – | – | 81.11 | <0.0001 | 83.20 | <0.0001 |
| % >10 | 22.94 | <0.0001 | 22.62 | <0.0001 | – | – | – | – |
| InLT | – | – | 4.71 | 0.0335 | – | – | 3.84 | 0.0541 |
| In4–5 | 8.73 | 0.0043 | – | – | 7.97 | 0.0063 | – | – |
| PModel | R2 | PModel | R2 | PModel | R2 | PModel | R2 | |
| <0.0001 | 0.3442 | <0.0001 | 0.3084 | <0.0001 | 0.6224 | <0.0001 | 0.6000 |
There are three degrees of freedom
R2 coefficient of determination, LE life expectancy, % >10 percentage of dogs examined over age 10, InLT lifetime cataract incidence, In4–5 Cataract incidence between ages 4 and 5
Table 6.
Generalized Linear Model (type III Sum of Squares) for variables explaining size class, now incorporating cataract incidences between ages 7 and 10, as well as for age 7 and above
| Variable | F Value | P Value |
|---|---|---|
| % Dogs seen >10 years | 22.65 | <0.0001 |
| Incidence 4 to 5 years | 0.02 | 0.8751 |
| Incidence 7 to 10 years | 3.22 | 0.0773 |
| Incidence >7 years | 8.45 | 0.0050 |
| Lifetime Incidence | 12.62 | 0.0007 |
There are five degrees of freedom. Coefficient of determination (R2) = 0.45, PModel <0.0001
Body size groups were defined as small (less than 20 lb, n = 17 breeds), lower medium (40 to 60 lb, n = 21), upper medium (65 to 85 lb, n = 20), and giant (90 lb and over, n = 11). Lower and upper medium groups were merged for some of our analyses (see the “Results” section). No breeds weighing between 20 and 40 lb were available for analysis in sufficient numbers. Body mass was chosen as a surrogate for body size because it has been shown to be more closely related to life span than height at shoulder, a measure commonly used for stature (Greer et al. 2007).
Data were stored and managed in Microsoft Excel® 2007, which was also used to create bar graphs and scatter plots. Merging and statistical analysis were performed using The SAS System® version 8.02. Linear regression was carried out using PROC GLM, with types I and III Sum of Squares according to the data distribution. Tests for normality were carried out in PROC UNIVARIATE using Kolmogorov–Smirnov values. All other statistical tests were carried out using PROC NPAR1WAY. A P = 0.05 was considered statistically significant.
Results
Population structure
Our study population consisted of n = 102,235 dogs from n = 72 breeds. There were 20 breeds weighing less than 20 lb, totaling 15,482 dogs; 21 breeds weighing 40 to 60 lb, totaling 32,110 dogs; 20 breeds weighing 65 to 85 lb, totaling 49,727 dogs; and 11 breeds weighing over 90 lb, totaling 4,916 dogs. Table 1 shows the 72 breeds included in this study and indicates size groupings, life expectancy, cataract incidence between ages 4 and 5, cataract incidence between ages 7 and 10, lifetime cataract incidence, and the percentage of dogs examined at ages over 10 in relation to all dogs examined.
Table 1.
Dog breeds used for this study, divided into three and four body mass classes, with mean life expectancy, percentage of dogs diagnosed with cataract between ages 4 and 5 in relation to all dogs examined at this age, percentage of dogs diagnosed with cataract between ages 7 and 10 in relation to all dogs examined at this age, percentage of dogs diagnosed with cataract at age 7 and above in relation to all dogs examined at this age, percentage of dogs diagnosed with cataracts throughout life in relation to all dogs examined, and percentage of dogs examined over age 10 in relation to all dogs examined
| Breed | Mass Cl. (4) | Mass Cl. (3) | Mean Life Expectancy | Cataract 4–5 (%) | Cataract 7–10 (%) | Cataract over 7 (%) | Lifelong Cataract (%) | % Seen Over 10 |
|---|---|---|---|---|---|---|---|---|
| Alaskan Malamute | 65–85 lb | Medium | 12 | 4.54 | 13.45 | 13.00 | 6.28 | 2.76 |
| American Water Spaniel | 40–60 lb | Medium | 12 | 5.00 | 5.88 | 5.00 | 4.96 | 2.48 |
| Australian Cattle Dog | 40–60 lb | Medium | 13.5 | 5.50 | 16.67 | 18.61 | 10.38 | 5.63 |
| Australian Shepherd | 40–60 lb | Medium | 13.5 | 3.47 | 9.64 | 10.55 | 6.36 | 3.20 |
| Australian Terrier | Small | Small | 13 | 5.13 | 9.52 | 9.38 | 4.46 | 9.82 |
| Bearded Collie | 40–60 lb | Medium | 13 | 14.54 | 19.00 | 18.18 | 15.80 | 5.21 |
| Belgian Malinois | 65–85 lb | Medium | 11 | 2.50 | 6.67 | 10.13 | 5.65 | 5.65 |
| Belgian Sheepdog | 40–60 lb | Medium | 12 | 3.01 | 9.09 | 9.91 | 6.22 | 6.45 |
| Belgian Tervueren | 40–60 lb | Medium | 12 | 4.26 | 18.73 | 20.72 | 9.92 | 6.04 |
| Bernese Mountain Dog | Giant | Giant | 7 | 8.55 | 23.03 | 20.93 | 13.41 | 2.40 |
| Bichon Frisé | Small | Small | 13.5 | 5.50 | 17.51 | 18.79 | 10.67 | 5.58 |
| Border Collie | 40–60 lb | Medium | 13.5 | 6.38 | 13.00 | 14.74 | 9.88 | 4.69 |
| Borzoi | 65–85 lb | Medium | 13 | 3.08 | 10.31 | 9.92 | 5.34 | 4.27 |
| Bouvier des Flandres | 65–85 lb | Medium | 11 | 8.11 | 19.28 | 19.64 | 13.40 | 4.74 |
| Boxer | 65–85 lb | Medium | 11 | 2.11 | 8.70 | 5.56 | 3.16 | 6.84 |
| Briard | 65–85 lb | Medium | 11.5 | 2.17 | 14.49 | 12.22 | 4.97 | 5.80 |
| Bullmastiff | Giant | Giant | 8 | 3.85 | 33.33 | 16.67 | 7.02 | 5.26 |
| Cairn Terrier | Small | Small | 13.5 | 1.91 | 6.52 | 10.70 | 7.13 | 13.73 |
| Chesapeake Bay Retriever | 65–85 lb | Medium | 11 | 4.91 | 12.33 | 13.66 | 7.18 | 2.73 |
| Chinese Crested | Small | Small | 11 | 0.77 | 9.47 | 10.74 | 4.45 | 4.13 |
| Collie (Rough) | 40–60 lb | Medium | 14 | 2.91 | 5.29 | 6.55 | 4.14 | 6.26 |
| Curly Coated Retriever | 65–85 lb | Medium | 10 | 2.56 | 13.85 | 15.12 | 6.55 | 6.25 |
| Doberman Pinscher | 65–85 lb | Medium | 10 | 5.99 | 12.94 | 12.62 | 8.24 | 2.97 |
| English Mastiff | Giant | Giant | 8 | 14.53 | 30.51 | 26.47 | 17.30 | 1.42 |
| English Setter | 40–60 lb | Medium | 12 | 3.57 | 9.09 | 8.89 | 7.32 | 7.32 |
| English Springer Spaniel | 40–60 lb | Medium | 13 | 3.71 | 12.01 | 13.53 | 7.23 | 5.93 |
| English Toy Spaniel | Small | Small | 11 | 4.17 | 5.88 | 4.76 | 4.29 | 5.71 |
| Field Spaniel | 40–60 lb | Medium | 11 | 3.49 | 25.93 | 30.00 | 16.30 | 1.63 |
| Flat-Coated Retriever | 65–85 lb | Medium | 12 | 10.24 | 35.98 | 35.05 | 17.58 | 1.93 |
| German Shepherd | 65–85 lb | Medium | 11.5 | 5.91 | 12.82 | 13.16 | 7.41 | 5.77 |
| German Shorthaired Pointer | 40–60 lb | Medium | 13 | 7.76 | 16.33 | 17.74 | 10.24 | 4.44 |
| Giant Schnauzer | 65–85 lb | Medium | 13.5 | 12.90 | 16.67 | 12.50 | 14.58 | 4.17 |
| Golden Retriever | 65–85 lb | Medium | 11 | 5.83 | 20.31 | 20.29 | 9.73 | 2.42 |
| Gordon Setter | 65–85 lb | Medium | 11 | 2.36 | 2.33 | 1.54 | 2.99 | 6.59 |
| Great Dane | Giant | Giant | 8.5 | 6.02 | 2.70 | 4.26 | 5.90 | 2.95 |
| Great Pyrenees | Giant | Giant | 11 | 8.62 | 14.29 | 10.53 | 9.46 | 6.76 |
| Greater Swiss Mountain Dog | Giant | Giant | 11 | 16.98 | 40.00 | 31.25 | 24.49 | 3.06 |
| Greyhound | 65–85 lb | Medium | 13 | 4.76 | 7.41 | 12.90 | 7.63 | 3.05 |
| Havanese | Small | Small | 14.5 | 7.41 | 27.20 | 27.10 | 11.71 | 1.91 |
| Irish Setter | 65–85 lb | Medium | 13.5 | 5.97 | 15.38 | 16.67 | 11.56 | 3.06 |
| Irish Wolfhound | Giant | Giant | 7 | 5.56 | 7.69 | 6.25 | 8.64 | 1.85 |
| Italian Spinone | 65–85 lb | Medium | 13 | 4.00 | 23.08 | 21.05 | 6.70 | 3.35 |
| Jack Russell Terrier | Small | Small | 15 | 4.11 | 9.73 | 10.66 | 6.36 | 3.88 |
| Keeshond | 40–60 lb | Medium | 14 | 9.29 | 13.24 | 17.44 | 10.05 | 4.89 |
| Kuvasz | Giant | Giant | 12 | 2.22 | 0.00 | 0.00 | 3.36 | 2.52 |
| Labrador Retriever | 65–85 lb | Medium | 13 | 6.67 | 21.13 | 21.91 | 11.28 | 2.15 |
| Leonberger | Giant | Giant | 8 | 14.81 | 41.67 | 40.00 | 17.54 | 2.63 |
| Lhasa Apso | Small | Small | 16.5 | 4.26 | 11.76 | 9.88 | 8.85 | 15.63 |
| Löwchen | Small | Small | 13 | 3.49 | 18.75 | 17.95 | 6.76 | 3.15 |
| Miniature Dachshund | Small | Small | 13.5 | 6.25 | 18.92 | 21.43 | 8.70 | 8.26 |
| Miniature Poodle | Small | Small | 13.5 | 3.85 | 12.00 | 15.07 | 8.05 | 6.05 |
| Miniature Schnauzer | Small | Small | 15 | 3.26 | 11.63 | 12.45 | 5.98 | 6.06 |
| Newfoundland | Giant | Giant | 9 | 4.63 | 19.35 | 20.00 | 8.33 | 3.95 |
| Norwegian Elkhound | 40–60 lb | Medium | 12 | 4.67 | 15.63 | 13.41 | 7.98 | 4.79 |
| Norwich Terrier | Small | Small | 13.5 | 0.00 | 2.56 | 10.00 | 4.29 | 9.01 |
| Old English Sheepdog | 65–85 lb | Medium | 11 | 8.10 | 22.22 | 21.62 | 13.98 | 4.24 |
| Papillon | Small | Small | 16 | 1.85 | 11.33 | 13.46 | 5.71 | 6.05 |
| Pointer | 40–60 lb | Medium | 13.5 | 10.00 | 0.00 | 0.00 | 9.23 | 9.23 |
| Portugese Water Dog | 40–60 lb | Medium | 12 | 4.58 | 25.29 | 27.61 | 12.49 | 5.36 |
| Pug | Small | Small | 13.5 | 0.00 | 0.00 | 0.00 | 2.31 | 5.78 |
| Rottweiler | Giant | Giant | 10.5 | 8.09 | 15.63 | 15.33 | 10.05 | 1.60 |
| Saluki | 40–60 lb | Medium | 12 | 0.00 | 0.00 | 6.67 | 8.77 | 7.02 |
| Samoyed | 40–60 lb | Medium | 13.5 | 3.69 | 19.20 | 18.86 | 7.96 | 4.57 |
| Schipperke | Small | Small | 15 | 5.63 | 10.53 | 17.14 | 8.76 | 5.99 |
| Scottish Terrier | Small | Small | 12 | 3.57 | 3.85 | 11.90 | 7.69 | 15.38 |
| Siberian Husky | 40–60 lb | Medium | 13 | 2.04 | 5.39 | 6.09 | 3.30 | 2.40 |
| Silky Terrier | Small | Small | 14 | 15.38 | 13.33 | 16.00 | 14.81 | 18.52 |
| Tibetan Spaniel | Small | Small | 13.5 | 3.16 | 5.56 | 5.43 | 4.18 | 4.40 |
| Viszla | 40–60 lb | Medium | 13.5 | 3.00 | 6.56 | 8.75 | 4.97 | 6.29 |
| Weimaraner | 65–85 lb | Medium | 11 | 4.23 | 11.11 | 10.26 | 4.94 | 1.85 |
| Welsh Springer Spaniel | 40–60 lb | Medium | 13 | 4.17 | 20.00 | 22.86 | 10.00 | 1.92 |
| Yorkshire Terrier | Small | Small | 15 | 0.00 | 14.29 | 14.00 | 7.44 | 18.18 |
Lifetime cataract incidence
No significant differences in cataract incidence between lower and upper medium size dogs could be found when their whole life span was considered (P = 0.5958, Wilcoxon 2-sided z; P = 0.2979, Wilcoxon 1-sided z). These two groups were therefore merged into one single category for analysis. The analyzed data were thus divided into the three categories “small” (under 20 lb), “medium” (40 to 85 lb), and “giant” (over 90 lb).
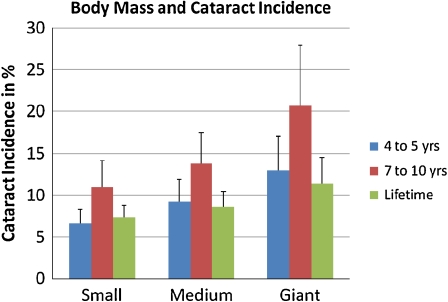
Lifetime cataract incidence in the total studied population was 9.07 ± 5.69%. Cataract incidences in the three groups were 6.59 ± 3.53% in small dogs, 9.23 ± 5.29% in medium-sized dogs, and 12.99 ± 8.08% in giant dogs. Figure 1 shows the results for these groupings for ages 4 to 5.
Fig. 1.
Body mass and cataract incidence between ages 4 and 5, ages 7 to 10, and during overall life span for small, medium, and giant dog breeds. Error bars indicate standard error
Lifetime cataract incidence was not normally distributed in small and medium dogs and borderline in giant dogs. When comparing all three size groups, there was a significant influence of size group on lifelong cataract incidence, which increased as dog mass increased (χ2 = 8.7468, P = 0.0126, Kruskal–Wallis test). When comparing only small and giant breeds, this effect became considerably more significant (P = 0.0032, Wilcoxon rank sum test).
Cataract incidence between ages 4 and 5
No significant differences in cataract incidence between lower and upper medium size dogs between ages 4 and 5 could be found (P = 0.5573, Wilcoxon 2-sided z; P = 0.2787, Wilcoxon 1-sided z). These two groups were therefore merged into one single category for analysis as above.
Cataract incidence during the fourth to fifth year of life was 8.68 ± 4.12% in the overall studied population. Cataract incidences in the three groups were 7.33 ± 2.91% in small dogs, 8.60 ± 3.67% in medium-sized dogs, and 11.41 ± 6.20% in giant dogs. This is also shown in Fig. 1.
Cataract incidence during years 4 to 5 was normally distributed in all three groups. When comparing all three groups, there was a significant influence of group on cataract incidence during years 4 to 5, which increased as dog mass increased (P = 0.0286, ANOVA). When comparing only small and giant breeds, this effect became more significant (P = 0.0185, ANOVA).
Cataract incidence between ages 7 and 10
No significant difference in cataract incidence between lower and upper medium size dogs between ages 7 and 10 could be found (P = 0.3612, Wilcoxon 2-sided z; P = 0.1806, Wilcoxon 1-sided z). These two groups were therefore merged into one single category for analysis as above.
Cataract incidence during the seventh to tenth year of life was 14.10 ± 8.96% in the overall studied population. Cataract incidences in the three groups were 11.02 ± 6.37% in small dogs, 13.81 ± 7.38% in medium-sized dogs and 20.75 ± 14.37% in giant dogs. This is also shown in Fig. 1.
Cataract incidence during years 7 to 10 was normally distributed in all three groups. When comparing all three groups, there was a significant influence of size group on cataract incidence between ages 7 and 10, which increased as dog mass increased (P = 0.0125, ANOVA). When comparing only small and giant breeds, this effect stayed roughly as significant as before (P = 0.0138, ANOVA).
Life expectancy
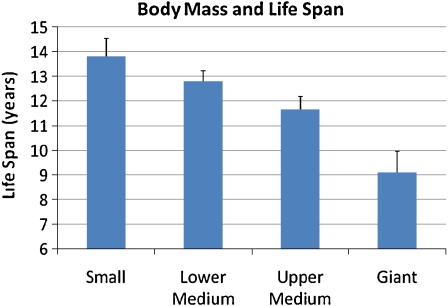
Life expectancies between the two medium-size classes were significantly different (P = 0.0013, Wilcoxon 2-sided z). Therefore, these groups could not be merged for the analysis of life expectancy, which was conducted using four size groups. Differences in life expectancy between the four groups were highly significant (P < 0.0001, Kruskal–Wallis χ2 = 39.46). Results are provided in Table 2 and Fig. 2.
Fig. 2.
Mean life span (in years) between the different body mass groups. Error bars indicate standard error
The percentages of dogs examined over age 10 in relation to the overall number of examined dogs per breed were also significantly different between the two medium-sized groups, which allowed them to be tested separately. Differences between all four groups were also highly significant for this measure (P = 0.0004, Kruskal–Wallis χ2 = 18.46). These results are also provided in Table 2.
Generalized linear models
Having established both the correlation between cataract incidence and breed size and between breed size and life expectancy, several Generalized Linear Models (type III Sum of Squares) were constructed to examine the interaction between these variables.
We initially designed two models: The first one was set up to show how much of the difference between size classes was explained by life expectancy and cataract incidence at different ages. The second one was set up similarly, but replaced life expectancy with the percentage of dogs examined over age 10 in relation to the total number of dogs examined per breed. Given that there were significant differences in both life expectancy and the percentage of dogs examined over age 10 between the two medium size groups, these were kept separate for both analyses, leading to four size classes in the overall models (Tables 3 and 4).
Table 3.
Generalized Linear Model (Type III Sum of Squares) showing the influence of the variables beneath on size class
| Variable | F Value | P value |
|---|---|---|
| Life Expectancy | 80.19 | <0.0001 |
| Incidence 4 to 5 year | 4.05 | 0.0484 |
| Lifetime Incidence | 0.19 | 0.6615 |
There are four degrees of freedom. Coefficient of determination (R2) = 0.56, PModel < 0.0001
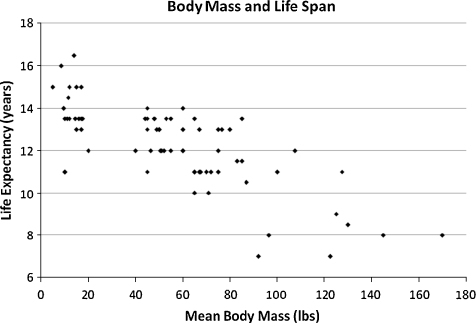
Given the results of these two models, we determined that there were correlations between both body mass and cataract incidence and life span and cataract incidence. In order to examine the interactions between all three of these variables, four additional models considering either life expectancy or percentage of dogs seen over age 10 and either cataract incidence between ages 4 and 5 or lifetime cataract incidence were constructed to further examine the interaction of these variables. Their results are rendered in Table 5. A scatter plot of life expectancy versus body mass is provided in Fig. 3.
Fig. 3.
Scatter plot of mean body mass in pounds and life expectancy in the 72 studied breeds
As evident in Table 5, life expectancy and percentage of dogs examined over age 10 are solid predictors of size class in all variants, even though the percentage of dogs seen over age 10 explains less variability than life expectancy, as expressed in the coefficients of determination (R2). This comparison also shows that cataract incidence between ages 4 and 5 is a more significant predictor of size class than lifetime cataract incidence. It is noted that the former included more dogs, as expected due to late life deaths in the latter list.
Given the previously cited concerns that dogs aged 4 to 5 may still be subject to a significant number of inherited cataracts (Gelatt and Mackay 2005), we also incorporated cataract incidence between ages 7 and 10 into a Generalized Linear Model, which resulted in the following:
As can be seen in Table 6, when considering dogs that are diagnosed with cataract above age 7, cataract incidence above age 7 and lifetime incidence (ages 4 and above) are better predictors of size class than cataract incidence between ages 4 and 5, while cataract incidence between ages 7 and 10 is borderline significant as a predictor of size class.
Discussion
Our results offer evidence as to the interrelationships of body mass, mean survival time, and the prevalence of non-hereditary canine cataract, with cataract proposed as a body size related biomarker of aging. To the best of our knowledge, this interaction has not been reported before: The prevalence of cataracts among breeds, but without reference to body size, has been reported previously (Gelatt and Mackay 2005). The giant breeds that we have included here were not present in this data set and the comparisons that we show here were not made. A report by Patronek et al. (Patronek et al. 1997) on breed body mass and longevity is extensive and includes two breeds of giant size dogs but did not report on cataracts. It is based on survival statistics taken from veterinary hospitals and lists the median age at death for all pure breed dogs as 6.7 years, which seems excessively low when compared to our data. Their report thus gives a short mean life span in a list of all of the breeds covered and this may be because of the source material used, with the listing skewed by dogs dying early. Williams et al. (2004) have reported a difference between three small and three medium size breeds in cataract prevalence with a higher percent of cataract in the larger breeds in this set of six entries, and they also noted an expected increase of cataracts with age in 2,000 individual dogs but without reference to their breed or size.
The present results clearly show that there are significant correlations between body size and related life span, the overall cataract prevalence at middle age, and its incidence throughout the full length of life, although the latter effect is less strong. However, CERF records indicate that fewer dogs are presented for eye exams with increasing dog ages, and the mean life span for the giant dogs in several breeds is approximately 7 years, thus changing their statistical and health profiles in this and later years. This could explain why life expectancy values taken from a previously published study (Greer et al. 2007) explained size variance in our study considerably better than did the percentage of dogs examined over age 10 in relation to the total number of dogs examined per breed.
While there were several outliers among the breeds in each of these size groups (Table 1), the comparisons between groupings showed robust statistically significant differences. The underlying importance of breed size for length of life span was also confirmed and extended. However, these several variances for ARC prevalence among the individual breeds in groupings of similar size (Table 1) indicate that mean breed body mass alone is not the sole factor affecting the cataract differences, but that individual genetic, epigenetic, and possibly environmental factors are also involved. These individual variances can nevertheless be overcome for study purposes when a large number of breeds are included in the survey, as seen here.
There is some controversy concerning the age after which a cataract can be considered age-related rather than hereditary in the domestic dog. Our results show that when examining a sample of cataract cases that occurred between ages 4 and 5 and were classified by board-certified veterinary ophthalmologists as non-hereditary, a clear correlation between cataract incidence, life expectancy, and body mass can be established (Table 5). However, this correlation is more significant when considering cataract incidence between ages 7 and 10 and body mass than when considering cataract incidence between ages 4 and 5 and body mass. It would therefore seem that cataract incidence between ages 4 and 5 is still influenced by a residual hereditary effect, although this effect was not strong enough to lead to non-significant P values in our case. This interpretation is further supported when we analyze these variables simultaneously using multiple linear regression (Table 6), which shows that cataract incidence in the sample of older dogs is a much more powerful predictor of body mass than cataract incidence in the sample of younger dogs when they are incorporated into the same model. Given that we are not aware of any studies linking hereditary cataracts to breed body mass, this indicates that there is some residual influence of hereditary cataracts in dogs aged 4 to 5, even when these cataracts have been classified as non-hereditary by veterinary ophthalmologists.
Age-related cataract is characterized by lens protein aggregation and is associated with an abnormal migration of lens epithelial cells, failure to eliminate their internal organelles in the progressive differentiation of lens surface cells to internal lens fiber cells, and the presence internally in the lens of large amounts of reactive oxygen species (ROS) in mice and rats (Babizhayev et al. 1992; Reddy et al. 2001; Wolf et al. 2005; Pendergrass et al. 2006; Greer et al. 2007). In our preliminary unpublished studies, these events also appear to occur in age-related cataracts in dogs and humans.
In our present study of a large dog population and within the limitations incurred by an individual breed’s and an individual animal’s overall genetic makeup, as well as environmental differences, it is seen that the timing and lifelong prevalence of ARC represents an informative biomarker that coexists with systemic age-related events that affect life span. These events include the accumulation of cellular damage, primarily of oxidative nature, and other occurrences that may affect both ARC and life span among dog breeds of differing sizes. The animals’ respective growth rates and body sizes suggest that the IGF pathway may be involved (Bartke et al. 2003; Tryfonidou et al. 2003). We note that a listing of the terminal diseases found in relationship to small and large dog sizes can be found in Deeb and Wolf (1994), and also with emphasis on cancer (Bronson 1982), and especially for diseases of large dogs (Lauten 2006).
The IGF relationship may be indirect, i.e., proceed through additional pathways, but it has been found that mice deficient in IGF-1 are not only smaller, but live longer and have a significantly reduced prevalence of cataracts when examined in late midlife (Bartke and Brown-Borg 2004; Wolf et al. 2005). It also has been shown in the mouse that receptors for IGF-1 and insulin are both present in the lens (Xie et al. 2007). A claim concerning the IGF-1 pathway input on life span has been made for humans (Samaras and Storms 1992), while Eigenmann has connected circulating IGF-1 levels with breed size (Eigenmann et al. 1988).
Our cataract studies reported here relate ARC timing with size and life expectancy in dogs, but without accompanying measurements of IGF-1 or its binding proteins and appropriate receptors, thus our proposals concerning IGF1 involvement are speculative. We suggest that the rate of growth during birth to adult size may be important for subsequent life span, as is also proposed by others (Miller et al. 2000; Samaras et al. 2003), and note that the initial birth weight of small versus large or giant dogs is not greatly different, requiring a greater turnover of cells during the period of more rapid body growth in the larger breeds (Tryfonidou et al. 2003).
The relationship between IGF1, the insulin receptor, and ROS presence causing cell and DNA oxidative damage is at present a subject of conflicting findings (Davies 1995; Bartke et al. 2003; Sanz et al. 2005; Speakman 2005; Papaconstantinou 2009). There is evidence for these life span-related events in rapidly growing animals (Deeb and Wolf 1994; Miller et al. 2002; Coile 2005; Galis et al. 2007; Samaras 2009).
In conclusion, our results show for the first time that the time of appearance of a specific observable aging-related lesion, canine ARC, serves as a biomarker of aging and that its incidence correlates directly with breed size and inversely with breed life span. ARC itself is a general biomarker for expected life span in the domestic dog, and both ARC and life span relate to body size when a large number of breeds (72) and individual animals (over 100,000) are considered in a retrospective study of eye examinations over a life span of 10+ years. Significantly, IGF-1 and its receptor alleles have been linked both directly and indirectly to cataract incidence (Wolf et al. 2005) and to body size, skeletal structure, and life span in dogs (Eigenmann et al. 1988; Greer et al. 2007; Sutter et al. 2007) and in other mammals (Pendergrass et al. 1993; Miller et al. 2002; Bartke et al. 2003; Samaras et al. 2003; Suh et al. 2008; Taguchi and White 2008).
Increasing the pathological, hormonal, and molecular data for the canine breeds selected for body size, rate of early growth, and evidence of oxidative damage sustained should provide new information on the effects of the rate of growth and the IGF-1 pathway on life span, age-related lesions, and the rate and extent of cell replication that accompanies these. The knowledge of breed and group-specific ARC prevalence and its relationship to body size and life span provides one baseline for such studies.
Acknowledgements
The authors express their appreciation to Ms. Debbie Folks-Huber and CERF for help in obtaining the data for all breeds in the CERF listing and censoring of the data for the limitations listed above. This study was supported by the Swiss National Science Foundation (project number PBBEB-119243) and by NIH grant R01 EY 11733.
Contributor Information
Silvan R. Urfer, Email: urfers@u.washington.edu
Kimberly Greer, Email: kagreer@iue.edu.
Norman S. Wolf, Phone: +1-206-5438376, FAX: +1-206-5433644, Email: normwolf@u.washington.edu
References
- Ackermann L. The genetic connection—a guide to health problems in purebred dogs. Lakewood: American Animal Hospital Association; 1999. Cataracts. [Google Scholar]
- Anonymous (1996–2005). Canine Eye Registry Foundation (CERF) Database, Veterinary Medical Database (VMDB).
- Austad SN. Diverse aging rates in metazoans: targets for functional genomics. Mech Ageing Dev. 2005;126(1):43–49. doi: 10.1016/j.mad.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Dainyak BA, et al. ESR spin label and ultrastructural monitoring of protein-lipid interactions in the lens fiber-cell plasma membranes in relation to human ageing and cataractogenesis. Mech Ageing Dev. 1992;64(1–2):133–147. doi: 10.1016/0047-6374(92)90102-J. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, et al. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4(1):1–8. doi: 10.1023/A:1022448532248. [DOI] [PubMed] [Google Scholar]
- Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88(2):195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson RT. Variation in age at death of dogs of different sexes and breeds. Am J Vet Res. 1982;43(11):2057–2059. [PubMed] [Google Scholar]
- Brosnahan MM, Paradis MR. Demographic and clinical characteristics of geriatric horses: 467 cases (1989–1999) J Am Vet Med Assoc. 2003;223(1):93–98. doi: 10.2460/javma.2003.223.93. [DOI] [PubMed] [Google Scholar]
- Coile D. Encyclopedia of dog breeds. Barron’s: Hauppauge, NY; 2005. [Google Scholar]
- Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- Deeb B, Wolf N. Studying longevity and morbidity in giant and small breeds of dogs. Vet Med. 1994;89:702–713. [Google Scholar]
- Eigenmann JE, Amador A, et al. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol Copenh. 1988;118(1):105–108. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- Galis F, Sluijs I, et al. Do large dogs die young? J Exp Zool B Mol Dev Evol. 2007;308(2):119–126. doi: 10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- Gelatt KN, Mackay EO. Prevalence of primary breed-related cataracts in the dog in North America. Vet Ophthalmol. 2005;8(2):101–111. doi: 10.1111/j.1463-5224.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Greer KA, Canterberry SC, et al. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci. 2007;82(2):208–214. doi: 10.1016/j.rvsc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Lauten SD. Nutritional risks to large-breed dogs: from weaning to the geriatric years. Vet Clin N Am Small Anim Pract. 2006;36(6):1345–1359. doi: 10.1016/j.cvsm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chrisp C, et al. Differential longevity in mouse stocks selected for early life growth trajectory. J Gerontol A Biol Sci Med Sci. 2000;55(9):B455–B461. doi: 10.1093/gerona/55.9.B455. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harper JM, et al. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1(1):22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol. 2009;299(1):89–100. doi: 10.1016/j.mce.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, et al. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52(3):B171–B178. doi: 10.1093/gerona/52A.3.B171. [DOI] [PubMed] [Google Scholar]
- Pendergrass WR, Li Y, et al. Decrease in cellular replicative potential in "giant" mice transfected with the bovine growth hormone gene correlates to shortened life span. J Cell Physiol. 1993;156(1):96–103. doi: 10.1002/jcp.1041560114. [DOI] [PubMed] [Google Scholar]
- Pendergrass WR, Penn PE, et al. Cellular debris and ROS in age-related cortical cataract are caused by inappropriate involution of the surface epithelial cells into the lens cortex. Mol Vis. 2006;12:712–724. [PubMed] [Google Scholar]
- Reddy VN, Giblin FJ, et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Invest Ophthalmol Vis Sci. 2001;42(13):3247–3255. [PubMed] [Google Scholar]
- Samaras TT. Should we be concerned over increasing body height and weight? Exp Gerontol. 2009;44(1–2):83–92. doi: 10.1016/j.exger.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Samaras TT, Elrick H, et al. Birthweight, rapid growth, cancer, and longevity: a review. J Natl Med Assoc. 2003;95(12):1170–1183. [PMC free article] [PubMed] [Google Scholar]
- Samaras TT, Storms LH. Impact of height and weight on life span. Bull World Health Organ. 1992;70(2):259–267. [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Gredilla R, et al. Effect of insulin and growth hormone on rat heart and liver oxidative stress in control and caloric restricted animals. Biogerontology. 2005;6(1):15–26. doi: 10.1007/s10522-004-7380-0. [DOI] [PubMed] [Google Scholar]
- Slatter DH, Bradley JS, et al. Hereditary cataracts in canaries. J Am Vet Med Assoc. 1983;183(8):872–874. [PubMed] [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208(Pt 9):1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9(12):1173–1182. [PubMed] [Google Scholar]
- Suh Y, Atzmon G, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105(9):3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter NB, Bustamante CD, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316(5821):112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- Tryfonidou MA, Holl MS, et al. Hormonal regulation of calcium homeostasis in two breeds of dogs during growth at different rates. J Anim Sci. 2003;81(6):1568–1580. doi: 10.2527/2003.8161568x. [DOI] [PubMed] [Google Scholar]
- Heemst D, Beekman M, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4(2):79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Heath MF, et al. Prevalence of canine cataract: preliminary results of a cross-sectional study. Vet Ophthalmol. 2004;7(1):29–35. doi: 10.1111/j.1463-5224.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Wolf N, Penn P, et al. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res. 2005;81(3):276–285. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Xie L, Chen H, et al. Elevated insulin signaling disrupts the growth and differentiation pattern of the mouse lens. Mol Vis. 2007;13:397–407. [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Zubenko WN, et al. Reduced age-related cataracts among elderly persons who reach age 90 with preserved cognition: a biomarker of successful aging? J Gerontol A Biol Sci Med Sci. 2007;62(5):500–506. doi: 10.1093/gerona/62.5.500. [DOI] [PubMed] [Google Scholar]