Abstract
The purpose was to determine the influence of movement variability and level of muscle activation on the accuracy of targeted movements performed with the index finger by young and older adults. Twelve young (27.4 ± 4.4 years) and 12 older adults (74.5 ± 8.9 years) attempted to match the end position of an index finger movement to a target position when lifting and lowering a light load (10% of the maximum). Visual feedback was provided after each trial. Movement error was calculated as the absolute distance from the target. Movement variability was quantified as the standard deviation of finger acceleration and the variability of end position across trials. The EMG activity of first dorsal interosseus (FDI) and second palmar interosseus (SPI) muscles was measured with intramuscular electrodes. Older adults exhibited greater spatial and temporal errors and greater variability in finger acceleration and end position during both the lifting and lowering tasks. Older adults lifted the load by activating FDI less but SPI the same as young adults, whereas they lowered the load by activating SPI less and FDI the same as young adults. In addition, older adults exhibited lower variability across trials in SPI activation when lifting the load and lower variability for FDI activation when lowering the load. The findings demonstrate that the decrease in spatial and temporal accuracy observed in older adults when lifting and lowering a light load to a target position was due to greater movement variability and differences in antagonistic muscle activity.
Keywords: Motor output variability, Older adults, Movement control, EMG, Muscle synergy, Antagonist muscles
Introduction
Accuracy in lifting and lowering light loads is an essential skill for daily activities. Older adults are more variable than young adults when performing such tasks (Christou and Tracy 2005), as exemplified by greater variability in displacement and acceleration when lowering light inertial loads with the first dorsal interosseus muscle (Burnett et al. 2000; Laidlaw et al. 2000) and with the elbow flexor muscles (Graves et al. 2000). Due to the more variable trajectories during each movement, older adults are also more variable when they repeat a task, such as tracing a line with the index finger (Christou et al. 2003), reproducing a force–time parabola with the knee extensor muscles (Christou and Carlton 2001, 2002a), and performing tests of manual dexterity (Marmon et al. 2010).
Age-associated differences in movement variability are presumably the consequence of differences in muscle activation, both within and across muscles. The activation of motor units in agonist muscles, for example, can differ between young and older adults and contribute to greater trajectory variability (Kornatz et al. 2005; Laidlaw et al. 2000) and impairments in force control (Vaillancourt et al. 2003) in older adults. The accuracy of targeted movements can also be influenced by the synergistic activation of antagonistic muscles (Berardelli et al. 1996; Christou et al. 2007; Corcos et al. 1989; Ghez and Gordon 1987; Gottlieb et al. 1992), and the increased coactivation often observed in older adults (Darling et al. 1989; Seidler-Dobrin et al. 1998) can impair movement accuracy.
Little is known about the relative contributions of changes in movement variability and level of muscle activation to the decline in movement accuracy observed in healthy older adults. Although the reduced accuracy of older adults when attempting to achieve a target force with isometric contractions was associated with differences in the activation of antagonistic muscles and not with greater variability in the force trajectory (Christou et al. 2007), the extent to which this finding generalizes to lifting and lowering movements remains unknown as isometric and anisometric contractions are controlled by different strategies (Franklin and Wolpert 2008; Scott 2008; Todorov and Jordan 2002). The purpose of the study was to determine the influence of movement variability and level of muscle activation on the accuracy of targeted movements performed with the index finger by young and older adults. The approach was to compare the ability of young and older adults to lift and lower a load by abducting and adducting the index finger, which requires controlling the activation of single pair of antagonistic muscles (Chao et al. 1989; Li et al. 2003). The ability to control the force exerted by these muscles is associated with differences in manual dexterity across the lifespan (Jones and Lederman 2006; Marmon et al. 2010). Preliminary results have been reported in abstract form.
Methods
Subjects
Twelve young adults (27.4 ± 4.44 years) and 12 older adults (74.5 ± 8.95 years) volunteered to participate in the study. All subjects reported being healthy without any known neurological problems and were right-handed according to a standardized survey (Oldfield 1971). Subjects provided written informed consent prior to participating in the study and the Human Research Committee at the University of Colorado in Boulder approved the protocol.
Experimental arrangement
Each subject was seated and faced a 17-in. monitor that was located 1 m away at eye level. All subjects affirmed that they could clearly see the information displayed on the monitor. The left arm was abducted by 45° and the left elbow was flexed to 90°. The left forearm and hand were kept in a prone position with a custom-made device that only allowed movement of the index finger about the metacarpophalangeal joint in the abduction–adduction plane. The forearm and wrist were immobilized by metal plates and velcro straps that minimized the influence of the arm muscles on abduction of the index finger. The thumb, middle, ring, and fifth fingers of the left hand were restrained with metal plates, and there was approximately an 80° angle between the index finger and thumb. Only the left index finger was free to move, but it was placed in an adjustable finger orthosis to maintain the middle and distal interphalangeal joints in an extended position. The left hand was used so the results could be compared with previous studies (Burnett et al. 2000; Christou et al. 2003, 2007; Laidlaw et al. 2000).
Measurement of index finger displacement
Loads were lifted (abduction) and lowered (adduction) over a 10° range of motion about the metacarpophalangeal joint. The lifting and lowering actions involved the first dorsal interosseus (FDI) and second palmar interosseus (SPI) muscles. The abduction–adduction displacement of the index finger was measured with a low-friction potentiometer (Helipot 7239-44-0) that was located directly under the metacarpophalangeal joint. The coefficient of sliding friction for the device was estimated as less than 9.8 mN. The index finger position was digitized at 1,000 samples/s with a Power 1401 data acquisition system (Cambridge Electronic Design, Cambridge, UK) and stored on a computer.
Muscle activation
Abduction of the index finger is produced almost exclusively by FDI (Chao et al. 1989; Li et al. 2003; Zijdewind and Kernell 1994), and the primary antagonist muscle is SPI. Activation of the two muscles was measured with intramuscular bipolar electrodes to ensure that the recordings were obtained from these muscles. Each electrode comprised two stainless steel wires (50 μm diameter) that were insulated with Formvar (California Fine Wire Company, Grover Beach, CA, USA). The electrodes were inserted into the belly of each muscle with a 30-gauge hypodermic needle; the needle was removed after the wires were inserted. Reference electrodes were placed on the styloid process of the ulna for FDI and on the dorsal surface of the fifth metacarpophalangeal joint for SPI. The EMG signals were amplified (×5,000) and band-pass filtered (13–5,000 Hz; Coulbourn Instruments, Allentown, PA, USA). The EMG signals were sampled at 10,000 samples/s with a Power 1401 data acquisition system (Cambridge Electronic Design, Cambridge, UK) and stored on a personal computer.
Experimental procedures
Subjects participated in one experimental session that lasted approximately 2 h. Each subject began the session by completing several questionnaires and then was familiarized with the experimental procedures. The familiarization included a demonstration of the lifting and lowering movements and an explanation of the feedback provided on the monitor. After the familiarization, each subject performed the following procedures: (1) maximal voluntary contractions (MVC) with the FDI (abduction of the index finger) and SPI (adduction of the index finger) muscles and (2) in a counterbalanced order, 30 lifting movements and 30 lowering movements (three blocks of ten contractions) to the target position.
MVC task
Subjects were instructed to exert maximal abduction (FDI) and adduction (SPI) forces with the index finger in the shortest time possible. Index finger force was measured with a compression transducer (Model 41, Sensotec). The maximal force achieved in 600 ms was used to determine the load to be lifted and lowered. Prior to each MVC, subjects were required to maintain a constant abduction (or adduction) force of 0.05 N (∼1.5% of the MVC force) for 3–5 s to minimize the electromechanical delay and to use procedures that were similar to the experimental task. The start of the MVC was denoted as the time when force was 0.1 N (∼3% of the MVC). Three to five trials were recorded for each muscle, with a 60-s rest between consecutive trials. The EMGs for FDI and SPI were normalized to the peak EMG recorded during the MVC task.
Accuracy task
The task was to match the displacement of the index finger to a target that comprised a thick black line on a white background. The target line was displayed on the bottom half (15 × 30 cm) of the monitor (Fig. 1, bottom row). The endpoint of the line had the target coordinates of 600 ms (time target) and 10° (displacement target). The size of the target was 0.1 cm2. The load was 10% of the force achieved at 600 ms during the MVC task. A light load (10%) was selected because older adults are least steady when exerting low forces (Christou and Tracy 2005) and the 600-ms target represents a moderate movement speed similar to many activities of daily living. Subjects were instructed to match the endpoint of the movement trajectory (finger position endpoint) to the end of the target line (displacement and time targets).
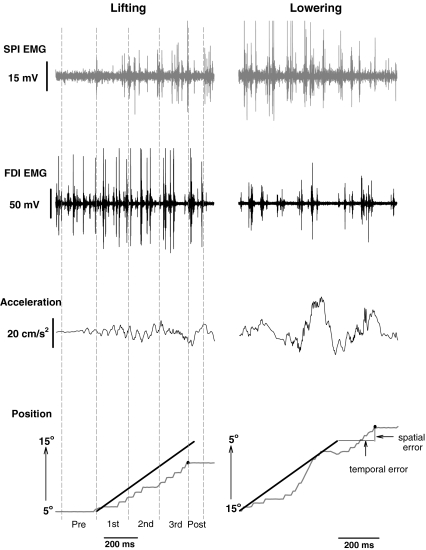
Fig. 1.
The lifting and lowering tasks and the methods used to assess endpoint accuracy and to quantify EMG activity of the antagonistic muscles. Representative data from one young subject when lifting (left column) and lowering (right column) the light load. The top row represents the SPI interference EMG, the second row indicates the FDI interference EMG, the third row shows the unfiltered acceleration of the index finger, and the bottom row displays the target (black line) and position of the index finger (gray line). The target was displayed as a thick black line on a white background. The coordinates of the target were time (X; equal to 600 ms) and displacement (Y; equal to 10°). The endpoint error was quantified for both the temporal and spatial targets. The acceleration and EMG activity were quantified over five phases (dotted vertical lines shown only for the lifting movement): (1) Pre—200 ms before the movement started, (2) 1st—the initial one third of the movement, (3) 2nd—the middle third of the movement, (4) 3rd—the final third of the movement, and (5) Post—50 ms after the end of the movement
Subjects were required to begin the trial by holding the index finger at either 5° (abduction movement) or 15° (adduction movement) of abduction from the neutral position for 3–5 s. The target for the initial condition was presented to the subject in the top-half of the monitor as a thin black line and the position of the index finger was shown as a green line. Subjects were instructed to perform the accuracy task when ready (no reaction was required) after a “GO” cue from one of the investigators. The accuracy task was performed 30 times in each direction with a 5-s rest between trials. Subjects received visual feedback of the performance 0.1 s after each trial by displaying the displacement of the index finger as a red line superimposed on the target line (black line on white background) on the bottom half of the monitor. In addition, one of the investigators provided verbal feedback about the performance by describing it as (1) short movement, short time; (2) large movement, short time; (3) large movement, long time; or (4) short movement, long time. The knowledge of results provided by this feedback was intended to improve performance in subsequent trials.
Data analysis
Data were acquired with the Spike2 software (Version 5.07; Cambridge Electronic Design, Cambridge, UK) and analyzed off-line using programs written in Matlab (Mathworks Inc., Natick, MA, USA). The force was digitized at 1,000 samples/s and the EMG signals were acquired at 10,000 samples/s.
Finger displacement and movement performance
Displacement of the finger was characterized with the following measurements: (1) peak displacement, (2) time-to-peak displacement, (3) range of motion, and (4) average finger velocity. The accuracy of goal-directed movements was quantified in the spatial (degrees) and temporal (milliseconds) domains. The spatial error was the absolute difference between the target position and peak displacement achieved during the trial (Fig. 1, bottom row). The temporal error was the absolute difference between the target time and the time-to-peak displacement. The variability in performance was quantified for each set of 30 trials as the average standard deviation of acceleration (trajectory variability) and the standard deviations for peak displacement (degrees) and time-to-peak displacement (milliseconds).
Antagonistic EMG activity
The EMG and acceleration signals were quantified for the five phases shown in the left column of Fig. 1. The five phases were intended to indicate (Berardelli et al. 1996; Corcos et al. 1989) (1) postural control before the movement, (2) feedforward control at the beginning of the movement, (3) approximate time of peak velocity, (4) approach to the target, and (5) target acquisition. The EMG was quantified as the average EMG and the trial-to-trial variability of the EMG. The average activity was represented by the root mean square of the interference signal (Merletti et al. 2001). The trial-to-trial variability was expressed as the standard deviation (SD) and coefficient of variation (CV = (SD/mean) × 100) of each parameter for all 30 trials.
In addition, coactivation of FDI and SPI during each movement was quantified with the index developed by Olney and Winter (1985): 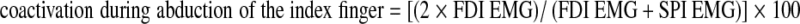 and
and 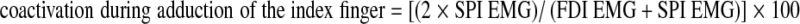 .
.
Statistical analysis
The rate of improvement in performance of the two tasks was similar for young and older adults; thus, the data analysis focused on the average performance across 30 trials. Three ANOVA models (SPSS version 14.0) were used to compare young and older adults. A mixed, two-way ANOVA (2 age groups×2 movements) with repeated measures on movement type was used to compare the accuracy (displacement and time accuracy) of the two groups of subjects. A mixed, three-way ANOVA (2 age groups×2 movements×5 movement phases) with repeated measures on movement type and phases was used to compare trajectory variability (SD of acceleration) and coactivation index. A mixed, four-way ANOVA (2 age groups×2 movements×5 movement phases×2 muscles) with repeated measures on movement type, phases, and muscles was used to compare the muscle activity (average EMG and variability of EMG). Significant main effects and interactions from the ANOVAs were examined with post hoc analyses. Differences between the five phases of each trial were examined with one-way ANOVA and Tukey’s honestly significant difference test. The differences between the two age groups were identified with independent t tests, whereas differences between muscles and between movement types were identified with a dependent t test. Pearson correlations (r) were used to determine significant associations between endpoint error, motor output variability, and EMG variables.
Multiple linear regression models were used to establish statistical models that could predict the spatial and temporal errors (criterion variables) from the variability in peak displacement, variability in time-to-peak displacement, SD of acceleration, and FDI and SPI muscle activity (predictor variables). Predictor variables were included in the multiple regression models only when they were significantly associated (bivariate regressions; Pearson correlations) with the spatial or temporal error (criterion variable). Separate multiple linear regression models were used to predict spatial and temporal errors from the coactivation index.
The goodness-of-fit of the model, which indicates how well the linear combination of the variables predicted the spatial and temporal endpoint error, was given by the squared multiple correlation (R2) and the adjusted squared multiple correlation (adjusted R2). The adjusted R2 is reported because the R2 can overestimate the percentage of the variance in the criterion variable that can be accounted for by the linear combination of the predictor variables, especially when the sample size is small and the number of predictors is large (Green and Salkind 2002). The relative importance of the predictors was estimated with part correlations (part r), which provide the correlation between a predictor and the criterion after removing the effects of all other predictors in the regression equation from the predictor but not the criterion (Green and Salkind 2002). A positive sign of the part correlation indicates that the predictor and the criterion are directly related, whereas a negative sign denotes an inverse relation.
The alpha level for all statistical tests was 0.05. Data are reported as means ± SD within the text and tables and as means ± SEM in the figures. Only the significant main effects and interactions are presented, unless otherwise noted.
Results
The young adults (27.4 ± 4.4 years) were similar in height (P > 0.2; 170.0 ± 9.8 vs. 173.3 ± 12.3 cm, respectively) to the older adults (74.5 ± 8.9 years; P < 0.001), but they had a greater mass (P = 0.015; 67.4 ± 11.4 vs. 79.0 ± 13.1 kg). The young and older adults achieved a similar (P > 0.2) peak abduction MVC force (35.4 ± 11.3 and 31.8 ± 14.4 N, respectively) and peak adduction MVC force (14.6 ± 2.40 and 15.2 ± 4.70 N). Due to the similar peak forces, the 10% load for the goal-directed task was similar (P > 0.2) for the two groups of subjects (young, 5.52 ± 1.42 N; older, 6.52 ± 1.89 N). The two groups also had a similar (P > 0.1) range of motion when lifting (young, 11.1 ± 1.75°; older, 12.6 ± 2.96°) and lowering (young, 13.3 ± 1.40°; older, 13.6 ± 2.22°) the light load and used a similar (P > 0.1) average speed when lifting (young, 24.3 ± 5.2°/s; older, 22.0 ± 5.71°/s) and lowering (young, 26.2 ± 7.80°/s; older, 20.4 ± 5.90°/s) the load. Therefore, group differences in accuracy were independent of the strength and contraction-speed capabilities of the participants.
Spatial and time accuracy
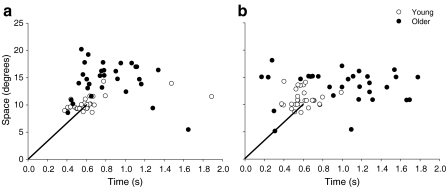
Spatial (P = 0.03) and temporal (P = 0.008) errors were significantly greater for the older adults compared with young adults when both lifting and lowering the light load. Representative endpoint locations for one young and one older adult when lifting and lowering the 10% load with the index finger are presented in Fig. 2, and the group results are reported in Table 1. On average, older adults exhibited ∼40% greater spatial errors when lifting and lowering the light load and ∼97% greater temporal errors compared with young adults. The interaction between age and movement direction (lifting or lowering) was not significant for either spatial or temporal errors (P > 0.1).
Fig. 2.
Representative endpoint locations for the lifting (a) and lowering (b) tasks with the 10% load performed by one young adult and one older adult. The older adult had greater spatial and temporal endpoint errors and endpoint variability compared with the young subject. The group data are reported in Table 1
Table 1.
Accuracy and trial-to-trial variability (SD) exhibited by young and older adults when lifting and lowering a light load
| Lifting | Lowering | |||
|---|---|---|---|---|
| Young | Older | Young | Older | |
| Displacement error (deg) | 2.16 ± 1.21 | 4.11 ± 2.20* | 3.60 ± 1.47 | 4.07 ± 2.04*,** |
| Time error (s) | 0.18 ± 0.05 | 0.33 ± 0.26* | 0.17 ± 0.06 | 0.36 ± 0.17* |
| SD displacement (deg) | 0.17 ± 0.02 | 0.26 ± 0.03 | 0.18 ± 0.03 | 0.34 ± 0.03** |
| SD time (s) | 0.16 ± 0.07 | 0.26 ± 0.09 | 0.18 ± 0.08 | 0.26 ± 0.12 |
Mean ± SD
*P < 0.05 older compared with young; **P < 0.05 lowering compared with lifting movements for both groups
Motor output variability
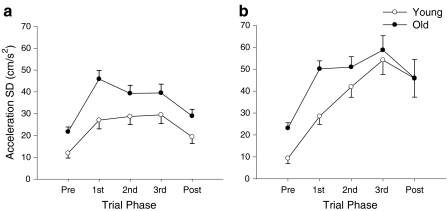
The acceleration SD (P = 0.015), trial-to-trial SD of time-to-peak displacement (P < 0.001), and trial-to-trial SD of displacement (P < 0.04) were significantly greater for the older adults compared with young adults. In addition, there was a significant interaction between age and trial phase for acceleration SD (P = 0.045) due to a greater value for the older adults at the beginning of the movement (Fig. 3). Furthermore, there was a significant interaction between age and task for spatial variability (P = 0.002) due to greater trial-to-trial SD in peak displacement during the lowering task for the older adults (Table 1).
Fig. 3.
Age differences in the acceleration SD of the index finger when lifting (a) and lowering (b) the 10% load. Older adults (filled circles) exhibited greater acceleration SDs than young adults (open circles), especially at the beginning of the movement. In addition, there was an effect due to movement type due to the greater acceleration SDs when lowering the load, especially at the end of the movement
There was a significant main effect for task (P < 0.001) due to a greater acceleration SD when lowering the load compared with lifting it (Table 1). There was also a significant interaction between task and trial phase (P < 0.001), which post hoc analyses indicated were attributable to differences at the mid-to-end of the movement (between second and post-phases; Fig. 3).
EMG activity
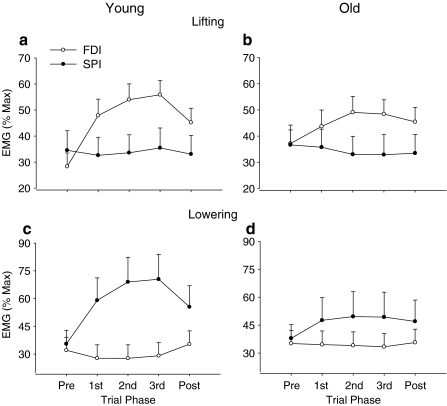
There was a significant (P < 0.001) four-way interaction (age×movement direction×trial phase×muscle) for EMG activity (Fig. 4). Post hoc analyses identified two differences: (1) greater normalized EMG for FDI compared with SPI when lifting the load and greater normalized EMG for SPI than FDI when lowering the load and (2) older adults used less normalized EMG for FDI when lifting the load and less normalized EMG for SPI when lowering the load. These differences were significant only during the movement and not either before or after the movement.
Fig. 4.
Age differences in the average EMG amplitude for FDI and SPI when lifting (top row) and lowering (bottom row) a light load. The older adults used less FDI activity than young adults when lifting the 10% load but similar levels of SPI activity, which indicates greater coactivation for the older adults. Similarly, the older adults used less SPI activity than young adults when lowering the load but similar levels of FDI activity, which again indicates greater coactivation for the older adults. As constrained by the biomechanics of each task, there was greater FDI activity when lifting the load and greater SPI activity when lowering the load
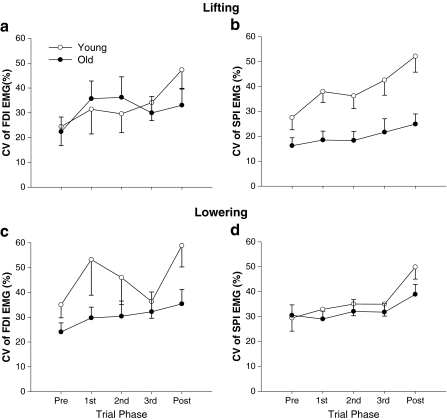
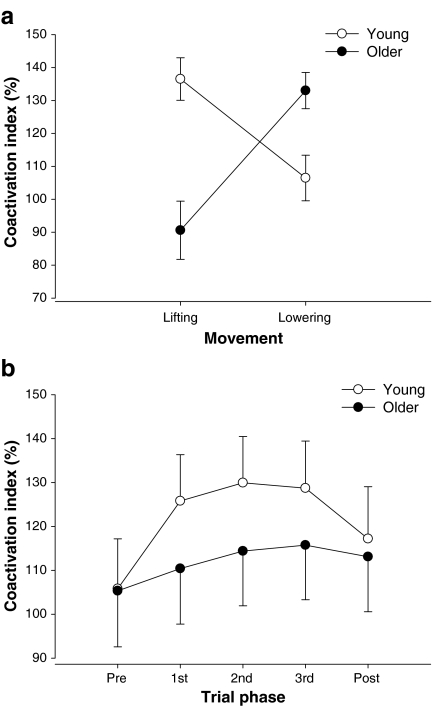
There were significant (P = 0.01) three-way (age×task×muscle) and two-way (age×trial phase) interactions (P < 0.001) for the coefficient of variation for EMG amplitude (Fig. 5). There was a similar effect for the SD of EMG amplitude (data not shown). Post hoc analysis indicated that the CV of EMG amplitude for SPI was lower for older adults than the young adults when lifting the load and for FDI when lowering the load. The young adults had similar values for the CV of EMG amplitude for FDI and SPI when lifting and greater CV of EMG amplitude for the FDI when lowering the light load. In contrast, the older adults had greater CV of EMG amplitude for the FDI when lifting the load and similar CV of EMG amplitude for the FDI and SPI when lowering the light load. These findings indicate fewer adjustments in EMG activity for the SPI muscle when lifting a load and FDI muscle when lowering a load by older adults and thus demonstrate that young and older adults activate their antagonistic muscles differently when lifting and lowering light loads. The index of coactivation varied with age, movement direction, and phase. The age×movement (P < 0.05), age×phase (P < 0.01), and movement×phase (P < 0.01) interactions were all significant. Post hoc analyses indicated that the significant age×movement interaction was due to older adults using less coactivation than young adults during abduction of the index finger, but greater coactivation during the adduction movements (Fig. 6a). The age×phase interaction indicated that older adults used less coactivation than young adults during the movement phases, but not during the postural phase or during the target acquisition phase (Fig. 6b). In contrast to these subject group differences, the movement×phase interaction suggested that all subjects used more coactivation during the adduction movement in all phases except during the target acquisition phase.
Fig. 5.
Age differences in the coefficient of variation (CV) of EMG amplitude for FDI (left column) and SPI (right column) when lifting (top row) and lowering (bottom row) the 10% load. The older adults lifted the load with similar CVs of EMG amplitude for FDI but lower values for SPI than young adults. Conversely, the older adults lowered the load with similar CVs for SPI but lower values for FDI than young adults
Fig. 6.
Age differences in coactivation as quantified with the coactivation index. a The older adults lifted the load with less coactivation and lowered the load with more coactivation than young adults. b On average, younger adults used more coactivation than young adults during the movement phases, but not during the postural phase prior to the movement (Pre) or during target acquisition phase (Post)
Prediction of error
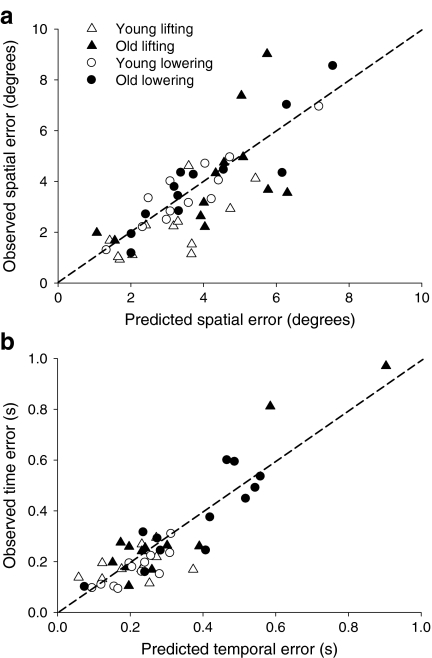
Multiple linear regression models were used to determine the contributions of motor output variability (spatial variability, variability in time-to-peak displacement, and acceleration SD) and EMG activity to the differences in spatial and temporal errors between the two groups of subjects. The spatial error when lifting the light load was predicted (R2 = 0.78; adjusted R2 = 0.63; P < 0.001; Fig. 7a) by the variability in peak displacement, EMG amplitude of the FDI, and CV of EMG of the SPI (Table 2). The temporal error when lifting the light load was predicted (R2 = 0.64; adjusted R2 = 0.55; P < 0.001; Fig. 7b) by the acceleration SD before the movement, EMG amplitude of the FDI and SPI, and CV of EMG of the SPI (Table 2).
Fig. 7.
Prediction of the spatial and temporal errors derived from the measurements of motor output variability and EMG activity of FDI and SPI. The dashed line represents the line of identity between predicted and observed values. a The greater spatial endpoint error when lifting the 10% load was predicted (R2 = 0.78; adjusted R2 = 0.63; P < 0.001) by one kinematic and three EMG parameters. The greater spatial endpoint error for the older adults when lowering the 10% load was predicted (R2 = 0.85; adjusted R2 = 0.7; P < 0.001) by two kinematic and nine EMG measures. b The greater temporal endpoint error when lifting the load was predicted (R2 = 0.64; adjusted R2 = 0.55; P < 0.001) by one kinematic and eight EMG measures. The greater temporal endpoint error when lowering the load was predicted (R2 = 0.83; adjusted R2 = 0.77; P < 0.001) by three kinematic and three EMG parameters. The corresponding predictive kinematic and EMG variables for the endpoint accuracy of lifting and lowering loads are listed in Table 2
Table 2.
Multiple regression predictions of the spatial and temporal errors for the lifting and lowering movements to a target
| Lifting | Lowering | |||
|---|---|---|---|---|
| Spatial error | Temporal error | Spatial error | Temporal error | |
| Overall prediction (R2) | 0.78 | 0.64 | 0.85 | 0.83 |
| Acceleration SD | ||||
| Before | – | 0.70 | – | 0.30 |
| First third | – | – | 0.26 | – |
| Last third | – | – | – | −0.23 |
| Variability in displacement | 0.60 | – | 0.60 | – |
| Variability in time to peak | – | – | – | 0.43 |
| EMG amplitude for FDI | ||||
| Before | – | – | – | −0.55 |
| First third | −0.41 | −0.55 | −0.49 | |
| Middle third | – | – | 0.51 | – |
| Last third | – | −0.33 | −0.47 | – |
| After | 0.41 | 0.52 | −0.36 | – |
| EMG amplitude for SPI | ||||
| First third | – | −0.37 | – | – |
| Middle third | – | 0.27 | – | – |
| Last third | – | – | −0.26 | – |
| CV of EMG for FDI | ||||
| Before | – | – | −0.30 | – |
| First third | – | – | 0.33 | – |
| Middle third | – | – | 0.43 | – |
| Last third | – | – | −0.55 | 0.22 |
| CV of EMG for SPI | ||||
| Before | – | 0.35 | – | – |
| Last third | −0.15 | −0.48 | – | – |
| After | – | 0.42 | – | −0.16 |
The data indicate the overall prediction (R2) of each equation (columns) and the relative contributions (r; part correlations) of the significant predictors for the spatial and temporal errors when lifting and lowering the light load
The spatial error when lowering the load was predicted (R2 = 0.85; adjusted R2 = 0.7; P < 0.001; Fig. 7a) by the variability in peak displacement, the acceleration SD during the first third of the movement, EMG amplitude of the FDI and SPI muscles, and CV of the FDI muscle (Table 2). The temporal error when lowering the load was predicted (R2 = 0.83; adjusted R2 = 0.77; P < 0.001; Fig. 7b) by the variability in time-to-peak displacement, acceleration SD prior to the movement and during the last third of the movement, EMG amplitude of the FDI, and CV of EMG of the FDI and SPI muscles (Table 2).
The coactivation index was able to predict only the temporal error when subjects lowered the light load (R2 < 0.1; P > 0.3). Specifically, the coactivation between the FDI and SPI muscles during the postural phase and the initial movement phase moderately predicted the temporal error (R2 = 0.36, P < 0.01). In contrast, the models with the coactivation index for predicting spatial and temporal error when lifting the light load and spatial error when lowering the light load were not significant (R2 < 0.1; P > 0.3).
Discussion
The current study found that the augmented motor output variability often exhibited by older adults (Christou and Tracy 2005; Christou et al. 2002) led them to produce greater spatial and temporal errors when lifting and lowering a light load with a hand muscle. This finding provides support for the predictions of the minimum variance theory (Faisal et al. 2008; Harris and Wolpert 1998) and extends its predictions to the accuracy of lowering loads. The differences in accuracy for the two groups of subjects were accompanied by differences in the relative activation of the two antagonistic muscles involved in the two tasks. These findings indicate that older adults are less accurate during targeted lifting and lowering movements due to both greater motor output variability (resulting from noise in the motor command) and altered activation of the antagonistic muscles.
Motor output variability and accuracy
Motor output variability was characterized by the SD of acceleration during the five phases of the movement and the variability in the magnitude and time-to-peak displacement across trials. The variability in motor output is attributed to noise being superimposed on the motor command as it progresses from the motor cortex to the muscle fibers (Faisal et al. 2008; Harris and Wolpert 1998; van Beers et al. 2004). The functional significance of this signal-dependent noise is that it increases trajectory variability (Hamilton et al. 2004) and consequently endpoint variance (Harris and Wolpert 1998), both of which impair movement accuracy. According to this scheme, the reduced accuracy for the older adults should be related to increases in the variability of the movement trajectory and an increase in the variability of peak displacement.
Consistent with the hypothesis, the reduction in spatial accuracy of the older adults, both when lifting and lowering a light load, was associated with greater trajectory variability (acceleration SD) and variability in peak displacement. Similarly, the reduced accuracy in the timing of the displacement for the older adults, again both when lifting and lowering a light load, was associated with increased variability in the movement trajectory and variability in timing (see Table 2). These results extend previous observations (Christou et al. 2003, 2007; Hamilton et al. 2004; Selen et al. 2005) and predictions (Faisal et al. 2008; Harris and Wolpert 1998) by demonstrating the following: (1) age-associated increases in trajectory variability are associated with increases in endpoint variability and decreases in the accuracy of targeted displacements and (2) endpoint accuracy when lowering a load is associated with both endpoint variability and trajectory variability.
EMG activity and accuracy
In addition to the influence of motor output variability on the decline in accuracy exhibited by the older adults, the predictive models (Table 2) indicated independent contributions by measures of EMG amplitude and variability for the two muscles. The older adults modulated the amplitude (Fig. 4) and the variability (Fig. 5) of EMG activity for the two muscles differently than the young adults during the two tasks examined in the current study.
A number of previous studies have reported that changes in the activation of the antagonistic muscles can significantly influence endpoint accuracy. In response to a reduction in target size during aiming movements, for example, the CNS increases coactivation and thereby increases joint impedance, minimizes the trajectory variability, and improves the accuracy in reaching a target (Gribble et al. 2003). Conversely, coactivation of antagonistic muscles decreases with practice and is accompanied by an improvement in the accuracy of targeted movements (Gribble et al. 2003; Osu et al. 2002). In addition, older adults and individuals with neurological disorders exhibit impaired endpoint accuracy due to changes in the requisite amplitude ratio and timing of activation between the antagonistic muscles during aiming tasks (Berardelli et al. 1996; Christou et al. 2007; Darling et al. 1989; Seidler-Dobrin et al. 1998).
Findings from the current study suggest that older adults activate the involved muscles less (FDI when lifting a load and SPI when lowering a load) than young adults and modulate EMG amplitude less across trials, which may contribute to the declines in accuracy. Accordingly, the regression models included both the EMG amplitude and CV for FDI and SPI as predictors of the spatial and temporal errors. The variability in the observed spatial and temporal error (range of error) was due to age-associated differences in accuracy for both the lifting and lowering movements (see Fig. 7). It is also evident that the predicted values for spatial and temporal error from motor output variability and EMG parameters were strongly associated with the observed errors (see symbols and line of identity in Fig. 7). As the measures of motor output variability and EMG activity contributed independently to movement error (see Fig. 7 legend and Table 2), these findings demonstrate that the age-associated differences in movement accuracy are attributable to both motor output variability and altered activation of the antagonistic muscles. Nonetheless, it is not clear whether the changes in the activation of antagonistic muscles are responsible for the reduction in endpoint accuracy or reflects an adaptation in muscle activity due to a decline in accuracy.
The greater motor output variability and altered muscle activity of the antagonistic muscles displayed by the older adults, which reduced the accuracy of both the timing and amplitude of the targeted displacements, may be due to a number of physiological changes that accompany aging, including (1) alterations in various structures of the brain (Dinse 2006), (2) death of cortical neurons (Eisen et al. 1996; Henderson et al. 1980) and spinal motor neurons (Masakado et al. 1994; Roos et al. 1997), (3) the slowing of the signal transmitted from the corticospinal and reflex pathways to the motor neurons (Henderson et al. 1980), and (4) changes in the mechanical characteristics of muscle (Narici et al. 2008). These changes may influence the planning and execution of the motor command and consequently alter the precision of the motor output (Harris and Wolpert 1998).
Aging and predictions from the minimum variance theory
Although our results are consistent with the minimum variance theory that noisier output (e.g., greater trajectory variability) impairs movement accuracy, the findings also indicate that the EMG amplitude of the antagonistic muscles was less variable in older adults compared with young adults. The EMG results might be interpreted as contradicting the minimum variance theory; however, it must be acknowledged that the measure of EMG variability used in the current study is a rather crude index of variability in the activation signal. For example, Negro et al. (2009) demonstrated that ∼60% of the fluctuations in force could be explained by the first principal component of the smoothed motor unit discharge rates. Furthermore, actions that involve relatively sparse motor unit activity (Fig. 1) are strongly influenced by the variability in motor unit discharge times (Enoka et al. 2003). Similar to our current findings, others have reported that greater endpoint variability in older adults during goal-directed contractions is associated with altered activation of antagonistic muscles (Christou et al. 2007; Darling et al. 1989).
The impaired ability of older adults to perform accurate limb movements, therefore, may be due to a noisier descending command or an impaired ability to change the antagonistic muscle activity based on the demands of the task. The noisier command may be evident as greater trajectory variability during the movement (caused by greater motor unit discharge variability), whereas the inability to change the antagonistic muscle activity may be evident as lower trial-to-trial variability in the EMG amplitude of the antagonistic muscles (caused by impaired planning). The regression analysis suggests that these two mechanisms are independent.
Control of lifting and lowering light loads
When subjects attempt to match a constant-velocity template while slowly (<5°/s) lifting and lowering light inertial loads, the trajectory is more variable when lowering a load compared with lifting it (Burnett et al. 2000; Christou and Tracy 2005; Graves et al. 2000; Laidlaw et al. 2000; Tracy and Enoka 2002). The influence of task on trajectory variability increases with movement speed (Christou et al. 2003) and augments the trial-to-trial variability of the movement trajectory (Christou and Carlton 2001, 2002a, b; Christou et al. 2003). The more variable trajectory when lowering a load is attributed to the lesser EMG activity (Christou et al. 2002) and more variable motor unit activity (Kornatz et al. 2005; Laidlaw et al. 2000) during the lengthening contraction compared with the shortening contraction (Duchateau and Enoka 2008). The current findings also included greater trajectory variability and greater trial-to-trial variability when lowering the 10% load compared with lifting it.
Previous studies demonstrate that trajectory variability is greater in older adults when lowering a light load with the index finger (Burnett et al. 2000; Laidlaw et al. 2000) and elbow flexor muscles (Graves et al. 2000), but not when lifting and lowering loads with the knee extensor muscles (Tracy and Enoka 2002). Older adults are also more variable across trials when they attempt to repeat the same lengthening contraction. This is evident when they lift or lower a load with the index finger (Christou et al. 2003) or when they resist forces imposed by a torque motor with the knee extensors (Christou and Carlton 2002a). The current findings provide evidence that both age groups exhibit greater trajectory variability and trial-to-trial variability when lowering a light load. Thus, our study is consistent with previous studies (Christou and Carlton 2001, 2002a; Christou et al. 2002) that have demonstrated greater variability by both young and older adults when lowering a load compared with lifting it. However, the current findings indicate that trial-to-trial variability in peak displacement and not trajectory variability was exacerbated in older adults when lowering a load (see Fig. 3).
Lowering the light load exacerbated only endpoint variability, but not the movement trajectory variability, in older adults perhaps due to the coactivation strategy they used. We found that older adults exhibited greater coactivation of the antagonistic muscles than young adults when they lowered the light load, but lower coactivation when they lifted the load. The greater coactivation when lowering the load may have minimized trajectory variability (Gribble et al. 2003) but impaired endpoint variability (Darling et al. 1989). It is also possible that young adults took into account the effects of gravity while lowering the load, whereas the older adults did not (Rao et al. 2009). These findings underscore the interaction between aging and control of lifting and lowering light loads and provide evidence that the accuracy of older adults is impaired when lifting and lowering a light load with the index finger.
In summary, the older adults were less accurate and more variable than young adults when they lifted and lowered a light load with the index finger to a prescribed target in space and time. The worse performances by the older adults were predicted by differences in movement variability and altered EMG activity of the involved antagonistic muscles. The less accurate performances of the older adults, therefore, are attributable to more than differences in movement variability and include an independent contribution by changes in antagonistic muscle activity.
Acknowledgments
Awards to Evangelos Christou (AG024662, AG031769) and Roger Enoka (AG09000) from the National Institute of Aging supported this work.
References
- Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119:661–674. doi: 10.1093/brain/119.2.661. [DOI] [PubMed] [Google Scholar]
- Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol. 2000;89:61–71. doi: 10.1152/jappl.2000.89.1.61. [DOI] [PubMed] [Google Scholar]
- Chao EYS, An KN, Cooney WP, Linschied RL. Biomechanics of the hand. A basic research study. Teaneck: World Scientific; 1989. [Google Scholar]
- Christou EA, Carlton LG. Old adults exhibit greater motor output variability than young adults only during rapid discrete isometric contractions. J Gerontol A Biol Sci Med Sci. 2001;56:B524–B532. doi: 10.1093/gerona/56.12.B524. [DOI] [PubMed] [Google Scholar]
- Christou EA, Carlton LG. Age and contraction type influence motor output variability in rapid discrete tasks. J Appl Physiol. 2002;93:489–498. doi: 10.1152/japplphysiol.00335.2001. [DOI] [PubMed] [Google Scholar]
- Christou EA, Carlton LG. Motor output is more variable during eccentric compared with concentric contractions. Med Sci Sports Exerc. 2002;34:1773–1778. doi: 10.1097/00005768-200211000-00013. [DOI] [PubMed] [Google Scholar]
- Christou EA, Tracy BL. Aging and motor output variability. In: Davids K, Bennett S, Newell K, editors. Movement system variability. Champaign: Human Kinetics; 2005. pp. 199–215. [Google Scholar]
- Christou EA, Tracy BL, Enoka RM. The steadiness of lengthening contractions. In: Latash ML, editor. Progress in motor control, volume II: structure–function relations in voluntary movements. Champaign: Human Kinetics; 2002. pp. 195–207. [Google Scholar]
- Christou EA, Shinohara M, Enoka RM. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. J Appl Physiol. 2003;95:373–384. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- Christou EA, Poston B, Enoka JA, Enoka RM. Different neural adjustments improve endpoint accuracy with practice in young and old adults. J Neurophysiol. 2007;97:3340–3350. doi: 10.1152/jn.01138.2006. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Agarwal GC. Organizing principles for single-joint movements. II. A speed-sensitive strategy. J Neurophysiol. 1989;62:358–368. doi: 10.1152/jn.1989.62.2.358. [DOI] [PubMed] [Google Scholar]
- Darling WG, Cooke JD, Brown SH. Control of simple arm movements in elderly humans. Neurobiol Aging. 1989;10:149–157. doi: 10.1016/0197-4580(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Dinse HR. Cortical reorganization in the aging brain. Prog Brain Res. 2006;157:57–80. doi: 10.1016/S0079-6123(06)57005-0. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Enoka RM. Neural control of shortening and lengthening contractions: influence of task constraints. J Physiol. 2008;586:5853–5864. doi: 10.1113/jphysiol.2008.160747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology. 1996;46:1396–1404. doi: 10.1212/wnl.46.5.1396. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/S1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DW, Wolpert DM. Specificity of reflex adaptation for task-relevant variability. J Neurosci. 2008;28:14165–14175. doi: 10.1523/JNEUROSCI.4406-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Gordon J. Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res. 1987;67:225–240. doi: 10.1007/BF00248545. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Latash ML, Corcos DM, Liubinskas TJ, Agarwal GC. Organizing principles for single joint movements: V. Agonist–antagonist interactions. J Neurophysiol. 1992;67:1417–1427. doi: 10.1152/jn.1992.67.6.1417. [DOI] [PubMed] [Google Scholar]
- Graves AE, Kornatz KW, Enoka RM. Older adults use a unique strategy to lift inertial loads with the elbow flexor muscles. J Neurophysiol. 2000;83:2030–2039. doi: 10.1152/jn.2000.83.4.2030. [DOI] [PubMed] [Google Scholar]
- Green SB, Salkind NJ. Using SPSS for the windows and Macintosh: analyzing and understanding data. Upper Saddle River: Prentice Hall; 2002. [Google Scholar]
- Gribble PL, Mullin LI, Cothros N, Mattar A. Role of cocontraction in arm movement accuracy. J Neurophysiol. 2003;89:2396–2405. doi: 10.1152/jn.01020.2002. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Jones KE, Wolpert DM. The scaling of motor noise with muscle strength and motor unit number in humans. Exp Brain Res. 2004;157:417–430. doi: 10.1007/s00221-004-1856-7. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Henderson G, Tomlinson BE, Gibson PH. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. J Neurol Sci. 1980;46:113–136. doi: 10.1016/0022-510X(80)90048-9. [DOI] [PubMed] [Google Scholar]
- Jones LA, Lederman SJ. Human hand function. Oxford: Oxford University Press; 2006. [Google Scholar]
- Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98:2072–2080. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600–612. doi: 10.1002/(SICI)1097-4598(200004)23:4<600::AID-MUS20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Li ZM, Pfaeffle HJ, Sotereanos DG, Goitz RJ, Woo SL. Multi-directional strength and force envelope of the index finger. Clin Biomech (Bristol, Avon) 2003;18:908–915. doi: 10.1016/S0268-0033(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Marmon AR, Pascoe MA, and Enoka RM (2010) Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc (in press). doi:10.1249/MSS.0b013e3181f3f3ab [DOI] [PubMed]
- Masakado Y, Noda Y, Nagata MA, Kimura A, Chino N, Akaboshi K. Macro-EMG and motor unit recruitment threshold: differences between the young and the aged. Neurosci Lett. 1994;179:1–4. doi: 10.1016/0304-3940(94)90920-2. [DOI] [PubMed] [Google Scholar]
- Merletti R, Rainoldi A, Farina D. Surface electromyography for noninvasive characterization of muscle. Exerc Sport Sci Rev. 2001;29:20–25. doi: 10.1097/00003677-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disab Rehab. 2008;30:1548–1554. doi: 10.1080/09638280701831058. [DOI] [PubMed] [Google Scholar]
- Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol. 2009;587:5925–5938. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Winter DA. Predictions of knee and ankle moments of force in walking from EMG and kinematic data. J Biomech. 1985;18:9–20. doi: 10.1016/0021-9290(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, Kawato M. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol. 2002;88:991–1004. doi: 10.1152/jn.2002.88.2.991. [DOI] [PubMed] [Google Scholar]
- Rao G, Amarantini D, Berton E. Influence of additional load on the moments of the agonist and antagonist muscle groups at the knee joint during closed chain exercise. J Electromyogr Kinesiol. 2009;19:459–466. doi: 10.1016/j.jelekin.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. doi: 10.1002/(SICI)1097-4598(199706)20:6<679::AID-MUS4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol. 2008;586:1217–1224. doi: 10.1113/jphysiol.2007.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler-Dobrin RD, He J, Stelmach GE. Coactivation to reduce variability in the elderly. Mot Control. 1998;2:314–330. doi: 10.1123/mcj.2.4.314. [DOI] [PubMed] [Google Scholar]
- Selen LP, Beek PJ, Dieen JH. Can co-activation reduce kinematic variability? A simulation study. Biol Cybern. 2005;93:373–381. doi: 10.1007/s00422-005-0015-y. [DOI] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5:1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol. 2002;92:1004–1012. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging. 2003;24:25–35. doi: 10.1016/S0197-4580(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Beers RJ, Haggard P, Wolpert DM. The role of execution noise in movement variability. J Neurophysiol. 2004;91:1050–1063. doi: 10.1152/jn.00652.2003. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Index finger position and force of the human first dorsal interosseus and its ulnar nerve antagonist. J Appl Physiol. 1994;77:987–997. doi: 10.1152/jappl.1994.77.2.987. [DOI] [PubMed] [Google Scholar]