Abstract
The purpose of this study was to determine changes in klotho, endothelin (ET) receptors, and superoxide production in kidneys of aged rats and whether these changes are exacerbated in aged rats with cognitive impairment. Twenty aged rats (male, 27 months) were divided into an Old Impaired group (n = 9) and an Old Intact group (n = 11) according to a cognitive function test. A group of 12-month-old rats (n = 10) was used as a Young Intact group. Serum creatinine was increased significantly in the Old Impaired group, suggesting impaired renal function. Aged rats showed glomerulosclerosis and tubulointerstitialfibrosis. These pathological changes were markedly aggravated in the old cognitively impaired than in the old cognitively intact animals. Notably, aged rats demonstrated a significant decrease in klotho protein expression in renal cortex and medulla. Protein expression of IL-6, Nox2, ETa receptors and superoxide production were increased whereas mitochondrial SOD (MnSOD) and ETb receptors expression were decreased in kidneys of the aged rats. Interestingly, these changes were more pronounced in the old impaired than in the old intact rats. In conclusion, the aging-related kidney damage was exacerbated in aged rats with cognitive impairment. Klotho, ETB, and MnSOD were downregulated but ETa, IL-6, Nox2, and superoxide production were upregulated in the aging-related kidney damage. These changes were more pronounced in rats with cognitive impairment.
Keywords: Aging, Klotho, Glomerusclerosis, ET receptor, Superoxide, Interleukin-6
Introduction
Aging is defined as the age-related deterioration of physiological functions necessary for the survival and fertility of an organism (EJ 1995). Many processes undergo modifications to a different extent as a function of age; noteworthy modifications are observed in renal, respiratory, cardiovascular and cognitive functions. The renal function declines with aging. Cognitive deficits such as memory impairment and amnesia are the debilitating consequences of aging. However, little is known if old cognitively impaired animals are associated with exacerbated aging-related kidney damage. In this study, we assessed if cognitive impairment parallels with kidney damage in the aged rats.
Klotho is a recently identified anti-aging gene (Wang and Sun 2009a). It encodes a single-pass transmembrane protein with a long extracellular domain and a short cytoplasmic tail. Klotho protein is predominantly expressed in distal convoluted tubules in the kidneys and in the choroid plexus in the brain (Kurosu et al. 2005; Kuro-o 2008; Zhang and Zheng 2008). However, a secreted form of the protein has been described and is found in the blood and cerebrospinal fluid. Genetic mutation of klotho (Kl−/−) results in extensive aging phenotypes resembling human aging, including shortened life span, growth retardation, infertility, arteriosclerosis, skin and muscle atrophy, osteoporosis, pulmonary emphysema (Kuro-o et al. 1997), and cognition impairment (Nagai et al. 2003). Conversely, overexpression of the klotho gene extends the life span in mice (Kurosu et al. 2005). Klotho expression in the kidneys has been shown to be reduced in patients with chronic renal failure (Koh et al. 2001) and with acute renal failure in ischemia reperfusion injury murine models (Sugiura et al. 2005). These findings would imply that the reduction of klotho protein may be relevant to the pathophysiology of renal failure. It is not known, however, if klotho expression is altered in kidneys of aged animals. In this study, we examined klotho expression in kidney cortex and medulla of aged rats.
The kidney is one of the most important organs in the regulation of systemic hemodynamics and is a target organ of endothelin-1 (ET-1) (Abassi et al. 2001). ET-1 exerts its function via binding to ETa and ETb receptors (Abassi et al. 2001; Chen and Sun 2006). Activation of ETa receptors increases NADPH oxidase activity and superoxide production (Dammanahalli and Sun 2008b; Sun 2010). Activation of ETb receptors, however, stimulates the release of nitric oxide (NO) and suppresses oxidative damage in kidneys (Abassi et al. 2001). An increase in superoxide production or oxidative stress may contribute to aging (Krause 2007; Mendoza-Nunez et al. 2007). The superoxide level is determined by NADPH oxidases and anti-oxidant enzymes (e.g., superoxide dismutase-SOD).
The objective of this study was to determine changes in klotho, ET receptors, superoxide production, and SOD in aging-related kidney damage and whether these changes were exacerbated in rats with cognitive impairment.
Materials and methods
Animal study protocols
This study was carried out according to the guidelines of the National Institute of Health on the care and use of laboratory animals. The project was approved by the Institutional Animal Care and Use Committee. Fisher 344xBrown Norway (F1) male rats (10 and 27 months of age) were obtained from the NIA colony at Harlan Industries (Indianapolis, IN, USA). Animals were singly housed in polycarbonate cages and provided with standard rat chow and tap water ad libitum throughout the experiment.
After adaptation to the animal facility for 2 weeks, acquisition was assessed using a variation of the Morris (Morris et al. 1982) and Maze. Based on the cognitive performance test (Freeman et al. 2009), old animals were divided into Old Intact (N = 11, 27 months) and Old Impaired groups (N = 9, 27 months). Old animals with cognitive performance in the same range as the Young animals (N = 10, 10 months) were categorized as Old Intact, and those with cognitive performance below the range of Young animals were placed in the Old Impaired group. The cognitive performance data were published recently (Freeman et al. 2009).
At the end of the cognitive test, all animals were perfused transcardially with heparinized saline under deep anesthesia (sodium pentobarbital, 100 mg/kg, IP). Blood was collected for measuring plasma levels of klotho and endothelin-1 (ET-1) before perfusion. A small part of the kidney was excised and processed for immediate determination of the in situ superoxide production. A part of kidneys were fixed in paraformaldehyde (4% PFA) overnight and embedded in OCT for histological and immunohistochemical analysis. Kidney collagen deposition was assayed using a HT15-trichrome Stain (Masson) kit (Sigma-Aldrich, St Louis, MO) according to the manufacturer’s instruction. The remaining tissues were snapped in liquid nitrogen for Western blot analysis.
Immunohistochemical analysis of IL-6 and MnSOD expression in kidneys
The immunohistochemical procedure was described in our previous studies (Chen and Sun 2006; Wang et al. 2006; Bello Roufai et al. 2007; Wang and Sun 2009a; Wang and Sun 2009b; Crosswhite and Sun 2010). Briefly, following perfusion, kidneys were fixed overnight with 4% paraformaldehyde (4°C) and then embedded with paraffin. Tissue sections (5 μm) were incubated with peroxidase-blocking solution (Dako North America, Inc. Carpinteria, CA, USA) for 5 min followed by protein blocker (Biocare Medical, Concord, CA, USA) for 15 min. The sections were then incubated with monoclonal Anti-mouse IL-6 Antibody (dilution 1:800, BD Transduction Laboratories Inc., Mississauga, ON, Canada) and mitochondrial SOD (MnSOD; 1:200dilution; Millipore, Billerica, Massachusetts) for 1 h followed by a goat anti-mouse secondary antibody (dilution 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 30 min. The sections were then examined and photographied using a Nikon Eclipse Ti-U microscope coupled with a digital color camera. The expression of IL-6 and SOD was evaluated by measuring the areas of positive staining density using the Nikon software. To demonstrate antibody specificity, sections were stained without the primary antibody which served as negative controls.
Histological evaluation of renal tissue
For histological examination, kidneys were fixed in 4% buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin. Periodic acid-Schiff staining was performed to evaluate the extent of glomerulosclerosis. The extent of glomerulosclerosis was analyzed according to Raij et al. (Raij et al. 1985). Glomerulosclerosis was defined by the presence of increased amounts of PAS positive material in the glomeruli. A minimum of 200 glomeruli in each specimen was randomly examined in a series of 15 tissue sections. The severity of glomerulosclerosis was scored as follows: 0, no sclerosis; 1, <25% sclerotic changes in glomerulus; 2, 25%–50%; 3, 50%–75%; and 4, >75%. All tissue samples were evaluated independently by two investigators in a blinded manner.
The severity of interstitial matrix collagen deposition was evaluated using Masson–Trichrome staining (Yukawa et al. 2005). Severity of tubulointerstitial damage was evaluated by examining ten randomly selected fields in each tissue sample. Blue-stained scarred areas were quantified by using a color image analyzer. Collagen deposition was expressed as the percentage of the blue-stained interstitial area (connective tissue) to the total interstitial area of the whole tissue section (collagen area fraction).
Western blot analysis
Western blot was performed as described previously. (Wang et al. 2006; Sun et al. 2008). Briefly, tissues were homogenized in lysis buffer (50 mM Tris, 150 mM NaCl, 1% sodium dodecyl sulfate, 1% sodium deoxycholic acid, 1 mM phenylmethylsulfonyl fluoride, 1 mM ethylene diaminetetracetic, and 1% Triton X-100) containing a protease inhibitor and centrifuged for 5 min at 10,000 g. The supernatant was collected and mixed immediately with an equal volume of electrophoresis loading buffer for Western blot analysis of klotho, ETa, ETb, IL-6, MnSOD, and Nox2 protein expression. The equal amount of protein was loaded in a 4–20% gradient SDS–PAGE gel, the protein was transferred onto nitrocellulose filters after separated in the gel. Blots were blocked in 2% BSA in TBST for 1 h, the membranes were incubated overnight (4°C) with antibodies against klotho (dilution 1:300, R&D Systems, Inc. Minneapolis, MN, USA), ETa (1:200 dilution) or ETb (dilution 1:500; Alomone Labs, Jerusalem, Israel), IL-6 (dilution 1:1,000; BD Transduction Laboratories Inc., Mississauga, ON, Canada), NOX2 (dilution 1:1,000; BD Transduction Laboratories Inc.), MnSOD (dilution 1:1,000; Millipore, Billerica, MA) and β-actin (dilution 1:10,000; Abcam Inc., Cambrige, MA, USA). The membranes were incubated with HRP conjugated secondary anti-goat, anti-mouse, or anti-rabbit antibodies (dilution 1:2,000–1:5,000), respectively, for 1 h at room temperature. Proteins were visualized by ECL, exposed to an X-ray film and developed with a X-ray processor. The protein band intensities were quantified as described previously (Chen and Sun 2006; Bello Roufai et al. 2007; Sun et al. 2008). Protein expression was normalized with the expression of β-actin. Serum level of klotho was also measured using Western blot. The antibodies were fully tested and were used in our previous publications (Chen and Sun 2006; Dammanahalli and Sun 2008a; Wang and Sun 2009b; Wang and Sun 2010). The ET receptor antibody specificity was confirmed by using the blocking peptide (ET-1).
Measurement of in situ superoxide production
The in situ superoxide production was measured in kidneys using the oxidation sensitive dye dihydroethidium (DHE, Sigma-Aldrich, Atlanta, GA, USA). Briefly, kidneys were embedded in OCT, frozen at −80°C, and cut at 10 μm using a cryostat. Sections were incubated in PBS (37°C) in a humidified chamber for 30 min followed by incubation with DHE (10−5 M in PBS) in the dark for 30 min. The preparations were counterstained with the nuclear stain 4,5,6-diamidin-2-phenylindol dichlorohydrate (DAPI, 3 × 10−7 M, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 37°C for 5 min and mounted on slides. The images were captured with FITC filter using Nikon fluorescence microscopy, the average intensity was measured at 400× magnification in three randomly chosen fields from three independent experiments. DAPI fluorescence were quantified using Image J (Dammanahalli and Sun 2008a)
Analysis of serum creatinine and endothelin-1
Serum creatinine concentration was measured by a kinetic colorimetric method using a creatinine assay kit (QuantiChrom™ Creatinine, BioAssay Systems,CA,USA). Serum ET-I concentration was measured using an Endothelin-1 ELISA kit (Correlate-EIA™ kit, Assay Designs Inc., MI, USA).
Statistical analyses
Data were analyzed using a one-way ANOVA. The Newman–Keuls procedure was used to assess the significance of differences between means. Significance was set at the 95% confidence limit.
Results
Aging was associated with glomerular collapse and impaired creatinine excretion
As indicated in the “Materials and methods”, animals were categorized as Young Intact, Old Intact, and Old Impaired groups based on their cognitive performance (Freeman et al. 2009). The cognitive performance data were published recently (Freeman et al. 2009). Briefly, the Young Intact animals showed significant improvement over the training blocks. Similar improvement was found for the Old Intact animals. In contrast, poorer cognitive performance was noted in the Old Impaired animals, indicating impaired cognitive function (Freeman et al. 2009).
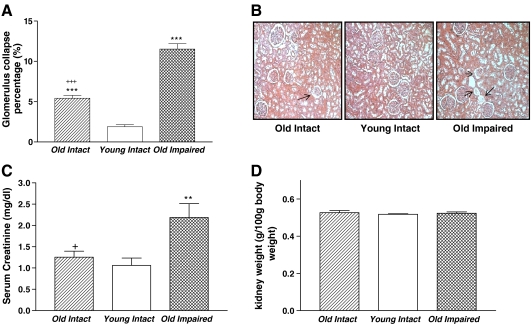
The percentage of the collapsed glomeruli was increased significantly in both the Old Intact and the Old Impaired groups compared with the Young Intact group (Fig. 1a, b), suggesting that aging was associated with glomerular collapse. The impaired glomerular damage was exacerbated in the Old Cognitively Impaired group as evidenced by a greater increase in the percentage of glomerular collapse in this group than in the Old Intact group. Concomitantly, serum level of creatinine was increased significantly in the Old Impaired group although it was not altered in the Old Intact group (Fig. 1c), suggesting that renal excretion of creatinine was impaired in the old cognitively impaired group. Kidney weights were not significantly different between groups (Fig. 1d).
Fig. 1.
Aging was associated with glomerular collapse and impaired creatinine excretion. a The percentage of collapsed glomeruli. b Photomicrograph of glomerulus collapse (HE staining). Arrow indicated glomerulus collapse, 100×. c Serum creatinine. d Kidney weight. Data = mean ± SE. **p < 0.01;***p < 0.001 versus the Young Intact Group; addition symbol, p < 0.05; triple addition symbols, p < 0.001 versus the Old Impaired Group. N = 11 in the Old Intact Group, n = 10 in the Young Intact Group, n = 9 in the Old Impaired Group
Aging was associated with glomerulosclerosis and interstitial fibrosis in kidneys
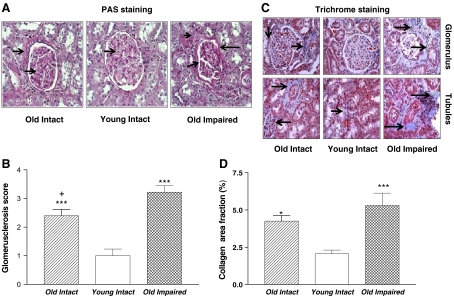
PAS staining showed extracellular matrix protein expansion in the Old Intact and the Old Impaired rats (Fig. 2a), indicating glomerulosclerosis. Glomerulosclerosis was more pronounced in the Old Impaired rats than in the Old Intact rats (Fig. 2a, b). Trichrome staining revealed increased collagen deposition in and around glomeruli in kidneys of the Old Intact and the Old Impaired rats (Fig. 2c, d), suggesting interstitial fibrosis. These data indicated that renal structures were damaged in aged rats and that the damages were aggravated in the old impaired rats.
Fig. 2.
Aging was associated with glomerusclerosis and tubular interstitial fibrosis PAS (periodic acid-Schiff) staining (400×) and trichrome staining (200×) in kidneys. a Photomicrograph of PAS staining in renal cortex. Arrows indicated glomerulosclerosis (Pink). b Glomerulosclerosis score. c Photomicrograph of kidney trichrome staining. Arrows indicated interstitial collagen staining (blue) around the glomeruli. d Semi-quantitative analysis of collagen staining. Data = mean ± SE. *p < 0.05, ***p < 0.001 vs the Young Intact group; addition symbol, p < 0.05 versus Old. Impaired group. N = 11 animals in the Old Intact group, n = 10 animals in the Young, Intact group, n = 9 animals in Old Impaired group
Aging-related kidney damage was associated with a decrease in renal klotho protein expression
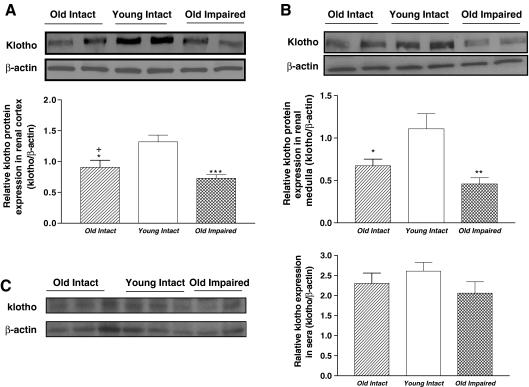
Western blot analysis showed that klotho protein expression were decreased significantly (p < 0.05, p < 0.01) in both renal cortex (Fig. 3a) and medulla (Fig. 3b) in aged animals. Serum level of klotho tended to be decreased in the Old Impaired group although no statistical significance was found among the three groups (Fig. 3c).
Fig. 3.
Aging was associated with downregulation of klotho expression in kidneys. a Representative Western blot bands and quantitative analysis of klotho protein expression in renal cortex (≈130 kDa). b Representative Western blot bands and quantitative analysis of klotho protein expression in renal medulla. c Representative Western blot bands and quantitative analysis of klotho protein level in sera. Data = mean ± SE, *p < 0.05, **p < 0.01, ***p < 0.001 versus young intact group; addition symbol, p < 0.05 versus Old Impaired group. N = 11 animals in the Old Intact group, n = 10 animals in the Young Intact group, n = 9 animals in the Old Impaired group
Aging-related kidney damage was associated with upregulation of ETa receptor expression and an increase in the ratio of ETa/ETb receptors
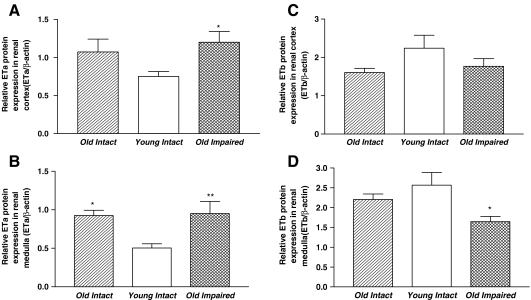
ETa receptor protein expression was increased significantly in both renal cortex (Fig. 4a) and medulla (Fig. 4b) in the aged groups than in young intact group. In contrast, ETb receptor expression in renal cortex was not altered in either of the aged groups (Fig. 4c). ETb receptor expression in renal medulla was decreased significantly in the Old Impaired group although it was not altered in the Old Intact group (Fig. 4d).
Fig. 4.
Aging was associated with upregulation of ETa receptors and downregulation of ETb expression in kidneys (Western blot bands not shown). a Quantitative analysis of ETa protein expression in renal cortex. b Quantitative analysis of ETa protein expression in renal medulla. c Quantitative analysis of ETb protein expression in renal cortex. d quantitative analysis of ETb protein expression in renal medulla. Data = mean ± SE. *p < 0.05, **p < 0.01 versus the Young Intact group. N = 11 animals in the Old intact group, n = 10 animals in the Young Intact group, n = 9 animals in the Old Impaired group
The ratio of ETa to ETb in renal medulla was increased significantly (doubled) in both aged groups although it was not altered in renal cortex (Figures not shown). The serum level of ET-1 was not significantly different between any two groups (Figure not shown).
Aging-related kidney damage was associated with an increase in IL-6 expression
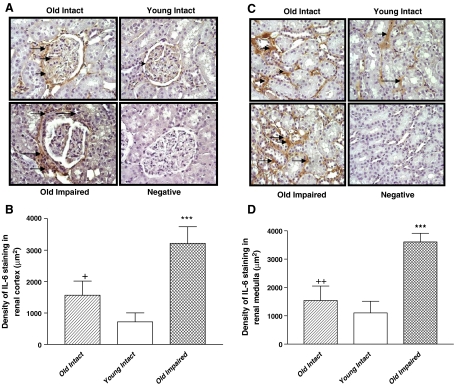
Immunohistochemical (IHC) analysis showed that IL-6 expression (brown staining) was increased in and around renal glomeruli in both aged groups compared with the Young Intact group (Fig. 5a, b). A significant increase in IL-6 expression was also found in the basal membrane of renal tubules of the aged animals (Fig. 5c, d). The increase in IL-6 expression was more evident in the Old Impaired than in the Old Intact animals (Fig. 5b, d).
Fig. 5.
Aging was associated with an increase in IL-6 immunostaining in kidneys. a Immunohistochemical (IHC) analysis of IL-6 expression in renal cortex. Brown staining (arrows) indicated positive IL-6 expression. IL-6 expressed in and around the nucleus. Negative control, without the first antibody (400×). b Semi-quantification of IL-6 expression in renal cortex. c IHC analysis of IL-6 expression in renal medulla. Brown staining (arrows) indicated positive IL-6 expression. IL-6 expressed in the basal membrane of renal tubules. Negative control, without the first antibody (400×). d Semi-quantification of IL-6 expression in renal medulla. Data = mean ± SE. ***p < 0.001 versus the Young Intact group; addition symbol, p < 0.05, double addition symbols, p < 0.01 versus the Old Impaired group. N = 11 animals in the Old Intact group, n = 10 animals in the young intact group, n = 9 animals in the Old Impaired group
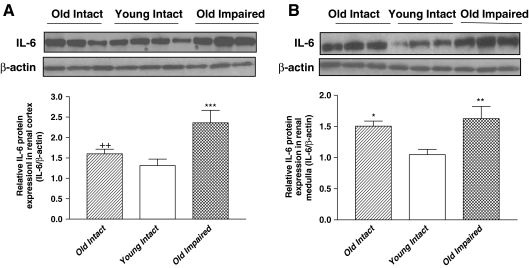
Western blot analysis showed that IL-6 protein expression in renal cortex (Fig. 6a) and medulla (Fig. 6b) was increased significantly in the Old Impaired group. Although IL-6 expression was not altered in the cortex of the Old Intact group (Fig. 6a), it was increased significantly in the medulla (Fig. 6b).
Fig. 6.
Aging was associated with upregulation of IL-6 protein expression in kidneys. a Representative Western blot bands and quantitative analysis of IL-6 protein expression in renal cortex. b Representative Western blot bands and quantitative analysis of IL-6 protein expression in renal medulla. Data = mean ± SE. *p < 0.05, **p < 0.01, ***p < 0.001 versus the Young Intact group; double addition symbols, p < 0.01 versus the Old Impaired group. N = 11 animals in the Old Intact group, n = 10 animals in the Young Intact group, n = 9 animals in the Old Impaired group
Aging-related kidney damage was associated with an increase in superoxide production and upregulation of Nox2 protein expression
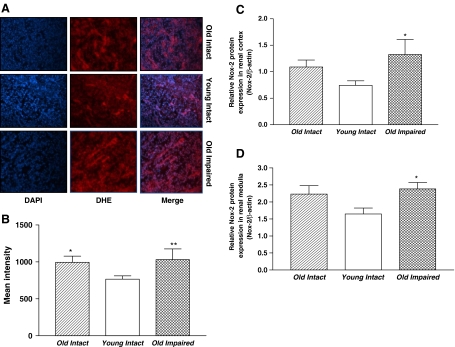
Kidney segments of aged rats showed an increase in superoxide production (DHE staining) compared with those of the Young Intact rats. Nox2 protein expression was increased in renal cortex and medulla in both aged groups but it reached a significant level only in the Old Impaired group (Fig. 7c, d).
Fig. 7.
Aging was associated with increases in superoxide production and NADPH oxidase expression in kidney cortex. a Renal segments showing DAPI staining (blue), DHE staining (red), and a merge of DAPI and DHE staining, respectively. An overlap of DAPI and DHE staining (light yellow) indicated superoxide production in the nuclei (200×). b Quantification of superoxide production in kidney cortex. c Quantitative analysis of Nox-2 protein expression in renal cortex (Western blot bands not shown). d Quantitative analysis of Nox-2 protein expression in renal medulla (Western blot bands not shown). Data = mean ± SE. *p < 0.05, **p < 0.01 vs the Young Intact group. N = 11 animals in the Old Intact group, n = 10 animals in the Young Intact group, n = 9 animals in the Old Impaired group
Aging-related kidney damage was associated with a decrease in MnSOD expression in kidneys
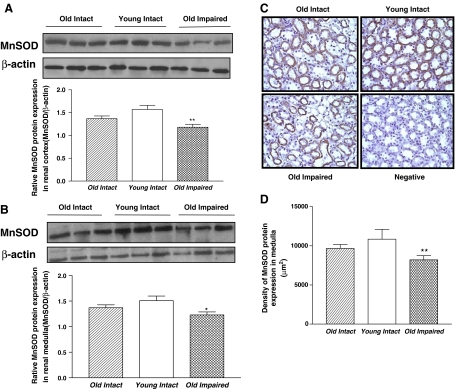
MnSOD protein expression was decreased significantly in both renal cortex and medulla in the Old Impaired group compared with the Young Intact group (Fig. 8a, b). The IHC analysis revealed that MnSOD protein expression (brown staining) was localized in renal tubule epithelial cells (Fig. 8c). A semi-quantitative analysis of confirmed that MnSOD protein expression was decreased in the medulla the Old Impaired rats (Fig. 8d). SOD expression was decreased slightly but not significantly in the Old Intact group (Fig. 8a–d).
Fig. 8.
MnSOD expression was downregulated in kidneys of the Old Impaired rats. a Representative Western blot bands and quantitative analysis of MnSOD protein expression in renal cortex. b Representative Western blot bands and quantitative analysis of MnSOD protein expression in renal medulla. c IHC analysis of MnSOD expression in renal medulla. Brown staining indicated positive MnSOD protein expression. MnSOD expressed in renal tubule epithelial cells. Negative control, without the first antibody (200×). d Semi-quantification of MnSOD expression in renal medulla. Data = mean ± SE. *p < 0.05, **p < 0.01, ***p < 0.001 versus the young intact group; addition symbol, p < 0.05 vs the Old Impaired group. N = 11 animals in the Old Intact group, n = 10 animals in the Young Intact group, n = 9 animals in the Old Impaired group
Discussion
Aging is a degenerative biologic process that affects multiple organs or tissues including the kidneys. The aging-related decline in renal function is attributed, in part, to a progressive loss of functioning nephrons which eventually results in a decrease in glomerular filtration rate (GFR). The present study further demonstrated that the aged animals were associated with glomerulus collapse and glomerular and tubulointerstitial fibrosis. These changes would impair glomerular and tubular function, systemic hemodynamics, and, as a whole, body homeostasis, contributing to the aging process. The renal function may be damaged in the Old Impaired animals as evidenced by a significant increase in serum creatinine. Glomerulosclerosis is characterized by excessive accumulation of extracellular matrix in the kidney glomerulus. The glomerulosclerotic process is usually progressive in nature, eventually impairing the function of neighboring structures due to the gradual expansion of scar tissue and the collapse of glomeruli. The degree of glomerulosclerosis correlates with a progressive loss of renal function, and eventually end-stage renal disease (Taguchi et al. 2005).
The cognitive performance declines with increasing age. Interestingly, the aged rats with impaired cognition showed exaggerated glomerular collapse (atrophy). These findings suggest, for the first time, that the aging-related kidney damage (e.g., glomerusclerosis) parallels with the cognitive function impairment. One possibility is that the same aging mechanism may affect both brain and kidneys in some aged animals. For instance, overactivation of the endothelin system may damage both brain and kidneys by impairing microvascular function in these organs (Zhang et al. 1994; Kuiper et al. 2008; Seccia et al. 2008; Soleman et al. 2010). On the other hand, the impaired kidney function results in accumulation of metabolic toxic products which could impair brain function (e.g., cognition). The mechanistic link of the aging-related kidney damage and cognitive impairment is an interesting topic to pursue.
Klotho is a recently-discovered anti-aging gene (Kuro-o et al. 1997; Wang and Sun 2009a). Klotho protein is predominantly expressed in renal tubule epithelial cells (Wang and Sun 2009a). A decrease in renal klotho expression has been found in kidneys of hypertensive animals (Aizawa et al. 1998; Wang and Sun 2009b). The klotho production was severely reduced in human chronic failure kidneys (Koh et al. 2001). Klotho deficiency causes kidney damage (Aizawa et al. 1998; Koh et al. 2001; Wang and Sun 2009b), suggesting that klotho is essential in maintaining normal kidney structure and function. Interestingly, in vivo expression of klotho in kidneys prevents renal damage in spontaneous hypertensive rats (Wang and Sun 2009b) and ameliorates angiotensin II-induced renal injury (Mitani et al. 2002). Klotho reduces apoptosis in acute experimental ischemic renal failure (Sugiura et al. 2005). Genetic manipulation of klotho ameliorates progressive renal injury (Haruna et al. 2007). Klotho increases nitric oxide availability and SOD activity (Wang and Sun 2009a). Klotho gene delivery suppresses superoxide production in kidneys (Wang and Sun 2009b). There are two forms of klotho, the membrane-form (renal) klotho and the secreted form (circulating) klotho due to alternative RNA splicing (Wang and Sun 2009a). The present study demonstrated that klotho protein expression was downregulated in kidneys while the circulating klotho level remained unchanged in aged rats. This finding is new because it may open a new avenue for studying the aging-related kidney damage. An impairment in the RNA splicing for generating the membrane-form klotho could lead to a decrease in the renal klotho, but the exact mechanism remains to be found. The tissue level of klotho may be more important than the circulating klotho in determining its function in the kidney. The present study, however, cannot determine if klotho is involved in kidney damage. Therefore, a further study is required to assess if the downregulation of renal klotho expression contributes to the aging-related kidney damage by in vivo kidney-specific expression of klotho in aged animals.
The action of ET-1 is mediated by ETa and ETb receptors. Activation of ETa receptors increases NADPH oxidase activity and superoxide production (Dammanahalli and Sun 2008b) which may contribute to aging-related kidney damage. In contrast, activation of ETb receptors increases the NO release in kidney medulla (Abassi et al. 2001) which may protect the kidneys. Therefore, the effects of ET-1 in kidney depend on the location and regulation of ETa and ETb receptors. Although aging-related changes in ET-1 and ET receptors were reported in hearts and bladders (Afiatpour et al. 2003; Yono et al. 2004; Del Ry et al. 2008), the regulation of ET receptors in the aged kidneys was poorly studied. Lattmann et al. reported that both ETa and ETb mRNA levels were decreased in aged kidneys (Lattmann et al. 2005). The present finding revealed that ETa receptor protein expression was upregulated in renal cortex and medulla while ETb receptor protein expression was downregulated in medulla. Interestingly, the ratio of ETa:ETb was increased in renal medulla in aged rats although the level of the circulating ET-1 was not altered. These changes may contribute to the aging-related kidney damage. The suppression of medullary ETb expression was more pronounced in the Old Impaired animals, which may contribute to the aggravation of the kidney damage in this group.
Another important finding is that kidney aging was associated with increased inflammation as evidenced by increased IL-6 expression in both glomeruli and tubules in aged rats. The exacerbated kidney damage in the Old Impaired rats may be attributed, in part, to aggravated inflammation because the aging-related increase in IL-6 expression was more pronounced in the Old Impaired rats. Inflammation could activate TGF-β leading to tissue remodeling (Izad et al. 2010; Wang et al. 2010). The mechanism of aging-related increase in IL-6 is not fully understood. IL-6 is secreted from macrophages (Suzuki et al. 2004) and is the main cytokine involved in the induction of inflammation (Czarkowska-Paczek et al. 2005). It was reported that IL-6 expression was increased with age in rats (Franchini and Ottaviani 2007) and humans (Friedman et al. 2007). Its production is enhanced by renal mesangial cells, which may be associated with mesangial cell proliferation or interstitial tissue injury as proposed by Suzuki et al. (Suzuki et al. 1995). These cells adhere to the glomerular capillary wall, between endothelial cells and the basal lamina. The present data showed significant mesangial expansion based on the PAS staining. IL-6 protein expression was increased in the peritubular spaces in renal medulla of aged rats, suggesting that inflammation may be involved in interstitial fibrosis.
Cumulative oxidative injury is believed to play a major role in the process of cell aging. Oxidative stress and generation of free radicals increase with aging (Mendoza-Nunez et al. 2007). Persistent oxidative damage to cytosolic structures leads to cross-linking of oxidized proteins and deposition of lipofuscin with functionally and structurally impaired mitochondria (Jung et al. 2007). Recent data suggest that chronic oxidant stress plays a contributory role in telomere shortening and thus in senescent changes (Houben et al. 2008). The mechanism of aging-related kidney damage is not fully understood. In a rat model, kidney aging was associated with a 60% decline in GFR, a threefold increase in renal F2 isoprostanes, and an increase in oxidant-sensitive heme oxygenase (Thomas et al. 1998). The NADPH oxidases may be involved in the pathogenesis of age-associated diseases (Krause 2007). The level of oxidative stress is determined by superoxide production and dismutation. The present study demonstrated that NADPH oxidase Nox2 expression and superoxide production were increased in kidneys of aged animals, which could contribute to aging-related oxidative stress.
On the other hand, MnSOD expression was suppressed in kidneys of the Old Impaired rats which may contribute to the aging-related kidney damage. The mitochondria-derived superoxide is the major source of reactive oxygen species. MnSOD is the primary anti-oxidant enzyme that scavenges superoxide formed within the mitochondria and protects against oxidative stress. Overexpression of MnSOD protects against hyperglycemia-induced renal injury using normal rat renal proximal tubular cells (NRK) (Munusamy and MacMillan-Crow 2009). The present study demonstrated that NOX-2 protein expression was upregulated but the MnSOD protein expression was downregulated in renal cortex and medulla of aged rats. These changes increase the superoxide level contributing to oxidative injury in kidney aging.
The aging-related kidney damage may involve multiple molecular pathways. The present study revealed aging-related changes in klotho, ET receptors, Nox2, and MnSOD, providing a basis for assessing the roles and the relationship of these factors in kidney aging. ET-1 stimulates the production of superoxide via activation of NADPH oxidases (Dammanahalli and Sun 2008b). On the other hand, NADPH oxidase-derived ROS appear to stimulate ET-1 production (Dammanahalli and Sun 2008b). A decrease in klotho expression was associated with an increase in Nox2 expression and superoxide production in kidneys in spontaneous hypertensive rats (SHRs) (Wang and Sun 2009b). Oxidative stress may decrease expression of klotho in a mouse inner medullary collecting duct (mIMCD3) cell line (Mitobe et al. 2005). Klotho gene delivery suppressed Nox2 expression and superoxide production and attenuated kidney damage in SHRs (Wang and Sun 2009b). There is growing evidence that the klotho protein induces the expression of MnSOD (Kuro-o 2008) and suppression of NADPH oxidases (Wang and Sun 2009b; Wang and Sun 2009a) to protect against oxidative stress. Genetic mutation of klotho decreases SOD expression while overexpression of klotho increases SOD expression (Wang and Sun 2009a), indicating that klotho may regulate SOD expression. Klotho gene delivery enhanced IL-10 level in the blood in SHRs (Wang and Sun 2009b), suggesting that klotho may suppress inflammation. It is expected that a decrease in klotho may upregulate IL-6 in kidneys. This hypothesis, however, needs to be tested.
In summary, the aging-related kidney damage was associated with downregulation of klotho, ETb and MnSOD expression and upregulation of ETa, IL-6, and Nox2 expression and superoxide production. These changes were more pronounced in the Old Impaired animals. The findings raise the necessity to evaluate the roles of these factors and their relationship in aging-related kidney damage. The novel aspect of this study is that it reveals that the aging-related cognitive impairment paralleled with the kidney damage and that kidney aging was associated with a reduction of klotho.
Acknowledgements
This work was supported by the National Institute of Health (R01 HL077490) and the Reynolds Oklahoma Center on Aging.
Disclosures Nothing to disclose.
References
- Abassi ZA, Ellahham S, Winaver J, Hoffman A. The intrarenal endothelin system and hypertension. News Physiol Sci. 2001;16:152–156. doi: 10.1152/physiologyonline.2001.16.4.152. [DOI] [PubMed] [Google Scholar]
- Afiatpour P, Latifpour J, Takahashi W, Yono M, Foster HE, Jr, Ikeda K, Pouresmail M, Weiss RM. Developmental changes in the functional, biochemical and molecular properties of rat bladder endothelin receptors. Naunyn Schmiedebergs Arch Pharmakol. 2003;367:462–472. doi: 10.1007/s00210-003-0715-6. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- Bello Roufai M, Li H, Sun Z. Heart-specific inhibition of protooncogene c-myc attenuates cold-induced cardiac hypertrophy. Gene Ther. 2007;14:1406–1416. doi: 10.1038/sj.gt.3302995. [DOI] [PubMed] [Google Scholar]
- Chen GF, Sun Z. Effects of chronic cold exposure on the endothelin system. J Appl Physiol. 2006;100:1719–1726. doi: 10.1152/japplphysiol.01407.2005. [DOI] [PubMed] [Google Scholar]
- Crosswhite P, Sun Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension. 2010;55:1484–1491. doi: 10.1161/HYPERTENSIONAHA.109.146902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska-Paczek B, Bartlomiejczyk I, Gabrys T, Przybylski J, Nowak M, Paczek L. Lack of relationship between interleukin-6 and CRP levels in healthy male athletes. Immunol Lett. 2005;99:136–140. doi: 10.1016/j.imlet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Dammanahalli JK, Sun Z. Endothelin (ET)-1 inhibits nicotinamide adenine dinucleotide phosphate oxidase activity in human abdominal aortic endothelial cells: a novel function of ETB1 receptors. Endocrinology. 2008;149:4979–4987. doi: 10.1210/en.2008-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammanahalli KJ, Sun Z. Endothelins and NADPH oxidases in the cardiovascular system. Clin Exp Pharmacol Physiol. 2008;35:2–6. doi: 10.1111/j.1440-1681.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- Ry S, Maltinti M, Giannessi D, Cavallini G, Bergamini E. Age-related changes in endothelin-1 receptor subtypes in rat heart. Exp Aging Res. 2008;34:251–266. doi: 10.1080/03610730802070233. [DOI] [PubMed] [Google Scholar]
- Ej M. Aging. In: Ej M, editor. Handbook of physiology. New York: Oxford University Press; 1995. pp. 3–24. [Google Scholar]
- Franchini A, Ottaviani E. IL-6 immunoreactivity changes during aging in the polychaete Ophryotrocha labronica (Polychaeta: Dorvilleidae) Tissue Cell. 2007;39:27–34. doi: 10.1016/j.tice.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Vanguilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159:183–195. doi: 10.1016/j.neuroscience.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Hayney M, Love GD, Singer BH, Ryff CD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci USA. 2007;104:2331–2336. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben JM, Moonen HJ, Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Izad M, Vodjgani M, Niknam MH, Amirzargar A, Shahbeigi S, Heidari AB, Keramatipour M. Cytokines genes polymorphisms and risk of multiple sclerosis. Am J Med Sci. 2010;339:327–331. doi: 10.1097/MAJ.0b013e3181cef1a1. [DOI] [PubMed] [Google Scholar]
- Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann NY Acad Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol. 2007;42:256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, Versteilen AM, Niessen HW, Vaschetto RR, Sipkema P, Heijnen CJ, Groeneveld AB, Plotz FB. Production of endothelin-1 and reduced blood flow in the rat kidney during lung-injurious mechanical ventilation. Anesth Analg. 2008;107:1276–1283. doi: 10.1213/ane.0b013e31818067a2. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattmann T, Shaw S, Munter K, Vetter W, Barton M. Anatomically distinct activation of endothelin-3 and the l-arginine/nitric oxide pathway in the kidney with advanced aging. Biochem Biophys Res Commun. 2005;327:234–241. doi: 10.1016/j.bbrc.2004.11.160. [DOI] [PubMed] [Google Scholar]
- Mendoza-Nunez VM, Ruiz-Ramos M, Sanchez-Rodriguez MA, Retana-Ugalde R, Munoz-Sanchez JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213:261–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–843. doi: 10.1161/01.HYP.0000013734.33441.EA. [DOI] [PubMed] [Google Scholar]
- Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101:e67–e74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Munusamy S, MacMillan-Crow LA. Mitochondrial superoxide plays a crucial role in the development of mitochondrial dysfunction during high glucose exposure in rat renal proximal tubular cells. Free Radic Biol Med. 2009;46:1149–1157. doi: 10.1016/j.freeradbiomed.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med. 1985;79:37–41. doi: 10.1016/0002-9343(85)90078-6. [DOI] [PubMed] [Google Scholar]
- Seccia TM, Maniero C, Belloni AS, Guidolin D, Pothen P, Pessina AC, Rossi GP. Role of angiotensin II, endothelin-1 and L-type calcium channel in the development of glomerular, tubulointerstitial and perivascular fibrosis. J Hypertens. 2008;26:2022–2029. doi: 10.1097/HJH.0b013e328309f00a. [DOI] [PubMed] [Google Scholar]
- Soleman S, Yip P, Leasure JL, Moon L. Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Exp Neurol. 2010;222:13–24. doi: 10.1016/j.expneurol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant. 2005;20:2636–2645. doi: 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- Sun Z. Cardiovascular responses to cold exposure. Front Biosci (Elite Ed) 2010;2:495–503. doi: 10.2741/e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Bello-Roufai M, Wang X. RNAi inhibition of mineralocorticoid receptors prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;294:H1880–H1887. doi: 10.1152/ajpheart.01319.2007. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Miyazaki M, Naka R, Koji T, Yagame M, Jinde K, Endoh M, Nomoto Y, Sakai H. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes. 1995;44:1233–1238. doi: 10.2337/diabetes.44.10.1233. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Won KJ, Horiguchi K, Kinoshita K, Hori M, Torihashi S, Momotani E, Itoh K, Hirayama K, Ward SM, Sanders KM, Ozaki H. Muscularis inflammation and the loss of interstitial cells of Cajal in the endothelin ETB receptor null rat. Am J Physiol Gastrointest Liver Physiol. 2004;287:G638–G646. doi: 10.1152/ajpgi.00077.2004. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107–121. doi: 10.1159/000086055. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ. Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol. 1998;9:231–242. doi: 10.1681/ASN.V92231. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sun Z. RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol Genomics. 2010;41:120–126. doi: 10.1152/physiolgenomics.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Skelley L, Cade R, Sun Z. AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther. 2006;13:1097–1103. doi: 10.1038/sj.gt.3302768. [DOI] [PubMed] [Google Scholar]
- Wang B, Dileepan T, Briscoe S, Hyland KA, Kang J, Khoruts A, Cleary PP. Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc Natl Acad Sci USA. 2010;107:5937–5942. doi: 10.1073/pnas.0904831107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yono M, Latifpour J, Takahashi W, Pouresmail M, Afiatpour P, Weiss RM. Age-related changes in the properties of the endothelin receptor system at protein and mRNA levels in the rat vas deferens. J Recept Signal Transduct Res. 2004;24:53–66. doi: 10.1081/RRS-120034106. [DOI] [PubMed] [Google Scholar]
- Yukawa K, Kishino M, Goda M, Liang XM, Kimura A, Tanaka T, Bai T, Owada-Makabe K, Tsubota Y, Ueyama T, Ichinose M, Maeda M, Takeda K, Akira S. STAT6 deficiency inhibits tubulointerstitial fibrosis in obstructive nephropathy. Int J Mol Med. 2005;15:225–230. [PubMed] [Google Scholar]
- Zhang R, Zheng F. PPAR-gamma and aging: one link through klotho? Kidney Int. 2008;74:702–704. doi: 10.1038/ki.2008.382. [DOI] [PubMed] [Google Scholar]
- Zhang WW, Ma KC, Andersen O, Sourander P, Tollesson PO, Olsson Y. The microvascular changes in cases of hereditary multi-infarct disease of the brain. Acta Neuropathol. 1994;87:317–324. doi: 10.1007/BF00296749. [DOI] [PubMed] [Google Scholar]