Abstract
The aged heart displays a loss of cardiomyocyte number and function, possibly due to the senescence and decreased regenerative potential that has been observed in some cardiac progenitor cells. An important cardiac progenitor that has not been studied in the context of aging is the cardiac side population (CSP) cell. To address this, flow cytometry-assisted cell sorting was used to isolate CSP cells from adult (6–10 months old) and aged (24–32 months old) C57Bl/6 mice that were fed either a control diet or an anti-aging diet (caloric restriction, CR). Aging caused a 2.3-fold increase in the total number of CSP cells and a 3.2-fold increase in the cardiomyogenic sca1+/CD31− subpopulation. Aging did not affect markers of proliferation or senescence, including telomerase activity and expression of cell cycle genes, in sca1+/CD31− CSP cells. In contrast, the aged cells had reduced expression of genes associated with differentiation, including smooth muscle actin and cardiac muscle actin (5.1- and 3.2-fold, respectively). None of these age effects were altered by CR diet. Therefore, it appears that the manner in which CSP cells age is distinct from the aging of post-mitotic tissue (and perhaps other progenitor cells) that can often be attenuated by CR.
Keywords: Adult stem cell, Cardiac regeneration, CR, CSP, Sca1, CD31
Introduction
Advanced age remains one of the strongest risk factors for cardiovascular disease (Lloyd-Jones et al. 2009). Data from rodent models suggest that during normal aging the number of cardiomyocytes declines leading to hypertrophy of the remaining cardiomyocytes, accelerated cardiomyocyte death, interstitial fibrosis, and a general pattern of maladaptive remodeling (Chimenti et al. 2003; Sussman and Anversa 2004).
A potential contributor to age-associated cardiomyocytes loss is a decrease in the quantity and/or quality of progenitor cells in the aged heart. Several reports have found that aged progenitor cells display evidence of inefficient proliferation, differentiation, or homing (Morrison et al. 1996; Pearce et al. 2007; Torella et al. 2004). In the aged mouse heart specifically, c-kit+ progenitors were found to have increased markers of senescence (Torella et al. 2004). This is an important issue for both understanding age-associated cardiomyocyte loss and for characterizing progenitor cells for potential in vivo activation in aged hearts (Urbanek et al. 2005).
To date, the effect of aging on cardiac progenitors has been studied only in c-kit+ cells. Another putative cardiac progenitor is the cardiac side population (CSP) cell. This cell type is identified by its ability to efflux the nuclear-staining dye Hoechst 33342 via transmembrane pumps of the ATP-binding cassette (ABC) family, specifically ABCG2 and ABCB1/MDR1 (Goodell et al. 1996; Pfister and Liao 2008). Side population cells exhibit stem/progenitor characteristics in a wide variety of tissues including bone marrow, brain, small intestine, and the heart (Asakura and Rudnicki 2002). CSP cells have been shown to be capable of differentiation into at least five cell types: cardiomyocyte, endothelial, smooth muscle, adipocyte, and osteocyte (Liang et al. 2009; Oyama et al. 2007; Pfister et al. 2005; Yamahara et al. 2008).
A sub-population of CSP cells (sca1+/CD31−) was identified and found to contain the majority of the CSP cardiomyocyte differentiation potential in vitro (Pfister et al. 2005). Subsequently, Liang et al. showed that sca1+/CD31− CSP cells were capable of in vivo differentiation into cardiomyocyte- and endothelial-like cells in a mouse model of myocardial ischemia (Liang et al. 2009). Because of their differentiation potential, sca1+/CD31− CSP cells could be of interest in circumstances where loss of cardiomyocyte number contributes to myocardial dysfunction, such as post-myocardial infarction and advanced age. However, it is unknown whether sca1+/CD31− CSP cells suffer the same age-associated increase in senescence as other progenitor cells (Morrison et al. 1996; Pearce et al. 2007; Torella et al. 2004). Therefore, the first goal of our study was to determine how normal aging affects these cells.
The second goal of our study was to determine how aged sca1+/CD31− CSP cells are affected by a well-known anti-aging diet (caloric restriction, CR) that has been reported to reverse many aspects of cardiac aging in rodents and humans, including cardiac contractile dysfunction, alterations in systemic hemodynamics, oxidative damage, and altered gene expression (reviewed in Saupe and Mulligan 2007 and Marzetti et al. 2009). CR treatments limit food intake to 50–75% of normal, but are supplemented with sufficient micronutrients to prevent malnutrition. The mechanisms by which CR delays aging and age-related diseases are not fully understood, but one possibility is that CR returns stem/progenitor cells to a more “youthful” phenotype. This has been demonstrated in hematopoietic stem cells, where CR enhanced function to the extent that hematopoietic stem cells from aged mice on a CR diet outperformed hematopoietic stem cells from young ad lib (AL) fed mice in a repopulation assay (Chen et al. 2003). Despite the observed effects of CR on cardiac aging, it has never been studied as a strategy for reversing the effects of aging on progenitor cells within the heart.
To address these questions, sca1+/CD31− CSP cells were isolated from adult (6–10 months old) and aged (24–32 months old) mice fed either an AL or CR diet. The effects of aging and CR on CSP cell abundance, composition, senescence, and gene expression were then assessed.
Methods
Animals Adult (6–10 months old) and aged (24–32 months old) male C57Bl/6 mice were purchased from a colony maintained by the National Institute on Aging (NIA) and housed singly in an AAALAC-accredited University of Wisconsin Animal Care Facility. The research protocols were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Mice were fed either an AL diet or CR diet (approximately 40% reduction in caloric intake since 16 weeks of age). Mice in the adult AL group consumed an average of 0.55 kcal/day/g body weight, while the aged AL group consumed an average of 0.70 kcal/day/g body weight of NIH-31 diet (3.4 kcal/g). All CR mice were maintained on the NIA CR feeding schedule and diet of 0.39 kcal/day/g body weight of NIH-31/NIA Fortified diet (3.33 kcal/g). The diets are essentially the same (18.5% protein, 4.5% fat, 4 kcal/g gross energy) except that the fortified diet has approximately 40% more of the vitamin component to ensure adequate intake of micronutrients. Mice were fed daily and food consumption and body weights measured weekly. Hearts were not pooled for the following experiments; each data point represents a single mouse.
Isolation of lineage-negative non-cardiomyocyte cells Hearts were minced finely, added to digestion base solution [DBS; Hank’s balanced salt solution (HBSS), 10 mM HEPES, 2% FBS] and incubated for 10 min at 37°C. Then, 1 mg/ml collagenase B (Roche), 2.4 U/ml dispase II (Roche), and 2.5 mM CaCl2 were added to the DBS/heart solution and incubated for 15 min at 37°C. The solution was then passed up and down through a 10-ml pipette, incubated for 15 min longer, and once again passed up and down through the 10-ml pipette. Ice-cold HBSS with 10 mM HEPES and 2% FBS was added, the solution was filtered through 70 μm and 40 μm cell strainers, and then centrifuged at 1,000×g at 4°C for 5 min. The pellet was resuspended in magnetic sorting buffer (MB; HBSS with 10 mM HEPES, 2 mM EDTA, 0.5% BSA) and centrifuged again. The lin− fraction (depleted of blood lineage cells) was collected from a MACS MS column (Miltenyi, No. 130-042-201) after using a Lineage Cell Depletion Kit (Miltenyi, No. 130-090-858). Cells were washed twice with MB and nucleated cells were counted using methylene blue stain.
Cell staining and FACS To stain for the side population, cells were incubated at 37°C for 90 min in Hoechst solution (DMEM/F12 with 5 mM HEPES, 2% FBS, and 5 μg/ml Hoechst 33,342) at a concentration of one million cells per milliliter (typically 3–5 million cells were used). Cells were then washed twice with MB. For cells sorted by sca1 and CD31, cells were incubated at 4°C for 10 min with 10 μg/ml CD31-FITC (BD Pharmingen, No. 558738) and sca-1-PE (BD Pharmingen, No. 553336), and washed twice with MB. Propidium iodide was added prior to flow cytometry to identify dead cells. Cells were analyzed and sorted on a FACSVantage SE instrument with FACSDiVa digital electronics (BD Biosciences).
Telomerase activity Telomerase activity in flow cytometry-sorted CSP cells was determined using a Quantitative Telomerase Detection Assay (Allied Biotech Inc., No. MT3011) according to the manufacturer’s instructions. Each reaction used 10,000 sorted cells. The assay was performed with an ABI Prism 7000 (Applied Biosystems) quantitative real-time PCR machine.
Gene expression analysis RNA was extracted from approximately 15,000 flow cytometry-sorted sca1+/CD31− CSP cells using a PicoPure RNA Isolation kit (Arcturus, No. KIT0204). RNA concentrations were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop). RNA integrity was measured by RNA6000 PicoChip (Agilent, No. 5067-1513) run on an Agilent 2100 BioAnalyzer. RNA was converted to cDNA using an RT2 Nano PreAMP cDNA Synthesis Kit (SABiosciences, No. C-06) and cDNA quality was tested by RT2 RNA QC PCR Array (SABiosciences, No. PAMM-999). The cDNA was then used on custom-designed RT2 Profiler PCR Arrays, based off of the RT2 Profiler Stem Cell Array (SABiosciences, No. PAMM-405). Gene expression was normalized to the average expression of three housekeeping genes: Hsp90ab, Gapdh, and Actb. All kits were used according to manufacturer’s instructions, and analysis was done using software available at www.sabiosciences.com. The adult mice used for this experiment were 3-month-old male C57BL/6 from Harlan. These mice were identical to the 6–10-month-old adult AL mice from NIA in physical parameters and side population characteristics.
Statistics Two-way ANOVA was used in Figs. 1 and 2, and Table 1. For Table 1, in cases where there was significant interaction between factors, Bonferroni post tests were used to assess significance of individual comparisons. A p value <0.05 was considered statistically significant. Gene expression data (Fig. 3) were adjusted for multiple testing by the positive false discovery rate (pFDR) method using QVALUE software (Storey and Tibshirani 2003). A q value cut-off of 0.05 indicates a 5% chance of false positives among the total number of genes with significant changes in expression.
Fig. 1.
Effect of aging and CR on CSP cell abundance and composition. a Representative flow cytometry plot used to distinguish cardiac side population (CSP) from cardiac main population (CMP) cells based on the efflux of Hoechst dye in lin− non-cardiomyocytes of adult and aged AL mice. ATP-binding cassette transporter activity is confirmed by inhibition with verapamil. b Aging increased CSP cell number (p < 0.0001), but CR had no effect (n = 6–8 per group). c Representative flow cytometry plots illustrating the division of CSP cells into sub-populations based on expression of sca1 and CD31 in an adult mouse heart. d Aging increased abundance of sca1+/CD31− CSP cells (p < 0.0001), but CR had no effect (n = 6–8 per group). Data are expressed as mean ± SE. Asterisk denotes significant effect of age
Fig. 2.
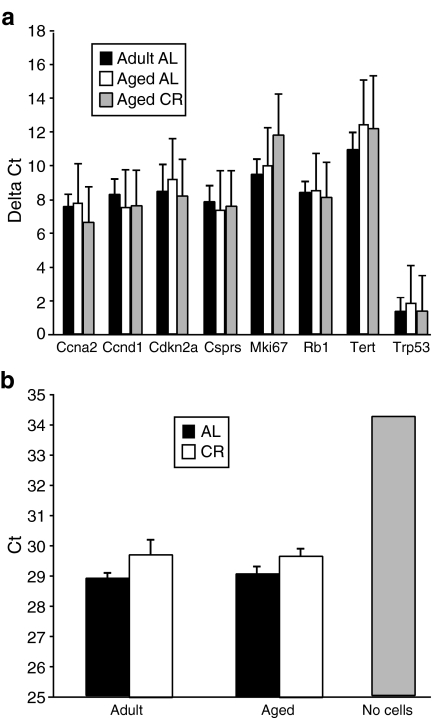
Effect of aging and CR on markers of proliferation and senescence in sca1+/CD31− CSP cells. a RNA was isolated from flow cytometry-isolated sca1+/CD31− CSP cells, and gene expression was analyzed by real-time RT–PCR gene panel. Data are expressed as mean delta Ct (=Ct sample − Ct average of housekeeping genes) ± SD; n = 4 per group. Gene expression did not differ between groups. b Telomerase activity in flow cytometry-isolated CSP cells was determined by quantitative real-time PCR and expressed as mean cycle threshold (Ct) ± SE; n = 4 per group. Activity was not different between groups
Table 1.
Effect of aging and CR on physical parameters of the heart
| Body weight (g) | Heart weight (mg) | HW/BW (mg/g) | Lin− cells/HW (thousand/mg) | |
|---|---|---|---|---|
| Adult AL | 29.1 ± 1.4 | 130.6 ± 3.8 | 4.5 ± 0.1 | 28.9 ± 2.2 |
| Adult CR | 20.2 ± 0.3b | 98.8 ± 2.7b | 4.9 ± 0.2 | 38.2 ± 3.7b |
| Aged AL | 32.3 ± 1.3 | 179.5 ± 6.9a | 5.7 ± 0.3a | 27.6 ± 1.3 |
| Aged CR | 20.6 ± 0.4b | 106.1 ± 1.9b | 5.2 ± 0.1 | 34.1 ± 2.1b |
Body weight was decreased by CR (p < 0.001). Heart weight was increased by aging in the AL group (p < 0.001) and decreased by CR (p < 0.01 for adult, p < 0.001 for aged). Ratio of heart weight to body weight (HW/BW) was increased by aging in the AL group (p < 0.01). Ratio of lineage-negative (lin−) non-cardiomyocytes to heart weight was increased by CR (p < 0.001). Data are expressed as mean ± SE; n = 6 for adult groups, n = 12 for aged groups
aSignificant effect of age
bSignificant effect of diet
Fig. 3.
Effect of aging and CR on sca1+/CD31− CSP cell gene expression. RNA was collected from flow cytometry-isolated sca1+/CD31− CSP cells and CMP cells, and gene expression was analyzed by real-time RT–PCR gene panel (n = 4 per group). a aged AL CSP vs. adult AL CSP, b aged CR CSP vs. aged AL CSP, and c adult AL CSP vs. adult AL CMP. Genes to the far left (downregulation) or far right (upregulation) of the bold vertical lines on the volcano plot indicate a fold change greater than 2-fold; genes above the bold horizontal line indicate a significant fold change (q < 0.05)
Results
Abundance of CSP cells and sca1+/CD31− CSP cells is altered with age but not CR
To determine whether CSP cell abundance is altered with age, lineage-negative non-cardiomyocyte (lin−) cells were isolated from hearts of adult and aged mice. ABCB1- and ABCG2-dependent efflux activity in the CSP fraction of these cells was confirmed by sensitivity to verapamil (Fig. 1a), and CSP cell number was determined by flow cytometry. We found that the number of CSP cells was 2.3-fold higher in aged mice than in adult mice (Fig. 1b, black bars). Therefore, any loss of progenitor potential in the aged heart could not be due to a decrease in the abundance of CSP cells.
The CSP is a heterogeneous population of cells, raising the possibility that while the total number of CSP cells increases with aging, the abundance of specific sub-populations within the CSP may be decreased. To determine if aging alters the abundance of a sub-fraction of the CSP that has been shown to form cardiomyocyte and endothelial cells in vivo, flow cytometry was used to measure the expression of the cell surface markers sca1 and CD31 on lin− CSP cells (Fig. 1c). Because the percentage of the CSP cells that were sca1+/CD31− (about 40–60%) was not significantly altered with aging, total sca1+/CD31− CSP cell number was elevated with age (3.2-fold; Fig. 1d, black bars).
Since CR is known to attenuate effects of aging in the heart, we hypothesized that CR might affect the age-associated increase in CSP and sca1+/CD31− CSP cell abundance. However, the number of CSP and sca1+/CD31− CSP cells was not significantly altered by CR in either age group (Fig. 1a, b, white bars). The effectiveness of the CR diet was confirmed by the expected anatomical time-of-sacrifice changes. Specifically, CR prevented the age-associated increase in body weight, heart weight, and ratio of heart weight to body weight (Table 1). CR also elevated the ratio of lin− non-cardiomyocyte cells to heart weight by 32% and 23% in adult and aged hearts, respectively (Table 1).
Markers of senescence in sca1+/CD31− CSP cells are not altered by aging or caloric restriction
It is possible that although sca1+/CD31− CSP cell number is increased with age, the cells may exhibit markers of senescence, as has been observed in other progenitor cells (Torella et al. 2004). To examine this, we looked at the expression of several genes associated with proliferation and senescence using a targeted real-time RT–PCR-based gene expression panel performed on sca1+/CD31− CSP cells. Neither aging nor CR altered gene expression of any of these genes (Fig. 2a).
We also measured telomerase activity (a marker of proliferative capacity) in CSP cells. Unfractionated CSP cells were used because the number of sca1+/CD31 CSP cells that could be isolated was insufficient for reliable detection. Telomerase activity was present in CSP cells from all four groups relative to a no-cell negative control, but there was no significant difference among the groups (Fig. 2b). This is consistent with our finding of unchanged proliferation and senescence marker gene expression.
Aging decreases expression of differentiation and immaturity genes in sca1+/CD31− CSP cells
While we did not observe age-associated senescence, it is possible that aged sca1+/CD31− CSP cells are altered in other pathways such as differentiation or maintenance of immaturity. Due to the uncertainties of maintaining the in vivo phenotypes of aging and CR in cell culture conditions for functional studies, we chose to examine the expression of differentiation- and immaturity-associated genes in the sca1+/CD31− CSP cells at the time of isolation. To do this, a targeted real-time RT–PCR-based gene expression array was performed on sca1+/CD31− CSP cells. Genes on this array were selected to cover a range of pathways, including immaturity, proliferation, and differentiation (Table 2).
Table 2.
Real-time RT–PCR panel gene list for the analysis of gene expression in sca1+/CD31− CSP cells and CMP cells
| Gene | Description | Gene | Description |
|---|---|---|---|
| Cellular immaturity | Differentiation | ||
| Abcb1a | ATP-binding cassette B1A/Mdr 1 | Acan | Aggrecan (chondrocyte) |
| Abcg2 | ATP-binding cassette G2 | Acta2 | Aorta actin alpha 2 (smooth muscle) |
| Aldh1a1 | Aldehyde dehydrogenase 1A1 | Actc1 | Cardiac actin alpha 1 (cardiac muscle) |
| Isl1 | Islet1 | Cd4 | CD4 antigen (T cell) |
| Pou5f1 | POU domain 5, factor 1/Oct4 | Cdh5 | Cadherin 5 (vascular endothelium) |
| Rexo1 | RNA exonuclease 1 homolog | Col1a1 | Collagen type 1 alpha 1 (bone) |
| Kdr | Kinase insert domain protein receptor/Flk1 | Cnn1 | Calponin 1 (smooth muscle) |
| Gata4 | GATA binding protein 4 (cardiac muscle) | ||
| Cell fate determination | Myod1 | Myogenic differentiation 1 (skeletal muscle) | |
| Hdac1 | Histone deacetylase 1 | Nes | Nestin (neuron) |
| Jag1 | Jagged 1 | Nkx2–5 | NK2 transcription factor related locus 5 (cardiac muscle) |
| Notch1 | Notch gene homolog 1 | Pah | Phenylalanine hydroxylase (liver) |
| Numb | Numb gene homolog | Pparg | Peroxisome proliferator-activated receptor gamma (adipose) |
| Pard6a | Partitioning defective 6 homolog alpha/Par-6 | ||
| Axin1 | Axin1 | Growth factors | |
| Wnt1 | Wingless-related MMTV integration site 1 | Bmp2 | Bone morphogenic protein 2 |
| Gja1 | Gap junction protein alpha 1/Connexin 43 | Cxcl12 | Chemokine C-X-C motif ligand 12 |
| Hgf1 | Hepatocyte growth factor | ||
| Cell cycle | Igf1 | Insulin-like growth factor 1 | |
| Ccna2 | Cyclin A2 | ||
| Ccnd1 | Cyclin D2 | Aging | |
| Cdkn2a | Cyclin-dependent kinase inhibitor 2A/p16INK4a | Csprs | Component of Sp 100-rs |
| Mki67 | Ki67 | ||
| Rb1 | Retinoblastoma 1 | ||
| Tert | Telomerase reverse transcriptase | ||
| Trp53 | Transformation related protein 53/p53 | ||
Genes are divided into six categories that cover a broad spectrum of relevant pathways: cellular immaturity, cell fate determination, cell cycle, differentiation, growth factors, and aging
Sca1+/CD31− CSP cell gene expression profiles were compared between adult and aged AL mice (Fig. 3a). Ten genes were differentially expressed between adult and aged AL sca1+/CD31− CSP cells. Aging caused the upregulation of three genes and the downregulation of seven genes. Three of the downregulated genes were markers for differentiation of cells into more mature cell types: Actca2a (smooth muscle), Kdr1 (early endothelial), and Actc1 (cardiac muscle). Two downregulated genes were markers of cellular immaturity: Isl1 and Pou5f1/Oct4.
No significant differences in expression were observed between aged AL and aged CR mice (Fig. 3b). Therefore, CR does not appear to have an obvious anti-aging effect on sca1+/CD31− CSP cells based on the parameters measured in this study.
The gene expression profile of sca1+/CD31− CSP cells differs greatly from CMP cells
To better characterize the sca1+/CD31− CSP cell relative to the other lin− non-cardiomyocytes, adult AL sca1+/CD31− CSP cells were compared to adult AL cardiac main population (CMP) cells (Fig. 3c). Seventeen genes were differentially expressed between sca1+/CD31− CSP and CMP cells; 11 were upregulated in sca1+/CD31− CSP cells, while six were downregulated. Of the 11 upregulated genes, at least five have been implicated in the notch pathway (Notch1, Hdac1, Pard6a, Numb, and Jag1). The expression of two genes, Myod1 (an early skeletal muscle marker) and Nkx2.5 (an early cardiac marker), was not detected in any samples. The absence of Nkx2.5 is consistent with a previous report that demonstrated that freshly isolated CSP cells did not express Nkx2.5 (Yamahara et al. 2008).
Discussion
The objective of this study was to determine if there is an effect of natural aging on CSP cells and if so, could the age-associated effect be attenuated by an anti-aging diet (CR). We found that aging elevated CSP cell number (Fig. 1b). Other groups have described the same effect of aging in various progenitor types (including cardiac), indicating that an increase in progenitor cell abundance may be a common response to aging (Morrison et al. 1996; Pearce et al. 2007; Torella et al. 2004). Therefore, it does not appear that age-associated cardiomyocyte loss is caused simply by a lack of progenitors in the aged heart.
The sca1+/CD31− sub-population of CSP cells is of particular interest since it has been shown to be capable of differentiation into both cardiomyocyte and endothelial cells in vitro and in vivo (Liang et al. 2009; Pfister et al. 2005). Therefore, these cells are an important sub-population to evaluate with respect to aging. We found that sca1+/CD31− CSP cell abundance was increased with age (Fig. 1d), ruling out the possibility that the age-associated increase in total CSP cells actually masked a decrease in this more relevant sub-population.
Some aged cardiac progenitor cell types have been reported to exhibit markers of senescence, interpreted as a possible reason why myocardial regeneration is not able to keep up with myocardial death, despite elevated total progenitor number (Chimenti et al. 2003; Rota et al. 2006; Torella et al. 2004). In contrast, we did not find this to be the case for sca1+/CD31− CSP cells (Fig. 2a). Specifically, aging did not increase expression of cell cycle arrest genes, such as p16ink4a/cdkn2a, Rb1, and p53. Likewise, aging did not decrease expression of proliferative markers such as ccna2, ccnd1, Mki67, or Tert, which codes for the protein component of telomerase. This finding was supported by a lack of age effect on telomerase activity in CSP cells (Fig. 2b). If sca1+/CD31− CSP cells do not exhibit markers of senescence with age, it is difficult to hypothesize that their level of proliferation could be a key determinant of age-associated cardiomyocyte loss. On the other hand, a progenitor cell that is not subject to elevated senescence could be an interesting target for in vivo activation in aged hearts.
We further characterized aged sca1+/CD31− CSP cells by determining their gene expression profile at the time of isolation (i.e., representative of the in vivo state). An alternate approach would be to observe proliferation and differentiation in vivo or in vitro. However, due to the low absolute cell number isolated from each heart, the experiments would require amplification of the CSP cells in culture. It must be emphasized that the systemic environment is critical to maintenance of the aging or CR phenotype and in vivo effects can be altered very quickly in culture, leading to ambiguous results (Conboy et al. 2005; Pignolo et al. 1992). This is a limitation of this study specifically, and of all aging/CR studies in general.
Discussing the effect of aging on sca1+/CD31− CSP cell gene expression is facilitated by first defining what makes sca1+/CD31− CSP cells unique from CMP cells. Compared to adult CMP cells, adult sca1+/CD31− CSP cells had altered expression of genes associated with cell immaturity and cell fate determination (Fig. 3c). Immature cells often feature increased protection from cytotoxic damage. In agreement with this, sca1+/CD31− CSP cells had elevated expression of the detoxification genes Abcb1, Abcg2, and Aldh1a1. Sca1+/CD31− CSP cells also expressed Isl1 at levels nearly 10-fold higher than CMP cells. It is thought that expression of this marker of cardiac immaturity decreases rapidly following birth (Cai et al. 2003), though to our knowledge this has never been examined in sca1+/CD31− CSP cells.
Many of the genes more highly expressed in sca1+/CD31− CSP cells were associated with the notch cell-fate signaling pathway, including Notch1, Jag1, Numb, Pard6, Gja1, and Hdac1. Hdac1 and notch1 have opposing actions in that notch1 induces gene expression by displacing hdac from its repressor complex (Kao et al. 1998). The simultaneous upregulation of Notch1 and Hdac1, coupled with upregulation of the asymmetric cell division genes Numb and Pard6, suggests a heterogeneous population in which multiple (sometimes opposing) processes are occurring simultaneously.
The gene expression profile of aged sca1+/CD31− CSP cells is similar to the adult profile except for a few key differences. First, aging decreased the expression of Notch1, and the immaturity markers Isl1 and Pou5f1/Oct4 (Fig. 3a). Second, aging reduced the expression of important differentiation markers of varied lineages (Fig. 3a). Specifically, Acta2a and Actc1 are genes for smooth muscle and cardiomyocyte-specific actins, respectively. Kdr1/Flk1 is first seen during development in the hemangioblast, and expression is retained in endothelial cells. There is also evidence that flk1+ cells can differentiate into cardiomyocytes (Baba et al. 2007).
The sca1+/CD31− CSP likely consists of varied progenitors and precursors that lie at intermediate stages along a spectrum of immaturity. Because of the decreased expression of both immaturity and differentiation markers, the aged sca1+/CD31− CSP cells do not seem to lie at either extreme end of this spectrum. One interpretation is that the aged cells are less efficient at moving through the intermediate stages toward a more mature phenotype, which could explain the accumulation of CSP cells with age. An alternate interpretation is that the aged cells move more rapidly through the intermediate stages to fully differentiated cells that do not isolate with the side population. In this case, accumulation of CSP with age would be due to increased proliferation. Since aged cells did not exhibit increased markers of proliferation, we favor the first interpretation.
While the anti-aging CR diet led to the expected changes in body weight and heart weight of aged mice, it did not alter sca1+/CD31− CSP cell abundance, markers of senescence, or expression of any of the genes on the RT–PCR panel. It remains a possibility that CR affects sca1+/CD31− CSP cells in ways that were not tested in this study, or that CR-mediated changes were affected by the cell isolation procedure. An alternate explanation is that sca1+/CD31− CSP cells do not age via pathways that are reversible by CR. For example, while aging of post-mitotic cells is characterized by a CR-reversible accumulation of oxidative damage and DNA mutations (Merry 2004), the expression of the detoxification genes Abcb1, Abcg2, and Aldh1a1 in sca1+/CD31− CSP cells may supercede the need for CR-dependent damage prevention. CR is also known to attenuate age-associated decline in cellular replicative capacity (Wolf and Pendergrass 1999). However, aged sca1+/CD31− CSP cells did not appear to show hallmarks of senescence, such as decreased telomerase activity, decreased expression of Mki67, and increased expression of p16INK4a/cdkn2a. Furthermore, while gene expression of Csprs was not changed with age in sca1+/CD31− CSP cells, its expression was nearly 6-fold lower in sca1+/CD31− CSP cells than in CMP cells. The gene expression of Csprs was the most altered in whole heart tissue by natural aging across seven mouse strains (Park et al. 2009). These results suggest that, depending on the “aging” markers, both adult and aged sca1+/CD31− CSP cells may seem young compared to post-mitotic tissue.
Aging increased the abundance of sca1+/CD31− CSP cells without a concomitant increase in markers of senescence. This positive property warrants further characterization of aged sca1+/CD31− CSP cells, despite the downregulation of select immaturity and differentiation genes. The lack of any CR-mediated change in sca1+/CD31− CSP cells suggests that the anti-aging effects of CR in the heart occur in other cell types such as the cardiomyocytes or different progenitor cells. CR may be of more use in studies where aged progenitors exhibit signs of senescence.
Acknowledgments
This work was supported by National Institutes of Health Grant R21HL092477 and an American Heart Association Greater Midwest Affiliate Post-doctoral Fellowship.
References
- Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–1345. doi: 10.1016/S0301-472X(02)00954-2. [DOI] [PubMed] [Google Scholar]
- Baba S, Heike T, Yoshimoto M, Umeda K, Doi H, Iwasa T, Lin X, Matsuoka S, Komeda M, Nakahata T. Flk1(+) cardiac stem/progenitor cells derived from embryonic stem cells improve cardiac function in a dilated cardiomyopathy mouse model. Cardiovasc Res. 2007;76:119–131. doi: 10.1016/j.cardiores.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/S1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Astle CM, Harrison DE. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Exp Hematol. 2003;31:1097–1103. doi: 10.1016/S0301-472X(03)00238-8. [DOI] [PubMed] [Google Scholar]
- Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SX, Tan TY, Gaudry L, Chong B. Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol. 2009;138:40–49. doi: 10.1016/j.ijcard.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med. 2009;25:715–732. doi: 10.1016/j.cger.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry BJ. Oxidative stress and mitochondrial function with aging—the effects of calorie restriction. Aging Cell. 2004;3:7–12. doi: 10.1046/j.1474-9728.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–495. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DJ, Anjos-Afonso F, Ridler CM, Eddaoudi A, Bonnet D. Age-dependent increase in side population distribution within hematopoiesis: implications for our understanding of the mechanism of aging. Stem Cells. 2007;25:828–835. doi: 10.1634/stemcells.2006-0405. [DOI] [PubMed] [Google Scholar]
- Pfister O, Liao R. Pump to survive: novel cytoprotective strategies for cardiac progenitor cells. Circ Res. 2008;102:998–1001. doi: 10.1161/CIRCRESAHA.108.176453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Masoro EJ, Nichols WW, Bradt CI, Cristofalo VJ. Skin fibroblasts from aged Fischer 344 rats undergo similar changes in replicative life span but not immortalization with caloric restriction of donors. Exp Cell Res. 1992;201:16–22. doi: 10.1016/0014-4827(92)90343-7. [DOI] [PubMed] [Google Scholar]
- Rota M, LeCapitaine N, Hosoda T, Boni A, Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- Saupe KW, Mulligan JD. Beyond obesity prevention: the anti-aging effects of caloric restriction. In: Bagchi D, Preuss H, editors. Obesity: epidemiology, pathophysiology, and prevention. Boca Raton: CRC; 2007. pp. 265–277. [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman MA, Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. doi: 10.1146/annurev.physiol.66.032102.140723. [DOI] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor–receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- Wolf NS, Pendergrass WR. The relationships of animal age and caloric intake to cellular replication in vivo and in vitro: a review. J Gerontol A Biol Sci Med Sci. 1999;54:B502–B517. doi: 10.1093/gerona/54.11.B502. [DOI] [PubMed] [Google Scholar]
- Yamahara K, Fukushima S, Coppen SR, Felkin LE, Varela-Carver A, Barton PJ, Yacoub MH, Suzuki K. Heterogeneic nature of adult cardiac side population cells. Biochem Biophys Res Commun. 2008;371:615–620. doi: 10.1016/j.bbrc.2008.04.021. [DOI] [PubMed] [Google Scholar]