Abstract
Of the acetylcholine muscarinic receptors, the type 1 (M1) and type 2 (M2) receptors are expressed at the highest levels in the prefrontal cortex (PFC) and hippocampus, brain regions important for cognition. As equivocal findings of age-related changes of M1 and M2 in the nonhuman primate brain have been reported, we first assessed age-related changes in M1 and M2 in the PFC and hippocampus using saturation binding assays. Maximum M1 receptor binding, but not affinity of M1 receptor binding, decreased with age. In contrast, the affinity of M2 receptor binding, but not maximum M2 receptor binding, increased with age. To determine if in the elderly cognitive performance is associated with M1 or M2 function, we assessed muscarinic function in elderly female rhesus macaques in vivo using a scopolamine challenge pharmacological magnetic resonance imaging and in vitro using saturation binding assays. Based on their performance in a spatial maze, the animals were classified as good spatial performers (GSP) or poor spatial performers (PSP). In the hippocampus, but not PFC, the GSP group showed a greater change in T2*-weighted signal intensity after scopolamine challenge than the PSP group. The maximum M1 receptor binding and receptor binding affinity was greater in the GSP than the PSP group, but no group difference was found in M2 receptor binding. Parameters of circadian activity positively correlated with the difference in T2*-weighted signal intensity before and after the challenge, the maximum M1 receptor binding, and the M1 receptor binding affinity. Thus, while in rhesus macaques, there are age-related decreases in M1 and M2 receptor binding, in aged females, hippocampal M1, but not M2, receptor function is associated with spatial learning and memory and circadian activity.
Keywords: M1 receptor, Scopolamine phMRI, Spatial maze
Introduction
Muscarinic acetylcholine receptors are associated with postsynaptic neurotransmission and memory function (Thathiah and De Strooper 2009). They are present in high concentrations in the prefrontal cortex (PFC) and the hippocampus (Nathanson 2008) and have an integral role in spatial learning and memory in rodents, nonhuman primates, and humans (Wisman et al. 2008; Gage et al. 1988; Fredrickson et al. 2008; Thomas et al. 2008). Scopolamine, a nonspecific muscarinic receptor antagonist (Elrod and Buccafusco 1988), impairs cognitive task performance in rats (Biggan et al. 1996), dogs (Araujo et al. 2005), rhesus monkeys (Savage et al. 1996; Taffe et al. 1999), and humans (Rosier et al. 1998). The most highly expressed muscarinic receptor in the PFC and hippocampus, regions critical for cognitive function, is the type 1 (M1) subtype (Gage et al. 1988; Fredrickson et al. 2008; Thomas et al. 2008; Flynn et al. 1995a,b; Tamminga 2006; Wisman et al. 2008). The muscarinic type 2 (M2) is the second most abundant muscarinic receptor in the PFC and hippocampus (Rouse et al. 2000; Jagoda et al. 2003) and might also contribute to cognitive function (Gautam et al. 2006). M1 receptor-deficient mice show impaired working memory and memory consolidation, and M2 receptor-deficient mice show impaired passive avoidance task performance (Matsui et al. 2004). Nonhuman primate studies show a decrease in M1 and M2 receptor number with age (Wagster et al. 1990). Other reports indicate an age-related decrease in M1 receptor affinity (Vannucchi and Goldman-Rakic 1991). In patients with Alzheimer’s disease, M1 receptor agonists are proposed to increase cognitive function and neurogenesis and decrease tau phosphorylation (Ma et al. 2009; Fisher et al. 2003; Van Kampen and Eckman 2009; Fisher 2008). Together, these findings suggest that the M1, and to a lesser extent M2, receptors may play a key role in the regulation of cognitive function in the elderly.
Cognitive function is negatively impacted by disruptions in circadian activity, especially in the elderly (Haimov et al. 2008; Morin et al. 1998). For example, circadian activity has been correlated with cognitive performance on a spatial task in old female rhesus monkeys (Haley et al. 2009). The M1 receptor might be involved in regulation of circadian activity (Gillette et al. 2001; Liu and Gillette 1996), and this, in turn, might contribute to the M1 receptor-mediated cognitive effects.
Neurotransmitter function can be readily assessed in vivo using pharmacological magnetic resonance imaging (phMRI). Although similar to functional MRI (fMRI), which measures blood oxygen level-dependent (BOLD) signal changes associated with cognitive performance, phMRI measures BOLD signal changes following exposure to pharmacological stimuli (Wise and Tracey 2006). This technique has been successfully used in humans and nonhuman primates to study the function of the dopamine system (Knutson and Gibbs 2007). For example, in experimentally 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned rhesus monkeys, the BOLD signal was found to be positively correlated with the number of surviving dopaminergic neurons (Zhang et al. 2006). Importantly, because the response of these neurons to dopaminergic agonists is lesion-dependent, phMRI can be utilized clinically to assess the efficacy of therapies in Parkinson’s disease patients (Luan et al. 2008). Although fewer phMRI studies have focused on the acetylcholine system (Wink et al. 2006; Thiel 2003), it is plausible that they also will yield clinical applications.
In this study, muscarinic receptor function was assessed in rhesus macaques in brain areas important for spatial learning and memory. First, to determine potential age-related changes, M1 and M2 receptor function was assessed in the PFC, hippocampus, and temporal cortex (TC) of noncognitively tested adult, middle-aged, and aged rhesus macaques. Then, muscarinic receptor function was assessed in a cohort of cognitively tested elderly female rhesus macaques in vivo using phMRI and in vitro using saturation binding assays. Based on the number of trials to reach a criterion performance in the food port spatial maze (Haley et al. 2009), the monkeys were classified into two groups: good spatial performers (GSP) and poor spatial performers (PSP). In the phMRI experiment, the animals were given scopolamine and the difference between baseline and challenge T2*-weighted image intensities in the PFC and hippocampus were assessed. Subsequently, postmortem M1 and M2 receptor binding assays were performed using the PFC and hippocampus tissues of these animals. Finally, the potential relationships between circadian activity, the difference in T2*-weighted image intensities and M1 and M2 receptor binding were explored.
Methods
Receptor binding assays: cross-sectional aging study
Postmortem tissues from the PFC, hippocampus, and TC were obtained from adult (5–9 years; n = 5; 3 male, 2 female, reproductively cycling), middle-aged (11–16 years; n = 5; 2 male, 3 female, reproductively cycling), and aged (22–30 years; n = 5; female, not reproductively cycling) male and female rhesus macaques (Macaca mulatta), through the Oregon National Primate Research Center (ONPRC) Tissue Distribution Program at Oregon Health and Science University (OHSU). At death, the average weight was 6.26 ± 0.85 kg for adult, 6.6 ± 0.79 kg for middle-aged, and 6.78 ± 0.59 kg for aged animals. All animals for the cross-sectional study were healthy during their lifetime and behaviorally naive. The brains were perfused with saline at room temperature, immediately extracted from the skull, dissected, flash frozen with liquid nitrogen, and stored in −80°C.
The tissues were homogenized in 1 ml of ice-cold buffer solution (140 mM NaCl, 0.2 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, 50 mM Tris, pH 7.5), centrifuged at 16,000 × g for 25 min, washed in ice-cold buffer, and recentrifuged. This was repeated for a total of four times, and the pellet was stored at −80°C until assayed. Pellets were re-suspended in ice-cold buffer (10 mM NaH2PO4, 10 mM Na2HPO4, 1 mM MgCl2, pH 7.5), and the protein concentration of each pellet was measured using the bicinchoninic acid (BCA) (Pierce Pharmaceuticals, Rockford, IL) protein assay kit. Specific binding and nonspecific binding were measured in duplicate. Eight different increasing concentrations of the M1 receptor ligand [3H]pirenzepine (312.5 pM to 80 nM) or the M2 receptor ligand [3H]AFDX-384 (312.5 pM to 80 nM) were used. Nonspecific binding was assessed using 2.5 μM of atropine for both M1 and M2 receptors. All samples were assayed for M1 and M2 receptor binding in separate experiments. Once radioligand was added to the tissue samples, they were incubated for 1 h at room temperature. The incubation was terminated by adding 5 ml of ice-cold binding buffer. Bound and free radioligand tissues were separated using Whatman filter paper and a Brandell cell harvester. Subsequently, the filters were placed in vials and after adding 5 ml of scintillation fluid counted in a beta-scintillation counter (LS 6000SC Beta counter, Beckman, Fullerton, CA).
phMRI procedures
Animal procedures were approved by the Institutional Animal Care and Use Committee of the ONPRC at OHSU. Monkeys [n = 17 females rhesus macaques (Macaca mulatta), age range: 19–25 years, mean age = 21.25 years, reproductively inactive; maintained as previously described (Haley et al. 2009)] were fasted the night before and the morning of MRI experiments, and phMRI was completed in the morning (before 1100 h). Monkeys were sedated with ketamine (1 mg/kg) and transported from their home cages to a Siemens whole-body 3-T trio MRI system (Erlinger, Germany) located at the ONPRC campus. Animals were immediately intubated and respirated, free-breathing, with 0.9–1.1% isoflurane, and an MRI-compatible catheter was inserted into the cephalic vein. Physiological parameters, including blood oxygen levels, carbon dioxide, blood pressure, heart rate, temperature, and respiration rate, were monitored continuously throughout the experiment using an MRI-compatible Precess monitor (Invivo, Orlando, FL).
The animal’s head was placed inside a quadrature transmit/eight-channel receive human extremity RF coil (Invivo) and fitted with pads to eliminate head movement. After acquisition of a series of “localizer images”, 20 contiguous T2*-weighted multi-shot echo planar images were obtained over the course of 7 min. (TR = 1,800 ms, TE = 30 ms, 4 segments, 2 mm slices, flip angle 90°, 24 slices, 20 volumes). These images were designated “baseline” herein for BOLD measurements. Immediately following the baseline scans, scopolamine (0.4 mg in 1 ml of isotonic saline i.v., n = 12; or 1 ml of isotonic saline for control animals, n = 5) was infused over 1 min using an infusion pump (Harvard Apparatus, Holliston, MA). Following the intravenous infusion, a T2- (TR = 5,280 ms, TE = 57 ms, 1 mm slice thickness, flip angle 120°, 50 slices) and 2 T1- (TR = 2,500 ms, TE = 4.38 ms, 0.6 mm slice thickness, flip angle 12°, 88 slices) weighted anatomical images were acquired. The total time for acquisition of anatomical scans was 46 min, sufficient time for scopolamine to have CNS effects (Ali-Melkkila et al. 1993). Immediately following the anatomical scans, a second BOLD series of T2*-weighted images was acquired using the exact parameters as the baseline scan (“challenge scan”).
Using the MRI analysis software AFNI [National Institutes of Health (NIH; Gold et al. 1998)], the difference in T2*-weighted image intensities between the baseline to challenge scan (scopolamine or vehicle) was determined. Isotonic saline control was used to ensure that the change observed between the baseline and challenge scan was due to a scopolamine effect and not an artifact from signal drift of the magnet. Slice-dependent time shifts were interpolated, odd–even slice intensity differences were removed, motion was corrected, and T1 and T2 anatomical scans were co-registered. Baseline and challenge phMRI scans were averaged across the 20 volumes, and the single image was normalized to the co-registered anatomical scan of each individual animal. Regions of interest (ROIs) were drawn for the PFC and hippocampus based on the anatomical scan using key neuroanatomical landmarks from anatomical scans: medial temporal lobe and the hippocampal sulcus (Small et al. 2004) for the hippocampus and the frontal pole and the emergence of the anterior horn of the lateral ventricle for the PFC. The ROI was drawn for each animal and was the same ROI for the baseline and scopolamine scan, both of which were co-registered to the anatomical scans.
The cohort of cognitively characterized animals was separated into two groups, GSP and PSP, based on performance in the spatial food port maze (Haley et al. 2009). Briefly, the spatial food port maze consisted of a bank of ten ports in a testing room, each port containing a one-way, opaque swinging door. Freely moving monkeys were placed in the room and were allowed to search the ports. One port was repeatedly baited with a preferred candy reward until the animal learned the location of the reward (first location) and the number of trials to criterion recorded. Once the first location was learned, the location of the baited food port was moved (second location) and the new port was repeatedly baited until the animal learned the location. The cohort that participated in the spatial food port maze was classified based on the performance of the whole group. GSP was defined as animals that completed the task in less than 30 trials in either the first or second position (n = 6;  ; 18.7 ± 4.8 trials, n = 4 for the first location; 7 and 14 trials, n = 2 for the second location). PSP was defined as animals that completed the task in more than 30 trials (n = 6;
; 18.7 ± 4.8 trials, n = 4 for the first location; 7 and 14 trials, n = 2 for the second location). PSP was defined as animals that completed the task in more than 30 trials (n = 6;  ; 81.8 ± 8.5 trials). Control animals were animals from the same cohort that did not participate in the spatial maze testing (n = 5;
; 81.8 ± 8.5 trials). Control animals were animals from the same cohort that did not participate in the spatial maze testing (n = 5;  ).
).
Circadian activity measures
Circadian activity measures were derived from the time points previously published (Haley et al. 2009) and were used in the current study for correlational purposes. In that study, animals were equipped with an Actiwatch activity recorder (Philips/Respironics, Bend, OR) which was worn on a nylon collar (Primate Products, Immokalee, FL) during the testing period. Activity counts were averaged over 2 weeks of measurement. Data was analyzed with Actiware-Sleep (Philips/Respironics) software for activity parameters and sleep quality.
Receptor binding assays: cognitively tested animals
Animals were euthanized within 1 week of completing the cognitive battery at ONPRC by trained pathologists according to Veterinarian approved procedures; postmortem tissues were made available to the investigators through the ONPRC Tissue Distribution Program. Saturation binding assays were performed on PFC and hippocampal wet tissues dissected from the right side of the brain; to ensure consistency, the same individual performed each of these dissections. The PFC and hippocampal tissue blocks were frozen in liquid nitrogen, and stored at −80°C. Tissues were assayed exactly as stated above under the “Receptor Binding Assays: Cross-Sectional Study” section. Maximum receptor binding (Bmax) and the equilibrium dissociation constant (Kd), the inverse of receptor binding affinity, in each brain region for each animal was determined using the Hill equation using nonlinear regression analysis.
Statistical analyses
For the phMRI analyses, statistical parametric mapping using general linear model and random effects analysis comparing signal intensities in voxels (Dumas et al. 2008), Student’s t tests for baseline T2*-weighted image intensity between GSP and PSP, and Mann–Whitney U tests for the difference in T2*-weighted image intensities before and after scopolamine challenge with MiniTab software (MiniTab, State College, PA) were used. For the receptor binding assay analyses, Prism software (GraphPad Software, La Jolla, CA) was used. Further analyses using Prism software involved Student’s t tests to determine potential statistical differences in Bmax, Kd, and circadian measures between the GSP and the PSP. Pearson’s product–moment correlations were computed using Prism software. Data are presented as group means ± SEM. Significance was considered at P < 0.05 for all analyses.
Results
Cross-sectional receptor binding assays
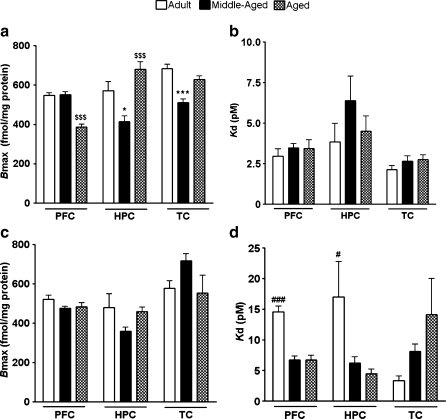
M1 Bmax was significantly lower in the aged than adult or middle-aged PFC [F(2, 14) = 46.88, P = 0.0001; Fig. 1a]. Conversely, in the TC, M1 Bmax was significantly lower in middle-aged than adult or aged animals [F(2, 14) = 21.58, P = 0.0001]. In the hippocampus, M1 Bmax was lower in the middle-aged than adult or aged animals. However, in aged animals, there was a significantly higher M1 Bmax than in adult animals [F(2, 14) = 12.31, P = 0.001]. M1 Kd was not significantly different between the age groups in the PFC (P = 0.6), hippocampus (P = 0.3), or TC (P = 0.3; Fig. 1b).
Fig. 1.
Age-related changes in M1 and M2 maximum receptor binding and receptor binding affinity. a Maximum M1 receptor binding (Bmax) for the PFC, hippocampus (HPC), and temporal cortex (TC) of adult, middle-aged, and aged rhesus macaques. b Dissociation constant (Kd) of M1. c Maximum M2 receptor binding. d Dissociation constant for M2. *P < 0.05 compared to adult and aged animals, $$$P < 0.001 compared to adult and middle-aged animals, ***P < 0.001 compared to adult and aged animals, #P < 0.05 compared to middle-aged and aged, and ###P < 0.001 compared to middle-aged and aged animals
M2 Bmax was not significantly altered with age in the PFC (P = 0.16), hippocampus (P = 0.15), or TC (P = 0.16; Fig. 1c). M2 Kd was not significantly different between age groups in the TC (P = 0.14). Yet, M2 Kd was significantly higher in the adult PFC [F(2, 14) = 35.17, P = 0.0001] and hippocampus [F(2, 14) = 4.00, P = 0.04] compared to those in brain region-matched middle-aged and aged animals (Fig. 1d).
Pharmacological magnetic resonance imaging
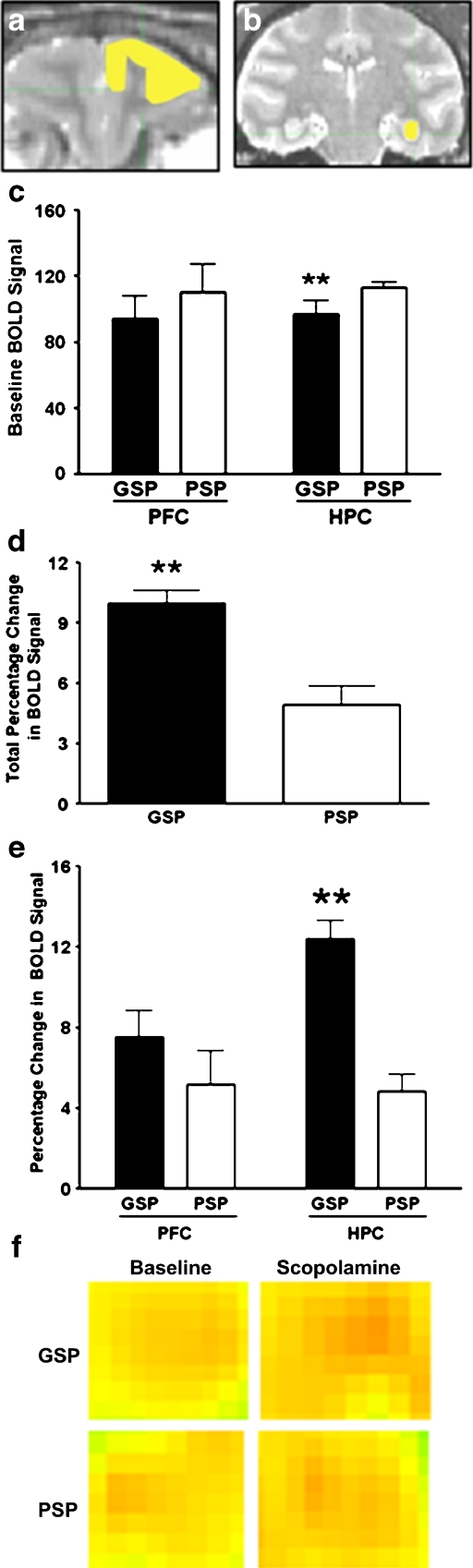
Control animals administered isotonic saline did not show a significant T2*-weighted image intensity change in the PFC (1.67 ± 0.57%; Fig. 2a) or hippocampus (1.53% ± 0.60%; Fig. 2b). In the PFC, the baseline T2*-weighted image intensity was not different between the GSP and the PSP groups (P = 0.9; Fig. 2c). However, in the hippocampus, the baseline T2*-weighted image intensity was significantly lower in the GSP compared to the PSP group [t(5) = 3.18; P = 0.01; Fig. 2c]. Following scopolamine challenge, the average T2*-weighted image intensity was not significantly different between GSP and PSP (P = 0.5). However, there was a significant difference in T2*-weighted image intensities following scopolamine administration in all the ROIs [U(5) = 19.5; P = 0.002; Fig. 2d and f] in the GSP and the PSP animals. In the hippocampus, the BOLD-mediated signal change from baseline to the scopolamine challenge scan was significantly greater in the GSP than the PSP group [U(5) = 21.0, P = 0.002; Fig. 2e and f]. In contrast, in the PFC, there was no difference in T2*-weighted signal intensity between PSP and GSP animals treated with scopolamine as compared to their baseline scan (P = 0.3).
Fig. 2.
Representative region of interest (ROI) drawings of a prefrontal cortex (PFC; yellow scale bar = 1.5 mm) and b hippocampus (HPC; yellow scale bar = 3 mm) on co-registered anatomical T1 and T2 coronal images. c Baseline BOLD signal (T2*-weighted signal intensity) for Good Spatial Performers (GSP; black bar) and Poor Spatial Performers (PSP; white bar) in the PFC and HPC of female rhesus macaques. d Total percentage BOLD signal change (T2*-weighted signal intensity before and after scopolamine challenge; overall change in the brain regions analyzed) following scopolamine challenge in GSP and PSP. e Percentage change in BOLD signal (T2*-weighted signal intensity) in the PFC and HPC of GSP and PSP. f Representative images of hippocampus ROI in baseline and scopolamine scans of a GSP and a PSP animal (black scale bar = 1.5 mm). Data are presented as group mean ± SEM. ** P < 0.01 compared to PSP
Receptor binding assays: cognitively tested animals
In the PFC, Bmax and Kd of M1 receptor binding (Fig. 3a and b; Bmax: P = 0.7; Kd: P = 0.2) and Bmax of M2 receptor binding (Fig. 3c and d; Bmax: P = 0.4; Kd: P = 0.3) was comparable in the GSP and the PSP groups. Conversely, in the hippocampus, the M1 Bmax was significantly higher [Bmax: t(5) = 3.6; P = 0.004; Fig. 3a] and M1 Kd was significantly lower in the GSP than the PSP group [Kd: t(5) = 2.6; P = 0.02; Fig. 3b]. M2 Bmax in the hippocampus was not significantly different between GSP and PSP (P = 0.6; Fig. 3c), but there was a trend toward a lower Kd in the GSP compared to the PSP (P = 0.06; Fig. 3d).
Fig. 3.
a Maximum receptor binding (Bmax) and b dissociation constants (Kd) from saturation binding curves of M1 from PFC and HPC tissue good spatial performers (GSP; black bar) and poor spatial performers (PSP; white bar). c Maximum receptor binding and d dissociation constants from saturation binding curves of M2 from PFC and HPC tissue GSP and PSP. Data presented as group means ± SEM. *P < 0.05 **P < 0.01 compared to PSP
Circadian activity measures
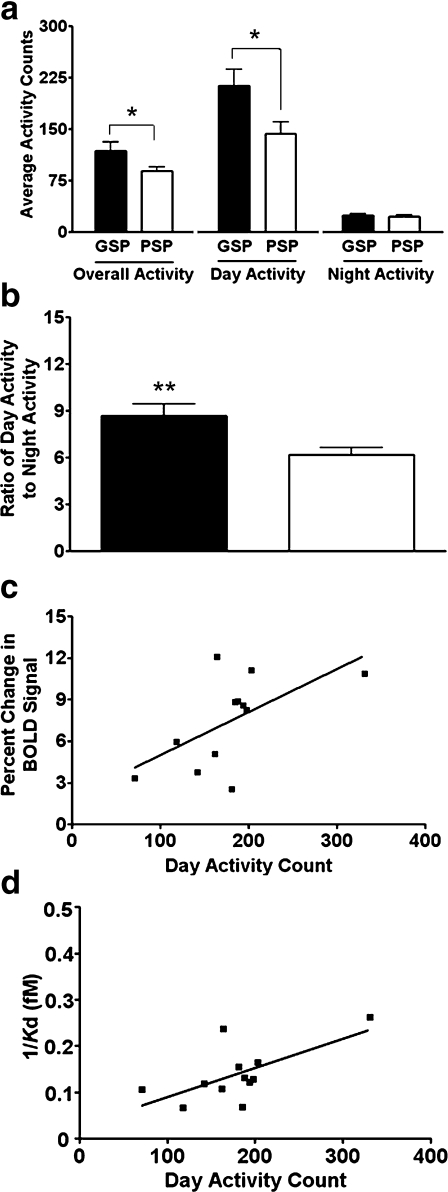
Overall [t(5) = 2.16; P = 0.04] and day [t(5) = 2.33; P = 0.04] activities were significantly greater in the GSP than the PSP group (Fig. 4a). Light-to-dark activity ratio was also greater in the GSP than the PSP group [t(5) = 2.79; P = 0.02; Fig. 4b]. Day activity was positively correlated with the difference in T2*-weighted signal intensity before and after scopolamine challenge (r = 0.70; r2 = 0.49; P = 0.007; Fig. 4c), negatively correlated with the inverse of Bmax (r = −0.54; r2 = 0.30; P = 0.03; data not shown), and positively correlated with the inverse of Kd (r = 0.65; r2 = 0.42; P = 0.02; Fig. 4d) of M1 receptor binding in the hippocampus.
Fig. 4.
a Overall, day, and night activity of good spatial performers (GSP; black bar) and poor spatial performers (PSP; white bar). b Ratio of day-to-night activity for the GSP and the PSP groups. Data are presented as mean ± SEM. *P < 0.05 and **P < 0.01. c Correlation of day activity and the percentage change in BOLD signal (T2*-weighted signal intensity before and after scopolamine challenge) in the hippocampus following scopolamine challenge (r2 = 0.49, P = 0.007). d Correlation of day activity and M1 affinity in the hippocampus (Kd; r2 = 0.42, P = 0.02)
Discussion
In this study, we show age-related changes in the maximum M1, but not M2, receptor binding in the PFC, hippocampus, and TC, of rhesus macaques. Maximum M1 receptor binding in the PFC decreases with age. Compared to adult animals, there is a decrease in the hippocampus and TC in middle-aged animals, but an increase in aged animals. Together, these data suggest that the timeline of age-related muscarinic receptor changes is brain region-dependent. Other papers have reported an age-related change in M1 receptors in the elderly. The potential discrepancy in these studies is the age range used in each study. Previous studies used animals that were only 20 years old (Wagster et al. 1990) or did not include middle-aged animals (Vannucchi and Goldman-Rakic 1991). In contrast, in the current study, we used adult, middle-aged, and aged animals, and our aged animals were 22–30 years. Therefore, with the present age range, we were able to identify more subtle age-related changes not only from adult to middle age but also those that occur in the oldest-old. The increase in maximum M1 receptor binding in the aged population could possibly be part of a compensatory mechanism in response to age-related decreases in acetylcholine (Costall et al. 1990; Sarter and Turchi 2002). Although this particular study did not have a sufficient animal number to compare male and female monkeys, there might be sex differences in M1 and M2 muscarinic receptor binding. It is also possible that the estrogen cycle in the monkeys affected M1 expression patterns (Tinkler and Voytko 2005). However, inconsistent results in the literature indicate that estrogen effects are likely determined by many factors including age, individual animal, and integrity of the muscarinic system, and that there is no clear consistent effect of estrogen on the muscarinic receptor system (Bartholomeusz et al. 2008). Due to the hypothesized role of muscarinic receptors in cognitive function, and the highly expressed M1, and to a lesser extent M2, in brain areas highly associated with cognitive function, it is conceivable that elderly cognitively tested animals have a different pattern of M1 and M2 expression, dependent on their cognitive performance. Therefore, it was important to include behaviorally naive animals in the current study.
The phMRI and receptor binding results suggest that the activity of the neurons in the hippocampus has a greater reliance on muscarinic receptors than the neurons of the PFC. However, it is possible that prior exposure to a hippocampal-dependent task contributed to the difference observed between the hippocampus and PFC. Following scopolamine challenge, there was a significantly greater increase in BOLD signal in the hippocampus of the GSP than PSP group, which appears to be related to cognitive performance and not age because there was no age difference between the groups. A similar pattern was observed in the PFC but it did not reach statistical significance. As there is a decrease in cognitive performance following scopolamine injection (Thiel 2003; Klinkenberg and Blokland 2010; Ebert and Kirch 1998), the increase in BOLD signal might be counterintuitive. However, antagonism of the muscarinic receptor system could initiate downstream effects on neurotransmitters, including acetylcholine, to be released locally to compensate (Day et al. 1991; Scali et al. 1995), thereby increasing the BOLD signal. The neurotransmitters and the neurons releasing them could initiate the recruitment of other neuronal populations, increasing neuronal activity, which could contribute to the increase in BOLD signal. Because scopolamine targets other muscarinic receptors, including presynaptic muscarinic receptors, blockade of those receptors can initiate the local release of acetylcholine, measured in the brain with microdialysis (Day et al. 1991). Thus, it is feasible that a more specific antagonist for the M1 receptor might have an alternate BOLD effect than scopolamine. Alternatively, the scopolamine dose used might have saturated muscarinic receptors in the hippocampus of PSP animals, but was not sufficient to saturate the muscarinic receptors in the hippocampus of GSP animals, and this might account for the difference between the groups. Based on the data, we hypothesize that the higher maximum muscarinic receptor binding in the GSP than the PSP group causes a greater compensatory effect, resulting in greater difference in T2*-weighted signal intensity before and after a scopolamine challenge. Thus, the BOLD signal might indicate the flexibility of the brain to compensate for a temporary blockade of the muscarinic receptor system, and the brains showing more flexibility might be those showing better cognitive performance. Receptor binding assays are consistent with this hypothesis and corroborate the phMRI findings. In the hippocampus, the maximum M1 receptor binding and M1 receptor binding affinity are higher in the GSP than the PSP group. Thus, a greater increase in BOLD signal in the hippocampus is associated with a greater maximum receptor binding and binding affinity of M1 receptors. The maximum M2 receptor binding and M2 receptor binding affinity was not different between the two groups, suggesting that M2 have a minor role in spatial learning and memory (Matsui et al. 2004).
In the PFC, there was more variability in the difference in T2*-weighted image intensities as well as in the baseline image intensities than in the hippocampus. Because the groups were defined based on performance in the spatial maze, this anatomical difference might be related to the fact that spatial learning and memory heavily relies on the hippocampus (Raber et al. 2004; Cho et al. 1999; Banta Lavenex and Lavenex 2009; Lavenex et al. 2006). Interestingly, there was lower baseline T2*-weighted image intensity in the hippocampus of the GSP than the PSP group. This lower baseline BOLD signal in the GSP hippocampus might have contributed to a greater response measured by scopolamine injection, while there was no difference in the T2*-weighted image intensity after scopolamine challenge between the GSP and the PSP groups. Furthermore, the lower baseline T2*-weighted image intensity in the hippocampus of the GSP group might indicate a different neuronal connectivity (Fransson 2004), as observed when functional connectivity MRI studies are performed during a resting state (Qiu et al. 2010; Pereira et al. 2010) and, in this way, contribute to their enhanced cognitive performance in the spatial maze.
There are many variables to consider with phMRI in animals including pharmacological intervention and anesthesia. Scopolamine has a half-life of 17 min in plasma (Frey et al. 1985); it crosses the blood brain barrier quickly (Frey et al. 1991), and the effects in the CNS are present for up to 8 h (Ali-Melkkila et al. 1993). In the brain, scopolamine binds to muscarinic receptors (Wamsley et al. 1980; Frey et al. 1985) and alters EEG activity in healthy resting humans (Ebert et al. 2001). We used isoflurane to anesthetize the animals in the present phMRI experiment and were able to measure BOLD signal changes. Other studies support this finding, demonstrating that the brain responds to stimuli during anesthesia, even at high doses (Porkkala et al. 1997; Hartikainen and Rorarius 1999), and that isoflurane does not fully suppress EEG activity (Tsushima et al. 1998; Ogawa et al. 1992; Murrell et al. 2008). Experimental fMRI studies demonstrate that isoflurane anesthesia between the range of 0.9% and 1.25% preserves a measurable BOLD signal (Vincent et al. 2007) level in the anesthetized monkey brain (Logothetis et al. 1999; Vincent et al. 2007; Small et al. 2004). Isoflurane was appropriate for our study because its effects are not mediated through the cholinergic system nor does it suppress acetylcholine activity or receptor function (Eger et al. 2002; Nakayama et al. 2006).
In the food port spatial maze (Haley et al. 2009), circadian activity was correlated with performance; animals with higher day activity and lower sleep latencies, wake bouts, and dark-to-light activity ratios performed better on the spatial maze. Here, we report that circadian parameters, using the same time points as previously reported, positively correlated with the difference in T2*-weighted image intensities before and after scopolamine challenge and with receptor binding affinity of M1 receptors in the hippocampus and negatively correlated with the inverse maximum M1 receptor binding. Although these results seem counterintuitive, both correlations suggest that more day activity is observed in animals with greater maximum receptor binding, as it was an inverse negative correlation, and higher receptor binding affinity (1/Kd). These data support an interconnected relationship between hippocampal M1 receptor function, spatial learning and memory, and circadian activity. Therefore, it is possible that the negative circadian effect on cognition is due to a negative circadian effect on muscarinic receptor function which results in decreased cognitive function.
In summary, the present study demonstrates that (a) age-related changes are observed in maximum M1 receptor binding, but not M1 receptor binding affinity, as well as M2 receptor affinity, but not maximum M2 receptor binding; and (b) the BOLD response to scopolamine and alterations in M1 receptor function in the hippocampus of rhesus monkeys previously characterized as showing superior spatial learning and memory is enhanced. These data support an important role for hippocampal M1 receptors in spatial learning and memory in elderly female primates. These results demonstrate that, in aged female rhesus monkeys, M1 receptor function is associated with performance in a spatial maze. Furthermore, these data demonstrate the value of using scopolamine phMRI for assessment of muscarinic receptor function in the context of cognitive performance and circadian activity.
Acknowledgments
The authors would like to thank Dominique Eghlidi, Sharon Kryger, Laurie Renner, Alison Weiss, and Vince Warren for their technical assistance. This work was supported by NIH grants RR-000163, AG-023477, and AG-029612 and an OHSU Tartar Fellowship.
References
- Ali-Melkkila T, Kanto J, Iisalo E. Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scand. 1993;37(7):633–642. doi: 10.1111/j.1399-6576.1993.tb03780.x. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(3):411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Banta Lavenex P, Lavenex P. Spatial memory and the monkey hippocampus: not all space is created equal. Hippocampus. 2009;19(1):8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz CF, Wesnes KA, Kulkarni J, Vitetta L, Croft RJ, Nathan PJ. Estradiol treatment and its interaction with the cholinergic system: effects on cognitive function in healthy young women. Horm Behav. 2008;54(5):684–693. doi: 10.1016/j.yhbeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Biggan SL, Ingles JL, Beninger RJ. Scopolamine differentially affects memory of 8- and 16-month-old rats in the double y-maze. Neurobiol Aging. 1996;17(1):25–30. doi: 10.1016/0197-4580(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Cho YH, Friedman E, Silva AJ. Ibotenate lesion of the hippocampus impair spatial learning but not contextual fear conditioning. Behav Brain Res. 1999;98:77–87. doi: 10.1016/S0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Costall B, Barnes JM, Hamon M, Muller WE, Briley M. Biochemical models for cognition enhancers. Pharmacopsychiatry. 1990;23(Suppl 2):85–88. doi: 10.1055/s-2007-1014540. [DOI] [PubMed] [Google Scholar]
- Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38(4):723–729. doi: 10.1016/0091-3057(91)90233-R. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Saykin AJ, McDonald BC, McAllister TW, Hynes ML, Newhouse PA. Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. Am J Geriatr Psychiatry. 2008;16(4):272–282. doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U, Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Investig. 1998;28(11):944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynapic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41:51–60. doi: 10.1177/00912700122009836. [DOI] [PubMed] [Google Scholar]
- Eger EI, Zhang Y, Laster M, Food P, Kendig JJ, Sonner JM. Acetylcholine receptors do not mediate the immobilization produced by inhaled anesthetics. Anaesth Analg. 2002;94:1500–1504. doi: 10.1097/00000539-200206000-00023. [DOI] [PubMed] [Google Scholar]
- Elrod K, Buccafusco JJ. An evaluation of the mechanism of scopolamine-induced impairment in two passive avoidance protocols. Pharmacol Biochem Behav. 1988;29(1):15–21. doi: 10.1016/0091-3057(88)90267-5. [DOI] [PubMed] [Google Scholar]
- Fisher A. Cholinergic treatments with emphasis on M1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):433–442. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A, Pittel Z, Haring R, Bar-Ner N, Kliger-Spatz M, Natan N, Egozi I, Sonego H, Marcovitch I, Brandeis R. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: implications in future therapy. J Mol Neurosci. 2003;20(3):349–356. doi: 10.1385/JMN:20:3:349. [DOI] [PubMed] [Google Scholar]
- Flynn DD, Ferrari-DiLeo G, Levey AI, Mash DC. Differential alterations in muscarinic receptor subtypes in alzheimer’s disease: implications for cholinergic-based therapies. Life Sci. 1995;56(11–12):869–876. doi: 10.1016/0024-3205(95)00022-X. [DOI] [PubMed] [Google Scholar]
- Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer’s disease. J Neurochem. 1995;64(4):1888–1891. doi: 10.1046/j.1471-4159.1995.64041888.x. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency bold signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2004;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson A, Snyder PJ, Cromer J, Thomas E, Lewis M, Maruff P. The use of effect sizes to characterize the nature of cognitive change in psychopharmacological studies: an example with scopolamine. Hum Psychopharmacol. 2008;23(5):425–436. doi: 10.1002/hup.942. [DOI] [PubMed] [Google Scholar]
- Frey KA, Ehrenkaufer RLE, Beaucage S, Agranoff BW. Quantitative in vivo receptor binding: I. Theory and application to the muscarinic cholinergic receptor. J Neurosci. 1985;5(2):421–428. doi: 10.1523/JNEUROSCI.05-02-00421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey KA, Ciliax B, Agranoff BW. Quantitative in vivo receptor binding: IV. Detection of muscarinic receptor down-regualtion by equilibrium and by tracer kinetic methods. Neurochem Res. 1991;16(9):1017–1023. doi: 10.1007/BF00965845. [DOI] [PubMed] [Google Scholar]
- Gage FH, Chen KS, Buzsaki G, Armstrong D. Experimental approaches to age-related cognitive impairments. Neurobiol Aging. 1988;9(5–6):645–655. doi: 10.1016/S0197-4580(88)80129-5. [DOI] [PubMed] [Google Scholar]
- Gautam D, Duttaroy A, Cui Y, Han SJ, Deng C, Seeger T, Alzheimer C, Wess J. M1–M3 muscarinic acetylcholine receptor-deficient mice: novel phenotypes. J Mol Neurosci. 2006;30(1–2):157–160. doi: 10.1385/JMN:30:1:157. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Buchanan GF, Artinian L, Hamilton SE, Nathanson NM, Liu C. Role of the M1 receptor in regulating circadian rhythms. Life Sci. 2001;68(22–23):2467–2472. doi: 10.1016/S0024-3205(01)01040-2. [DOI] [PubMed] [Google Scholar]
- Gold S, Christian B, Arndt S, Zeien G, Cizadlo T, Johnson DL, Flaum M, Andreasen NC. Functional MRI statistical software packages: a comparative analysis. Hum Brain Mapp. 1998;6(2):73–84. doi: 10.1002/(SICI)1097-0193(1998)6:2<73::AID-HBM1>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217(1):55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen K, Rorarius M. Cortical responses to auditory stiuli during isoflurane burst suppression. Anaesthesia. 1999;54:210–214. doi: 10.1046/j.1365-2044.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Jagoda EM, Kiesewetter DO, Shimoji K, Ravasi L, Yamada M, Gomeza J, Wess J, Eckelman WC. Regional brain uptake of the muscarinic ligand, [18f]fp-tztp, is greatly decreased in M2 receptor knockout mice but not in M1, M3 and M4 receptor knockout mice. Neuropharmacology. 2003;44(5):653–661. doi: 10.1016/S0028-3908(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev (in press) [DOI] [PubMed]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191(3):813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26(17):4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Gillette MU. Cholinergic regulation of the suprachiasmatic nucleus circadian rhythm via a muscarinic mechanism at night. J Neurosci. 1996;16(2):744–751. doi: 10.1523/JNEUROSCI.16-02-00744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat Neurosci. 1999;2(6):555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Luan L, Ding F, Ai Y, Andersen A, Hardy P, Forman E, Gerhardt GA, Gash DM, Grondin R, Zhang Z. Pharmacological MRI (phMRI) monitoring of treatment in hemiparkinsonian rhesus monkeys. Cell Transplant. 2008;17(4):417–425. [PMC free article] [PubMed] [Google Scholar]
- Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci USA. 2009;106(37):15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Yamada S, Oki T, Manabe T, Taketo MM, Ehlert FJ. Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci. 2004;75(25):2971–2981. doi: 10.1016/j.lfs.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Morin CM, Blais FC, Mimeault V. Sleep disturbances in late life. In: Hersen M, Hasselt VB, editors. Handbook of clinical geropsychology. New York: Plenum; 1998. pp. 273–299. [Google Scholar]
- Murrell JC, Waters D, Johnson CB. Comparative effects of halothane, isoflurane, sevoflurane, and desflurane on the electroencephalogram of the rat. Lab Anim. 2008;42:161–170. doi: 10.1258/la.2007.06019e. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Penheiter AR, Penheiter SG, Chini EN, Thompson M, Warner DO, Jones KA. Differential effects of volatile anesthetics on M3 muscarinic receptor coupling to the gαq heterotrimeric g protein. Anesthesiology. 2006;105:313–324. doi: 10.1097/00000542-200608000-00014. [DOI] [PubMed] [Google Scholar]
- Nathanson NM. Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther. 2008;119(1):33–43. doi: 10.1016/j.pharmthera.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Shingu K, Shibata M, Osawa M, Mori K. The divergent actions of volatile anaesthetics on background neuronal activity and reactive capability in the central nervous system in cats. Can J Anaesth. 1992;39:862–872. doi: 10.1007/BF03008298. [DOI] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkkala T, Kaukinen S, Hakkinen V, Jantti V. Median nerve somatosenory evoked potentials during isoflurane anaesthesia. Can J Anaesth. 1997;44:963–968. doi: 10.1007/BF03011968. [DOI] [PubMed] [Google Scholar]
- Qiu A, Tuan TA, Woon PS, Abdul-Rahman MF, Graham S, Sim K (2010) Hippocampal-cortical structural connectivity disruptions in schizophrenia: an integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. NeuroImage (in press) [DOI] [PubMed]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/RR3206. [DOI] [PubMed] [Google Scholar]
- Rosier A, Cornette L, Orban GA. Scopolamine-induced impairment of delayed recognition of abstract visual shapes. Neuropsychobiology. 1998;37(2):98–103. doi: 10.1159/000026486. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Edmunds SM, Yi H, Gilmor ML, Levey AI. Localization of m(2) muscarinic acetylcholine receptor protein in cholinergic and non-cholinergic terminals in rat hippocampus. Neurosci Lett. 2000;284(3):182–186. doi: 10.1016/S0304-3940(00)01011-9. [DOI] [PubMed] [Google Scholar]
- Sarter M, Turchi J. Age- and dementia-associated impairments in divided attention: psychological constructs, animal models, and underlying neuronal mechanisms. Dement Geriatr Cogn Disord. 2002;13(1):46–58. doi: 10.1159/000048633. [DOI] [PubMed] [Google Scholar]
- Savage UC, Faust WB, Lambert P, Moerschbaecher JM. Effects of scopolamine on learning and memory in monkeys. Psychopharmacology (Berl) 1996;123(1):9–14. doi: 10.1007/BF02246275. [DOI] [PubMed] [Google Scholar]
- Scali C, Vannucchi MG, Pepeu G, Casamenti F. Peripherally injected scopolamine differentially modulates acetylcholine release in vivo in the young and aged rats. Neurosci Lett. 1995;197(3):171–174. doi: 10.1016/0304-3940(95)11914-I. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA. 2004;101(18):7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8(3):203–212. doi: 10.1016/S0926-6410(99)00021-X. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67(9):e11. doi: 10.4088/JCP.0906e11. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Strooper B. G protein-coupled receptors, cholinergic dysfunction, and A beta toxicity in Alzheimer’s disease. Sci Signal. 2009;2(93):re8. doi: 10.1126/scisignal.293re8. [DOI] [PubMed] [Google Scholar]
- Thiel CM. Cholinergic modulation of learning and memory in the human brain as detected with functional neuroimaging. Neurobiol Learn Mem. 2003;80:234–244. doi: 10.1016/S1074-7427(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Thomas E, Snyder PJ, Pietrzak RH, Jackson CE, Bednar M, Maruff P. Specific impairments in visuospatial working and short-term memory following low-dose scopolamine challenge in healthy older adults. Neuropsychologia. 2008;46(10):2476–2484. doi: 10.1016/j.neuropsychologia.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(3):423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Tsushima K, Shingu K, Ikeda S, Kimura H, Yamada K, Murao K. Supressive actions of volatile anaesthetics on the response capability in cats. Can J Anaesth. 1998;45:240–245. doi: 10.1007/BF03012909. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB (2009) Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology (in press) [DOI] [PMC free article] [PubMed]
- Vannucchi MG, Goldman-Rakic PS. Age-dependent decrease in the affinity of muscarinic M1 receptors in neocortex of rhesus monkeys. Proc Natl Acad Sci USA. 1991;88(24):11475–11479. doi: 10.1073/pnas.88.24.11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, VanEsen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wagster MV, Whitehouse PJ, Walker LC, Kellar KJ, Price DL. Laminar organization and age-related loss of cholinergic receptors in temporal neocortex of rhesus monkey. J Neurosci. 1990;10(9):2879–2885. doi: 10.1523/JNEUROSCI.10-09-02879.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK, Zarbin MA, Birdsall NJM, Kuhar MJ. Muscarinic cholinergic receptors: autoradiographic localization of high and low affinity agonist binding sites. Brain Res. 1980;200:1–12. doi: 10.1016/0006-8993(80)91089-6. [DOI] [PubMed] [Google Scholar]
- Wink AM, Bernard F, Salvador R, Bullmore E, Suckling J. Age and cholinergic effects on hemodynamics and functional coherence of human hippocampus. Neurobiol Aging. 2006;27:1395–1404. doi: 10.1016/j.neurobiolaging.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23(6):862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- Wisman LA, Sahin G, Maingay M, Leanza G, Kirik D. Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci. 2008;28(31):7797–7807. doi: 10.1523/JNEUROSCI.1885-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Andersen AH, Ai Y, Loveland A, Hardy PA, Gerhardt GA, Gash DM. Assessing nigrostriatal dysfunctions by pharmacological MRI in Parkinsonian rhesus macaques. Neuroimage. 2006;33(2):636–643. doi: 10.1016/j.neuroimage.2006.07.004. [DOI] [PubMed] [Google Scholar]