Abstract
The precise restriction of proteins to specific domains within a cell plays an important role in early development and differentiation. An efficient way to localize and concentrate proteins is by localization of mRNA in a translationally repressed state, followed by activation of translation when the mRNA reaches its destination. A central issue is how localized mRNAs are derepressed. In this study we demonstrate that, when oskar mRNA reaches the posterior pole of the Drosophila oocyte, its translation is derepressed by an active process that requires a specific element in the 5′ region of the mRNA. We demonstrate that this novel type of element is a translational derepressor element, whose functional interaction with the previously identified repressor region in the oskar 3′ UTR is required for activation of oskar mRNA translation at the posterior pole. The derepressor element only functions at the posterior pole, suggesting that a locally restricted interaction between trans-acting factors and the derepressor element may be the link between mRNA localization and translational activation. We also show specific interaction of two proteins with the oskar mRNA 5′ region; one of these also recognizes the 3′ repressor element. We discuss the possible involvement of these factors as well as known genes in the process of localization-dependent translation.
Keywords: Localization-dependent translation, oskar mRNA, Drosophila
The targeting of newly synthesized proteins to specific intracellular locations plays a major role in the establishment of cell polarity. Membrane-associated and -secreted proteins are targeted cotranslationally to the endoplasmatic reticulum as mRNA–ribosome complexes, and they are translocated to the appropriate compartment through the budding and sorting of vesicles (Pfeffer 1996; Teasdale and Jackson 1996). In contrast, cytoplasmic proteins appear to be targeted mainly by prelocalization of their transcripts. As a single mRNA can be translated many times, localizing transcripts is an efficient mechanism for generating a concentrated source of localized protein. There is increasing evidence that RNA localization is often combined with local translational control (St Johnston 1995). This allows simultaneous spatial and temporal control of protein synthesis within a particular region of the cell. In the case of neurons it is speculated that localized mRNAs may be regulated translationally in response to synaptic activity (Crino and Eberwine 1996; Steward 1997). The combination of translational control with RNA localization can also serve to restrict protein activities to defined regions in the cytoplasm, thereby preventing deleterious interactions from occurring elsewhere in the cell. This seems to be the case for myelin basic protein, which causes membranes to compact (Ainger et al. 1993), and for developmental determinants whose activities specify the basic body axes and early differentiation of the embryo (St Johnston 1995; Wickens et al. 1996).
Most of our current knowledge of how localized mRNAs are controlled translationally has come from studies in Drosophila of the determinants of embryonic polarity encoded by bicoid (bcd) (Frohnhöfer and Nüss-lein-Volhard 1986), nanos (nos) (Lehmann and Nüsslein-Volhard 1991), and oskar (osk) (Lehmann and Nüsslein-Volhard 1986). In all three examples, the mRNA is made in nurse cells, transported into the adjacent oocyte, and subsequently localized within the cell (Berleth et al. 1988; Macdonald and Struhl 1988; Ephrussi et al. 1991; Wang and Lehmann 1991). Translation of these mRNAs is silenced transiently during their transport and until the protein is required (Driever and Nüsslein-Volhard 1988; Ephrussi and Lehmann 1992; Smith et al. 1992; Wang et al. 1994). The importance of controlling translation during mRNA transport is underlined by the fact that premature or ectopic translation leads to severe developmental defects (Ephrussi and Lehmann 1992; Gavis and Lehmann 1992; Smith et al. 1992; Kim-Ha et al. 1995).
In a wide range of organisms, including Drosophila, Xenopus, and mouse, many mRNAs appear to be silenced by underadenylation, and their translation is activated by cytoplasmic elongation of their poly(A) tail (Richter 1996). In Drosophila, the poly(A) tail of anteriorly localized bcd mRNA increases from ∼50 nucleotides in oocytes, where it is translationally silent, to 150 nucleotides in early embryos, coincident with its activation (Sallés et al. 1994). Experiments with injected bcd mRNAs show that a poly(A) tail of 150 nucleotides rescues the bcd phenotype of embryos, whereas a mere 50 nucleotides, as present in the oocyte, do not suffice.
For nos, osk, and very likely a number of other localized mRNAs, translational regulation does not involve modulation of the length of the poly(A) tail (Sallés et al. 1994; Gavis et al. 1996a; Lieberfarb et al. 1996; Webster et al. 1997). In contrast to bcd, localization of osk and nos mRNAs is required for their translation (Gavis and Lehmann 1994; Markussen et al. 1995; Rongo et al. 1995). Upon fertilization, nos mRNA is activated only if it resides at the posterior pole (Gavis and Lehmann 1994). Similarly, osk mRNA remains repressed translationally in mutants that prevent osk RNA localization to the posterior pole (Markussen et al. 1995; Rongo et al. 1995).
Localization and translational repression of nos and osk transcripts require regulatory sequences in the 3′ UTR (Kim-Ha et al. 1993, 1995; Dahanukar and Wharton 1996; Gavis et al. 1996a,b; Smibert et al. 1996). RNA-binding proteins that mediate repression have been identified. A 130-kD protein named Smaug is thought to prevent translation of nos transcripts that have failed to become localized (Smibert et al. 1996). Smaug repression is mediated by multiple sites, namely the SREs or TCE (Smaug response elements or translation control element) within the nos 3′ UTR (Smibert et al. 1996; Dahanukar and Wharton 1996; Gavis et al. 1996a, respectively). The TCE mediates localization and activation of nos mRNA, indicating that these aspects of nos translational regulation are tightly linked and perhaps interdependent (Dahanukar and Wharton 1996; Gavis et al. 1996a). In the case of osk, premature translation is prevented by Bruno, a 68-kD protein encoded by the arrest (aret) locus (Kim-Ha et al. 1995; Webster et al. 1997). Bruno recognizes a repeated conserved sequence (BRE, for Bruno response element) in the osk 3′ UTR, and colocalizes with osk mRNA to the posterior pole. The aret mutant phenotype (Schüpbach and Wieschaus 1991; Castrillon et al. 1993) and the colocalization of the protein with other mRNAs in the oocyte suggest that Bruno-mediated repression is not limited to osk mRNA (Schüpbach and Wieschaus 1991; Castrillon et al. 1993; Webster et al. 1997).
Controlling the translation of localized mRNAs is central to the establishment of polarity in embryos and most likely also in somatic cells. Other than cytoplasmic polyadenylation, surprisingly little is known about the mechanisms that cause translational activation of localized transcripts. In this report we show that translation of localized osk mRNA is activated specifically through a discrete element situated at the 5′ end of the transcript. This element is only active at the posterior pole and is only required when the transcript is repressed through the BRE, suggesting that it functions as a derepressor rather than as a simple enhancer of translation. We show a direct correlation between translational derepression and the binding of a 50-kD (p50) and a 68-kD protein (p68) to this element. One of the 5′ binding proteins, p50, also interacts with the BRE in the 3′ UTR, and this binding appears to be required for full translation repression. Our data demonstrate that translational activation of localized osk mRNA is caused not by the local inactivation of repressor, but rather by an active and specific derepression event mediated by a prelocalized machinery. Our findings add the notion of “derepressor element”, in addition to repressor removal and poly(A) tail lengthening, as means to achieve translational activation.
Results
An element in the 5′ end of osk mRNA is required for translation
Regulatory elements for both RNA localization and translational repression are situated in the 3′ UTR of osk, as they are in nos. In contrast to nos, however, 3′ UTR-mediated localization at the posterior pole is not sufficient for translation, as heterologous transcripts localized under the control of the full-length osk 3′ UTR are not translated (Rongo et al. 1995; Serano and Cohen 1995; A. Ephrussi, unpubl.). This indicates that the osk 3′ UTR, although it may participate, is not sufficient for translational activation, and that sequences elsewhere in the transcript are required for translation of osk mRNA.
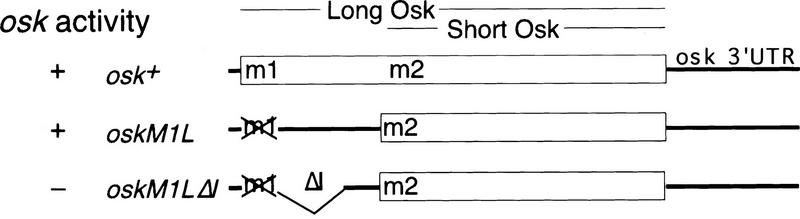
To identify possible translation regulatory signals in osk mRNA, we generated mutant osk transgenes and measured their translation capacity by their ability to suppress the abdominal and germ-line defects of osk embryos. Because the 5′ end of a transcript is a likely location for translational control elements, we first analyzed this region of osk mRNA in some detail. The wild-type osk RNA contains a 15-nucleotide 5′ UTR, followed by an exon containing two in-frame start codons (Fig. 1, here designated m1 and m2) that generate two Oskar isoforms (Markussen et al. 1995; Rongo et al. 1995). Previously we have shown that an osk transgene in which m1 is mutated and thereby the sequence upstream of m2 is transformed into an elongated 5′ UTR, is fully active (oskM1L, Fig. 1; Markussen et al. 1995). A deletion in the oskM1L transgene removing 249 nucleotides from this extended 5′ UTR abolishes its rescuing activity (oskM1LΔ1; Fig. 1), with respect to both abdomen and germ-line formation. Because the full-length osk 3′ UTR is sufficient for efficient RNA localization, and the mRNA produced by the transgene is present at normal levels (data not shown), we attribute the failure to rescue the osk phenotype to impaired translation rather than reduced RNA levels at the posterior pole. Taken together, these results indicate that the region between m1 and m2 osk functions as an RNA element necessary for its translation.
Figure 1.
Identification of a translation control element between the first and second start codons of osk mRNA. The cartoon shows the alternative usage of the two start codons, m1 and m2, in wild-type osk, which gives rise to a long and a short isoform of Oskar (Markussen et al. 1995). Full rescue activity of the wild-type construct was used as a standard and is indicated (+). Failure to rescue is also indicated (−). (oskM1L) Mutation of m1 into a leucine (CTG) turns the sequence upstream of m2 into a 5′ UTR. This transcript encodes short Oskar, which shows full rescue activity with respect to abdomen formation and fertility (Markussen et al. 1995). (oskM1LΔ1) Deletion of 249 nucleotides from the 5′ UTR of oskM1L abolishes osk activity; the two independent lines tested, oskM1LΔ1–2 and oskM1LΔ1–9 produced 0% and 2% hatchers, respectively, and none of the hatched females were fertile (males were not tested).
The 5′ end of osk mRNA mediates derepression of translation
To analyze this new translation element in more detail, we fused the 5′ end of osk, including m1, in-frame to a lacZ reporter gene under localization and repression control of the osk 3′ UTR (Fig. 2). Monitoring the translational status of a series of reporter gene constructs seemed preferable to an equivalent mutagenesis study in the context of the entire osk gene, as osk mRNA translation not only requires gene activities that localize the RNA, but also involves additional positive feedback involving Oskar protein itself, as well as downstream pole plasm components (Markussen et al. 1995, 1997; Rongo et al. 1995).
Figure 2.
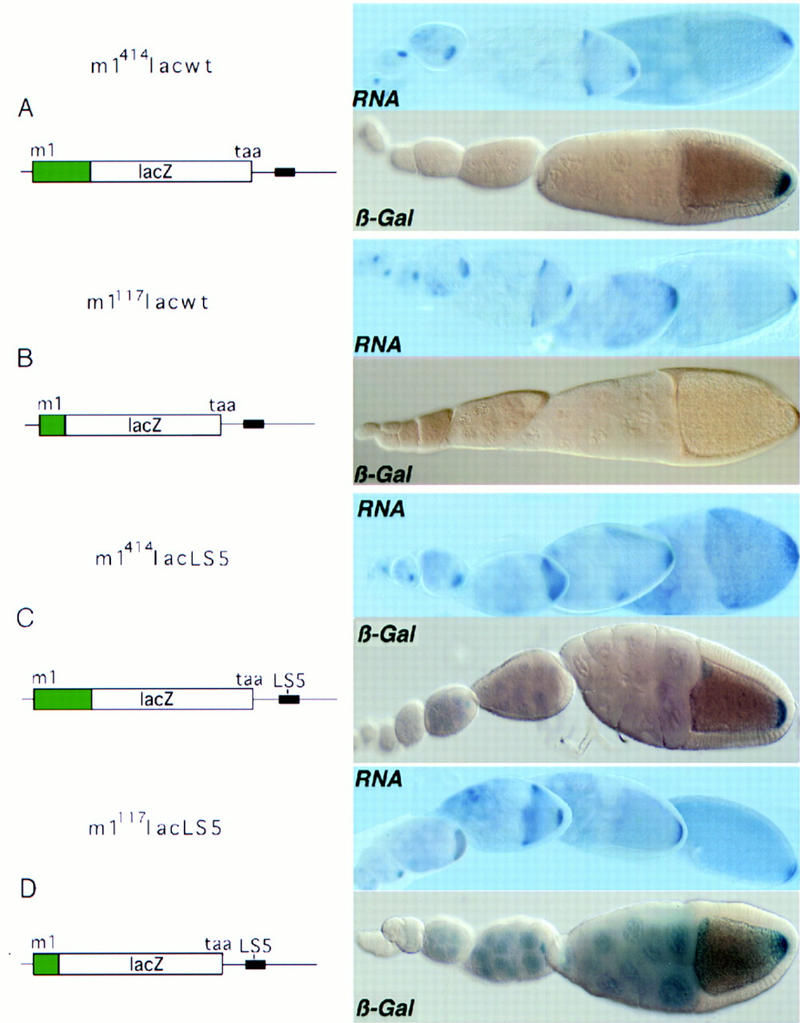
The 5′ end of osk mRNA contains sequences required to alleviate translational repression at the posterior pole. Functional analysis of the 5′ end of osk mRNA in chimeric reporter transgenes by analysis of RNA distribution and reporter gene activity. In the schematic representation of the transcripts, sequences derived from the osk 5′ end are indicated in green, the osk 3′ UTR is presented as a line, and the TAA proximal translation repressor element is indicated as a black box (region AB, Kim-Ha et al. 1995). Panels on the right shows in situ transcript distribution (RNA) and translation profile for the lacZ reporter as β-galactosidase staining (β-Gal). (A) m1414lacwt RNA, containing 414 nucleotides downstream of m1, is localized and regulated translationally like wild-type osk mRNA. (B) m1117lacwt RNA, consisting of the osk 15-nucleotide 5′ UTR and 117 nucleotides downstream of m1 fused in-frame to lacZ reading frame, and followed by the wild-type osk 3′ UTR, is localized and repressed like wild-type osk RNA, but fails to be translated at the posterior pole. (C) m1414lacLS5 contains a 5-nucleotide substitution (see Materials and Methods) in the second Bruno-binding consensus sequence (Kim-Ha et al. 1995) in an otherwise wild-type reporter transcript. This mutation causes moderate premature translation, detectable as light blue β-galactosidase staining in nurse cells. (D) m1117lacLS5 is identical to m1117lacwt, with the exception of the 5-nucleotide substitution (LS5) in the BRE. This RNA is translated efficiently in spite of the absence of the 5′ element. The LS5 mutation was identified in a linker-scanning mutagenesis series spanning what we find to be the essential part of the proximal BRE. Premature translation of LS5-containing transcripts is detected by blue β-galactosidase staining in the nurse cells and young oocytes. Mutation of additional Bruno-binding consensus sequences results in stronger staining of nurse cells and early oocytes (data not shown). Interestingly, premature translation in m1414lacLS5 is always less pronounced than in m1117lacLS5. All transgenes were assayed in the w1118 background and support similar steady-state levels of RNA (see Fig. 7, lanes 4–7).
A construct with the 15-nucleotide osk 5′ UTR and the translation start codon m1 as well as 414 nucleotides downstream of m1 allows translation of the localized reporter mRNA (m1414lacwt; Fig. 2A). The lacZ reporter transcript m1414lacwt recapitulates normal osk regulation with respect to mRNA localization, repression of premature translation, and translational activation at the posterior pole. Truncation of the osk mRNA 5′ region to only the first 117 nucleotides downstream of m1 does not allow translation of the localized reporter transcripts (m1117lacwt; Fig. 2B). We failed to detect any β-galactosidase activity from m1117lacwt, even in overstained samples, indicating that translational repression is efficient and that an element required for activation is lacking. RNA quantification confirms the results of in situ mRNA hybridization and indicates that the steady-state amounts of reporter mRNA produced by m1414lacwt and m1117lacwt are essentially identical (Fig. 7, lanes 4,5, below). These results confirm that the 5′ end of the osk transcript contains signals required for translation of localized osk mRNA. Furthermore, these experiments reveal that the positive element not only acts downstream, on m2, as demonstrated in M1LΔ1osk, but also upstream, on m1.
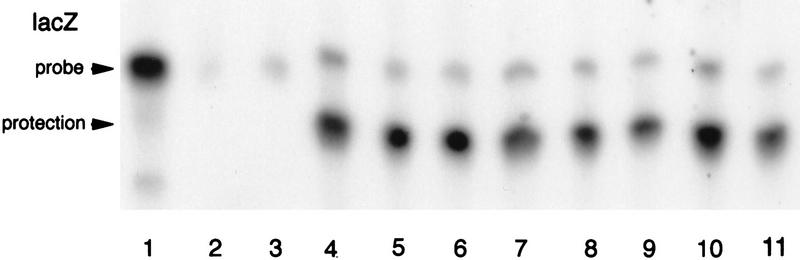
Figure 7.
Comparative quantification of reporter transcripts by RNase protection. The amounts of input mRNA, isolated from ovaries, were first adjusted to equalize endogenous osk mRNA and then quantified with respect to reporter transcripts. (Lane 1) Antisense Ribo probe alone; (lane 2) probe digested; (lane 3) probe digested in the presence of 10 μg total ovarian RNA (Oregon-R); (lane 4) m1414lacwt; (lane 5) m1117lacwt; (lane 6) m1117lacLS5; (lane 7) m1414lacLS5; (lane 8) m1m2lacwt; (lane 9) m1INVm2lacwt; (lane 10) m1414lacBREbcd; (lane 11) m1414lac5′Δ24/3′Δ25.
The positive element could be essential for osk mRNA translation in general, or it could be required to overcome repression at the posterior pole. Therefore, we asked whether the element is necessary for translation of a transcript that is not repressed. To address this question, we constructed a transgene (m1414lacLS5; Fig. 2C; Fig. 7, lane 7, below) lacking a functional repressor region, by introducing point mutations (LS5) into one of the Bruno-binding consensus sequences of the proximal BRE in the 3′ UTR, thereby weakening the translational block. This mutation causes slight premature translation in that moderate β-galactosidase staining is detected in the nurse cells, where the transcript is synthesized, and in young oocytes. The level of posterior translation is not affected by the LS5 mutation, as determined by a time course of β-galactosidase stainings comparing m1414lacwt and m1414lacLS5 (data not shown). Truncation of the 5′ element, which in the case of a functional BRE abolishes translation completely (Fig. 2B), does not affect posterior translation when the BRE is mutated (Fig. 2D, m1117lacLS5; Fig. 7, lane 6, below). Hence, this positive element is required to activate posterior expression of transcripts that are repressed translationally by the BRE. The element functions as a true derepressor, rather than a general enhancer of translation (Leathers et al. 1993; Tanguay and Gallie 1996), as LS5 mutant transcripts that retain the positive element (m1414lacLS5) do not appear to be translated more efficiently at the posterior pole than those that lack the element (m1117lacLS5).
A discrete region at the 5′ end of osk mRNA is bound specifically by a 50- and a 68-kD protein
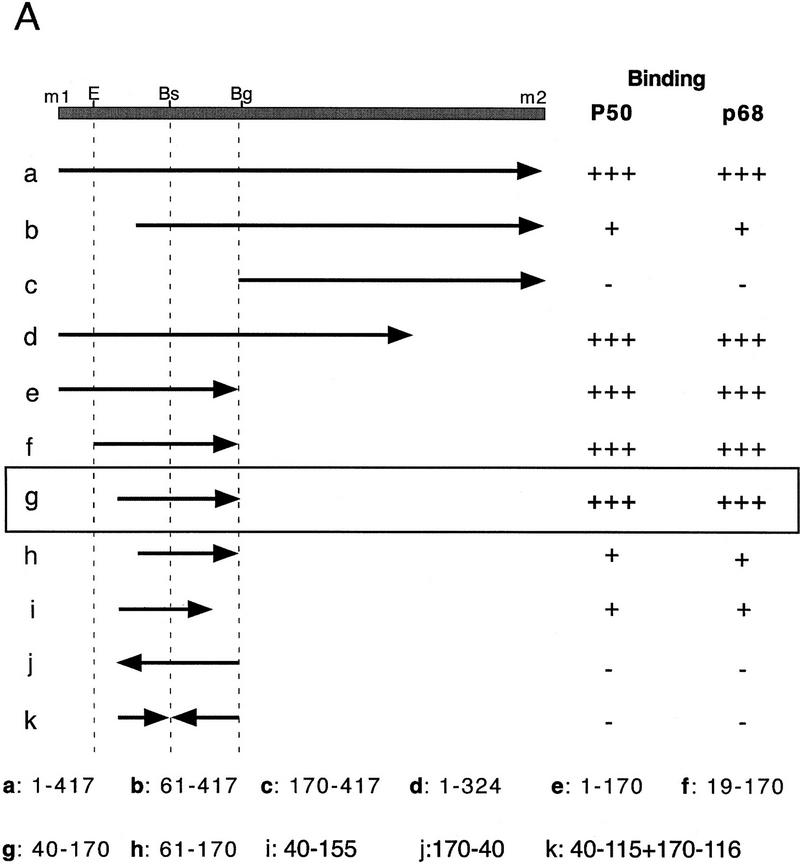
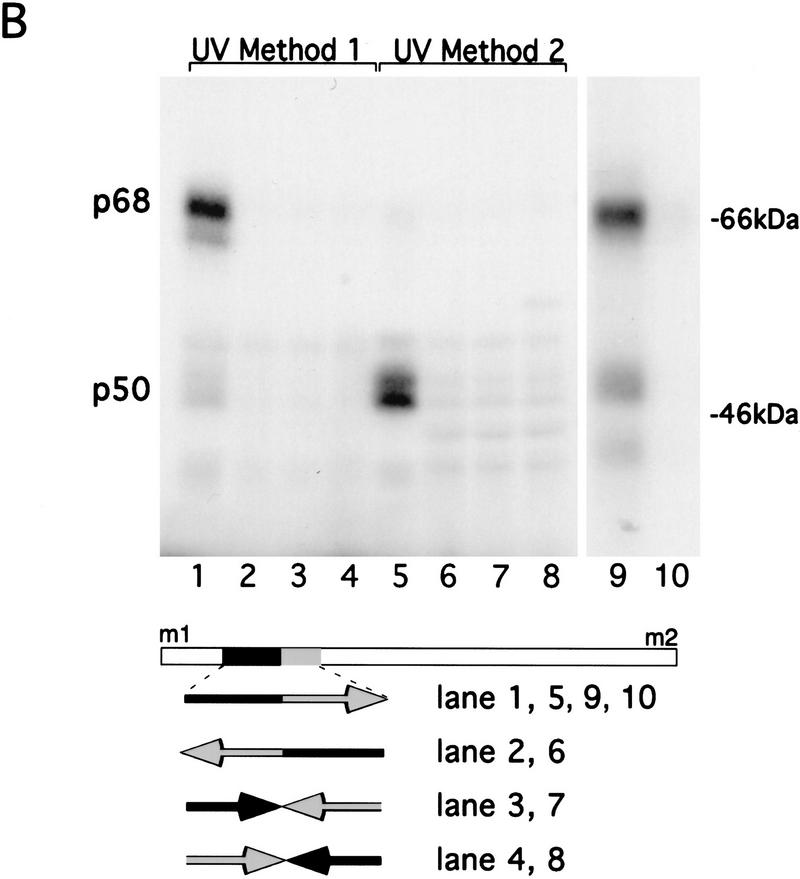
Assuming that translational derepression of osk mRNA is mediated by RNA-binding proteins, we decided to localize the regulatory element by first mapping the sites of specific protein interactions within the 5′ region and subsequently testing the effect of mutations in these sites in vivo. Initial UV cross-linking experiments, in which the entire sequence between m1 and m2 was labeled, revealed a protein doublet with an apparent molecular mass of 50 kD (p50) and a 68-kD (p68) protein (Fig. 3B). p50 binding is detected in extracts of ovaries, embryos, and Schneider cells, whereas p68 binding is restricted to the ovary (data not shown). Although both proteins interact specifically with the m1–m2 region under the same buffer conditions, distinct UV cross-linking protocols were used for optimal visualization of these two interactions (see Materials and Methods for details). To map the binding sites of p50 and p68 more precisely, subfragments of the entire 417 nucleotide m1–m2 region were generated and tested for protein binding (Fig. 3A). This analysis revealed that the region recognized by both p50 and p68 resides within a 130-nucleotide stretch beginning 40 nucleotides downstream of m1 (Fig. 3A, fragment g and Fig. 3B, lanes 1,5,9,10). Sequences outside of this 130-nucleotide fragment were not recognized by p50 or p68 (Fig. 3A, fragment c), nor did we detect any other specific protein binding. Further reduction of the 130-nucleotide RNA fragment from either the 5′ or the 3′ end reduced binding of both p50 and p68 (Fig. 3A, fragments h,i). Inversion of either the 5′ or 3′ half abolishes both p50 and p68 binding, indicating that both halves are essential for RNA recognition by these proteins (Fig. 3, A, fragment k, and lanes 3,4,7,8). Sequence inspection of this region failed to reveal any repeated motifs, binding sites for known RNA-binding proteins, or obvious secondary structure. Linker scanning mutations within the 130-nucleotide fragment so far have failed to show any differential effect on the binding of p50 and p68 (data not shown). This indicates that the proteins either recognize the same element or bind to different elements localized within the same higher order RNA structure. The recognition sites for p50 and p68 are entirely deleted from oskM1LΔ1 mRNA (see Fig. 1) and partially deleted from m1117lacwt and m1117lacLS5 (see Fig. 2B,D), indicating that these protein/RNA interactions may be involved in translational derepression.
Figure 3.
Mapping specific protein/RNA interactions in the m1–m2 fragment of osk mRNA that is essential for its translation. (A) The 5′ end of osk mRNA, including the first and second start codons, m1 and m2, respectively, is diagrammed schematically. A partial restriction map of the region, showing sites used for subcloning is shown at the top. (E) EcoRI; (Bs) BstEII; (Bg) BglII. Transcripts used as probes for the UV cross-linking assay are identified by lowercase letters. The coordinates of the subfragments are indicated at the bottom. Position 1 is the first nucleotide of m1, position 417 is the last nucleotide of m2. The entire m1–m2 region (a) or subfragments of the region, created with available restriction sites or by PCR were labeled radioactively and incubated with oocyte extract. Proteins cross-linked to labeled RNA were separated by SDS-PAGE and visualized by autoradiography. The signal intensity obtained with the full-length m1–m2 probe was used as a standard and is indicated by (+++). Significantly reduced but detectable binding, and complete loss of binding are indicated (+) and (−), respectively. (B) UV cross-linking assay revealing the specific interaction of p50 and p68 with RNA fragment g. RNA probes (bottom) were incubated with oocyte extract and cross-linked by method 1 (lanes 1–4 and 9,10) or method 2 (lanes 5–8). (Lane 10) p68 complex with RNA fragment g was incubated with anti-Bruno antiserum and subjected to immunoprecipitation. The minimal region recognized (fragment g) was determined as shown in A. Probes used in each lane are indicated below. Arrows pointing rightward indicate RNA in sense orientation; arrows pointing leftward indicate RNA in antisense orientation.
The size of the p68 protein was intriguing in its similarity to what we estimate to be the size of the translation repressor Bruno, as extrapolated from the mobility of the latter in SDS-PAGE after UV cross-linking to a 3′ UTR probe containing the proximal BRE (see below). Therefore, we used an antiserum directed against Bruno, which efficiently precipitates a Bruno/RNA complex (Fig. 5, lane 6, below), to test whether p68 is in fact Bruno. p68 is not recognized by the Bruno antiserum (Fig. 3B, lane 10), demonstrating that p68 and Bruno are two distinct proteins. Furthermore, binding of p68 to the derepressor element is not affected by competition with RNA molecules containing the proximal BRE, showing that the two proteins have distinct binding specificities.
Figure 5.
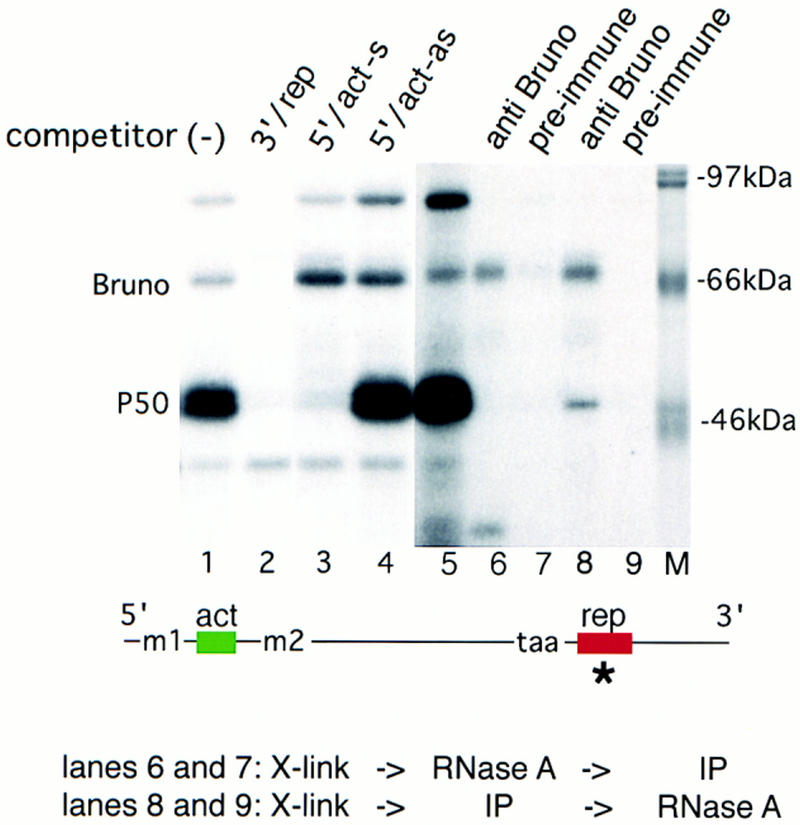
p50 binds both to the 5′ end of osk mRNA and to the repressor element in the 3′ UTR. An RNA competition assay was performed to determine the binding specificity of p50. A radioactive RNA probe consisting of the essential subfragment of the proximal repressor element (region AB; Kim-Ha et al. 1995) was incubated and UV cross-linked to proteins in the oocyte extract in the absence of competitor RNA (lane 1). Competitions were carried out with a 100-fold excess of either repressor fragment (rep, lane 2), EcoRI–BglII fragment containing the 5′ activator in sense orientation (5′/act-s, lane 3), or the same EcoRI–BglII fragment in antisense orientation (5′/act-as, lane 4). Immunoprecipitation of proteins UV cross-linked to radiolabeled repressor fragment (lane 5) by anti-Bruno antiserum (lane 6) or preimmune serum (lane 7). Simultaneous binding of p50 and Bruno was tested by treating with RNase A only after the immunoprecipitation. (Lane 8) Anti-Bruno antiserum; (lane 9) preimmune serum. We noted that in some cases (as shown here) the intensity of the upper band of the p50 doublet was reduced after coprecipitation. The locations of the 5′ and 3′ competitors in the osk transcript are indicated below. The radiolabeled probe is indicated by an asterisk. The reverse experiment, with activator element RNA as a radioactive probe, and cold activator and repressor RNA elements as unlabeled competitors, yielded the same result with respect to p50 (data not shown).
To determine whether the element we identified in vitro indeed corresponds to the functionally defined derepressor element, we introduced into an otherwise wild-type reporter construct a 65-bp inversion that disrupts protein binding to this RNA region in vitro (Fig. 3B, lanes 3,7). The inversion introduces neither stop or additional start codon or splice sites, nor does it create any obvious RNA secondary structure that might obstruct translation. Furthermore, the reporter construct contains both m1 and m2, so as to mimic osk mRNA regulation as accurately as possible. The protein expression pattern of the wild-type control transgene m1m2lacwt (Fig. 4A) recapitulates that of endogenous osk in that the mRNA is repressed transiently during transport to the posterior pole but is translationally active from stage 10 onward. The inversion m1INVm2lacwt (Fig. 4B) causes the mRNA to remain repressed at stage 10 and later, although the RNA is localized efficiently. Thus the region delimited in vitro by mapping protein/RNA interactions mediates the posterior release of the translational block imposed by Bruno. We will discuss the individual involvement of p50 and p68 in derepression below.
Figure 4.
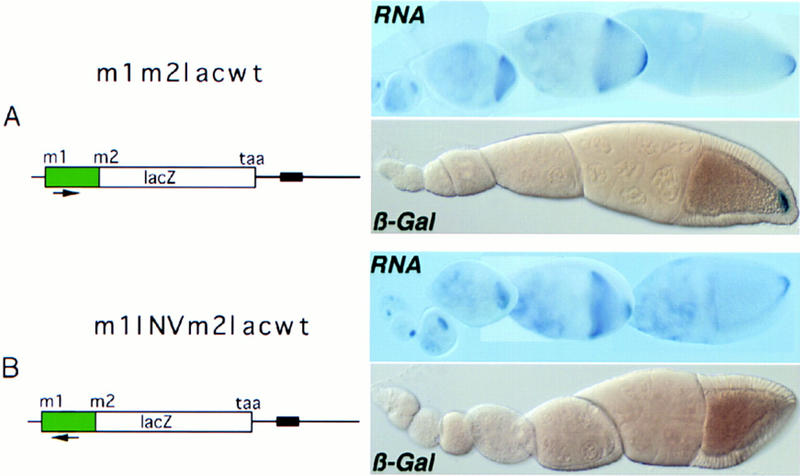
Inversion of the 3′ half of the p50/p68-binding RNA fragment prevents posterior derepression in vivo. The orientation of the 3′ half of the fragment is indicated by an arrow. (A) m1m2lacwt, wild-type m1–m2 region, fused 3 nucleotides downstream of m2 to the lacZ reporter, under the regulatory control of wild-type osk 3′ UTR. Reporter RNA and protein distributions, as detected in situ by RNA hybridization and β-galactosidase activity, are virtually indistinguishable from those of endogenous osk. (B) m1INVm2lacwt, the p50/p68-binding fragment in the m1–m2 region of the reporter transgene was mutated by inversion of the 3′ half (see probe c). As in the wild-type construct, the RNA is efficiently localized; however, translation is not derepressed.
The 50-kD protein also interacts with the 3′ repressor region
We also examined the entire osk 3′ UTR in our cross-linking assay and detected the binding of a 50-kD protein doublet, this time to the two regions known to function as translation repressor elements (Fig. 5, showing protein interaction with region AB; Kim-Ha et al. 1995). In addition, we detected the interaction of Bruno. The interactions of both Bruno and p50 with the RNA are specific. Both proteins are equally affected by linker scanning mutations, such as LS5, in or close to the Bruno-binding consensus sequences, but are unaffected by point mutations elsewhere in the repressor element (N. Gunkel and A. Ephrussi, unpubl.), suggesting that the two proteins recognize a similar sequence or structure. Immunoprecipitation of proteins UV cross-linked to the repressor element, using an anti-Bruno antiserum raised against a central part of the Bruno protein, shows that the 50-kD protein is not a breakdown product of Bruno (Fig. 5, lanes 6,7).
Competition with the 5′ element severely reduces binding of the 50-kD protein doublet (Fig. 5, lane 3), indicating that the same protein interacts with both the 5′ activator and the 3′ repressor RNA element. The same competitor does not reduce the interaction of Bruno with the BRE, but rather increases its binding (Fig. 5, cf. lane 1 with lane 3). This suggests that access of Bruno to the RNA is increased because of a reduction in free p50 by competition with the 5′ element. This is also consistent with our linker scanning experiments, which show that p50 and Bruno compete for binding to similar sites in the repressor element. To test whether p50 and Bruno can bind simultaneously to the BRE, which contains multiple Bruno-binding consensus sequences, we modified the immunoprecipitation assay such that the RNA probe was digested only after precipitation with anti-Bruno antiserum, rather than before. This allows coprecipitation, by way of Bruno, of proteins bound to the same RNA molecule. Under such conditions, p50 is recovered (Fig. 5, lane 8), showing that RNA molecules exist to which both p50 and Bruno are simultaneously bound. Compared with simple cross-linking, after immunoprecipitation the Bruno/p50 ratio is shifted in favor of Bruno (cf. lanes 5 and 8). Because the experimental design is biased toward detection of Bruno-containing complexes and endogenous ribonucleolytic activity in the oocyte extract may cleave the RNA before precipitation, this experiment does not allow us to evaluate the relative amounts of p50 and Bruno bound in vivo to a single BRE.
The 5′ and 3′ RNA elements show no obvious similarity in sequence or secondary structure. Nevertheless, p50 recognizes both elements specifically. The fact that these two regions compete efficiently for p50 binding suggests that the same domain in the protein interacts with the two RNAs, perhaps recognizing similar higher order RNA structures. It is also possible that p50 recognizes the two regions through distinct domains whose affinities for RNA are modulated allosterically.
Correlation of p50 binding to the BRE with translational repression
The fact that all linker scanning mutations tested have a similar effect on p50 and Bruno binding suggests that the proteins recognize similar sequence motifs or structures in the repressor element. However, deletion of sequences flanking the Bruno-binding consensus sequences within the proximal BRE, including the fourth consensus motif, affects the binding of the two proteins in vitro very differently. p50 binding is reduced, whereas Bruno binding is either unchanged or even slightly increased when 24 nucleotides from the 5′ end and 25 nucleotides from the 3′ end of the EcoRI–DraI repressor fragment are deleted (5′Δ24/3′Δ25, Fig. 6A, cf. lanes 1 and 4). To determine the possible function of p50 in translational regulation, we examined in vivo the expression of a transgene bearing these deletions.
Figure 6.
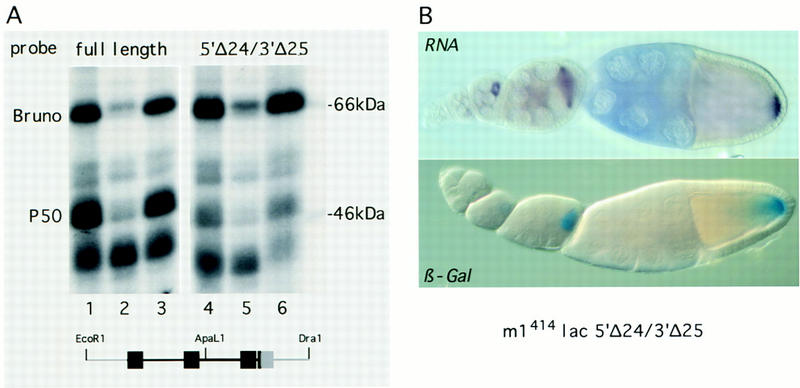
Reduction of p50 binding correlates with premature translation. (A) A truncated EcoRI–DraI repressor RNA fragment shows a >10-fold decrease in p50 binding but Bruno binding is unaffected. A radioactive probe consisting of either the full-length repressor region (lanes 1–3) or a truncated form with 24 nucleotides deleted from the 5′ and 25 nucleotides from the 3′ (lanes 4–6) were incubated with oocyte extract in the presence of 100-fold molar excess of specific competitor (EcoRI–DraI fragment, lanes 2,5) or nonspecific competitor (polylinker of pSP72, lanes 3,6) and subsequently UV cross-linked. The same deletion mutation in the context of the first 380 nucleotides of the osk 3′ UTR had essentially the same effect on p50 and Bruno binding. The locations of the Bruno-binding consensus sequences with respect to the 5′ and 3′ truncations (light gray) are indicated below. (B) In vivo analysis of the effect of the 5′Δ24/3′Δ25 truncation of the proximal repressor element in an otherwise wild-type lacZ reporter gene (m1414lac5′Δ24/3′Δ25). The mutation does not affect RNA localization or levels of reporter transcripts (cf. with Fig. 2A, and see Fig. 7, lane 4,9); however, translation is detectable already from stage 6/7 onward.
p50 was identified originally through its binding to the 5′ derepressor RNA element. One possible function of p50 could be to act in derepression by a mechanism requiring its interaction with both the 5′ and the 3′ elements, perhaps resulting in displacement of Bruno. If this were the case, a mutation interfering selectively with p50 binding to the BRE should perturb translational derepression and thus no β-galactosidase staining should be detected at the posterior pole. Alternatively, p50 could be a Bruno corepressor and act to prevent premature translation of osk mRNA. If this were the case, the mutation 5′Δ24/3′Δ25 should weaken the translational block and β-galactosidase staining should be detected before stage 9/10.
The mutation 5′Δ24/3′Δ25 in an otherwise wild-type transgene (m1414lac5′Δ24/3′Δ25) causes the RNA to be translated at stage 7 (Fig. 6B), when wild-type transcripts are still silent (Fig. 2A). The observed premature translation is not caused by elevated amounts of transcript or aberrant RNA localization, as both the level and the distribution of reporter mRNA are indistinguishable from those of the wild-type control, m1414lacwt (see Figs. 2A and 7, lanes 4,11). This result suggests that Bruno binding to the BRE is alone not sufficient to repress premature translation. Furthermore, it indicates that p50 could act as a corepressor of osk translation through its interaction with the 3′ repressor RNA element.
Translational derepression requires activities localized to the posterior pole
Mutations in the genes involved in osk mRNA localization to the posterior pole also affect osk translation, leading to the idea that osk RNA localization is required for translation (Markussen et al. 1995; Rongo et al. 1995). Our results demonstrating the existence of a mechanism for active derepression of osk mRNA at the posterior pole suggest that RNA localization serves to bring osk mRNA into an environment containing prelocalized activities required for derepression. We wished to test this notion in a wild-type genetic background, to exclude the possibility that the mutations affecting localization affect derepression directly.
To this end we constructed a chimeric gene containing sequences involved both in translational repression and derepression, linked to the bcd 3′ UTR (Fig. 8, m1414lacBREbcd). The transcript of this gene is localized to the anterior pole like endogenous bcd transcript. In contrast to a posteriorly localized counterpart (m1414lacwt) expressed in the same oocyte, the anteriorly localized RNA remains repressed despite the presence of the 5′ derepressor element. Endogenous bcd is translated only after fertilization, hence the lack of anterior translation of the chimeric transcript at stage 9/10 could be explained if it were regulated like bcd mRNA. However, the m1414lacBREbcd transcript is not subject to bcd translational control, as equivalent chimeric constructs that lack the osk BRE show efficient translation at stage 10 (Ephrussi and Lehmann 1992; Rongo et al. 1995; N. Gunkel and A. Ephrussi, unpubl.). Hence, although the cis-acting derepressor element is essential for translational activation of osk mRNA, other components of the derepression machinery are required that do not colocalize with the RNA but are localized and restricted to the posterior pole.
Figure 8.
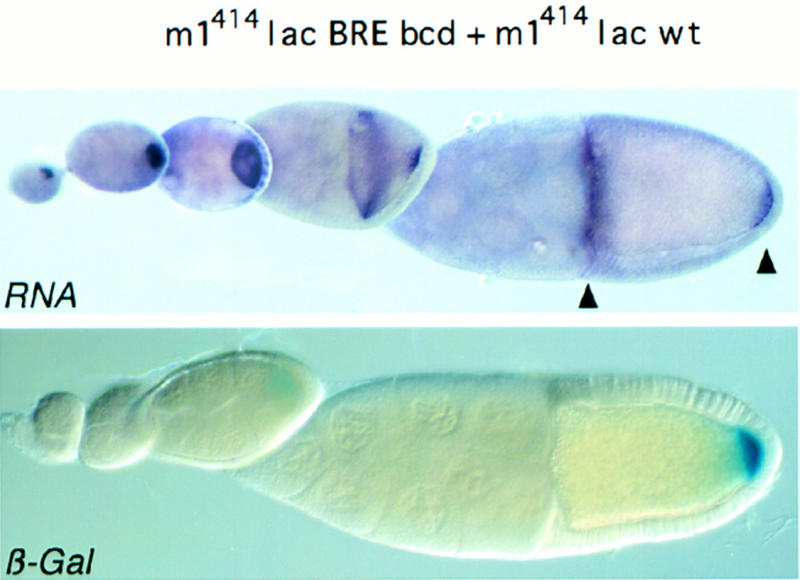
The derepressor element functions at the posterior but not at the anterior pole of the oocyte. Flies were generated that bear two distinct reporter transgenes: m1414lacBREbcd and m1414lacwt. m1414lacBREbcd has the osk mRNA 5′ end (m1414), including the derepressor element, and the first 370 nucleotides of the osk 3′ UTR, including the proximal repressor element (BRE). The bcd 3′ UTR, which directs the chimeric transcript to the anterior pole of the oocyte was fused downstream of the BRE. m1414lacwt was described in Fig. 2A. It is localized to the posterior pole of the oocyte and repressed translationally until stage 9, because of the presence of a wild-type osk 3′ UTR (wild type). The amount of transcript produced by each transgene was assessed separately (see Fig. 7, lanes 4,10).
Discussion
Translation of osk mRNA is tightly linked to its localization at the posterior pole. osk mRNA is repressed translationally in the nurse cells where it is produced, during its translocation into the growing oocyte, and within the oocyte until stage 8/9 when it is localized at the posterior pole. Once osk mRNA is at the posterior pole, its translation is activated. Despite growing evidence that the mode of regulation of osk mRNA applies, at least in part, to other localized mRNAs (Standart and Jackson 1994; Micklem 1995; Macdonald and Smibert 1996; Seydoux 1996), so far little is known about the mechanisms by which translation is repressed before and during transport, or about how translation is activated. Furthermore, it is unclear how translational activation is linked to localization of the mRNA.
We have investigated the mechanism of translational recruitment of localized osk mRNA and obtained evidence for an active derepression event that is controlled by a discrete RNA element situated between the first and second start codons of osk. This element was defined by a combination of in vitro protein-binding experiments and functional tests in vivo. The correspondence between protein binding and derepressor function suggests that a locally restricted interaction between trans-acting factors and the derepressor element may be the link between mRNA localization and translational activation. In addition to this novel activator element, we have found evidence that the binding of the previously identified Bruno protein to the osk 3′ UTR is not sufficient for repression of premature osk translation.
Repression of osk transcripts prior to localization
Translational repression of osk mRNA at developmental stages when RNA localization is incomplete is mediated by discrete RNA elements, the BREs. These RNA elements, which contain redundant sequence motifs, are recognized by a specific RNA-binding protein of 70 kD, Bruno. Therefore, Bruno was postulated to mediate translational repression of unlocalized osk mRNA (Kim-Ha et al. 1995).
In the present study we have identified an additional BRE-binding protein, p50, that recognizes motifs similar to those recognized by Bruno. Linker-scanning analysis of the BRE shows that the Bruno-binding consensus sequences are crucial for p50 binding (N. Gunkel and A. Ephrussi, unpubl.). However, deletion mutations, which are likely to have a greater effect than base substitutions on the overall structure of the BRE, reveal that the proteins are differentially sensitive to the manner in which the consensus sequences are presented within the mRNA (Fig. 6A). p50 and Bruno can bind the BRE independently, as the BRE is bound by p50 in extracts that lack Bruno protein (Kim-Ha et al. 1995), and a mutant RNA containing BRE consensus sequences binds Bruno efficiently but not p50. The fact that p50 was not identified in earlier studies is most likely attributable to differences in the binding assays used to detect the proteins. Only when using probes whose cross-linking efficiency was enhanced by incorporation of thio-UTP were we able to detect significant p50 binding using the conditions that permitted the identification of Bruno (Kim-Ha et al. 1995). The conditions developed for our study do not require thio-UTP incorporation into the probe for detection of p50 (or Bruno).
Several lines of evidence suggest that, independently of Bruno, p50 plays a role in translational repression at the 3′ end. First, a mutant BRE, which in vitro binds p50 poorly and Bruno as well or better than the wild-type BRE, does not efficiently repress osk mRNA translation in vivo during the early stages of oogenesis (Fig. 6B, m1414lac5′Δ24/3′Δ25). Second, transcripts lacking the 5′ derepressor element remain translationally repressed even during the later stages when osk mRNA is localized at the posterior pole and Bruno protein is no longer detected (Webster et al. 1997). These observations suggest that during several stages of oogenesis, p50 is present and competent to repress osk translation, both when the mRNA is in transit and when it is localized at the posterior pole. The fact that premature translation of a reporter transcript containing a p50-compromised BRE is only observed in young oocytes but not in nurse cells (Fig. 6B) could indicate that p50 is not required for translational repression in nurse cells, or that p50 is present only in oocytes.
The involvement of a second repressor protein in osk translational control is not unexpected. Indeed aubergine (aub), a gene required for efficient osk mRNA translation, is required even when Bruno-mediated repression is alleviated by mutations in the BRE (Wilson et al. 1996), leading the investigators to speculate that the aub gene product enhances translation by counteracting the action of a second repressor. It is interesting to note that the requirement for aub function in osk translation is conferred not only by the osk 3′ UTR but also involves the 5′ end of osk mRNA. Consistent with this possible involvement of the osk 5′ end in translational repression, we find that in transgenic flies containing an inefficient BRE (LS5), premature translation increases when the 5′ end is truncated (Fig. 2, cf. m1414lacLS5 with m1117lacLS5). Understanding the extent to which the 5′ end of the osk transcript might contribute to overall translational repression will require mutations that selectively disrupt 5′ repressor function without simultaneously affecting derepressor function. However, so far it has not been possible to define a p50-binding specificity distinct from that of p68 and to abolish selectively the binding of one or the other protein. Hence, our data do not allow us to affirm that p50 functions as a repressor not only by binding to the BRE but also through its interaction with the osk 5′, or that p68 is the derepressor protein.
A novel RNA element mediates translational activation of osk mRNA at the posterior pole
The functional linkage of translation and RNA localization suggests several mechanisms by which osk translation could be activated at the posterior pole. The translation repressor proteins Bruno and p50 could be degraded by an activity localized at the posterior pole or else be displaced competitively by a derepressor protein. Alternatively, Oskar protein expression could be activated by concentration of the mRNA, resulting in the accumulation of small amounts of Oskar protein by leaky translation, thus initiating a positive feedback loop in which Oskar protein stimulates its own translation (Markussen et al. 1997). Our experiments indicate that none of these mechanisms is involved in the initial event of translational derepression. In the absence of the derepressor element, osk transcripts remain repressed (Figs. 1, 2B, and 4B), arguing against a passive, local repressor inactivation model. Therefore, the mode of action of the derepressor element is distinct from that of previously described cases, in which repression is released passively by inactivation of a repressor protein and no additional RNA elements are required (Kwon and Hecht 1993; Walker et al. 1996). The derepressor element does not coincide with the BRE, suggesting that a competitive displacement of the repressor protein from the BRE is unlikely to be the mechanism leading to derepression. Finally, a combination of leaky translation and positive feedback of Oskar protein on its own translation as a mechanism for derepression is unlikely, as reporter transcripts can be derepressed in the absence of endogenous Oskar (data not shown).
The mechanisms by which 3′ UTR-binding proteins repress translation are still not understood and it is unclear how the 5′ derepressor element overcomes translational repression. The fact that transcripts lacking the derepressor element are localized but not translated demonstrates that the element plays little or no role in RNA localization and that localization does not suffice for translational derepression. Therefore, the derepressor element is distinct from the nos translation control elements, which mediate both localization and derepression (Dahanukar and Wharton 1996; Gavis et al. 1996a).
The derepressor element could open an alternative route for translation initiation that is not affected by a repressor protein (e.g., by a cap-independent mechanism). However, preliminary experiments using bicistronic transcripts indicate that the region between m1 and m2 does not contain an internal ribosome entry site (N. Gunkel, A. Jenny, and A. Ephrussi, unpubl.). In addition, derepression of osk mRNA translation appears to be independent of cytoplasmic polyadenylation, as transcripts that remain repressed because of a mutation in the derepressor element and those that are translated have poly(A) tails of the same length (M. Muckenthaler and A. Ephrussi, unpubl.).
Properties of a derepression machinery: evidence and hypothesis
Translational recruitment of osk mRNA is always accompanied by posterior localization of the mRNA, indicating that localization may trigger the release from translational repression. We imagine that RNA localization directs osk transcripts into a cytoplasmic subcompartment containing trans-acting factors that interact specifically with the 5′ element to mediate derepression. The spatial restriction of the derepression machinery could be achieved by prelocalization of at least some of the components to the posterior pole, or by the localized activation of uniformly distributed factors. During the early stages of oogenesis, osk mRNA initially fills the entire cytoplasm of the growing oocyte and yet no Oskar protein is detected, even in the posterior region. This suggests that the derepressor proteins are expressed or activated only at certain stages of oocyte development, possibly through signals from the posterior pole. The existence of localized derepressors is supported by our observation that reporter transcripts bearing the bcd 3′ UTR into which the osk repressor element is inserted are localized to the anterior of oocytes and embryos and not derepressed, even when they contain the derepressor element (Fig. 8).
The DEAD-box RNA helicase Vasa (whose SDS-PAGE mobility is similar to that of p68; Hay et al. 1988; Lasko et al. 1988; Liang et al. 1994), the 120-kD double-stranded RNA-binding protein Staufen (St Johnston et al. 1991, 1992), and Aubergine, whose gene has not yet been cloned, play a role in translation of osk mRNA (Kim-Ha et al. 1995; Markussen et al. 1995; Rongo et al. 1995; Wilson et al. 1996). We have examined ovary extracts from vasQ7 (which produces only a truncated Vasa peptide of 35 kD; Tomancak et al. 1998), stauD3, and aub mutants and find no alteration in the binding of p50 and p68 to the osk 5′ RNA element (N. Gunkel, T. Yano, and A. Ephrussi, unpubl.). One explanation is that vas, stau, and aub might indeed be involved in enhancement as was proposed, rather than in derepression of translation. However, our results cannot exclude that the mutant Aubergine or Staufen proteins, although inactive in vivo, still bind RNA in vitro. Alternatively, the vas, stau, or aub gene products may act downstream of the protein that interacts directly with osk mRNA. Therefore, it is still an open question whether vas, stau, or aub play any additional role in derepression. On the basis of the data presented in this report, Staufen and Aubergine could be required to overcome p50-mediated repression, as both are necessary for osk translation even in the absence of BRE-mediated repression (Kim-Ha et al. 1995; Wilson et al. 1996).
The importance of communication between the 5′ and 3′ ends of mRNAs in translational regulation has been hypothesized for some time. Only recently have such proposed interactions begun to receive experimental proof, with the demonstration of a direct interaction between RNA-bound poly(A)-binding protein Pab1p and eIF-4G, a component of the 40S initiation complex (Tarun and Sachs 1996). Our data expand the closed loop model of translation initiation (Jacobson 1996), by implicating the functional interaction of a specific 5′ RNA element and a 3′ element as a prerequisite for proper temporal and spatial regulation of translation.
Materials and methods
DNA constructs
osk+ and oskM1L transgenes are as described (Markussen et al. 1995). Nucleotide positions refer to the A in the first start codon (m1) of osk, for mutations in the 5′ end, and to the T in the stop codon of osk, for mutations in the 3′ UTR (EMBL database M65178). All mutations were verified by DNA sequencing. oskM1LΔ1 has a deletion of 249 bp from the m1–m2 region starting at position 24. Reporter transgenes were made in the context of osk+. m1414lacwt was constructed by fusing in-frame the 430-bp osk 5′ region lacking m2 (including the 15-bp 5′ UTR) to a 3-kb lacZ fragment, equivalent to the PstI fragment of pMC1871 (Pharmacia). Downstream of lacZ, a 1040-bp wild-type genomic osk fragment, beginning with the stop codon of osk and containing the 3′ UTR, was added. m1117lacwt contains only the first 157 bp of the osk 5′ region of m1414lacwt. In m1117lacLS5 and m1414lacLS5 one Bruno motif has been mutated to ATcTaTTgaTcC (starting at position 179 of the osk 3′ UTR; lowercase letters indicate substituted nucleotides). m1m2lacwt was constructed by fusing in-frame the 436-bp m1–m2 region of osk to the lacZ fragment. m1INVm2lacwt is identical to m1m2lacwt except for the inversion of a 62-bp fragment starting at position 105. m1414lac5′Δ24/3′Δ25 contains a truncated form of the EcoRI–DraI repressor element in an otherwise unaltered osk 3′ UTR. The two deletions in this construct remove nucleotides 130–154 and 245–270 of the osk 3′ UTR. m1414lacBRE bcd is identical to m1414lacwt with respect to the 5′ translation control elements and the proximal BRE in the 3′ UTR. However, the RNA transport, localization, and 3′ processing signals downstream of position 376 have been replaced by a 726-bp bcd 3′ UTR fragment, starting at bp 87 downstream of the bcd stop codon.
Transgenic flies
Transgenic flies were generated by P-element-mediated transformation (Spradling and Rubin 1982) using w1118 flies as recipients. Wild-type and mutant osk transgenes were tested in the maternal mutant background osk54/oskDf(3R)p-XT103 (Lehmann and Nüsslein-Volhard 1986). Rescue of the osk phenotype was determined as follows: Eggs were collected on apple juice plates and hatch rates scored by counting hatched and unhatched eggs after aging for 30 hr at 25°C. Fertility of hatchers was tested by mating the female offspring with wild-type males. For each reporter construct, at least four independent transgenic lines were characterized. All reporter constructs produced transcripts that were both transported to the oocyte and localized to the posterior pole equally efficiently. The effect of RNA elements on translation was assessed by comparison of lines that produced the same amount of RNA as determined by an RNase protection assay.
Analysis of RNA and protein expression
In situ labeling of RNA was performed essentially as described (Ephrussi et al. 1991), but with several modifications to improve tissue integrity and signal-to-noise ratio (available upon request). β-Galactosidase activity in ovaries of 2- to 3-day-old virgins was determined in situ as described (Clark et al. 1994). Each set of compared constructs was stained for the same length of time. Ovaries were mounted in Aqua Poly/Mount (Polysciences Inc.).
For the RNase protection assay, the mRNA was isolated from ovaries of ∼50 2-day-old virgins/lane by three rounds of poly(A) selection with Dynabeads (Dynal). The template used to produce antisense lacZ RNA probe was a BbsI-linearized pSP72 plasmid containing a 3-kb BamHI lacZ fragment (in antisense orientation with respect to the T7 promoter) derived from pDF313 (Ferrandon 1994). The unprotected probe spans 347 nucleotides and contains 55 nucleotides of pSP72 polylinker and 292 nucleotides encoding the β-galactosidase carboxyl terminus. The template for the internal osk control probe was a BclI-linearized pSP73 plasmid containing a PCR fragment of the osk open reading frame (in antisense orientation with respect to the T7 promoter) spanning the coding region from nucleotide 1795 to 2070 (Ephrussi et al. 1991). The 250-nucleotide unprotected probe comprises 200 nucleotides of osk sequence and 50 nucleotides of polylinker sequence. The RNase protection assay was done essentially as described by Ausubel et al. (1987–1998). The annealing temperature was 45°C for lacZ probe and 50°C for osk probe. Fifty micrograms of RNase A and 4 units of RNase T1 (Worthington Biochemical) were used. The amounts of input RNA for reporter RNA quantification were normalized to endogenous osk mRNA.
In vitro RNA–protein interaction assay
RNA–protein-binding assays were performed using fresh extracts. The ratios of p50, p68, p90, and Bruno are variable in different extract preparations. Protein extracts were prepared from ovaries of well-fed 2- to 3-day-old females (20°C), dissected in cold Ringer’s solution. After removal of excess Ringer’s, two to three volumes of ice-cold homogenization buffer [(25 mm HEPES (pH 7.5), 400 mm NaCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm PMSF, 10% glycerol] were added and the ovaries were homogenized with a plastic pestle in a 1.5-ml microcentrifuge tube on ice. The lysate was cleared by centrifugation at 20,000 rpm for 10 min at 4°C. Protein extract equivalent to one ovary (5–10 μg) was incubated in a 10-μl reaction mix containing 2 μl of 5× reaction buffer [250 mm Tris-HCl (pH 8.3), 375 mm KCl, 15 mm MgCl2], 0.5 μl of 100 mm DTT, 1 μl of 10 mg/ml heparin, and 5 fmoles of 32P-labeled RNA probe for 30 min on ice. Optimal UV cross-linking conditions for p68 were achieved using a Ultra Lum HRI-100 UV X-linker (1 min at 15°C, UV method 1) and for p50 using a Stratalinker (800 mJ on ice, UV method 2). Complexes were digested with 10 μg of RNase A for 20 min at 37°C to improve resolution of the RNA/protein complexes in subsequent gel electrophoresis. After addition of loading buffer, the samples were heated to 90°C for 5 min and subjected to SDS-PAGE (10%) and autoradiography. For the experiment in Figure 5, the binding mix was preincubated with competitor RNA for 10 min on ice before addition of the radioactive probe. The exact concentration of the competitor RNA was determined by UV spectroscopy and electrophoresis of a dilution series of RNAs on an ethidium bromide-stained agarose gel. Efficient cross-linking of p68 required the incorporation of thio-UTP into the labeled probe. Cross-linking of p50 did not require this modification. However, in experiments in which p50 an p68 were compared, thio-probes were used.
Immunoprecipitation assay
Either before or after RNase A digestion of UV cross-linking reaction mixtures were incubated with anti-Bruno polyclonal antibody (Webster et al. 1997) for 1 hr at 4°C. The resulting immunocomplexes were recovered with protein A–Sepharose (Pharmacia), and analyzed by SDS-PAGE (10%) electrophoresis followed by autoradiography.
Acknowledgments
We thank A. Guichet for an oskΔI cDNA and for initial analysis of ΔI, P. Lasko for the Bruno antiserum, A.-M. Voie for germ-line transformation, and P. Závorszky for improvements on the RNA in situ hybridization protocol. We also thank S. Cohen, F. Gebauer, I. Mattaj, P. Gönczy, M. Hentze, A. Jenny, M. Muckenthaler, and T. Preiss for critical comments on manuscript. N.G. was supported by a fellowship from the Deutsche Forschungsgemeinschaft, and by a Human Frontier Science Program Organization grant to A.E., T.Y. by fellowships from the Cell Science Research Foundation and the TOYOBO Biotechnology Foundation, F.-H.M. by an EMBL predoctoral fellowship and a short-term fellowship from the Norwegian Research Council, and L.C.O. by a fellowship from the Norwegian Research Council.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL ephrussi@embl-heidelberg.de; FAX (49) 6221 387 166.
References
- Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993;123:431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, F., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, eds. 1987–1998. Current protocols in molecular biology. Wiley & Sons, New York, NY.
- Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon D, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: Characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN. Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol. 1994;4:289–300. doi: 10.1016/s0960-9822(00)00068-3. [DOI] [PubMed] [Google Scholar]
- Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: Regulated mRNA transport and local protein synthesis. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Wharton R. The Nanos gradient in Drosophila embryos is generated by translation regulation. Genes & Dev. 1996;10:2610–2620. doi: 10.1101/gad.10.20.2610. [DOI] [PubMed] [Google Scholar]
- Driever W, Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Ferrandon D. “Mise en place de la polarité antéro-postérieure de la Drosophile au cours de l’ovogénèse: Étude de la localisation de l’ARNm du morphogène bicoid au pôle antérieur de l’embryon.” Ph.D. thesis. Strasbourg, France: Université Louis Pasteur Strasbourg; 1994. [Google Scholar]
- Frohnhöfer HG, Nüsslein-Volhard C. Organization of the anterior pattern in the Drosophila embryo by the maternal gene bicoid. Nature. 1986;324:120–125. [Google Scholar]
- Gavis ER, Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992;71:301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- ————— Translational regulation of nanos by RNA localization. Nature. 1994;369:315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lunsford L, Bergsten SE, Lehmann R. A conserved 90 nucleotide element mediates translational repression of nanos RNA. Development. 1996a;122:2791–2800. doi: 10.1242/dev.122.9.2791. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Curtis D, Lehmann R. Identification of cis-acting sequences that control nanos RNA localization. Dev Biol. 1996b;176:36–50. doi: 10.1006/dbio.1996.9996. [DOI] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55:577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Poly (A) metabolism and translation: The closed loop model. In: Hershey WB, Mathews M, Sonenberg N, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 451–480. [Google Scholar]
- Kim-Ha J, Webster PJ, Smith JL, Macdonald PM. Multiple RNA regulatory elements mediate distinct steps in localization of oskar mRNA. Development. 1993;119:169–178. doi: 10.1242/dev.119.1.169. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Kwon YK, Hecht NB. Binding of a phosphoprotein to the 3′ untranslated region of the mouse protamine 2 mRNA temporally represses its translation. Mol Cell Biol. 1993;13:6547–6557. doi: 10.1128/mcb.13.10.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988;335:611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Leathers V, Tanguay R, Kobayashi M, Gallie DR. A phylogenetically conserved sequence within viral 3′ untranslated RNA pseudoknots regulates translation. Mol Cell Biol. 1993;13:5331–5347. doi: 10.1128/mcb.13.9.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R, Nüsslein-Volhard C. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell. 1986;47:141–152. doi: 10.1016/0092-8674(86)90375-2. [DOI] [PubMed] [Google Scholar]
- ————— The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development. 1991;112:679–691. doi: 10.1242/dev.112.3.679. [DOI] [PubMed] [Google Scholar]
- Liang L, Diehl-Jones W, Lasko PF. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- Lieberfarb ME, Chu T, Wreden C, Theurkauf W, Gergen JP, Strickland S. Mutations that perturb poly(A)-dependent maternal mRNA activation block the initiation of development. Development. 1996;122:579–588. doi: 10.1242/dev.122.2.579. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Smibert CA. Translational regulation of maternal mRNAs. Curr Opin Genet Dev. 1996;6:403–407. doi: 10.1016/s0959-437x(96)80060-8. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Struhl G. Cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988;336:595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Markussen F-H, Michon A-M, Breitwieser W, Ephrussi A. Translational control of oskar generates Short OSK, the isoform that induces pole plasm assembly. Development. 1995;121:3723–3732. doi: 10.1242/dev.121.11.3723. [DOI] [PubMed] [Google Scholar]
- Markussen FH, Breitwieser W, Ephrussi A. Efficient translation and phosphorylation of Oskar require Oskar protein and the RNA helicase Vasa. Cold Spring Harbor Symp Quant Biol. 1997;62:13–17. [PubMed] [Google Scholar]
- Micklem DR. mRNA localisation during development. Dev Biol. 1995;172:377–395. doi: 10.1006/dbio.1995.8048. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNAREs and associates. Ann Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Richter J. Dynamics of poly (A) addition and removal during development. In: Hershey WB, Mathews M, Sonenberg N, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 481–503. [Google Scholar]
- Rongo C, Gavis ER, Lehmann R. Localization of oskar RNA regulates oskar translation and requires Oskar protein. Development. 1995;121:2737–2746. doi: 10.1242/dev.121.9.2737. [DOI] [PubMed] [Google Scholar]
- Sallés FJ, Lieberfarb ME, Wreden C, Gergen JP, Strickland S. Coordinate initiation of Drosophila development by regulated polyadenylation of maternal messenger RNAs. Science. 1994;266:1996–1999. doi: 10.1126/science.7801127. [DOI] [PubMed] [Google Scholar]
- Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serano TL, Cohen RS. Gratuitous mRNA localization in the Drosophila oocyte. Development. 1995;121:3013–3021. doi: 10.1242/dev.121.9.3013. [DOI] [PubMed] [Google Scholar]
- Seydoux G. Mechanisms of translational control in early development. Curr Opin Genet Dev. 1996;6:555–561. doi: 10.1016/s0959-437x(96)80083-9. [DOI] [PubMed] [Google Scholar]
- Smibert CA, Wilson JE, Kerr K, Macdonald PM. Smaug protein represses translation of unlocalized nanos mRNA in the Drosophila embryo. Genes & Dev. 1996;10:2600–2609. doi: 10.1101/gad.10.20.2600. [DOI] [PubMed] [Google Scholar]
- Smith J, Wilson J, Macdonald P. Overexpression of oskar directs ectopic action of nanos and presumptive pole cell formation in Drosophila embryos. Cell. 1992;70:849–859. doi: 10.1016/0092-8674(92)90318-7. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Standart N, Jackson JR. Regulation of translation by specific protein/mRNA interactions. Biochimie. 1994;76:867–879. doi: 10.1016/0300-9084(94)90189-9. [DOI] [PubMed] [Google Scholar]
- St Johnston D. The intracellular localization of messenger RNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Beuchle D, Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. mRNA localization in neurons: A multipurpose mechanism? Neuron. 1997;18:9–12. doi: 10.1016/s0896-6273(01)80041-6. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Gallie DR. Isolation and characterization of the 102-kilodalton RNA-binding protein that binds to the 5′ and 3′ translational enhancers of tobacco mosaic virus RNA. J Biol Chem. 1996;271:14316–14322. doi: 10.1074/jbc.271.24.14316. [DOI] [PubMed] [Google Scholar]
- Tarun S, Sachs A. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- Teasdale RD, Jackson MR. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Ann Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- Tomancak P, Guichet A, Závorszky P, Ephrussi A. Oocyte polarity depends on regulation of gurken by Vasa. Development. 1998;125:1732–1732. doi: 10.1242/dev.125.9.1723. [DOI] [PubMed] [Google Scholar]
- Walker J, Dale M, Standart N. Unmasking mRNA in clam oocytes: Role of phosphorylation of a 3′ UTR masking element-binding protein at fertilization. Dev Biol. 1996;173:292–305. doi: 10.1006/dbio.1996.0024. [DOI] [PubMed] [Google Scholar]
- Wang C, Lehmann R. Nanos is the localized posterior determinant in Drosophila. Cell. 1991;66:637–642. doi: 10.1016/0092-8674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- Wang S, Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994;369:400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- Webster PJ, Liang L, Berg CA, Lasko P, Macdonald PM. Translational repressor Bruno plays multiple roles in development and is widely conserved. Genes & Dev. 1997;11:2510–2521. doi: 10.1101/gad.11.19.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M, Kimble J, Strickland S. Translational control of developmental decisions. In: Hershey WB, Mathews M, Sonenberg N, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 411–450. [Google Scholar]
- Wilson JE, Connell JE, Macdonald PM. aubergine enhances oskar translation in the Drosophila ovary. Development. 1996;122:1631–1639. doi: 10.1242/dev.122.5.1631. [DOI] [PubMed] [Google Scholar]