Abstract
Hyperkinetic Jak2 tyrosine kinase signaling has been implicated in several human diseases including leukemia, lymphoma, myeloma and the myeloproliferative neoplasms. Using structure-based virtual screening, we previously identified a novel Jak2 inhibitor named G6. We showed that G6 specifically inhibits Jak2 kinase activity and suppresses Jak2-mediated cellular proliferation. To elucidate the molecular and biochemical mechanisms by which G6 inhibits Jak2-mediated cellular proliferation, we treated Jak2-V617F expressing human erythroleukemia (HEL) cells for 12 hours with either vehicle control or 25 μM of the drug and compared protein expression profiles using two-dimensional gel electrophoresis. One differentially expressed protein identified by electrospray mass spectroscopy was the intermediate filament protein, vimentin. It was present in DMSO treated cells, but absent in G6 treated cells. HEL cells treated with G6 showed both time- and dose-dependent cleavage of vimentin as well as a marked reorganization of vimentin intermediate filaments within intact cells. In a mouse model of Jak2-V617F mediated human erythroleukemia, G6 also decreased the levels of vimentin protein, in vivo. The G6-induced cleavage of vimentin was found to be Jak2-dependent and calpain-mediated. Furthermore, we found that intracellular calcium mobilization is essential and sufficient for the cleavage of vimentin. Finally, we show that the cleavage of vimentin intermediate filaments, per se, is sufficient to reduce HEL cell viability. Collectively, these results suggest that G6-induced inhibition of Jak2-mediated pathogenic cell growth is concomitant with the disruption of intracellular vimentin filaments. As such, this work describes a novel pathway for the targeting of Jak2-mediated pathological cell growth.
Keywords: Janus kinase 2 (Jak2), Vimentin, Calpain
Jak2 is a member of the Janus family of cytoplasmic tyrosine kinases. Other members of this family include Jak1, Jak3 and Tyk2 (1). Jak2 is activated by a variety of cytokines, growth factors and G Protein-coupled Receptor (GPCR) ligands, resulting in signaling cascades that regulate cell growth, proliferation and death (1). Upon binding of the ligand to its specific receptor, the receptor-associated Jak proteins are activated via a phosphorylation event. An activated Jak can in turn phosphorylate and activate the Signal Transducers and Activators of Transcription (STAT) family of transcription factors. Phosphorylated STATs dimerize and translocate to the nucleus where they modulate gene transcription (2, 3). Thus, the Jak/STAT pathway results in a signal cascade from binding and activation at the plasma membrane to changes in gene transcription in the nucleus.
Hyperkinetic Jak2 promotes cell growth and prevents apoptosis. Hence, constitutively active Jak/STAT signaling pathway has been implicated in a variety of neoplastic disorders. Jak2 can become constitutively active by several different gene alterations including specific chromosomal translocations and point mutations. Jak2 chromosomal translocations such as TEL-Jak2, REL-Jak2, BCR-Jak2 and PCM1-Jak2 lead to the development of a variety of leukemias, lymphomas and myelomas (4–10). Additionally, an activating Jak2 point mutation (Jak2-V617F) has been linked to the myeloproliferative neoplasms (MPN) such as polycythemia vera, essential thrombocythemia and primary myelofibrosis (11–15). This valine to phenylalanine substitution mutation present in codon 617 of the autoinhibitory pseudokinase domain of Jak2 allows the kinase to evade negative regulation thereby making it constitutively active. MPN patients bear this mutation in their marrow derived stem cells and are characterized by the overproduction of terminally differentiated blood cells of the myeloid lineage such as red cells or platelets. Current therapies for MPN patients include phlebotomy and hydroxyurea. While these treatments alleviate some disease symptomologies, they are not curative in any way. Therefore, there is an unmet clinical need for these patients.
Using structure-based virtual screening, our group recently identified a novel stilbenoid small molecule inhibitor of Jak2 named G6 (16). We subsequently showed that G6 specifically inhibits Jak2 mediated human pathologic cell growth in vitro, ex vivo, and in vivo (17, 18). We also demonstrated that G6 inhibits Jak2 mediated cell proliferation via the suppression of key signaling molecules of the Jak/STAT pathway; the consequence of this inhibition is G1/S cell cycle arrest and apoptosis (17, 18).
Here, we sought to elucidate the molecular and biochemical mechanisms by which G6 inhibits Jak2-mediated cellular proliferation. For this, we compared protein expression profiles between vehicle treated and G6 treated cells using two-dimensional gel electrophoresis. The intermediate filament protein, vimentin, was one protein that was differentially expressed between the two conditions. We therefore hypothesized that the mechanism by which G6 inhibits Jak2-dependent cell proliferation involves modification of this protein. In this study, our data support this hypothesis as we show that G6-induced inhibition of Jak2-mediated pathogenic cell growth correlates with the specific cleavage and cellular reorganization of vimentin.
EXPERIMENTAL PROCEDURES
Drugs
G6, obtained from the National Cancer Institute/Developmental Therapeutics Program (NCI/DTP), was solublized in dimethyl sulfoxide (DMSO) at a concentration of 10 mM and stored at −20°C.
Reagents
AG490, Jak Inhibitor I, PD98059 and PP2 were purchased from Calbiochem. Cycloheximide was purchased from Fisher Scientific. Caspase Inhibitor I (Z-VAD (OMe)-FMK), Calpain Inhibitor V (Mu-Val-HPh-CH2F, Mu = morpholinoureidyl; HPh = homophenylalanyl), Verapamil, BAPTA-AM, A23187 and 3′,3′-iminodipropionitrile (IDPN) were also purchased from Calbiochem.
Cell Culture
Human Erythroleukemia (HEL) cells were purchased from the American Type Culture Colletion (ATCC) and maintained in RPMI 1640 (Mediatech) supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, and L-glutamine at 37°C and 5% CO2.
2-D Differential In Gel Electrophoresis (2-D DIGE)
HEL cells treated either with vehicle control DMSO or 25 μM G6 for 12 hours. The cell pellets were resuspended in ice cold buffer containing 0.3% SDS, 20 mM Tris pH 8.0, 100 mM DTT, 10 μl protease inhibitor cocktail III (Calbiochem), 5 mM MgCl2 and 250 units of benzonase. The cell suspension was homogenized by sonication. Protein was precipitated with 9 volumes of ice cold 10% trichloroacetic acid in acetone overnight at 4°C. Precipitated proteins were then dissolved in solubilization buffer (7 M urea, 2 M thiourea, 4% CHAPS, 0.2% SDS and 20 mM Tris, pH 8.0). After centrifugation at 43,000 rpm for 30 min, solubilized protein in the supernatant was quantified using the EZQ Protein Assay Kit (Invitrogen). Proteins (100 μg per sample) were minimally labeled with CyDye (GE Healthcare) as per the manufacturer’s protocol. An internal standard, which is loaded on every gel, was created by mixing equal amounts of protein from all samples. Proteins from the DMSO treated samples were labeled with Cy3 (green) and the G6 treated samples were labeled with Cy5 (red). The internal standard was labeled with Cy2 (blue).
100 μg of Cy2 labeled internal standard, 100 μg of Cy3 labeled sample, 100 μg of Cy5 labeled sample were mixed with 200 ug unlabeled internal standard. The mixture was used to rehydrate a 24 cm pH 3 to 11 nl IPG strip (GE Healthcare) overnight in a rehydration buffer (solubilization buffer with 100 nM DTT containing Orange G as tracking dye) in the dark at room temperature. Three independent replicates of each sample were run on three strips. IEF was carried out in IPGphor3 unit (GE Healthcare) as per manufacturer’s recommendation. Temperature was maintained at 19°C throughout focusing.
After completion of IEF, strips were first reduced in 15 ml of 50 mM Tris-HCl pH 6.8, 6 M Urea, 30% (v/v) glycerol, 2% (w/v) SDS, 100 mM DTT for 20 min in the dark at room temperature, then alkylated in 15 ml of 50 mM Tris-HCl pH 6.8, 6 M Urea, 30% (v/v) glycerol, 2% SDS, and 2.5% idoacetamide for 20 min. After equilibration, strips were transferred and mounted on an 8% – 16% precast Tris Glycine polyacrylamide gel (Jule). Electrophoresis was carried out initially at 12ºC at 10 mA/gel for one hour and then at constant current overnight at 12 mA/gel and a limit of 150 V until dye front reached the bottom of the plate.
Gels were then scanned with Typhoon 9400 Variable Mode Imager (GE Healthcare). The excitation/emission wavelengths for Cy2, Cy3 and Cy5 were 488/520, 532/580 and 633/670 nm, respectively. For each gel, images for the internal standard as well as the control and experimental conditions were acquired. The digital images were then analyzed with DeCyder 2D version 7.0 (GE Healthcare). Information from replicate gels was analyzed with BVA Module (Biological Variation Analysis). Spots were selected by setting the fold difference threshold to 1.6-fold. Statistical significance was estimated using Student’s t-test. Protein identification using electrospray mass spectroscopy was done at the Scripps Research Institute.
Cell Lysis
Cells (~ 107) were washed with two volumes of ice-cold PBS and then lysed in 0.8 ml of ice-cold RIPA buffer (20 mM Tris pH 7.5, 10% glycerol, 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 2.5 mM EDTA, 50 mM NaF, 10 mM Na4P2O7, 4 mM benzamidine, and 10 μg/mL aprotinin). Protein concentrations in the whole cell lysates were determined using a Bradford assay (Bio-Rad). Cell lysates were then resuspended in SDS sample buffer. Whole cell lysates (~ 30 μg) were separated by SDS-PAGE and then transferred onto nitrocellulose membranes for analysis by western blotting.
Western Blotting
Nitrocellulose membranes were first blocked with 5% milk/TBST solution (20 mM Tris pH 7.2, 150 mM NaCl, 0.05% Tween 20) at room temperature and then probed first with the different indicated primary antibodies overnight at 4°C followed by the respective secondary antibodies (1:4000, GE Healthcare). The immuno-reactive bands were then visualized using the enhanced chemi-luminescence system (Western Lightning Ultra, Perkin-Elmer). The following antibodies were used at the indicated dilutions: vimentin (Abcam and BD Biosciences, each at 1:500), STAT1 (Santa Cruz Biotechnology, 1:1000), and β-actin (Cell Signaling, 1:500).
Immunofluorescence
HEL cells were cultured in RPMI in 100 mm dishes and treated with 25 μM G6 for 24 hours. Following treatment, the cells were centrifuged, washed and resuspended in 1X PBS. Cells were then plated onto poly-L-lysine coated 8-chamber slides (Santa Cruz Biotechnology) and fixed at −20°C in a mixture of 50% methanol and 50% acetone for 10 minutes. The fixed cells were then permeabilized with 0.2% Triton X-100 and blocked with 5% goat serum for 30 minutes at room temperature. The samples were incubated overnight at 4°C with a primary antibody of mouse anti-vimentin (BD Biosciences, 1:100) or rabbit anti-β-actin (Cell Signaling, 1:100) and washed 4X with PBS the following morning. The samples were then incubated with a FITC-conjugated anti-mouse secondary antibody or a FITC-conjugated anti-rabbit secondary antibody (Santa Cruz Biotechnology) for one hour at room temperature. The cells were again washed with PBS, mounted with UltraCruz DAPI containing mounting media (Santa Cruz Biotechnology) and sealed with a cover slip. These cells were imaged using a 100X objective on an inverted fluorescence microscope (Olympus).
Cell Proliferation Assay
HEL cells were plated in 96-well plates and treated with either 0.25% DMSO, 30 μM G6 or 2% IDPN for the indicated periods of time. Cell viability was then assessed for each sample by trypan blue exclusion staining and hemocytometer.
In vivo Animal Model
The xenograft model of Jak2-V617F expressing HEL cells in NOD/SCID mice has been described previously (18). Briefly, 3 months old NOD/SCID mice were randomized into 5 groups (n=6 mice per group). One group consisted of naive animals that did not receive any treatment. All other groups received a single tail vein injection of 2 × 106 Jak2-V617F-positive HEL cells. Three weeks after HEL cell injection, the mice developed symptoms of a fully penetrant bone marrow malignancy. The mice then began receiving intraperitoneal injections of either vehicle control (DMSO) or G6 at doses of 0.1, 1, and 10 mg/kg/day for the next 21 days. At the end of the three week treatment period, all groups of mice were euthanized and bone marrow tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin.
Bone Marrow Immunohistochemistry
Paraffin embedded bone marrow sections from each treatment group were analyzed by anti-vimentin immunohistochemistry. Antigen retrieval was carried out first by microwaving at 95°C for 20 min in 1mM EDTA-NaOH solution, pH 8.0. The section were then cooled, blocked with Protein Block (DAKO), and incubated with anti-vimentin antibody (Abcam, 1:100) for 2 hours at room temperature. Antigen-antibody complexes were detected using biotinylated secondary antibodies and streptavidin-peroxidase substrate (DAKO). Stained sections were then analyzed via a standard light microscope (Nikon) at 40X and 100X magnifications.
Statistical Analysis
For statistical evaluation of time-dependent response of HEL cell viability to G6 and IDPN, a two-way analysis of variance was used. For analysis of differential expression of proteins in 2-D DIGE and G6-induced degradation of vimentin using densitometry, a Student’s t-test was employed. Data were assumed to be statistically significant when p < 0.05.
RESULTS
G6 treatment induces time- and dose-dependent degradation of vimentin
The human erythroleukemia (HEL 92.1.7) cell line is homozygous for the Jak2-V617F mutation (19, 20). The presence of this mutation induces constitutively active Jak/STAT signaling and promotes a G1/S phase transition thereby driving increased cellular proliferation (21). We previously demonstrated that the Jak2 inhibitor, G6, inhibits Jak2-V617F-mediated HEL cell proliferation and induces apoptosis (16,17). However, the specific mechanisms by which G6 does this are not known.
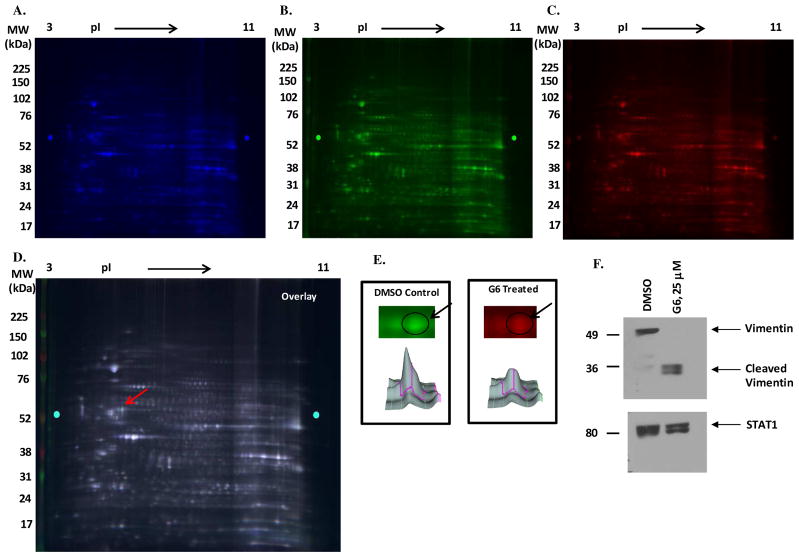
To gain some insight into the mechanism by which G6 reduces cell viability, HEL cells were treated for 12 hours with either vehicle control (0.25% DMSO) or 25 μM G6. The protein expression profiles of these two treatment conditions were compared using two-dimensional gel-electrophoresis (Fig. 1A, 1B, 1C & 1D). The two-dimensional gel images were then scanned and the staining intensities of the various protein spots were compared between the two treatment conditions. One spot in particular, identified from the scanning results, was highly expressed in the DMSO treated cells, but significantly reduced in the G6 treated cells (Fig. 1E; circled and marked by arrows). That spot was excised and identified using electro spray mass spectrometry as vimentin. In a separate two-dimensional gel electrophoresis study, we compared the protein expression profiles between HEL cells that had been treated with either DMSO or 25 μM G6 for 24 hours. Even at this longer time point, the spot representing vimentin was still significantly reduced in the G6 treated cells when compared to the DMSO treated cells (data not shown).
Figure 1. Identification of vimentin as a differentially expressed protein between vehicle treated and G6 treated HEL cells.
HEL cells were treated with either 0.25% DMSO or with 25 μM of G6 for 12 hr. Proteins from the internal standard (A), DMSO treated (B) and G6 treated (C) were labeled with Cy2 (blue), Cy3 (green) and Cy5 (red), respectively. (D) An overlay of the three colored images is shown. One protein spot, indicated by the arrow, was differentially expressed between the vehicle treated and G6 treated samples. (E) Analysis of the images obtained from the 2-D DIGE using DeCyder 2D predicted this differentially expressed protein to be significantly downregulated in the G6 treated samples (p=0.01). The indicated protein was excised and identified using electro spray mass spectrometry as vimentin. (F) HEL cells were treated either with DMSO or 25 μM of G6 for 24 hr and analyzed by western blotting using an anti-vimentin antibody. The same samples were also blotted with an anti-STAT1 antibody to confirm equal loading across all lanes. Shown is one of three representative images.
To confirm that vimentin protein levels were decreasing with G6 treatment, protein samples from both conditions were subjected to western blot analysis with an anti-vimentin antibody. Consistent with the mass spectroscopy data, treatment of HEL cells with G6 resulted in the disappearance of full-length vimentin (Fig. 1F). Of note, we also observed the appearance of low molecular weight fragments of vimentin in the G6 treated cells (Fig. 1F).
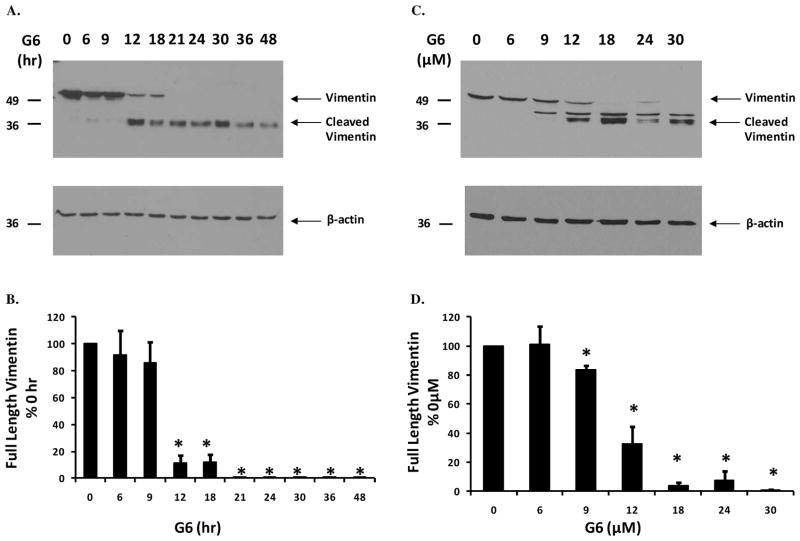
To determine whether this effect was time- and dose-dependent, HEL cells were treated either with 25 μM of G6 for increasing lengths of time or with varying doses of G6 for 24 hours. Whole cell lysates were then separated on SDS-PAGE and examined by immunoblotting with an anti-vimentin antibody. Fig. 2A is a representative blot showing that full-length vimentin was cleaved into low molecular weight fragments with G6 treatment as a function of time. The same samples were then reprobed with an anti-β-actin antibody to confirm equal protein loading and also to demonstrate the specificity of G6 for vimentin over other cytoskeletal proteins such as β-actin. Quantification of all blots using densitometry confirmed the total loss of full-length vimentin protein in response to G6 treatment over time (Fig. 2B). Similarly, Fig. 2C is a representative blot showing a dose-dependent cleavage of full-length vimentin in response to G6 and Fig. 2D is a quantification of all dose-dependent blots.
Figure 2. G6 treatment induces time- and dose-dependent degradation of vimentin.
HEL cells were treated either with 25 μM of G6 for varying lengths of time (A) or with increasing doses of G6 for 24 hours (C). Cell lysates were then separated on SDS-PAGE and immunoblottted with an anti-vimentin antibody. The same samples were then reprobed with an anti-β-actin antibody to confirm equal protein loading and also to demonstrate the specificity of G6 for vimentin over other cytoskeletal proteins such as β-actin. Shown is one of three independent results for each. Expression of full-length vimentin was quantified using densitometry and plotted as a function of either time (B) or dose (D) of G6 treatment. Data shown are the mean ± SE from three independent experiments. *p<0.05 with respect to 0 hr (B) or 0 μM (D).
Collectively, the data in Figs. 1 and 2 demonstrate the ability of G6 to induce specific cleavage of the intermediate filament protein vimentin. Furthermore, this effect is both time- and dose-dependent.
G6 treatment induces marked reorganization of vimentin intermediate filaments within cells
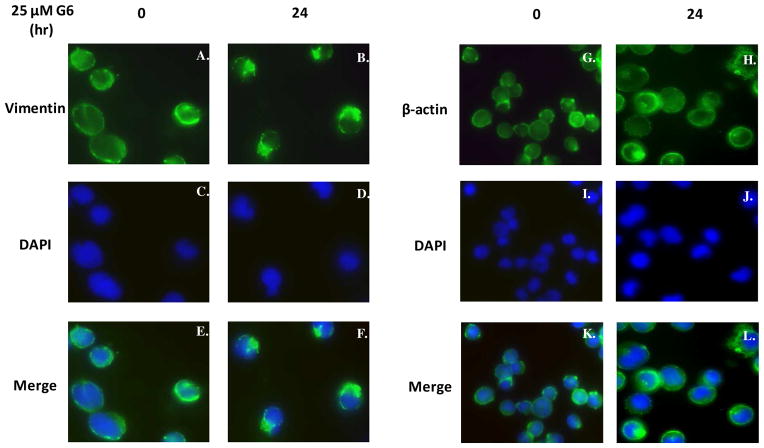
We next wanted to study the effect of G6 treatment on structure and cellular distribution of intracellular vimentin filaments. For this, HEL cells were treated with 25 μM G6 for 0 and 24 hours and then vimentin expression was analyzed via indirect immunoflorescence. For the 0 hr time point, we found that vimentin was largely distributed over the cytoplasm (Fig. 3A, 3C, & 3E). However, after 24 hr of G6 treatment, vimentin had an irregular staining pattern in the perinuclear region of the cell (Fig. 3B, 3D, 3F). As a control, similarly treated HEL cells were examined for changes in β-actin expression. We found that β-actin was uniformly distributed across the cytoplasm of the cell at the 0 hr time point and this pattern did not change with G6 treatment (Fig. 3G–L).
Figure 3. G6 treatment induces marked reorganization of vimentin intermediate filaments within cells.
HEL cells, treated with 25 μM of G6 for 0 or 24 h, were analyzed via indirect immunofluorescence for changes in the cellular distribution of vimentin and β-actin in response to drug treatment. Vimentin (A and B) and β-actin (G and H) were indirectly labeled with a FITC-conjugated secondary antibody. The nuclei were counter stained with DAPI (C, D and I, J). The images were then merged (E, F and K, L). Shown is one of two representative results.
As such, the data in Fig. 3 indicate that G6 treatment specifically induces cellular redistribution of vimentin intermediate filament within HEL cells while having no effect on the cellular distribution of β-actin microfilaments.
G6-induced cleavage of vimentin is Jak2-mediated
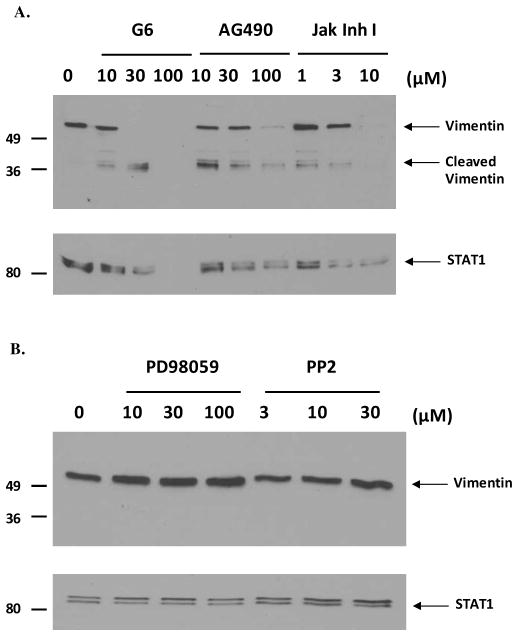
Having already demonstrated the ability of G6 to induce specific cleavage of vimentin (Fig. 2), we next wanted to determine if this G6-induced cleavage was Jak2-dependent. For this, HEL cells were treated for 24 hours with increasing concentrations of three different Jak2 inhibitors; G6, AG490 and Jak Inhibitor I. As a control, HEL cells were also treated with non-Jak2 inhibitors; namely, the MAPK inhibitor, PD98059 and Src family kinase inhibitor, PP2. Whole cell lysates were separated by SDS-PAGE and immunoblotted with an anti-vimentin antibody. We observed that the Jak2-specific inhibitors induced cleavage of vimentin dose-dependently (Fig. 4A) whereas the non-Jak2 inhibitors had no effect on full-length vimentin (Fig. 4B). The loading of total protein was determined by immunoblotting the same cellular lysates with an anti-STAT1 antibody (Fig. 4A & 4B). Treatment of HEL cells with higher concentrations of the different Jak2 inhibitors (such as 100 μM G6, 100 μM AG490 or 30 μM Jak Inhibitor I) potently induces apoptosis in these cells, thereby leading to the degradation of all cellular proteins, including vimentin and STAT1. Hence, there is a general reduction in the total protein that is extracted from these cells, which explains the lower level of expression or total absence of proteins observed in the samples that had been treated with high concentrations of the different Jak2 inhibitors.
Figure 4. G6-induced cleavage of vimentin is Jak2-mediated.
HEL cells were treated for 24 hrs with increasing concentrations of Jak2 specific inhibitors (G6, AG490 and Jak Inhibitor I) (A) or non-Jak2 inhibitors (MAPK inhibitor, PD98059 and c-Src inhibitor, PP2) (B). Whole cell lysates were separated by SDS-PAGE and immunoblotted with an anti-vimentin antibody. Loading of total protein across all lanes was determined using an anti-STAT1 antibody. Shown is one of three representative blots.
Thus, from Fig. 4, we conclude that G6-induced vimentin degradation is Jak2-mediated.
G6-induced cleavage of vimentin is independent of de novo protein synthesis and caspase activity, but calpain-dependent
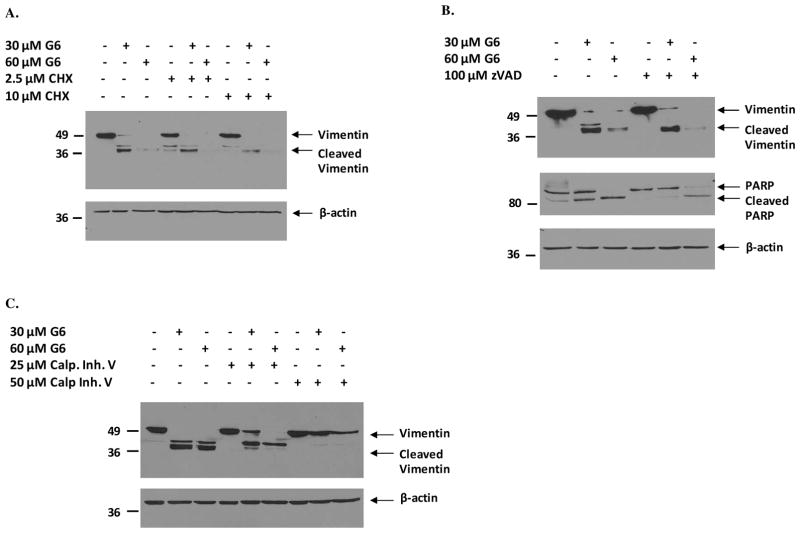
Given that G6 induces specific cleavage of vimentin (Fig. 2), we next wanted to determine whether this G6-induced vimentin cleavage is dependent on de novo protein synthesis. To assess this, HEL cells were first pretreated for 4 hours with increasing doses of cycloheximide (CHX), an inhibitor of protein biosynthesis, and then treated with increasing concentrations of G6 for 24 hours. Cycloheximide inhibits protein synthesis by interfering with the translation elongation process of protein biosynthesis (22). Western blot analysis of the cell lysates from the different treatment groups showed that exposure to increasing doses of G6 induced a dose dependent cleavage of vimentin in HEL cells which was not blocked by pretreatment with cycloheximide (Fig. 5A), indicating that this G6-induced cleavage process does not require de novo protein synthesis.
Figure 5. G6-induced cleavage of vimentin is independent of de novo protein synthesis and caspase activity, but calpain-dependent.
HEL cells were pretreated for 4 hours with either cycloheximide (CHX) (A), caspase inhibitor I (zVAD) (B) or calpain inhibitor V (C) and then treated with increasing doses of G6 for 24 hours. Whole cell lysates from the different treatment groups were then analyzed by western blotting with an anti-vimentin antibody. The same lysates were also probed with an anti-β-actin antibody to confirm equal protein loading across all lanes. Shown is one of three representative results for each.
Vimentin is cleaved in response to G6 treatment into low molecular weight fragments of vimentin (Fig. 2) suggesting that this process is mediated by a protease/preoteolytic enzyme. Caspases are a class of intracellular cysteine proteases with roles in cytokine maturation, inflammation and apoptosis (23). We previously showed that G6 induces caspase 3/7 activation in a time-dependent manner in HEL cells (18). It has also been reported that vimentin is a caspase substrate and can be cleaved by some caspases in vitro (24). Therefore, we wanted to determine if G6-induced vimentin cleavage is caspase-mediated. For this, we first pretreated HEL cells with the pan-caspase inhibitor, Caspase Inhibitor I (zVAD-fmk), for 4 hours before treating them with 30 μM and 60 μM G6 for 24 hours. The effect of caspase inhibition on G6-dependent vimentin cleavage was then studied by western blotting the cell lysates with an anti-vimentin antibody. We found that inhibition of caspases by zVAD-fmk was unable to prevent G6-induced cleavage of vimentin (Fig. 5B) but was able to significantly reduce the G6-induced cleavage of PARP (Fig. 5B), a substrate known to be cleaved by caspases (25), thereby indicating that G6-induced cleavage of vimentin is caspase-independent.
Calpain, a calcium-dependent neutral cysteine protease (26), is yet another protease that is known to cleave vimentin (27, 28). Hence, to examine whether G6-induced vimentin cleavage is calpain-mediated, we pretreated HEL cells with a calpain inhibitor, Calpain Inhibitor V (Mu-Val-HPh-CH2F), for 4 hours before exposing them to increasing doses of G6 for 24 hours. Immunoblotting analysis of the HEL cell lysates showed that calpain inhibition prevented G6-induced cleavage of vimentin in a dose-dependent manner (Fig. 5C), demonstrating that the protease involved in the cleavage of vimentin in response to G6 treatment is calpain.
Overall, the data in Fig. 5 indicate that the G6-induced cleavage of intermediate filament protein vimentin is independent of de novo protein synthesis and caspase activity, but dependent on calpain protease activity.
The mobilization of calcium is essential and sufficient for the cleavage of the intermediate filament protein vimentin
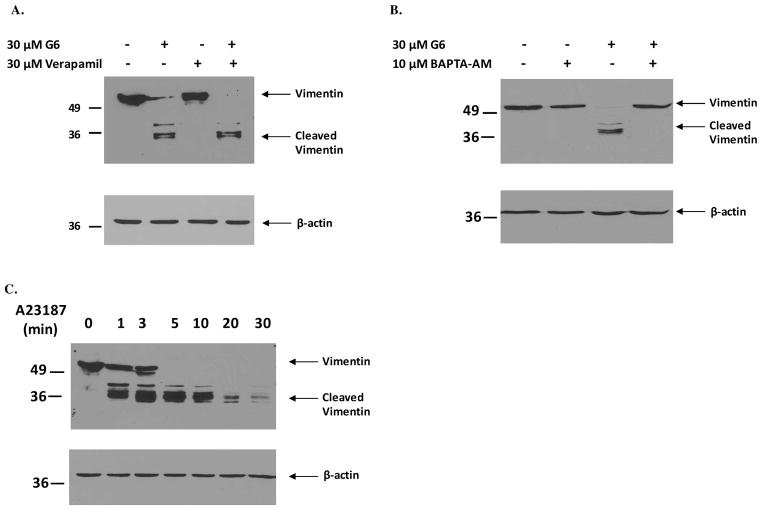
Given that calpain is a calcium-dependent cysteine protease, we next investigated the role of calcium in the G6-induced vimentin cleavage process. Specifically, we first examined the effect of inhibiting the flux of extracellular calcium into cells by pretreating the cells with verapamil. Verapamil blocks Ca2+ channels, principally the L-type channel, thereby interfering with the extracellular influx of calcium ions. HEL cells were pretreated with 30 μM verapamil for 4 hours before exposure to 30 μM G6 for 24 hours. Cell lysates were then immunoblotted with an anti-vimentin antibody. We found that inhibition of extracellular calcium ion influx into the cell via blockage of L-type calcium channels did not have any effect on G6-induced cleavage of vimentin (Fig. 6A). Therefore, we next studied the effect of chelating intracellular calcium on G6-mediated vimentin cleavage. For this, we pretreated HEL cells with 10 μM BAPTA-AM for 2 hours before treatment with 30 μM G6 for 24 hours. BAPTA-AM is membrane-permeable ester form of the calcium chelator BAPTA. Once inside the cell, it is hydrolyzed by cytosolic esterases into its active form and can chelate intracellular calcium. Results from the western blot analysis showed that chelation of intracellular calcium protected vimentin from G6-induced cleavage (Fig. 6B), indicating that intracellular calcium has a critical role to play in mediating the G6-induced cleavage of vimentin.
Figure 6. Mobilization of calcium is essential and sufficient for the cleavage of vimentin.
HEL cells were first pretreated with either 30 μM verapamil for 4 hours (A) or 10 μM BAPTA-AM for 2 hours (B) and then treated with 30 μM G6 for 24 hours. Post treatment, the cells were lysed, proteins were separated by gel electrophoresis and then immunoblotted with an anti-vimentin antibody (upper panel) or an anti-β-actin antibody (lower panel) to confirm equal protein across all lanes. (C) HEL cells were treated with 10 μM A23187 for the indicated periods of time. Cellular lysates were then probed with an anti-vimentin antibody (top panel). An anti-β-actin antibody was used as a loading control (bottom panel). Shown is a representative blot from three independent experiments for each.
In the next experiment, we examined the effect of the calcium ionophore, A23187, on vimentin protein levels within HEL cells. A23187 is a mobile ion-carrier that forms stable complexes with divalent cations, such as calcium, and can hence be used for increasing intracellular levels of calcium ions. Accordingly, HEL cells were treated with 10 μM A23187 for increasing periods of time and the cellular lysates were then western blotted using an anti-vimentin antibody. We found that increasing intracellular calcium levels via exposure to an ionophore is sufficient to induce cleavage of vimentin in HEL cells (Fig. 6C), further confirming the essential role that calcium ions play in the vimentin cleavage process.
Collectively, data in Fig. 6 demonstrate that mobilization of intracellular calcium ions is both essential and sufficient for the cleavage of the intermediate filament protein, vimentin.
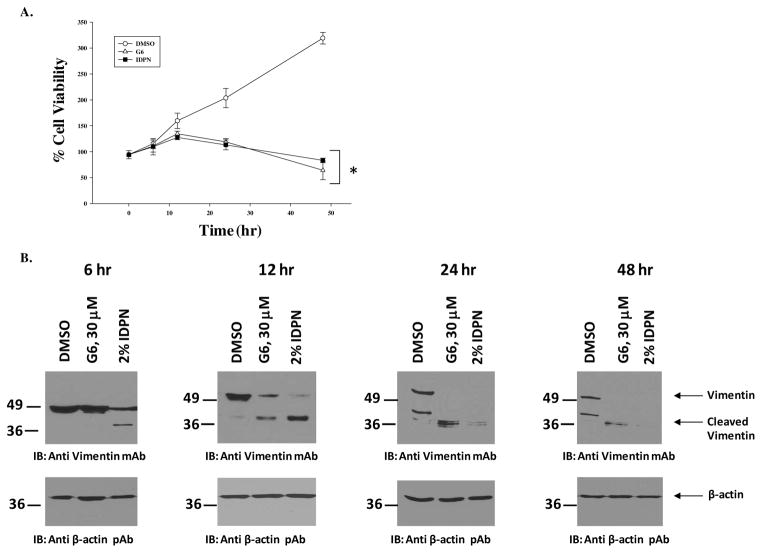
Cleavage of vimentin is sufficient to reduce HEL cell viability
To determine how critical vimentin is to the viability of cells, we studied the effect of vimentin cleavage on the survival of HEL cells. The drug, 3′,3′-iminodipropionitrile (IDPN), selectively disrupts vimentin intermediate filaments (29). Therefore, we treated HEL cells either with vehicle control DMSO, 30 μM G6 or 2% IDPN for 0, 6, 12, 24 or 48 hours. At each time point, the number of viable cells in each condition was determined and cell lysates from those same conditions were immunoblotted with an anti-vimentin antibody in order to correlate decreased cell numbers with increased vimentin cleavage. We found that treatment with both G6 and IDPN time-dependently decreased viable cell numbers (Fig. 7A) and this decrease in cell viability correlated with a corresponding time-dependent cleavage of full-length vimentin in the G6 and IDPN treated cells (Fig. 7B).
Figure 7. Cleavage of vimentin is sufficient to reduce HEL cell viability.
HEL cells were exposed to either vehicle control (DMSO), 30 μM G6 or 2% IDPN for 0, 6, 12, 24 or 48 hours. (A) The numbers of viable cells at each time point were determined and plotted as a function of treatment condition. (B) Cell lysates from each treatment group were collected simultaneously and analysed by immunoblotting with either an anti-vimentin antibody or an anti-β-actin antibody. Shown is one of three representative results. *p<0.05 with respect to DMSO.
Overall, the data in Fig. 7 demonstrate that the cleavage of vimentin intermediate filaments is sufficient to reduce the viability of Jak2-V617F expressing HEL cells.
G6 treatment decreases the levels of vimentin protein, in vivo
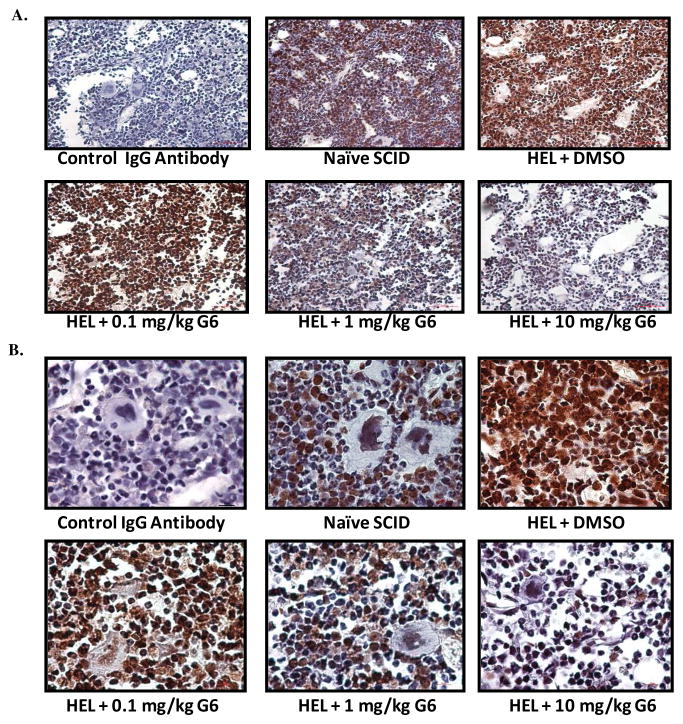
Our data thus far indicate that treatment of HEL cells with G6 results in the degradation and subsequent loss of vimentin protein, in vitro. To determine if this is conserved in vivo, HEL cells were injected into the tail vein of NOD/SCID mice and allowed to engraft into the bone marrow over the ensuing 21 days at which time the mice began receiving daily intraperitoneal injections of either vehicle control (DMSO) or G6 at doses of 0.1, 1, and 10 mg/kg/day, for the next 21 days. At the end of the three week treatment period, all groups of mice were euthanized and bone marrow was analyzed for vimentin protein levels via anti-vimentin immunohistochemistry. Representative stained sections from each treatment group are shown at 40X (Fig. 8A) and 100X (Fig. 8B) magnification. We found that when a negative control IgG antibody was used in place of the anti-vimentin primary antibody in the immuno-histochemical procedure, only the hematoxylin counter stain was observed. Probing the naïve bone marrow with the anti-vimentin antibody revealed strong staining in erythroid cells, but not myeloid cells. HEL cell injection followed by DMSO treatment resulted in a dramatic increase in the expression of vimentin protein when compared to naïve animals. Treatment with 0.1 mg/kg/day of G6 did not produce any observable change in the expression level of vimentin when compared to DMSO treated mice. However, treatment with 1 and 10 mg/kg/day of G6 clearly reduced the levels of vimentin protein to those seen in the completely naïve animals.
Figure 8. G6 treatment decreases the levels of vimentin protein, in vivo.
NOD/SCID mice were randomized into 5 groups (n=6 per group). One group consisted of naïve animals that did not receive any treatment whatsoever. All other mice received 2 × 106 HEL cells via a single tail vein injection. Three weeks after injection, the mice began receiving vehicle control solution (DMSO) or G6 at doses of 0.1, 1, and 10 mg/kg/day. After three weeks of treatment, the mice were euthanized. Anti-vimentin immuno-histochemistry was then carried out on bone marrow sections from the indicated groups of animals. Shown are representative stained bone marrow sections from each treatment group at 40X (A) and 100X (B) magnifications. Also shown is a negative control IgG antibody used in place of the anti-vimentin primary antibody in the immuno-histochemical procedure.
Hence, the data in Fig. 8 indicate G6 treatment reduces HEL cell-induced vimentin expression in a dose dependent manner, in vivo.
DISCUSSION
Our group recently identified a novel stilbenoid Jak2 small molecule inhibitor named G6 (16). We subsequently showed that it specifically inhibits Jak2 mediated human pathologic cell growth in vitro, ex vivo, and in vivo (17, 18). We have also demonstrated that G6 inhibits Jak2 mediated cell proliferation via the suppression of key signaling molecules of the Jak/STAT pathway and with the induction of G1/S cell cycle arrest and apoptosis (17,18). In this report, our objective was to determine the mechanisms by which G6 exerts its inhibitory actions. To this end, we found that G6 treatment induced a time- and dose-dependent cleavage of the intermediate filament protein, vimentin (Fig. 2). G6 treatment of HEL cells resulted in the movement of vimentin from a mostly cytoplasmic to a predominantly perinuclear distribution within the cell (Fig. 3). The G6-mediated cleavage of vimentin is Jak2-dependent (Fig. 4) and calpain-mediated (Fig. 5). The mobilization of intracellular calcium is critical for G6-mediated vimentin cleavage (Fig. 6) and the cleavage of vimentin, per se, is sufficient to reduce HEL cell viability (Fig. 7). Lastly, the ability of G6 to reduce vimentin levels is conserved in vivo (Fig. 8). Taken together, these results describe a novel mechanism by which G6 exerts its inhibitory actions.
Vimentin, a member of the Type III intermediate filament protein family, is a key component of the cytoskeleton of the cell. It plays an important role in maintaining cell shape and integrity as well as stabilizing cytoskeletal interactions such as adhesion, migration and signaling. Phosphorylation is a key regulator of the dynamics of vimentin assembly/disassembly and modulates the organization, subcellular distribution and function of these intermediate filaments. Vimentin can be phosphorylated on distinct serine/threonine residues by various protein kinases, including protein kinase A, protein kinase C, Cyclin-dependent kinase 1, Rho kinase, p21-activated kinase 1 and Aurora-B kinase. Site-specific phosphorylation is usually associated with disassembly of vimentin intermediate filaments. For example, phosphorylation of vimentin by protein kinase A or protein kinase C on specific serine residues results in disassembly of the vimentin filaments (30, 31). Site-specific phosphorylation of vimentin by other kinases during the various stages of mitosis, plays a critical role in the regulation/progression of mitotic events. Specifically, it has been reported that cyclin-dependent kinase 2 phosphorylates vimentin from prometaphase to metaphase (32), while Aurora-B kinase (33) and Rho-kinase (34) phosphorylate vimentin specifically at the cleavage furrow from anaphase to the end of mitosis. Disrupting the interaction between these protein kinases and vimentin filaments prevents effective separation of filaments during cytokinesis thereby resulting in abnormal cell division (35, 36). Another study (37) demonstrated that stimulation of cultured smooth muscle cells with serotonin resulted in p21-activation kinase 1-mediated phosphorylation of vimentin at serine-56. This phosphorylation event leads to the disassembly of vimentin intermediate filaments and alters the migratory and contractile properties of smooth muscle cells (38). Vimentin was recently identified as a protein which, in response to catecholamine stimulation, can directly interact with β-adrenergic receptor and activate extracellular signal-regulated kinases (Erk)-1 and -2 via direct recruitment and activation of Src kinases (29). It has also been suggested that the vimentin intermediate filament network can serve as platforms/scaffolds for signaling molecules (39). A group from France (40) previously reported that the Src family kinase, Yes, associates and localizes with vimentin filaments in amoeboid microglia. This suggests that vimentin might serve as a molecular support for Yes kinase and regulate its phosphorylation and subsequent signal transduction. Vimentin has also been shown to stabilize the phosphorylated form of Erk by protecting it from phosphatases via steric hindrance (41). Our lab has previously reported that Jak2 can associate with and directly phosphorylate the cytoskeleton protein tubulin (42). These studies suggest that it is possible that vimentin might have yet to be determined regulatory/stabilization associations with several other membrane associated protein complexes, including Jak2.
Epithelial-Mesenchymal Transition (EMT), a reprogramming process by which cells undergo a morphological switch from the epithelial phenotype to the mesenchymal fibroblast-like phenotype, is associated with normal embryonic development and is also activated during cancer invasion and metastasis (43). At a molecular level, during EMT, cells lose epithelial markers, such as E-cadherin and start expressing mesenchymal markers, such as N-cadherin and vimentin (44). As a result, epithelial cells lose their well-defined cell-cell/cell-substratum contacts/adhesion as well as their structural/functional polarity and gradually assume a spindle-shape morphology (45). Acquisition of mesenchymal characteristics allows the epithelial cells within a tumour to metastasize by migrating into surrounding tissues (45). EMT is also marked by an increase in nuclear localization of β-catenin (46), which in turn transcriptionally activates the expression of EMT-inducing genes, such as matrix metalloproteinase 7 (47), fibronectin (48) and vimentin (49). Vimentin overexpression is known to promote migration in epithelial cells that are involved in normal physiological processes, such as organogenesis, placentation, and wound healing (50). Increased de novo expression of this protein is also associated with invasive cancer cells that have higher chances of metastasizing and poor prognosis (51–54). Knockdown of vimentin expression in vimentin-expressing breast cell lines using antisense resulted in a corresponding decrease in the in vitro invasiveness/migration of these cells (53). It has also been reported that vimentin knockout mice show impaired wound healing ability (55). These studies strongly emphasize that vimentin has a functional role in inducing epithelial cell migration/invasion and EMT. However, the exact mechanism by which vimentin induces EMT is still elusive.
It is hypothesized that vimentin may destabilize E-cadherin mediated cell adhesion complexes, thereby leading to an increased migratory ability of cells overexpressing vimentin (49). There is evidence that phosphorylation of β-catenin on critical tyrosine residues can disrupt the interaction of the protein with E-cadherin, thereby rendering it free to translocate to the nucleus and regulate transcription of downstream target genes (56). For example, phosphorylation of β-catenin by Src kinase on tyrosine 654 causes a decrease in the binding affinity of the protein for E-cadherin (57). Similarly, studies have shown that blockade of Jak2 tyrosine kinase activity can supress the accumulation of β-catenin in leukemic cells (58). β-catenin can transactivate viemntin by directly interacting with the vimentin promoter (49), thereby suggesting that decreased accumulation of this protein can cause a corresponding decrease in the expression levels of vimentin protein. Another recent study has reported that blockade of Jak2 by AG490 inhibits migration and proliferation of human colon cancer cells (59). Specifically, AG490 decreased STAT3 phosphorylation and vimentin expression thereby suggesting that Jak2/STAT3/vimentin signaling participates in regulating the proliferation and migration of colon cancer cells (59). The group led by Ahmed (60, 61) demonstrated that in response to the stimulation of epidermal growth factor receptor, there is an activation of Jak2/STAT3 signaling pathway in high grade ovarian carcinomas. A corresponding transition to a migratory phenotype, marked by increased expression of mesenchyme –associated N-cadherin, vimentin and nuclear translocation of β-catenin, was also observed in these cells (60, 61). A separate study reported the role of STAT5a in RhoA-induced epithelial-to-mesenchymal transition. RhoA induces Jak2-dependent tyrosine phosphorylation of STAT5a with a concomitant increase in vimentin expression and cell motility (62). It is also known that STAT3 can directly regulate the expression of vimentin protein by interacting with its promoter region (63). When taken together, it is possible that the inhibition of Jak2 via G6 and the subsequent loss of vimentin expression that we report in this paper can either be due to a Jak2-mediated modulation of β-catenin activity/stability or via Jak2-mediated alteration in STAT3 trascriptional activity.
Apoptosis is associated with disruption of the cytoskeletal network and caspase and/or calpain-induced cleavage of cytoskeletal proteins, such as vimentin, is known to occur in response to various inducers of apoptosis (64–68). However, studies have reported that knockdown of vimentin does not induce significant apoptosis per se, but cleavage or degradation of vimentin potentiates the therapeutic effects of a drug (69, 70). These studies also report that higher levels of vimentin expression render cells more susceptible to drug-induced apoptosis. In agreement with these previous reports, we found that Jak2-V617F expressing HEL cells have readily detectable levels of vimentin protein and are very susceptible to drug inhibition and subsequent loss of cell viability. However, in contrast to the earlier reports, we found that the loss of vimentin, either by G6 or by IDPN treatment, was sufficient to significantly decrease cell viability (Fig. 7). Possible explanations for these observed differences include the fact that the earlier reports used siRNA to knockdown vimentin mRNA levels whereas we used pharmacological inhibitors that resulted in decreased vimentin protein levels. In addition, G6 also reduces Jak2 kinase activity (17, 18) which presumably vimentin siRNA treatment does not. These differences notwithstanding, our work here is significant in that we demonstrate that G6 treatment results in vimentin cleavage and the subsequent loss of cell viability.
With respect to the signaling pathway that facilitates G6-mediated vimentin cleavage, our data indicates that calcium plays a critical role in this process. For example, the G6-induced cleavage of vimentin is mediated by the calcium-dependent protease, calpain (Fig. 5C). Furthermore, we show that mobilization of intracellular calcium is both essential and sufficient for cleavage of vimentin. Our data therefore suggest that there is a close correlation between Jak2 kinase activity and the levels of intracellular calcium ions. This is supported by previous work which indicates that erythropoietin (Epo)-induced activation of its cognate receptor, EpoR, inhibits calcium-induced neurotransmitter release via the activation of Jak2 (71). This report also shows that this Epo-induced inhibition of calcium activity can be blocked by treatment with a tyrosine kinase inhibitor, such as genistein, further confirming the importance of Jak2 in mediating calcium-induced responses. Overall, these studies demonstrate that Jak2 can modulate intracellular calcium levels. However, the exact protein target(s) that Jak2 may phosphorylate in the calcium signaling pathway are not known.
We have previously reported that G6 treatment potently induces apoptosis in HEL cells via the modulation of various Bcl-2 family proteins, such as Bcl-xL, Bim and Bid (17, 18). Previous studies have reported that elevation of intracellular calcium levels can trigger apoptosis via the destabilization of the mitochondrial membrane and subsequent activation of calcium-dependent calpain proteases (72–74). Activation of calpains can further lead to the cleavage and activation of apoptotic regulators of the Bcl2 family, such as Bid (75). Therefore, these studies suggest that calcium and calcium-dependent proteases may have an important role to play in the G6-mediated cell death/apoptosis process.
The Jak2-V617F mutation is found in a large percentage on MPN patients (11–15). A previous study, which compared the mRNA expression profiles between healthy individuals and MPN patients, reported vimentin to be one gene that was differentially expressed in these two groups (76). Specifically, MPN patients were found to have elevated levels of vimentin mRNA when compared to non-diseased individuals. This increase in vimentin gene expression correlated positively with the presence of the Jak2-V617F mutation. In other words, significant over expression of vimentin was only observed in patients that were homozygous for the Jak2-V617F mutation. Thus, our data here, which describes vimentin as one protein that is down regulated in response to Jak2-V617F inhibition by G6, is noteworthy. Furthermore, our data which show that the cleavage of vimentin is Jak2-dependent (Fig. 4) imply that pharmacological inhibition of Jak2 is sufficient to induce cleavage of vimentin and subsequent loss of cell viability (Fig. 7). When taken together with the MPN microarray data (76), our work here suggests that there may be a link between hyper activation of Jak2 kinase and over expression of vimentin. Furthermore, vimentin expression might be a potential biomarker for the progression of Jak2-V617F mediated pathogenesis and for disease regression via Jak2 inhibitory therapy.
In summary, our data show that G6-induced inhibition of Jak2-mediated pathogenic cell growth correlates with decreased expression of vimentin in vitro and in vivo. As such, this work describes a novel mechanistic pathway for the targeting of Jak2-mediated pathological cell growth.
Acknowledgments
We thank Ms. Marjorie Chow for technical assistance. This work was supported by National Institutes of Health Award R01-HL67277, a University of Florida Opportunity Fund Award, and a University of Florida/Moffitt Cancer Center Collaborative Initiative Award.
Abbreviations
- Jak2
Janus Kinase 2
- GPCR
G Protein-coupled Receptor
- STAT
Signal Transducers and Activators of Transcription
- BCR
B-cell Receptor
- MPN
Myeloproliferative Neoplasms
- G1/S phase
Gap1/Synthesis phase
- HEL
Human Erythroleukemia
- DMSO
Dimethyl Sulfoxide
- DTT
Dithiothreitol
- SDS-PAGE
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
- CHAPS
(3-[(3-Cholamidopropyl)-dimethylammonio]-1-propane sulfonate)
- FITC
Fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
- IDPN
Beta, beta’-iminodipropionitrile
- EMT
Epithelial-Mesenchymal Transition
Footnotes
This work was supported by National Institutes of Health Award R01-HL67277, a University of Florida Opportunity Fund Award, and a University of Florida/Moffitt Cancer Center Collaborative Initiative Award.
References
- 1.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 3.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 4.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard OA. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 5.Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, Van Rompaey L, Baens M, Van den Berghe H, Marynen P. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 6.Joos S, Granzow M, Holtgreve-Grez H, Siebert R, Harder L, Martin-Subero JI, Wolf J, Adamowicz M, Barth TF, Lichter P, Jauch A. Hodgkin’s lymphoma cell lines are characterized by frequent aberrations on chromosomes 2p and 9p including REL and JAK2. Int J Cancer. 2003;103:489–495. doi: 10.1002/ijc.10845. [DOI] [PubMed] [Google Scholar]
- 7.Barth TF, Melzner I, Wegener S, Bucur AJ, Bruderlein S, Dorsch K, Hasel C, Leithauser F, Moller P. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in MedB-1 mediastinal lymphoma line. Verh Dtsch Ges Pathol. 2005;89:234–244. [PubMed] [Google Scholar]
- 8.Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, Pascutto C, Vanelli L, Arcaini L, Burcheri S, Malcovati L, Lazzarino M, Cazzola M. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–3682. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 9.Mc Lornan DP, Percy MJ, Jones AV, Cross NC, Mc Mullin MF. Chronic neutrophilic leukemia with an associated V617F JAK2 tyrosine kinase mutation. Haematologica. 2005;90:1696–1697. [PubMed] [Google Scholar]
- 10.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, Duffy A, Boyd EM, Bench AJ, Scott MA, Vassiliou GS, Milligan DW, Smith SR, Erber WN, Bareford D, Wilkins BS, Reilly JT, Harrison CN, Green AR. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 11.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 12.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 13.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 14.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, Zhao ZJ. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiss R, Polgar T, Kirabo A, Sayyah J, Figueroa NC, List AF, Sokol L, Zuckerman KS, Gali M, Bisht KS, Sayeski PP, Keseru GM. Identification of a novel inhibitor of JAK2 tyrosine kinase by structure-based virtual screening. Bioorg Med Chem Lett. 2009;19:3598–3601. doi: 10.1016/j.bmcl.2009.04.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumder A, Govindasamy L, Magis A, Kiss R, Polgar T, Baskin R, Allan RW, Agbandje-McKenna M, Reuther GW, Keseru GM, Bisht KS, Sayeski PP. Structure-function correlation of G6, a novel small molecule inhibitor of Jak2: indispensability of the stilbenoid core. J Biol Chem. 2010;285:31399–31407. doi: 10.1074/jbc.M110.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirabo A, Embury J, Kiss R, Polgar T, Gali M, Majumder A, Bisht KS, Cogle CR, Keseru GM, Sayeski PP. The stilbenoid tyrosine kinase inhibitor, G6, suppresses Jak2-V617F-mediated human pathological cell growth in vitro and in vivo. J Biol Chem. 2011;286:4280–4291. doi: 10.1074/jbc.M110.200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin P, Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982;216:1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- 20.Quentmeier H, MacLeod RA, Zaborski M, Drexler HG. JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders. Leukemia. 2006;20:471–476. doi: 10.1038/sj.leu.2404081. [DOI] [PubMed] [Google Scholar]
- 21.Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K, Griffin JD, Sattler M. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem. 2006;281:18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- 22.Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–217. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 24.Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001;8:443–450. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Harvey NL. Role of multiple cellular proteases in the execution of programmed cell death. FEBS Lett. 1995;375:169–173. doi: 10.1016/0014-5793(95)01186-i. [DOI] [PubMed] [Google Scholar]
- 26.Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Murachi T, Tsukahara I. Degradation of actin and vimentin by calpain II, a Ca2+-dependent cysteine proteinase, in bovine lens. FEBS Lett. 1984;170:259–262. doi: 10.1016/0014-5793(84)81324-1. [DOI] [PubMed] [Google Scholar]
- 28.Traub P, Scherbarth A, Willingale-Theune J, Paulin-Levasseur M, Shoeman R. Differential sensitivity of vimentin and nuclear lamins from Ehrlich ascites tumor cells toward Ca2+-activated neutral thiol proteinase. Eur J Cell Biol. 1988;46:478–490. [PubMed] [Google Scholar]
- 29.Kumar N, Robidoux J, Daniel KW, Guzman G, Floering LM, Collins S. Requirement of vimentin filament assembly for beta3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J Biol Chem. 2007;282:9244–9250. doi: 10.1074/jbc.M605571200. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki M, Nishi Y, Nishizawa K, Matsuyama M, Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987;328:649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- 31.Ando S, Tanabe K, Gonda Y, Sato C, Inagaki M. Domain- and sequence-specific phosphorylation of vimentin induces disassembly of the filament structure. Biochemistry. 1989;28:2974–2979. doi: 10.1021/bi00433a035. [DOI] [PubMed] [Google Scholar]
- 32.Tsujimura K, Ogawara M, Takeuchi Y, Imajoh-Ohmi S, Ha MH, Inagaki M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J Biol Chem. 1994;269:31097–31106. [PubMed] [Google Scholar]
- 33.Goto H, Yasui Y, Kawajiri A, Nigg EA, Terada Y, Tatsuka M, Nagata K, Inagaki M. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278:8526–8530. doi: 10.1074/jbc.M210892200. [DOI] [PubMed] [Google Scholar]
- 34.Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 35.Yasui Y, Goto H, Matsui S, Manser E, Lim L, Nagata K, Inagaki M. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene. 2001;20:2868–2876. doi: 10.1038/sj.onc.1204407. [DOI] [PubMed] [Google Scholar]
- 36.Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- 37.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006;281:34716–34724. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J. 2005;388:773–783. doi: 10.1042/BJ20050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE. 2006;2006:pe53. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- 40.Ciesielski-Treska J, Ulrich G, Chasserot-Golaz S, Aunis D. Immunocytochemical localization of protein kinases Yes and Src in amoeboid microglia in culture: association of Yes kinase with vimentin intermediate filaments. Eur J Cell Biol. 1995;68:369–376. [PubMed] [Google Scholar]
- 41.Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, Eisenstein M, Fainzilber M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol. 2006;364:938–944. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Sayeski PP. Identification of tubulin as a substrate of Jak2 tyrosine kinase and its role in Jak2-dependent signaling. Biochemistry. 2007;46:7153–7162. doi: 10.1021/bi700101n. [DOI] [PubMed] [Google Scholar]
- 43.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 44.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 45.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 46.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L. IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 47.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–2664. [PubMed] [Google Scholar]
- 50.Guarino M. Epithelial-to-mesenchymal change of differentiation. From embryogenetic mechanism to pathological patterns. Histol Histopathol. 1995;10:171–184. [PubMed] [Google Scholar]
- 51.Singh S, Sadacharan S, Su S, Belldegrun A, Persad S, Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306–2311. [PubMed] [Google Scholar]
- 52.Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci. 1999;112(Pt 24):4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- 53.Hendrix MJ, Seftor EA, Seftor RE, Trevor KT. Experimental co-expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol. 1997;150:483–495. [PMC free article] [PubMed] [Google Scholar]
- 54.Lang SH, Hyde C, Reid IN, Hitchcock IS, Hart CA, Bryden AA, Villette JM, Stower MJ, Maitland NJ. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate. 2002;52:253–263. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 55.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113(Pt 13):2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 56.Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem. 2001;276:20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- 57.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 58.Liu YC, Lai WC, Chuang KA, Shen YJ, Hu WS, Ho CH, Chen YB, Hsu MF, Hsu HC, Lieu CH. Blockade of JAK2 activity suppressed accumulation of beta-catenin in leukemic cells. J Cell Biochem. 2010;111:402–411. doi: 10.1002/jcb.22714. [DOI] [PubMed] [Google Scholar]
- 59.Xu JH, Zhang C, Tang B, Hao YX, Chen J, Liu T, Cui H. Effect of JAK2/STAT3/vimentin signaling pathway on proliferation and migration of human colon cancer cells. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:282–285. [PubMed] [Google Scholar]
- 60.Colomiere M, Findlay J, Ackland L, Ahmed N. Epidermal growth factor-induced ovarian carcinoma cell migration is associated with JAK2/STAT3 signals and changes in the abundance and localization of alpha6beta1 integrin. Int J Biochem Cell Biol. 2009;41:1034–1045. doi: 10.1016/j.biocel.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, Ackland L, Ahmed N. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100:134–144. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benitah SA, Valeron PF, Rui H, Lacal JC. STAT5a activation mediates the epithelial to mesenchymal transition induced by oncogenic RhoA. Mol Biol Cell. 2003;14:40–53. doi: 10.1091/mbc.E02-08-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y, Diab I, Zhang X, Izmailova ES, Zehner ZE. Stat3 enhances vimentin gene expression by binding to the antisilencer element and interacting with the repressor protein, ZBP-89. Oncogene. 2004;23:168–178. doi: 10.1038/sj.onc.1207003. [DOI] [PubMed] [Google Scholar]
- 64.Schutte B, Nieland L, van Engeland M, Henfling ME, Meijer L, Ramaekers FC. The effect of the cyclin-dependent kinase inhibitor olomoucine on cell cycle kinetics. Exp Cell Res. 1997;236:4–15. doi: 10.1006/excr.1997.3700. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto M, Inoue S, Ogawa S, Conrad C, Muramatsu M, Shackelford D, Masliah E. Rapid fragmentation of vimentin in human skin fibroblasts exposed to tamoxifen: a possible involvement of caspase-3. Biochem Biophys Res Commun. 1998;247:401–406. doi: 10.1006/bbrc.1998.8799. [DOI] [PubMed] [Google Scholar]
- 66.Prasad SC, Thraves PJ, Kuettel MR, Srinivasarao GY, Dritschilo A, Soldatenkov VA. Apoptosis-associated proteolysis of vimentin in human prostate epithelial tumor cells. Biochem Biophys Res Commun. 1998;249:332–338. doi: 10.1006/bbrc.1998.9137. [DOI] [PubMed] [Google Scholar]
- 67.Morishima N. Changes in nuclear morphology during apoptosis correlate with vimentin cleavage by different caspases located either upstream or downstream of Bcl-2 action. Genes Cells. 1999;4:401–414. doi: 10.1046/j.1365-2443.1999.00270.x. [DOI] [PubMed] [Google Scholar]
- 68.Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol. 2004;44:349–370. doi: 10.1146/annurev.pharmtox.44.101802.121804. [DOI] [PubMed] [Google Scholar]
- 69.Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby Ho Y, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Markovitz DM, Zhan CG, Kim KB, Mohan R. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lahat G, Zhu QS, Huang KL, Wang S, Bolshakov S, Liu J, Torres K, Langley RR, Lazar AJ, Hung MC, Lev D. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS One. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Kawakami M, Iwasaki S, Sato K, Takahashi M. Erythropoietin inhibits calcium-induced neurotransmitter release from clonal neuronal cells. Biochem Biophys Res Commun. 2000;279:293–297. doi: 10.1006/bbrc.2000.3926. [DOI] [PubMed] [Google Scholar]
- 72.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 73.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 74.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 75.Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kralovics R, Teo SS, Buser AS, Brutsche M, Tiedt R, Tichelli A, Passamonti F, Pietra D, Cazzola M, Skoda RC. Altered gene expression in myeloproliferative disorders correlates with activation of signaling by the V617F mutation of Jak2. Blood. 2005;106:3374–3376. doi: 10.1182/blood-2005-05-1889. [DOI] [PubMed] [Google Scholar]