Abstract
SHIP is a 145-kD SH2-containing inositol-5-phosphatase widely expressed in hemopoietic cells. It was first identified as a tyrosine phosphoprotein associated with Shc in response to numerous cytokines. SHIP has been implicated in FcγRIIB receptor-mediated negative signaling in B cells and mast cells and is postulated to down-regulate cytokine signal transduction in myeloid cells. To define further its role in the proliferation and differentiation of hemopoietic progenitors, as well as its function in mature cells, we have generated embryonic stem cells and mice bearing a targeted disruption of both SHIP alleles. Here we show that although SHIP null mice are viable and fertile, they fail to thrive and survival is only 40% by 14 weeks of age. Mortality is associated with extensive consolidation of the lungs resulting from infiltration by myeloid cells. Increased numbers of granulocyte–macrophage progenitors are observed in both the bone marrow and spleen of SHIP−/− mice, perhaps as a consequence of hyper-responsiveness to stimulation by macrophage–colony stimulating factor, granulocyte–macrophage colony stimulating factor, interleukin-3, or Steel factor as observed in vitro. In contrast, numbers of bone marrow lymphoid and late erythroid progenitors (CFU-E) are reduced. Thus, homozygous disruption of SHIP establishes the crucial role of this molecule in modulating cytokine signaling within the hemopoietic system and provides a powerful model for further delineating its function.
Keywords: SHIP, hemopoiesis, embryonic stem cells, knockout, signal transduction
SHIP (SH2-containing inositol-5-phosphatase) is a recently cloned 145-kD protein that is highly expressed in hemopoietic cells (Damen et al. 1996; Kavanaugh et al. 1996; Lioubin et al. 1996). It contains an amino-terminal src homology 2 (SH2) domain, a central 5′-phosphoinositol phosphatase domain, two phosphotyrosine binding (PTB) consensus sequences, and a proline-rich region at the carboxyl tail. SHIP becomes tyrosine phosphorylated following activation of the hemopoietic cell-surface receptors for numerous cytokines including erythropoietin (Epo), Steel factor (SF), interleukin-3 (IL-3) (Cutler et al. 1993; Damen et al. 1993), IL-2, granulocyte–macrophage colony-stimulating factor (GM-CSF), and macrophage colony stimulating factor (M-CSF) (Lioubin et al. 1994). Similarly, cross-linking of the B-cell antigen receptor (Saxton et al. 1994) or T-cell activation (Ravichandran et al. 1993) induce SHIP phosphorylation.
It has been postulated that SHIP may impact on many signal transduction pathways in hemopoietic cells. For example, SHIP selectively hydrolyzes the 5′-phosphate from inositol 1,3,4,5-tetraphosphate and phosphatidylinositol 3,4,5-trisphosphate (PIP3), the latter being a product of phosphatidylinositol 3′-kinase (PI-3-K) activity. PI-3-K activation in response to growth factor stimulation is implicated as a major step in mitogenic signaling (Kapeller and Cantley 1994). In addition, both phosphatidylinositol 3,4-bisphosphate and PIP3 may play a role in regulating the PKB/Akt kinase (Marte and Downward 1997). Interestingly, the catalytic activity of SHIP is not altered following cytokine stimulation, suggesting that its subcellular localization may be an important regulatory factor (Damen et al. 1996). In this regard, phosphorylated SHIP has been shown to associate with Shc (Liu et al. 1997a), Grb2 (Osborne et al. 1996), and the tyrosine phosphatase SHP-2 (Liu et al. 1997b; Sattler et al. 1997), which may change its proximity to the membrane and/or various substrates. Furthermore, association with these molecules suggests that SHIP may function in coupling cytokine receptor activation to the Ras signaling pathway in hemopoietic cells (Scharenberg and Kinet 1996; Liu et al. 1997c; Tridandapani et al. 1997). Thus, SHIP may regulate the proliferation and differentiation of hemopoietic cells by modulating PIP3 levels and Ras activity following cytokine stimulation.
SHIP has been implicated in the negative signaling pathways that abrogate activation in cells of the immune system. Coligation of the low-affinity Fc receptor for immunoglobulin G (IgG) (FcγRIIB) with the B-cell receptor (BCR) or the high-affinity mast cell IgE receptor (FcεRI) blocks the influx of extracellular calcium with minimal effects on the release of calcium from intracellular stores. This perturbation of calcium signaling may mediate the negative signals on B-cell growth and mast-cell degranulation. Studies with both mast and B cells derived from mice deficient in SHP-1 (me/me) suggest that SHIP is the primary mediator of inhibitory FcγRIIB signaling (Ono et al. 1996; Nadler et al. 1997). It is unclear however whether SHIP is an inhibitor of cytokine signal transduction pathways regulating the proliferation and differentiation of other hemopoietic progenitors. Ectopic expression of SHIP in the FDC-P1 cell line significantly reduces M-CSF-dependent growth (Lioubin et al. 1996) and increases apoptosis in the DA-ER murine hemopoietic cell line (Liu et al. 1997a). These observations suggest that SHIP may down-regulate signals required for survival and proliferation.
Targeted disruption using homologous recombination in embryonic stem (ES) cells was carried out to further define the role of SHIP in the proliferation and differentiation of hemopoietic progenitors, as well as its function in mature cells. The data presented suggest that SHIP plays an important role in down-regulating the mitogenic signals initiated through stimulation of numerous growth factor receptors. Absence of SHIP results in a myeloproliferative-like syndrome and consolidation of the lungs by infiltration of macrophages, with a consequent decrease in the survival of SHIP−/− mice.
Results
Targeted disruption of SHIP
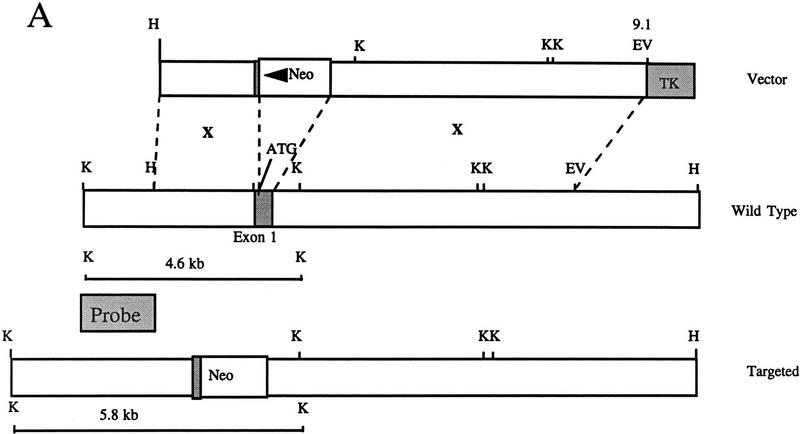
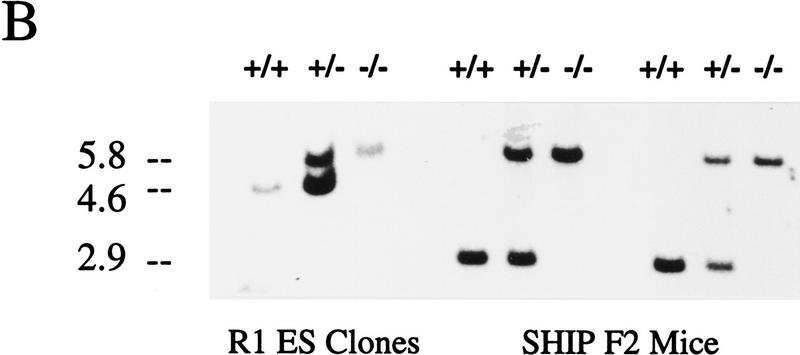
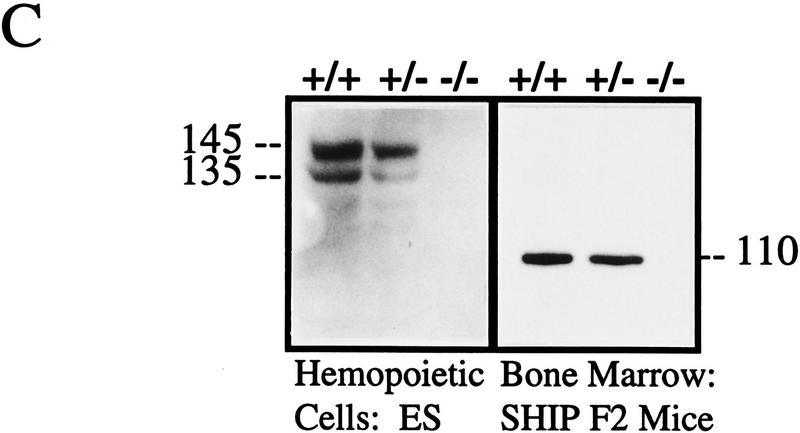
To examine the in vivo function of SHIP, targeted disruption of the murine SHIP gene was achieved using a strategy in which the entire 254-bp coding sequence of the first exon was replaced with the neomycin resistance gene in the antisense orientation using either of the two targeting vectors described in Figure 1A. Two lines of SHIP knockout mice, one generated with each targeting vector, were derived using standard techniques. Southern blot analysis of genomic DNA from both the targeted ES cells and representative F2 mice using a 2-kb KpnI–HindIII genomic probe confirmed the expected targeting event (Fig. 1B). Western blot analysis of hemopoietic cells derived from the in vitro differentiation of SHIP wild type (+/+), heterozygous (+/−), and null (−/−) R1 ES cells, as well as bone marrow cells derived from mice of each genotype, confirmed the absence of full-length or truncated protein products in the SHIP−/−cells (Fig. 1C).
Figure 1.
Targeted disruption of the murine SHIP gene. (A) Partial restriction map of the wild-type 129 SHIP gene (Wild type), the tk-containing targeting vector that replaces the coding sequence of the first exon with the Neo-resistance gene in the antisense orientation (Vector), and the organization of the targeted 129 allele (Targeted). (Dashed lines) Area of homology between the vector and the endogenous gene. The 2.0-kb genomic probe used for screening is indicated along with expected sizes of the wild-type and targeted KpnI fragments. (B) Genomic Southern blot analysis using a 2-kb KpnI–HindIII genomic probe. The nontargeted SHIP allele is visualized as a 4.6-kb band in the wild-type ES cells and a 2.9-kb C57Bl/6 band in the mice (because of a polymorphism at this locus), whereas the SHIP−/− cells and mice contain only the targeted 5.8-kb band. (C) Western blot analysis. R1 SHIP wild-type (+/+), heterozygous (+/−), and null (−/−) ES cells were differentiated in vitro as described previously (Helgason et al. 1996). Hemopoietic cells were derived from suspension cultures of day 10 embryoid bodies, and total cell lysates were probed with an antibody against the carboxy-terminal region spanning the two NPxY sequences of SHIP. The predominant 145- and 135-kD forms are indicated at left. Total cell lysates were also prepared from freshly isolated bone marrow of representative F2 mice and blots were probed with an antibody against the amino terminal SH2 domain. The predominant 110-kD protein is indicated at right.
SHIP−/−mice fail to thrive and have profound splenomegaly
Wild-type, heterozygous, and null F2 progeny were present at the expected Mendelian ratio of 1:2:1 (n = 360). Although both male and female SHIP−/− mice were viable and fertile, they failed to thrive and exhibited a 17% reduction in body weight at 4–5 weeks of age with a further 5% decrease by 8–10 weeks of age (Table 1). Splenomegaly was also a striking feature in SHIP−/− mice of both age groups, with nucleated cell counts increased two- to threefold above +/+ and +/− littermate controls. Total spleen cellularity (red + white cells) and weights were increased five- to sevenfold in the 8–10-week-old knockout animals (not shown). In contrast, the bone marrow of SHIP null mice became progressively hypocellular with age (Table 1). The cellularity of the thymus and lymph nodes obtained from SHIP−/− mice in these two age groups was highly variable and therefore the differences compared with the +/+ mice were not significant. In all cases the phenotype of the +/− mice more closely resembled that of the wild-type mice.
Table 1.
Characterization of SHIP F2 mice
| Parameter
|
Age (weeks)
|
+/+a
|
+/−a
|
−/−a
|
|---|---|---|---|---|
| Body weight (grams) | 4–5 | 20.5 ± 1.1 | 19.8 ± 1.3 | 17.0 ± 0.6* |
| 8–10 | 26.9 ± 1.6 | 25.3 ± 1.5 | 20.9 ± 1.0** | |
| Cellularityb | ||||
| spleen (×108) | 4–5 | 1.5 ± 0.1 | 2.5 ± 0.3 | 3.3 ± 0.4** |
| 8–10 | 2.0 ± 0.2 | 2.0 ± 0.3 | 6.0 ± 0.4** | |
| BM (no./femur × 107) | 4–5 | 1.5 ± 0.1 | 1.4 ± 0.2 | 1.2 ± 0.2 |
| 8–10 | 1.5 ± 0.2 | 1.3 ± 0.2 | 1.0 ± 0.1* | |
| thymus ×108) | 4–5 | 2.1 ± 0.5 | 1.9 ± 0.3 | 1.9 ± 0.1 |
| 8–10 | 1.3 ± 0.1 | 1.7 ± 0.3 | 1.9 ± 0.2 | |
| lymph nodesc (×107) | 4–5 | 1.4 ± 0.5 | 2.0 ± 0.6 | 4.8 ± 2.7 |
| 8–10 | 4.7 ± 1.2 | N.D. | 5.7 ± 0.5 |
Values represent the mean ± s.e.m. for at least three animals per determination. (N.D.) Not done. Statistical significance compared with the +/+ populations was determined using the Student’s t-test where P ≤ 0.05 (*) and P ≤ 0.005 (**).
Nucleated cell counts determined using acetic acid for cell dilutions.
Includes mesenteric, 2 popliteal, and 4 axial nodes.
SHIP−/− mice have a shortened life span associated with massive myeloid cell infiltration of the lungs
Homozygous knockout mice became moribund as early as 4 weeks of age and over 50% died by 10 weeks of age (Fig. 2). Gross anatomical examination of SHIP−/− mice at 8–10 weeks of age revealed, in addition to the pronounced splenomegaly, a uniform enlargement and patchy whitish discoloration of the pleural surfaces of the lungs compared to littermate controls (Fig. 3A). Histologic examination revealed a massive infiltration of the air spaces with distended lipid-laden macrophages, many of which were multinucleated (Fig. 3B,C). The histiocytic origin of these cells was corroborated by strong chloracetate esterase staining (Fig. 3D). The infiltrates were located primarily in alveolar spaces in the pattern of lipoid pneumonia. In addition, collections of neutrophils were distributed throughout the consolidated areas, and in some sections neutrophils were present in bronchiolar lumens. Although the degree of lung involvement was extensive and became progressively pronounced with age, the infiltrates were somewhat patchy in that areas of normal lung could be found adjacent to abnormal areas (Fig. 3B). Stains for bacteria and fungi were negative, and serological testing showed no evidence of viral pathogens or mycoplasma, ruling out obvious infectious causes. Similar lung histopathology was observed in the second line of SHIP−/− mice generated and housed in a separate pathogen-free animal facility (not shown).
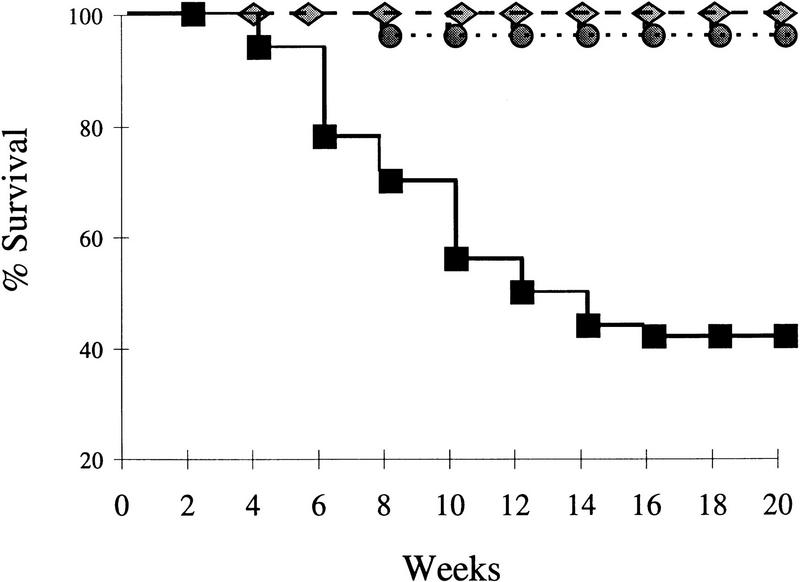
Figure 2.
Decreased survival of SHIP−/− mice. F2 littermates [14 +/+ (diamonds) , 22 +/− (circles), and 14 −/− (squares)] were followed over time and the percentage of surviving mice is indicated as a function of time.
Figure 3.
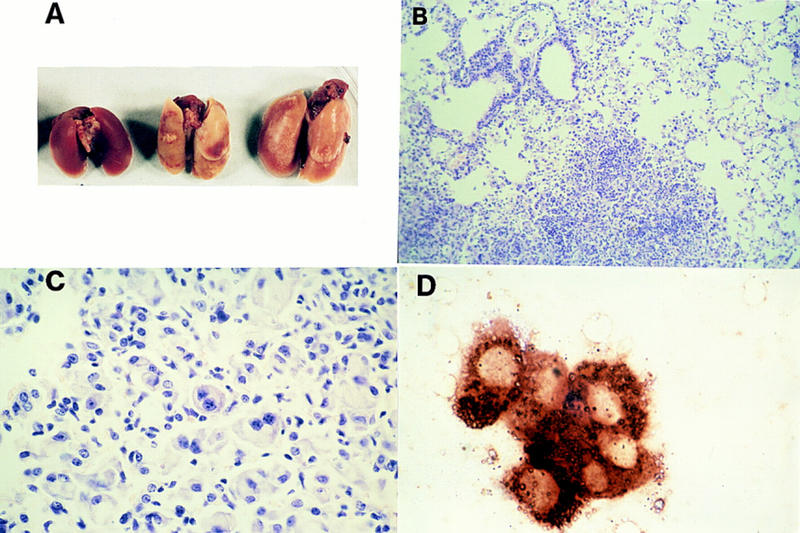
Lung pathology observed in SHIP−/− mice. (A) Representative gross appearance of lungs from a 4-week-old wild-type (left) and two 4-week-old SHIP−/− mice (right) showing diffuse enlargement and patchy pleural discoloration of the lungs from the knockout mice. (B) Low power micrograph of H&E stained sections from a representative 8-week-old SHIP−/− mouse showing patchy cellular infiltrates in alveolar airspaces adjacent to relatively normal appearing lung. (C) High power photomicrograph of H&E stained sections from an 8-week-old knockout mouse demonstrating alveolar infiltration by lipid-laden macrophages, including multinucleated forms, as well as collections of neutrophils mixed with the macrophages. (D). Chloracetate esterase stains for macrophages showing strong reactivity of alveolar cell infiltrates of the SHIP−/− lung.
Numerous hemopoietic abnormalities are observed in SHIP−/− mice
Because SHIP is believed to play an important role in the hemopoietic system, various hematological parameters of these mice were examined. No significant abnormalities were observed in hematocrits, peripheral blood white cell counts, or peripheral blood red cell counts. However, differential counts of peripheral blood smears revealed a substantial increase in the percentage of circulating monocytes and mature neutrophils with a concomitant decrease in the percentage of circulating lymphocytes (Table 2). Cytospins of bone marrow from SHIP−/− mice showed a similar increase in mature neutrophils, with a reduction in lymphoid and late erythroid cells (not shown).
Table 2.
Hematological parameters of SHIP F2 mice
| Parameter
|
Age (weeks)
|
+/+a
|
+/−a
|
−/−a
|
|---|---|---|---|---|
| Hematocrit (%) | 4–5 | 47.5 ± 1.3 | 49.0 ± 1.2 | 48.9 ± 2.8 |
| 8–10 | 50.7 ± 1.0 | 47.2 ± 2.6 | 42.4 ± 5.7 | |
| WBC (×107/ml) | 4–5 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.3 |
| 8–10 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | |
| RBC (×109/ml) | 4–5 | 9.9 ± 0.8 | 10.4 ± 0.3 | 10.9 ± 1.0 |
| 8–10 | 10.0 ± 0.3 | 10.1 ± 0.3 | 8.4 ± 0.7 | |
| Peripheral blood | ||||
| differential counts (%)a | 4–5 L | 68.8 ± 1.8 | 62.3 ± 2.4 | 45.3 ± 4.5** |
| M | 15.5 ± 0.8 | 13.3 ± 0.4 | 23.3 ± 3.0* | |
| N | 14.8 ± 1.9 | 19.0 ± 0.7 | 28.0 ± 2.1** | |
| E | 0 | 5.5 ± 2.4 | 3.3 ± 0.4 | |
| 8–10 L | 73.3 ± 2.6 | 67.3 ± 5.6 | 39.2 ± 3.2** | |
| M | 10.0 ± 1.4 | 10.0 ± 3.0 | 25.0 ± 4.2* | |
| N | 13.3 ± 1.1 | 23.3 ± 3.4* | 35.5 ± 7.3* | |
| E | 1.3 ± 0.9 | 0.8 ± 0.7 | 0.5 ± 0.3 |
Values represent the mean ± s.e.m. for at least three animals per determination. Statistical significance compared with the +/+ populations was determined using the Student’s t-test where P ≤ 0.05 (*) and P ≤ 0.005 (**).aWright–Giemsa-stained smears of peripheral blood were scored microscopically based on morphology. (L) lymphocyte; (M) monocyte; (N) neutrophil; (E) eosinophil and others.
Flow cytometric analysis of selected hemopoietic organs was carried out to further characterize the nature of the hemopoietic perturbations in the SHIP−/− mice. The percentage of cells staining positive for both Mac-1 and Gr-1 (Mac-1+Gr-1+), representing monocytes and granulocyte progenitors, was significantly elevated [1.69 ± 0.16-fold (P = 0.005; n = 5) above +/+ littermates] in the marrow of SHIP−/− mice (Fig. 4A, upper panel). In contrast, significant reductions in the percentage of bone marrow cells expressing the B220 antigen were noted (Fig. 4A, middle panel). In 8–10-week-old SHIP−/− mice the percentage of B220bright cells was reduced to ∼20% of wild-type levels (5.03 ± 0.58% for wild type vs. 0.95 ± 0.12% for SHIP−/−; P = 0.005; n = 3). Similarly, the percentage of bone marrow Ter119-positive erythroid cells was decreased to 70.0 ± 5.7% of wild-type levels in the 4-week-old animals (Fig. 4A, lower panel). The marrow erythroid component was reduced to 30% of normal (31.29 ± 4.94% Ter119+ for +/+ compared to 10.16 ± 1.92% for SHIP−/−; n = 3; P = 0.03) by 8–10 weeks of age. Because the bone marrow cellularity (Table 1), as well as the percentages of B220+ and Ter119+ cells, are reduced in these older SHIP−/− mice, total numbers of B-lymphoid and late erythroid cells are significantly reduced in the marrow compartment. In contrast, when the increased percentage of Mac-1+Gr-1+ cells is examined in the context of the decreased marrow cellularity, absolute numbers of these cells are not significantly different in the SHIP−/− mice compared to their +/+ littermates. Results of phenotypic analysis of bone marrow cells derived from +/− mice were similar to those of the wild-type cells (not show).
Figure 4.
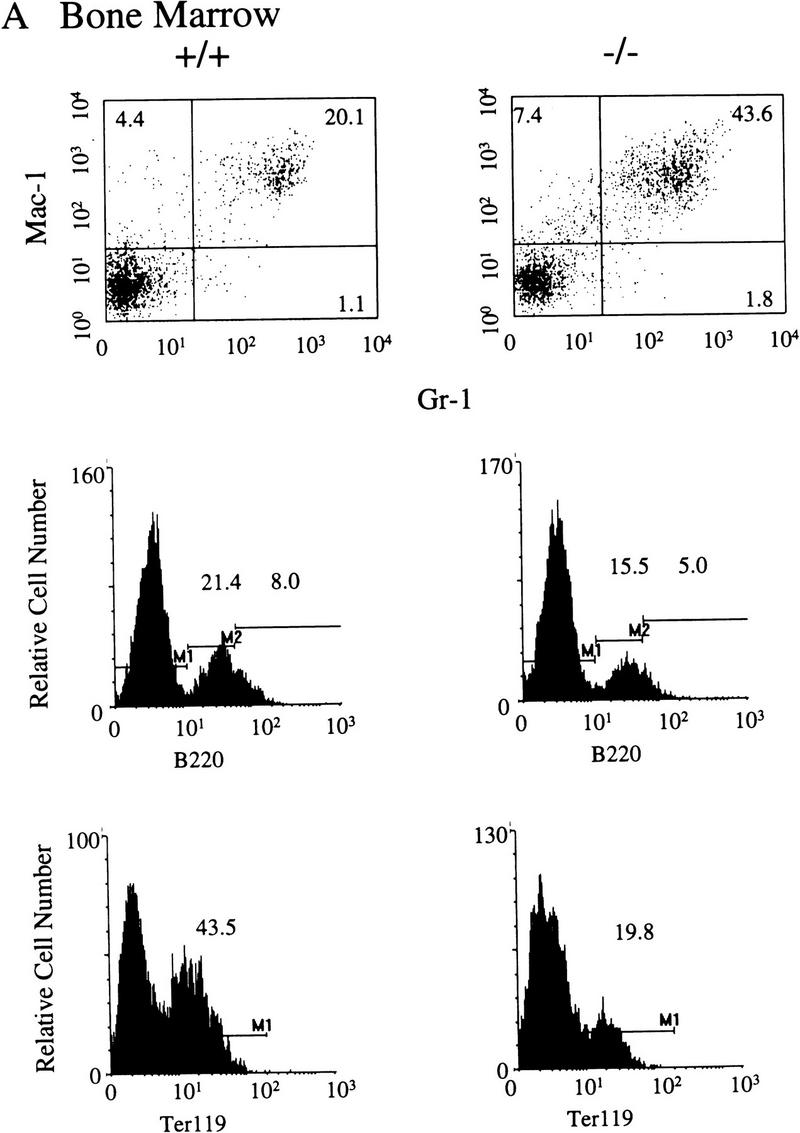
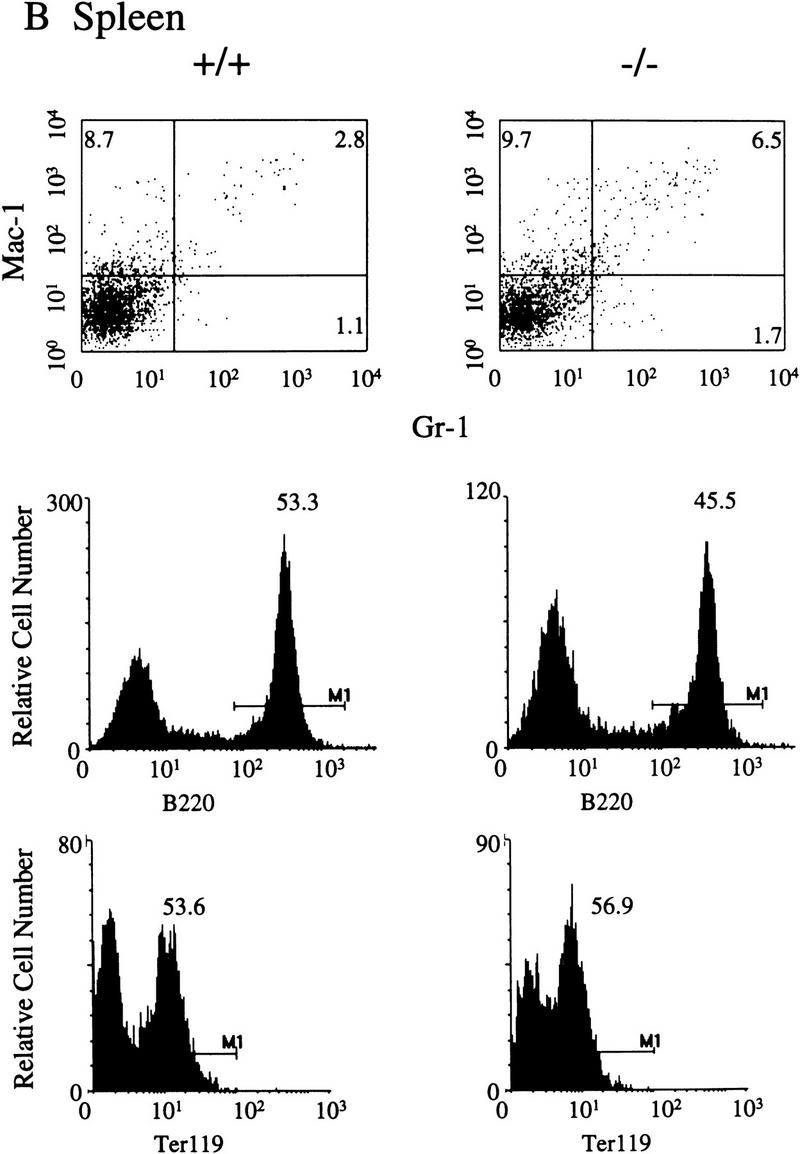
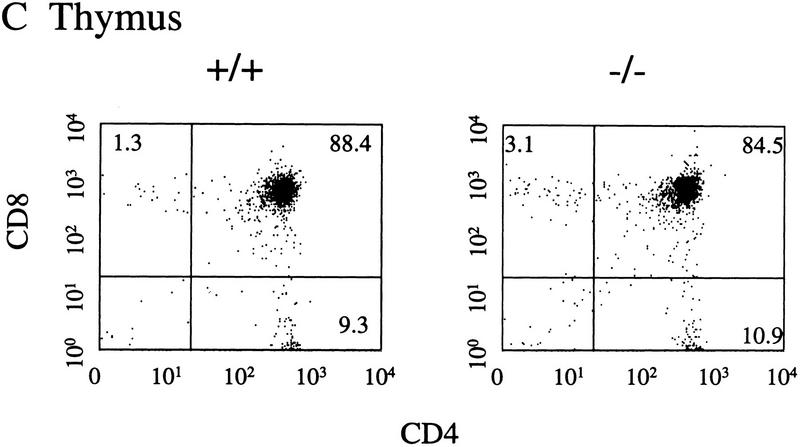
Representative FACS profiles of 4–5-week-old SHIP F2 mice. Five mice of each genotype were analyzed and the average percentages of positive cells determined. A FACS profile representative of each of these values is presented for bone marrow (A), spleen (B), and thymus (C) of SHIP wild-type (+/+) or null (−/−) mice. Numbers represent the percent of viable (PI−), positively stained cells within each marked region.
Representative FACS profiles from the analysis of splenic cells isolated from 4–5-week-old littermates are presented in Figure 4B. At this age there are no significant differences in the percentages of cells that are Mac-1+Gr-1+, B220+, or Ter119+ in the SHIP null mice (n = 5). However, because spleen cellularity of SHIP−/− mice is increased twofold relative to +/+ mice (Table 1), absolute numbers of cells expressing each of these markers are elevated ∼twofold. In spleens from 8–10-week-old SHIP−/− mice there are almost 4.5 times as many Mac-1+Gr-1+ cells (6.0 ± 0.2% in +/+ compared with 26.2 ± 5.3% for SHIP−/−; n = 3; P = 0.03). This translates into a 12-fold increase in absolute numbers of granulocyte progenitors and monocytes when the increased spleen cellularity is considered.
Normal proportions of CD4 and CD8 single-positive, double-positive, and double-negative thymocytes were seen in SHIP−/− mice at both 4–5 weeks of age (Fig. 4C) and 8–10 weeks of age (not shown). FACS analysis of peripheral blood cells revealed an increased percentage of Mac-1+Gr-1+ cells and a decreased percentage of cells expressing the B220 antigen (not shown). Thus, the FACS data are consistent with the changes observed in the counts of peripheral blood smears (Table 2).
SHIP−/− mice have decreased lymphoid and increased myeloid progenitor numbers
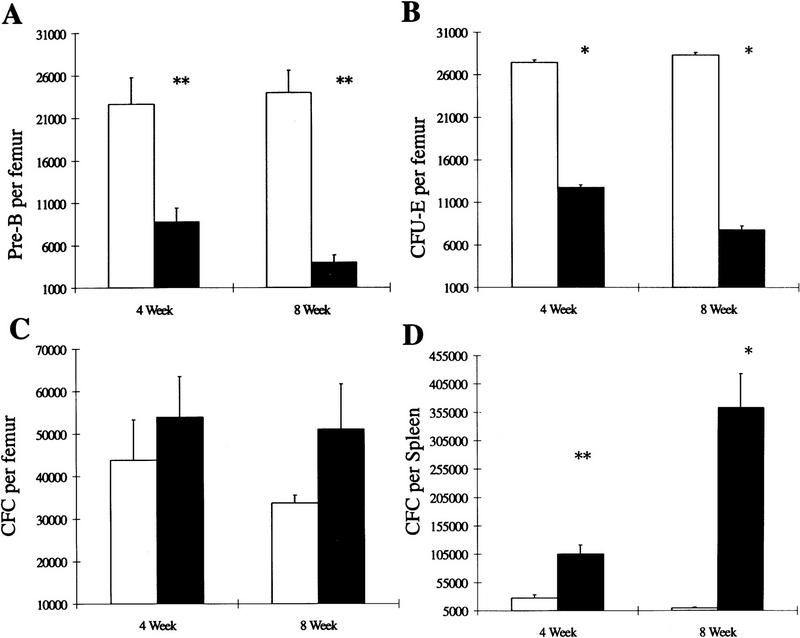
In order to determine if these perturbations in the hemopoietic organs and peripheral blood arise at the progenitor level, bone marrow cells from SHIP+/+, SHIP+/−, and SHIP−/− mice were plated in methylcellulose-based media and various assays performed. Absolute numbers of clonogenic progenitors observed with +/− cells were similar to wild-type levels (data not shown). Numbers of pre-B lymphoid (Fig. 5A) colony-forming cells were reduced to 39% of normal in mice 4–5 weeks of age, whereas numbers of day 2 colony-forming unit-erythroid (CFU-E) (Fig. 5B) progenitors were reduced to 47% of the levels observed in +/+ littermates. In both cases the reduction in progenitor numbers was more pronounced in animals 8–10 weeks of age. Neither day 3 burst forming unit (BFU)-erythroid nor day 10 BFU-E progenitor numbers were significantly reduced (results not shown), suggesting a block in the late stages of erythroid maturation. Although slight increases were observed in the number of granulocyte–macrophage colony forming cells (CFC) in SHIP−/− bone marrow (Fig. 5C), dramatic elevations were seen in the spleen (Fig. 5D). For example, CFC numbers were elevated almost 70-fold in the spleens of SHIP−/− mice 8–10 weeks of age. We observed similar increases in numbers of CFC-GM, and decreases in CFU-E, using the SHIP−/− ES cells in an in vitro differentiation system (not shown) suggesting that these variations in differentiation potential are intrinsic to the progenitor cells.
Figure 5.
Altered myelopoiesis, erythropoiesis, and lymphopoiesis in SHIP−/− mice. In vitro hematopoietic colony formation was examined in methylcellulose colony assays using cells from wild-type (open bars) or null (solid bars) SHIP F2 littermates at 4–5 weeks and 8–10 weeks of age. (A) Numbers of pre-B progenitors per femur detected in methylcellulose containing 10 ng/ml IL-7. (B) Numbers of day 2 CFU-E per femur detected in methylcellulose supplemented with 50 ng/ml SF and 3 U/ml Epo. Granulocyte–macrophage CFC per femur (C) or per spleen (D) were detected in methylcellulose containing 10 ng/ml IL-3, 10 ng/ml IL-6, 50 ng/ml SF, and 3 U/ml Epo. In all cases, values represent the mean ± s.e.m. of 3 or more duplicate determinations. Statistical significance compared to +/+ littermates was determined using the Student’s t-test. P ≤ 0.05 (*); P ≤ 0.01 (**).
Hemopoietic progenitors lacking SHIP are hyper-responsive to multiple cytokines
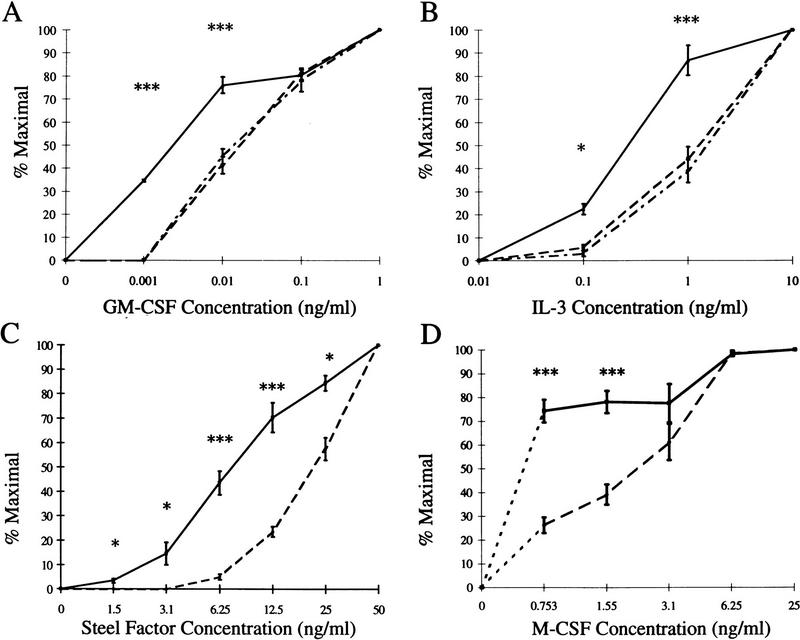
The generation and maturation of CFC can be achieved both in vitro and in vivo using a number of different growth factors. We therefore examined the colony-forming ability of SHIP−/− bone marrow cells in methylcellulose-based media containing various concentrations of cytokines that play a role in myeloid development. Under these conditions, SHIP−/− bone marrow progenitors exhibited an enhanced sensitivity to all growth factors examined (Fig. 6). Both GM-CSF (Fig. 6A) and IL-3 (Fig. 6B) yielded 50% maximal colony formation at a 10-fold lower concentration using SHIP−/− cells compared to those of +/+ and +/− littermates. These observations were confirmed using bone marrow derived from a second, independently derived line of SHIP null mice (not shown). In addition, SHIP−/− bone marrow cells were some two- to threefold more sensitive to SF (Fig. 6C) and M-CSF (Fig. 6D).
Figure 6.
Altered growth factor responsiveness of SHIP−/− CFC. Bone marrow derived from wild type (– –), heterozygous (– . –), or null (—) littermates was plated in methylcellulose containing the indicated concentrations of (A) GM-CSF, (B) IL-3, (C) Steel factor, or (D) M–CSF. Colonies of >20 cells were scored on day 10 of culture and the numbers of colonies present at each cytokine concentration were calculated as the percent of the number formed in the highest concentration of the cytokine indicated. Values represent the mean ± s.e.m. for duplicate determinations using at least three mice per group. Statistical significance compared to +/+ littermates was determined using the Student’s t-test. P ≤ 0.05 (*); P ≤ 0.008 (***).
In an attempt to determine if the absence of SHIP influenced colony size, suspension cultures were established with SHIP+/+ or SHIP−/− bone marrow cells in the presence of 0.01 ng/ml GM-CSF or 1.0 ng/ml IL-3 under conditions similar to those used to assess colony formation. SHIP−/− cultures yielded more cells in the presence of either GM-CSF or IL-3 than littermate control cultures. Moreover, if the cell yields from these suspension cultures were divided by the number of colonies generated in methylcellulose, it appears that the GM-CSF-stimulated SHIP−/− colonies were approximately double the size of those generated by the wild-type cells. In contrast, no difference in colony size was observed in the presence of IL-3.
Growth-factor-independent colony formation is one property of certain transformed cell populations. In order to evaluate the possibility that SHIP−/− progenitors possessed such potential, cells from all three genotypes of mice were plated in methylcellulose-based media containing 15% serum, but no growth factors. No colonies greater than 20 cells were observed in cultures from the wild-type, heterozygous, or SHIP null mice. However, 51.0 ± 3.9% of the SHIP−/− CFC detectable in cytokine-containing media were capable of giving rise to small clusters ranging in size from 5–20 cells. No such clusters were observed using cells from +/+ or +/− mice, suggesting that SHIP−/− progenitors exhibit enhanced survival, but only minimal proliferation, in the absence of exogenous growth factors.
Discussion
Tyrosine phosphorylation of SHIP is an early response to stimulation of many hemopoietic cell-surface receptors (Cutler et al. 1993; Damen et al. 1993; Lioubin et al. 1994). SHIP has been demonstrated to play a major role in FcγRIIB inhibitory signaling in both B cells (Ono et al. 1997) and mast cells (Ono et al. 1996). Thus, it has been postulated that SHIP may play a central role in negatively regulating the responses of both primitive and mature hemopoietic cells to external stimuli. We now demonstrate that the absence of SHIP in vivo results in a marked pathology exemplified by a shortened lifespan, pronounced splenomegaly, and massive myeloid cell accumulations in the lungs.
One of the most striking features of SHIP−/− mice was the decreased life span, with death observed as early as 4 weeks of age and only 40% of the animals surviving 14 weeks. We observed no major abnormalities in hematoxylin and eosin (H&E) stained sections of the brain, liver, or kidney (results not shown). In addition, peripheral blood abnormalities such as anemia, increased numbers of blast cells, or grossly elevated white cell counts were not seen. Thus, the most likely explanation for the early death of SHIP−/− mice would appear to be gross impairment of lung function caused by myeloid cell infiltration. Interestingly, some mice have now survived as long as six months, although they do exhibit decreases in body weight and a “scruffy” appearance. The variability in life span is most likely caused by the mixed genetic background inherent in the F2 populations examined in this study.
Phenotypically, SHIP−/− mice bear some resemblance to normal mice transplanted with bone marrow cells engineered for retroviral-mediated overexpression of GM-CSF. Splenomegaly, reductions in bone marrow cellularity, and patchy consolidation of the lungs, as well as decreased numbers of bone marrow erythroblasts and lymphocytes, were observed in these transplanted mice (Johnson et al. 1989). SHIP−/− mice also exhibit marked increases in the numbers of granulocyte–macrophage progenitors in the bone marrow, and especially the spleen. These increases are associated with an enhanced sensitivity of SHIP−/− granulocyte–macrophage progenitors to multiple cytokines including IL-3, GM-CSF, M-CSF, and SF. In this regard, we have also observed an enhanced degranulation of SHIP−/− bone-marrow-derived mast cells in response to IgE, suggesting that SHIP plays a major role in setting the threshold for cellular responses to external stimuli (M. Huber, C.D. Helgason, J.E. Damen, L. Liu, R.K. Humphries, and G. Krystal, in prep.). Because the lungs are a major site of GM-CSF production (Tazi et al. 1993) and SHIP−/− CFC exhibits a 10-fold increase in sensitivity to this cytokine, this may be sufficient to account for the abnormal accumulations of myeloid cells in the lung.
SHP-1, a protein-tyrosine phosphatase expressed primarily in hemopoietic cells (Yi et al. 1992), has been implicated as a negative regulator of signal transduction (Shultz et al. 1997). Mice homozygous for the mutated motheaten (Hcphme) or viable motheaten (Hcphme-v) alleles exhibit enhanced proliferation of monocyte/macrophage progenitors, splenomegaly, and a fatal hemorrhagic pneumonitis associated with accumulations of macrophages, granulocytes, and lymphocytes in the lungs (Tsui and Tsui 1994). Absence of functional SHP-1 increases the proliferative response of macrophages from these mice to GM-CSF (Jiao et al. 1997), leading to the suggestion that deregulated proliferation of myeloid cells may account for the phenotype of these mice.
The similarities between the phenotypes of SHIP−/− and viable motheaten mice raise an important question regarding the relative importance of SHIP and SHP-1 in negatively regulating cytokine signaling. That is, do these molecules function independently, within the same pathway, or in an overlapping manner? Studies using homologous recombination in the chicken DT-40 cell line suggest that SHP-1 and SHIP function independently in response to signaling through different receptors (Ono et al. 1997). For example, signals initiated by the killer cell inhibitory receptor are mediated by SHP-1, but not SHIP. Conversely, the proapoptotic signals initiated by FcγRIIB signaling are attenuated by SHIP but not SHP-1, suggesting that SHIP−/− mice should exhibit B-lymphoid perturbations. In fact, we observe decreased percentages of B220+ cells in the bone marrow and spleen of SHIP−/− mice and pre-B colony-forming cell numbers are significantly reduced. Further phenotypic and functional analyses of the B-lymphoid populations are currently in progress to evaluate the consequences of loss of SHIP-mediated negative signaling.
Several signal transduction pathways may be involved in the enhanced growth factor sensitivity of the SHIP−/− progenitors. One possible mechanism involves hyperactivation of the Ras pathway. SHIP associates with the protein tyrosine phosphatase SHP-2 in response to growth factors such as IL-3 and erythropoietin (Liu et al. 1997b; Sattler et al. 1997). Because the activity of SHP-2 is postulated to contribute to Ras-MAP kinase activation, association of SHIP with SHP-2 could modulate activity of the Ras pathway. In addition, SHIP may compete with Grb2 for Shc, thereby regulating the levels of Shc–Grb2 association within the cell (Liu et al. 1994; Tridandapani et al. 1997). In either case, absence of SHIP could theoretically lead to enhanced Ras activity. Although we have not yet examined the phosphorylation of Shc or activation of the Ras pathway in cytokine-stimulated bone marrow progenitors, studies with IgE-stimulated SHIP−/− mast cells suggest that Shc phosphorylation is markedly reduced compared to IgE-stimulated SHIP+/+ mast cells (M. Huber, C.D. Helgason, J.E. Damen, L. Liu, R.K. Humphries, and G. Krystal, in prep.). Experiments are now in progress to examine these biochemical pathways in more detail.
A second possible mechanism underlying the enhanced proliferative potential of SHIP−/− granulocyte–macrophage progenitors resides in the phosphatase activity of SHIP. The PI-3-K pathway plays an important role in signaling through cytokine receptors. For example, PIP3 is capable of stimulating members of the protein kinase C family (Toker et al. 1994), as well as modulating the activity of the Akt/PKB kinase (Marte and Downward 1997). In the absence of SHIP, which metabolizes this PI-3-K product, uncontrolled activation of these pathways could result in enhanced cell survival and proliferation.
In conclusion, we have provided evidence that supports the hypothesis that SHIP is an important negative regulator of cytokine signaling in cells of the hemopoietic system. SHIP−/− mice provide a powerful model in which to further explore SHIP function in hemopoietic stem and progenitor cell compartments, as well as in mature cells. In addition, studies with cells from these mice should further our understanding of the molecular mechanisms by which signaling thresholds are established in both mature cells and progenitors.
Materials and methods
SHIP gene targeting
A genomic DNA library derived from the 129 mouse strain was screened with a 600-bp fragment derived from the 5′ end of the SHIP cDNA. Positive clones were analyzed by restriction mapping and sequence analysis. The targeting vector was engineered to contain a 254-bp ApaI–BamHI deletion, thus effectively removing the transcriptional start site and most of the first exon. A Tk–neo cassette derived from pMC1TkNeoPolyA was subcloned into the deletion site in the opposite transcriptional orientation. The targeting vector contains 1.8 kb of SHIP homologous 5′ DNA and 6.0 kb of 3′ SHIP genomic DNA. A second targeting vector was constructed to include the HSV–Tk gene from pMC1–Tk at the 3′ end of the targeting sequence.
Each linearized targeting construct was electroporated into the R1 ES cell line and colonies were isolated following selection in either G418 alone or G418 plus gancyclovir. Genomic DNA was isolated from pools of 6–8 clones and analyzed by PCR analysis using Elongase (Life Technologies) to generate a 2662-bp amplicon derived from an internal Neo primer (5′-CAAGATGGATTGCACGCAGG) and a 5′ SHIP primer (G3: 5′-CCAGAAGTGTCTCTATCATGATAGT). Positive colonies were expanded and purified genomic DNA was digested with KpnI and analyzed by Southern blot analysis using a 2-kb KpnI–HindIII genomic probe. By Southern analysis, the nontargeted SHIP allele and the positively targeted allele were visualized as 4.6- and 5.8-kb bands, respectively. One clone out of 300 was positive using the double-selection protocol, whereas 7 positive clones were obtained with the single-selection vector. Similar blots were also probed with a Neor fragment to confirm single integrations (results not shown).
Germ-line transmission chimeras of clone 4.8B (double-selection vector) and clone 5.1 (single-selection vector) were generated by injection of C57BL/6J blastocysts, followed by breeding onto a C57BL/6J background. Genotype analysis was routinely done using Southern blot analysis and the probe described above. Animals were housed in microisolator units and provided with sterilized food and water. Routine testing for viral pathogens and mycoplasma was carried out on both animal colonies in which the two independent lines were maintained.
Homozygous deletion SHIP ES cells were generated by gene conversion in increasing concentrations of G418 using previously described techniques (Mortensen et al. 1992). Southern blot analysis of genomic DNA confirmed the absence of the SHIP allele. Karyotype analysis was carried out on all clones used both in vitro and in vivo in this study.
Western blot analysis
Equivalent numbers of nucleated cells, derived from hemopoietic suspension cultures of day 10 embryoid bodies (differentiated as described previously; Helgason et al. 1996) generated with SHIP wild-type, heterozygous, and null R1 ES cells, were washed once in phosphate-buffered saline, solubilized with 1.0% Triton-X 100 at 4°C, and subjected to Western blot analysis as described previously (Damen et al. 1993). Bone marrow cells from all three genotypes of mice were treated in a similar manner. The SHIP antibodies used for Western blot analysis were generated against the SH2 domain and the region spanning the two NPxY motifs using GST fusion proteins as described previously (Liu et al. 1997a).
Preparation of sections and slides
Selected organs were fixed in a buffered 4% paraformaldehyde solution, dehydrated in ethanol, and embedded in paraffin for sectioning. Sections were prepared and H&E stained at the Academic Pathology Laboratory, University of British Columbia, Vancouver using standard protocols. Cytospin preparations of bone marrow and spleen, as well as peripheral blood smears, were routinely stained with a modified Wright-Geimsa stain.
Assays to detect clonogenic progenitors
Nucleated cell counts were performed on bone marrow aspirates or cell suspensions of spleen, prepared using a nylon mesh screen. Appropriate cell numbers were plated in a 1.1 ml volume per Petri dish in standard conditions to detect the various clonogenic progenitors. All cell culture was carried out in a humidified incubator at 37°C with 5% CO2. Bone marrow pre-B progenitors were detected by culture in methylcellulose media containing 10 ng/ml IL7 for 5 to 7 days [StemCell Technologies Inc. (STI), Vancouver; Methocult M3630]. Methocult M3230 (STI) was supplemented with 50 ng/ml Steel factor (supplied as a supernatant from Cos cells engineered to express the protein) and 3 U/ml rhEpo for detection of day 2 CFU-E and day 3 BFU-E. Methocult M3434 (STI) containing 10 ng/ml rmIL-3, 10 ng/ml rhIL-6, 50 ng/ml rmSF, and 3 U/ml rhEpo was used for the detection of myeloid (CFC), late erythroid, and multipotential progenitors in bone marrow and spleen cell preparations. All colonies were scored microscopically using standard criteria.
Bone marrow cells derived from mice of all three genotypes were cultured in Methocult M3234 (STI) in the indicated concentrations of IL-3, rmGM-CSF (Peprotech), SF (supplied as a Cos supernatant), or rhM-CSF (Genetics Institute, Cambridge, MA) for growth factor response curves. Colonies containing 20 or more cells were scored. In all cases duplicate determinations were performed on each sample. Statistical significance compared with the +/+ populations was determined using the Student’s t-test.
Flow cytometry
Bone marrow, spleen, thymus, or lymph node cells at a density of 5–10 × 106 cells/ml were incubated on ice for 30 min with 3 μg/ml 2.4G2 (murine anti-IgG Fc receptor antibody) followed by incubation on ice for 40 min with the various FITC-labeled or phycoerythrine-conjugated antibodies. Cells were washed twice in Hank’s balanced salt solution containing 2% fetal bovine serum at 4°C and propidium iodide (Sigma Chemicals, St. Louis, MI) at a concentration of 1 ug/ml was included in the final wash. Cells were analyzed on a FACStar+ or FACSort (Becton-Dickinson, San Jose, CA).
The monoclonal antibodies used for analysis included: E13–161.7 (anti-Sca-1), RB6–8C5 (anti-Gr-1; granulocytes), M1/70 (anti-Mac-1; macrophages), Ter119 (anti-erythroid lineage) (the sources of these antibodies have been described elsewhere; Rebel et al. 1996). Antibodies against CD4, CD8, B220, and CD11b were purchased from Pharmingen (Mississauga, Ontario, Canada).
Acknowledgments
The authors thank James Ihle for helpful discussions regarding the targeting strategy. In addition, the authors wish to thank Gayle Thornbury and Giovanna Cameron for expert technical assistance on the FACStar+, Gloria Shaw for karyotype analysis of the ES clones, Malin Parmar for assistance with PCR and Southern blot analysis, and Julie Chow for preparation and staining of the lung sections, as well as Rosemary Hood, Christian Kalberer, Ling Liu, and Cindy Miller for helpful discussions. This work was supported by the National Cancer Institute of Canada (with funds from the Canadian Cancer Society and the Terry Fox Run) and the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL keith@terryfox.ubc.ca; FAX (604) 877-0712.
References
- Cutler RL, Liu L, Damen JE, Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993;268:21463–21465. [PubMed] [Google Scholar]
- Damen JE, Liu L, Cutler RL, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993;82:2296–2303. [PubMed] [Google Scholar]
- Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase. Proc Natl Acad Sci. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason CD, Sauvageau G, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood. 1996;87:2740–2749. [PubMed] [Google Scholar]
- Jiao H, Yang W, Berrada K, Tabrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: Detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol. 1997;25:592–600. [PubMed] [Google Scholar]
- Johnson GR, Gonda TJ, Metcalf D, Harihan IK, Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989;8:441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller R, Cantley LC. Phosphatidylinositol 3-kinase. Bioessays. 1994;16:565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Kavanaugh WM, Pot DA, Chin SM, Deuterreinhard M, Jefferson AB, Norris FA, Masiarz FR, Cousens LS, Majerus PW, Williams LT. Multiple forms of an inositol polyphosphate 5-phosphatase form signaling complexes with SHC and GRB2. Curr Biol. 1996;6:438–445. doi: 10.1016/s0960-9822(02)00511-0. [DOI] [PubMed] [Google Scholar]
- Lioubin MN, Myles GM, Carlberg K, Botwell D, Rohrschneider LR. SHC, GRB2, SOS1 and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994;14:5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold R, Rohrschneider LR. p150SHIP, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes & Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- Liu L, Damen JE, Cutler RL, Krystal G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol Cell Biol. 1994;14:6926–6935. doi: 10.1128/mcb.14.10.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Damen JE, Hughes MR, Babic I, Jirik FR, Krystal G. The Src homology 2 (SH2) domain of SH2-containing inositol phosphatase (SHIP) is essential for tyrosine phosphorylation of SHIP, its association with Shc, and its induction of apoptosis. J Biol Chem. 1997a;272:8983–8988. doi: 10.1074/jbc.272.14.8983. [DOI] [PubMed] [Google Scholar]
- Liu L, Damen JE, Ware MD, Krystal G. Interleukin-3 induces the association of the inositol 5-phosphatase SHIP with SHP2. J Biol Chem. 1997b;272:10998–11001. doi: 10.1074/jbc.272.17.10998. [DOI] [PubMed] [Google Scholar]
- Liu L, Damen JE, Ware M, Hughes M, Krystal G. SHIP, a new player in cytokine-induced signaling. Leukemia. 1997c;11:181–184. doi: 10.1038/sj.leu.2400559. [DOI] [PubMed] [Google Scholar]
- Marte BM, Downward J. PKB/Akt: Connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biol Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AAT, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler MJS, Chen B, Anderson JS, Wortis HH, Neel BG. Protein-tyrosine phosphatase SHP-1 is dispensable for FcγRIIB-mediated inhibition of B cell antigen receptor activation. J Biol Chem. 1997;272:20038–20043. doi: 10.1074/jbc.272.32.20038. [DOI] [PubMed] [Google Scholar]
- Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Osborne MA, Zenner G, Lubinus M, Zhang X, Songyang Z, Cantley LC, Majerus P, Burn P, Kochan JP. The inositol 5′-phosphatase SHIP binds to immunoreceptor signaling motifs and responds to high affinity IgE receptor aggregation. J Biol Chem. 1996;271:29271–29278. doi: 10.1074/jbc.271.46.29271. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, Lee KK, Songyang Z, Cantley LC, Burn P, Burakoff SJ. Interaction of shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993;262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- Rebel VI, Miller CL, Thornbury GR, Dragowska WH, Eaves CJ, Lansdorp PM. A comparison of long-term repopulating hematopoietic cells in fetal liver and adult bone marrow from the mouse. Expt Hematol. 1996;24:638–648. [PubMed] [Google Scholar]
- Sattler M, Salgia R, Shrikhande G, Verma S, Choi J-L, Rohrschneider LR, Griffin JD. The phosphatidylinositol polyphosphate 5-phosphatase SHIP and the protein tyrosine phosphatase SHP-2 form a complex in hematopoietic cells which can be regulated by BCR/ABL and growth factors. Oncogene. 1997;15:2379–2384. doi: 10.1038/sj.onc.1201422. [DOI] [PubMed] [Google Scholar]
- Saxton TM, Van Ostveen I, Botwell D, Aebersold R, Gold MR. B cell antigen receptor cross-linking induces tyrosine phosphorylation of the p21ras oncoprotein activators SHC and SOS1 as well as assembly of complexes containing SHC, GRB-2, mSOS1, and a 145-kD tyrosine-phosphorylated protein. J Immunol. 1994;153:623–636. [PubMed] [Google Scholar]
- Scharenberg AM, Kinet J-P. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Rajan TV, Greiner DL. Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends Biotechnol. 1997;15:302–307. doi: 10.1016/S0167-7799(97)01060-3. [DOI] [PubMed] [Google Scholar]
- Tazi A, Bouchonnet F, Grandsaigne M, Boumsell L, Hance AJ, Soler P. Evidence that granulocyte macrophage-colony-stimulating factor regulated the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J Clin Invest. 1993;91:566–576. doi: 10.1172/JCI116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker AM, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- Tridandapani S, Kelley T, Cooney D, Pradhan M, Coggeshall KM. Negative signaling in B cells: SHIP Grbs Shc. Immunol Today. 1997;18:424–427. doi: 10.1016/s0167-5699(97)01112-2. [DOI] [PubMed] [Google Scholar]
- Tsui FWL, Tsui HW. Molecular basis of the motheaten phenotype. Immunol Rev. 1994;138:185–206. doi: 10.1111/j.1600-065x.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Yi TL, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: Characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12:836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]