Abstract
The cAMP-dependent protein kinase A (PKA) is targeted to specific compartments in the cardiac myocyte by A-kinase anchoring proteins (AKAPs), a diverse set of scaffold proteins that have been implicated in the regulation of excitation-contraction coupling and cardiac remodeling. AKAPs bind not only PKA, but also a large variety of structural and signaling molecules. In this review, we discuss the basic concepts underlying compartmentation of cAMP and PKA signaling, as well as a few of the individual AKAPs that have been shown to be functionally relevant in the heart.
Keywords: PKA, AKAP, cAMP, compartmentation, scaffold, signaling
In the heart, stimulation of Gs-protein coupled receptors by catecholamines, prostanoids and peptide hormones all increase levels of the second messenger cyclic-adenosine monophosphate (cAMP). Through cAMP signaling pathways, these receptors differentially control inotropy (strength of muscle contraction), lusitropy (rate of relaxation), and chronotropy (heart rate), as well as myocyte hypertrophy (growth), metabolism, and cell survival. cAMP is synthesized by adenylyl cyclases (AC) and degraded by phosphodiesterases (PDE), both present in a large number of isoforms subject to diverse modes of regulation [1, 2]. Direct cAMP targets include protein kinase A (PKA), the small G protein guanine nucleotide exchange factor Epac, and hyperpolarization-activated and cyclic-nucleotide gated ion channels. PKA holoenzyme contains two regulatory “R” and two catalytic “C” subunits. cAMP binding to R-subunits causes release of the C-subunits and activation of the protein kinase. Over thirty years ago, it was recognized that stimulation of β-adrenergic receptors, but not prostanoid receptors, was inotropic, even though both increased intracellular cAMP levels and activated PKA [3–5]. Together, these fundamental observations raised a profound question which has been the subject of intense research to this day: how can a diffusible second messenger that activates a diffusible protein kinase differentially activate downstream signaling pathways that control distinct cellular processes?
One mechanism by which cAMP signals are spatially and temporally restricted is through the formation of multimolecular complexes or “signalosomes” by A-kinase anchoring proteins (AKAP) [6, 7]. There are over fifty known AKAPs (including alternative-spliced forms) that target PKA to different sites within the cell [8]. While AKAPs share in common their ability to bind PKA, they are remarkably diverse scaffold proteins. Within each signalosome, AKAPs couple PKA to different substrates, enhancing the rate and fidelity of their phosphorylation by the kinase. By incorporating select ACs, AKAPs direct the specific phosphorylation of these substrates in response to relevant stimuli [9]. In contrast, which phosphodiesterase is present in individual AKAP complexes affects the duration, amplitude, and spatial extent of cAMP signaling, as well as defining crosstalk with other signaling pathways [10, 11]. By bringing together different combinations of upstream and downstream signaling molecules, AKAPs provide the architectural infrastructure for specialization of the cAMP signaling network.
Compartmentation of the Second Messenger cAMP
Cyclic AMP is a small, freely diffusible hydrophilic molecule. While it is straightforward that through direct protein-protein interactions, scaffold proteins may physically organize the cAMP machinery within individual compartments, it is less intuitive how inappropriate signaling between adjacent compartments is prevented. This “compartmentation” can be imposed by either physical or functional barriers [12]. Functional compartmentation involves the production of areas of cAMP rarefaction by distinct pools of PDE anchored by AKAPs and other scaffold and adaptor proteins. Investigations using ion channels and fluorescence resonance energy transfer (FRET)-based cAMP and PKA activity sensors have demonstrated the importance of this mechanism in cardiac myocytes [12]. The importance of PDEs to cAMP compartmentation was first demonstrated in live cells in 1996 when Jurevicius and Fischmeister showed that local application of the β-adrenergic receptor agonist isoproterenol resulted in local L-type Ca2+ currents that were more generalized after addition of the general PDE inhibitor IBMX [13]. Of the PDEs expressed in myocytes, PDE3 and PDE4 family members account for the majority of activity [14–16]. In particular, PDE4 is responsible for limiting the distance that cAMP signals may diffuse when generated by β–adrenergic receptors [17, 18]. Type I PKA (PKAI) contains RIα or RIβ subunits, while type II PKA (PKAII) contains RIIα or RIIβ subunits; of these, RIα and RIIα are expressed in the heart [19]. Recently, Benedetto, et al., compared PKAI and PKAII compartments by fusing RI and RII-docking domains to the Epac1-camps FRET sensor [20]. “RII_epac” sensor was concentrated at the M-line and less so at the Z-line of the sarcomere, while “RI_epac” displayed a striated pattern that overlapped both the M and Z-lines. Although PKAI, but not PKAII, is typically soluble upon tissue fractionation [21], they showed using fluorescence recovery after photobleaching (FRAP) that PKAI is also anchored by AKAPs in myocytes, albeit more loosely than PKAII. Reminiscent of early data showing that particulate PKA is activated preferentially by β-adrenergic receptors [22], RII_epac was selectively activated by isoproterenol and inhibited by PDE4, while RI_epac was activated by isoproterenol, PGE1, glucagon and GLP-1 and preferentially inhibited by PDE2. Together, these results argue that multiple cAMP compartments regulated by distinct sets of receptors and PDEs may be present within the same organelle in a cell.
Physical compartmentation utilizes membrane barriers or the direct transfer of cAMP between AC and cAMP targets that are physically associated [23]. Although less well studied than PDEs, the differential association of ACs with select downstream pathways can contribute to specificity in cAMP signaling, promoting the generation of threshold cAMP fluxes near relevant targets. With the exception of soluble AC, all ACs are transmembrane proteins localized primarily at the cell membrane, providing close coupling of cAMP production to G-protein coupled receptors as well as calcium ion influx [24]. In myocytes, the plasma membrane is extended by the transverse tubule system deep into the interior of cell, thereby permitting the synthesis of cAMP within compartments close to intracellular targets. In particular, dyads composed of transverse tubules and either the sarcoplasmic reticulum or the outer nuclear membrane may facilitate the coupling of cAMP signaling to calcium ion release important for the contractile cycle and other functions such as hypertrophy [25, 26]. Of the nine AC isoforms known to be expressed in the heart, type 5 and type 6 cyclases account for the majority of AC activity [27]. These cyclases are similarly regulated, including activation by Gαs and Gβγ, and inhibition by Gαi, PKA feedback phosphorylation and Ca2+ [28]. However, AC5 and AC6 do not exhibit functional redundancy in vivo. For example, in response to transverse aortic constriction, a model for chronic pressure-overload, AC5−/− mice exhibited decreased myocyte apoptosis and increased Bcl2 expression, while AC6−/− mice exhibited decreased hypertrophy and unchanged Bcl2 expression [29, 30]. Together, these findings suggest that different cAMP compartments may be defined by different combinations of ACs and PDEs targeted by individual scaffold proteins. Like PDEs, clustering of ACs by AKAPs and other scaffolds within different plasmalemmal microdomains may account for their differential function [26, 31].
PKA binding to AKAPs
PKA R-subunits contain N-terminal docking (residues 1–23) and dimerization domains (24–44) followed by an inhibitor site and two cAMP binding domains (residues 158–426) that bind and inhibit a C-subunit [32]. AKAPs vary greatly in structure, localization, and the proteins for which they serve as a scaffold. What they share in common is an amphipathic, 14–18 amino acid residue motif capable of binding the PKA R-subunit docking domain. Most AKAPs show a preference for PKAII, while a few bind both PKAI and PKAII. The structure of PKA bound to AKAPs has been studied in great detail [33]. The N-terminal docking and dimerization domain of the R-subunit dimer forms a X-type, antiparallel four-helix bundle with a hydrophobic groove that binds the hydrophobic face of the AKAP amphipathic helix [34]. Differences in the depth of the groove and the conformation of the extreme N-terminal residues in the RI and RII dimer account for the differences in affinity for AKAPs between PKAI and PKAII [35].
Synthetic peptides based on AKAP sequences have proven instrumental in the elucidation of PKA anchoring-dependent events [8]. These peptides can either bind both PKAI and PKAII or show high specificity for a single type of PKA [36–38]. For example, expression by adenoviral infection of the Ht31 PKA-binding peptide that is selective for PKAII globally competed PKAII-AKAP complex formation in adult cardiac myocytes [39]. When introduced by in vivo adenoviral gene transfer into rat hearts, Ht31 inhibited isoproterenol-induced phosphorylation of troponin I (cTnI), phospholamban and the ryanodine receptor (RyR2) [40]. While baseline +dP/dtmax, −dP/dtmax and left ventricular end-diastolic pressure, but not ejection fraction, were decreased, at high doses of isoproterenol, ejection fraction and stroke volume were elevated. The authors suggest that the paradoxically increased inotropy in the face of inhibited PKA activity may be due to increased cTnI N-terminal proteolysis, which can in turn promote cardiac function. More recently, the cell-permeable peptide “TAT-AKAD” that binds both RI and RII with nanomolar affinity has been introduced into isolated myocytes and Langendorff-perfused hearts [41]. As with Ht31, cTnI and phospholamban phosphorylation were inhibited. Remarkably, TAT-AKAD inhibited both the contraction and relaxation rates and shortening of paced myocytes. Importantly, TAT-AKAD inhibited β-adrenergic induced ex vivo heart rate, peak pressure, +dP/dtmax and −dP/dtmax. Together, these results imply that AKAP anchoring of PKA is critical for cardiac function. There are at least 15 AKAPs expressed in the heart (see Table). In the discussion that follows, we now review what is known about some of these individual AKAPs. While global peptide disruptors are important research tools, future clinical therapy will likely target individual AKAP complexes dedicated to distinct cellular functions, thereby avoiding the complexities and side-effects of broad-based signaling inhibition.
Table.
AKAPs Expressed in the Heart.
| Gene Name | Protein Names and Isoforms | Binding Partners | Localization |
|---|---|---|---|
| AKAP1 | D-AKAP1, s-AKAP84, AKAP121, AKAP149 | PKA I and II, Protein tyrosine phosphatase PTPD1, Src, PKCα, Lfc, PDE7A, RSK1, PP1, PP2A, CaN, RNA, AMY-1 | Mitochondrion, Endoplasmic Reticulum, Nuclear Envelope |
| AKAP5 | AKAP79, AKAP75, AKAP150 | PKAII, PKC, CaN, KCNQ2, CaV1, β-AR, AC, SAP-97 | Plasmalemma and T-tubules |
| AKAP6 | mAKAPβ | PKAII, AC5, PDE4D3, PP2A B56δ, RyR2, CaNAβ, NFATc, HIF-1a, VHL, Siah2, Epac1, Rap1, ERK5, MEK5, RSK3, PDK1, NCX1, Nesprin-1α, myopodin | Nuclear Envelope |
| AKAP7 | AKAP15, AKAP18 (α,β,γ,δ) | PKAII, CaV1, Na Channel, phospholamban, Inhibitor-1, PP1 | Plasmalemma and sarcoplasmic reticulum |
| AKAP8 | AKAP95 | PKAII, PDE7A, MCM2, p68 RNA helicase, HDAC3, AMY-1, Cyclin D/E | Nucleus |
| AKAP9 | Yotiao, AKAP450, AKAP350, CG-NAP, Hyperion | PKAII, AC, PP1, PP2A, PDE4D3, KCNQ1, IP3R, PKCε, PKN, Casein kinase 1, chloride intracellular channel (CLIC) | Plasmalemma, Centrosomes, Golgi |
| AKAP10 | D-AKAP2 | PKA I and II, Rab11, Rab4, PDZK1 | Outer Mitochondrial Membrane |
| AKAP11 | AKAP220 | PKAII, PP1, GSK3β | Peroxisomes |
| AKAP12 | Gravin, SSeCKS | PKAII, PDE4D, PKC, Src, CaN, β2AR, Calmodulin, Cyclin D | Actin cytoskeleton, Plasma membrane |
| AKAP13 | AKAP-Lbc, Ht31, BRX-1 | PKAII, Gα12, Rho, PKNα, MLTK, MKK3, p38α, KSR1, Raf, MEK1/2, ERK1/2, 14-3-3, PKCη, PKD | Cytoskeleton |
| ARFGEF2 | BIG2 | PKAI and II, formin binding protein 3, PDE3A, TNFR1 | Cytoplasm, Internal Membranes including Golgi |
| EZR | Ezrin | PKAI and II, CFTR, EBP50/NHERF, NHE3, calmodulin, Rho kinase, Actin, α1AR, E-cadherin, β-catenin, EGFR, Fas-R, PKCα, S100, FAK, SAP-97, Moesin, Radixin | Cytoskeleton |
| MAP2 | MAP2B | PKAII, tubulin, Cav1 | microtubules |
| CMYA5 | Myospryn | PKAII, Dysbindin, Titin, Calpain-3 | Sarcomere |
| SPHKAP | SKIP | PKAI, sphingosine kinase type 1, | Cytosolic |
| SYNM | Synemin | PKAII, desmin, vimentin, dystrobrevin, desmuslin, zyxin, talin, vinculin, | Intercalated discs, sacrolemma, Z-lines, intermediate filaments |
| TNNT2 | Troponin T | Troponin I, Troponin C, Actin | Sarcomere Thin Filaments |
AC, AC; AMY-1, associate of myc-1; AR, adrenergic receptor; CaN, calcineurin (PP2B); CaV1, L-type Ca2+ channel; NCX1, sodium-calcium exchanger; PKC, protein kinase C; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; Siah2, seven in absentia homolog; RyR2, ryanodine receptor; VHL, von Hippel-Lindau tumor suppressor
AKAP79/150/75
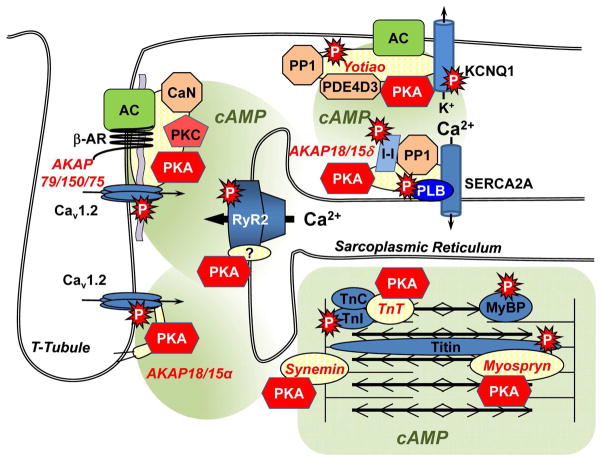
The AKAP5 gene product (AKAP79 in humans, AKAP150 in rodents, AKAP75 in bovine) is the best described AKAP and has recently been shown to be critical for sympathetic control of cardiac myocytes (Figure 1) [31]. It is a prime example of a scaffold protein that facilitates and integrates upstream signaling with far-reaching downstream effects on cellular function. Associated with a variety of ion channels and membrane receptors in excitable cells (neurons and myocytes), AKAP79/150/75 binds and integrates cAMP, calcium, and phospholipid signaling through PKA, protein kinase C and the Ca2+/calmodulin-dependent protein phosphatase calcineurin [42]. AKAP79/150/75 is targeted to the plasma membrane through the direct binding of phospholipids and is a major determinant of PKA anchoring at the neuronal post-synaptic density where it plays an important role in learning and memory [43–45]. Early on, it was shown that AKAP79/150/75 can bind both β1 and β2-adrenergic receptors in heterologous systems, facilitating both downstream signaling and PKA-regulated downregulation and desensitization [46–48]. Later, in the first demonstration of an AC-AKAP complex, it was discovered that AC5/6 associated directly with AKAP79/150/75, coupling cAMP production to PKA activation and negative feedback regulation of the cyclase [49]. Interestingly, AKAP79/150/75 is present in cardiac myocytes with β1-adrenergic receptors at caveolin-3-deficient synapses formed with sympathetic neurons in co-culture [50].
Figure 1. AKAPs Involved in Sympathetic Regulation of Excitation-Contraction Coupling.
AKAP79/150/75 and AKAP18/15α can regulate the L-type Ca2+ channel, yotiao the KNCQ1 slow outward potassium ion current, and AKAP18/15δ phospholamban and Ca2+ reuptake. Troponin T, myospryn, and synemin are sarcomeric AKAPs. The AKAP responsible for PKA phosphorylation of RyR2 at the sarcoplasmic reticulum is unclear, albeit AKAP5 knock-out mice have diminished RyR2 phosphorylation [31].
Although AKAP79/150/75 has been the subject of intense investigation of over 20 years, the specific function of this AKAP in the cardiovascular system has only recently been addressed. In the past, AKAP79/150/75 appeared in the cardiac literature mainly due to the use of its consensus PxIxIT calcineurin-binding domain as a potent inhibitor of calcineurin activity. Expression of the AKAP79/150/75 site, like other calcineurin-binding peptides, inhibits NFAT-dependent myocyte hypertrophy [51]. Experiments using a AKAP79/150/75 knock-out mouse have more directly tested whether this scaffold is important in the heart and vasculature. AKAP79/150/75 facilitates the association of the L-type Ca2+ channel with PKA and PKC in excitable cells, potentiating channel opening by direct phosphorylation of the channel [52–54]. In arterial smooth myocytes, AKAP79/150/75 expression was required for the generation of persistant Ca2+ “sparklets” that promote vascular tone, as well as angiotensin II-induced hypertension [55]. Interestingly, in this cell type, anchoring by AKAP79/150/75 of PKC, but not PKA, was critical for promoting Ca2+ influx through the channel. In contrast, Nichols et al, recently demonstrated that in cardiac myocytes, AKAP79/150/75 mediates the association of β1/2-adrenergic receptors, AC5/6, PKAII, calcineurin and a caveolin-3-associated sub-population of Cav1.2 L-type Ca2+ channels, facilitating the selective PKA phosphorylation of that pool of ion channels in response to β-adrenergic stimulation [31]. Importantly, β-adrenergic stimulation of Ca2+ transients was absent in myocytes from AKAP79/150/75 knock-out mice, even though the overall stimulated influx of Ca2+ through L-type Ca2+ channels remained intact. Further, β-adrenergic stimulation of Ca2+ transients was normal in myocytes isolated from an AKAP79/150/75 knock-in mouse in which the AKAP79/150/75 PKA binding domain is deleted, suggesting that overall Ca2+ transients depend on the scaffold, but not PKA-phosphorylation of Cav1.2. Remarkably, even though AKAP79/150/75 does not form a complex with RyR2 and phospholamban, AKAP79/150/75 knock-out resulted in decreased sarcoplasmic reticulum loading and spontaneous Ca2+ release through ryanodine receptors in response to β-adrenergic stimulation, as well as reduced PKA phosphorylation of both RyR2 and phospholamban. Although this study raises more questions than it answers, including the unclear relationship of Cav1.2 pools associated with or separate from AKAP79/150/75 to Ca2+ transients, it does establish the central role of a single AKAP in orchestrating the sympathetic stimulation of Ca2+ transients in adult myocytes [31].
AKAP18/15
AKAP79/150/75 is not the only AKAP associated with Cav1 channels. AKAP18/15 (AKAP7) is expressed in myocytes as multiple alternatively-spliced forms that are differentially localized to the plasmalemma and the sarcoplasmic reticulum. AKAP18/15α, the smallest AKAP with only 81 amino acids, is localized to the plasma membrane by dual N-terminal myristoylation and palmitoylation [56]. A leucine zipper motif mediates binding of the scaffold to the Cav1 family of voltage-gated channels, facilitating PKA phosphorylation and potentiation of the channels [56–58]. Interestingly, in myocytes, the distal C-terminal domain of Cav1.2 containing the AKAP18α binding site is often cleaved from the remainder of the pore-forming subunit, associating non-covalently and auto-inhibiting the channel in the absence of cAMP elevation [59]. Importantly, introduction of a competing leucine zipper peptide (AKAP18/15α residues 38–54) into adult cardiac myocytes that blocks association of the scaffold with the C-terminal Cav1.2 domain inhibits β-adrenergic stimulation of ICa [60].
AKAP18/15δ is a longer isoform of AKAP18/15 that is targeted to the sarcoplasmic reticulum [61]. When not phosphorylated, phospholamban binds and inhibits the sarco-endoplasmic reticulum Ca2+-ATPase 2A (SERCA2A). Upon sympathetic stimulation, PKA phosphorylation causes the dissociation of phospholamban from the pump, increasing Ca2+ re-uptake into internal stores (lusitropy) and cardiac contractility. AKAP18/15δ binds phospholamban and activates SERCA2 by promoting PKA phosphorylation of phospholamban [61]. The requirement for AKAP18/15δ for adrenergic-stimulated reuptake in cultured myocytes was confirmed by AKAP18/15δ RNA interference. Recently, we reported that AKAP18/15δ also binds protein phosphatase 1 and its inhibitor Inhibitor-1, facilitating PKA phosphorylation of the inhibitor and inactivation of the phosphatase [62]. This phosphatase is responsible for the inhibition of SERCA2A through phospholamban dephosphorylation [63]. Thus, by promoting Inhibitor-1 phosphorylation, AKAP18/15δ may further increase lusitropy. Taken together, in vitro evidence suggests that AKAP18/15 isoforms are important for both the regulation of Ca2+ influx through L-type Ca2+ channels and Ca2+ re-uptake through SERCA2A. In vivo evidence is, however, pending to support these hypotheses.
Yotiao
Repolarization of the cardiac myocyte after contraction is facilitated by the slow outward potassium ion current (IKs) contributed by the KCNQ1-KCNE1 channel. β-adrenergic stimulation increases IKs through PKA phosphorylation of KCNQ1, an event important for reducing action potential duration during states of increased chronotropy. Mutations in the subunits of this channel induce Long QT syndrome due to prolonged myocyte repolarization and are a cause of fatal cardiac arrhythmia. Through the binding of a leucine zipper motif on KCNQ1, Yotiao (AKAP9; AKAP350, AKAP450) is the AKAP responsible for targeting PKA to the IKs channel [64]. Importantly, mutations in either the human KCNQ1 leucine zipper (G589D) or the corresponding yotiao binding site (S1570L) block association of the scaffold with the channel, inhibiting KCNQ1 phosphorylation and inducing Long QT syndrome, and making yotiao the first AKAP known to be associated with the etiology of a human disease [64, 65].
Like AKAP79/150/75, yotiao binds other signaling molecules besides PKA, including AC (types 1–3 and 9), the phosphodiesterase PDE4D3 and protein phosphatase 1 that can affect localized PKA phosphorylation [64, 66, 67]. Interestingly, yotiao is also a PKA substrate and phosphorylation of yotiao affects IKs independently of PKA phosphorylation of the channel [68].
Sarcomeric AKAPs
Several sarcomeric proteins are phosphorylated in response to β-adrenergic stimulation, including cardiac troponin I (cTnI), myosin binding protein C and titin [69]. Recently, cardiac troponin T (cTnT) was shown to be dual AKAP capable to binding either PKAI or PKAII [69]. Remarkably, deletion of cTnT Lys-210, a mutation found in human familial dilated cardiomyopathy that reduces the phosphorylation of cTnI and myosin binding protein C, inhibited binding of PKA to cTnT.
The intermediate filament protein synemin is a PKAII AKAP that is localized to the Z-line, intercalated discs, and the sarcolemma [70]. The targets of PKA-anchored synemin remain unknown. In contrast, the large 449 kDa AKAP and PKA substrate myospryn is a calpain 3 and titin-binding protein [71]. A gene polymorphism for myospryn has been associated with left ventricular hypertrophy in hypertension [72].
AKAP-Lbc
AKAP-Lbc (AKAP13; Brx-1, and proto-Lbc) is essential for cardiac development and pathologic myocyte hypertrophy [73, 74]. AKAP-Lbc is perhaps better known for Ht31 (human thyroid clone 31), an AKAP-Lbc fragment containing its PKA-binding site [75]. The Ht31 peptide was used in early studies to define the determinants of PKA anchoring and to demonstrate the functional importance of anchored PKA in various cell types [6]. Later, full-length clones for this scaffold were isolated and the AKAP was recognized to be a longer splice variant of the Lbc proto-oncogene expressed most highly in the heart [76]. AKAP-Lbc is a homo-oligomeric Rho-selective guanine nuclear exchange factor (Rho-GEF) that binds Gα12/13, inducing Rho activity and stress fiber formation in lysophosphatidic acid-stimulated fibroblasts [76, 77]. One target for AKAP-Lbc activated Rho is PKNα, which in turn regulates a MLTK, MKK3 and p38α cascade associated with the scaffold [78]. Interestingly, when PKA phosphorylated, AKAP-Lbc activation of RhoA is inhibited by the binding of the small protein 14-3-3 [79, 80]. As a result, cAMP signaling inhibits RhoA activation through AKAP-Lbc.
In cardiac myocytes, AKAP-Lbc expression is required for α-adrenergic-induced hypertrophy and is itself increased in expression by hypertrophic stimuli [74]. Although AKAP-Lbc mediates α-adrenergic-stimulated RhoA activation, [74]. AKAP-Lbc Rho-GEF activity does not appear to be required for hypertrophic signaling, but instead depends on anchoring of PKD1 [81]. Besides being a Rho-GEF, AKAP-Lbc facilitates the activation of PKD1 by PKCη bound to the scaffold [82]. Together with PKA, activated AKAP-Lbc anchored PKD promotes the nuclear export of the class II histone deacetylase HDAC5 through 14-3-3 binding, resulting in increased MEF-2-dependent gene transcription [81]. The requirement for AKAP-Lbc in vivo in adult pathologic cardiac hypertrophy has not yet been tested, since the AKAP-Lbc knock-out mouse was embryonic lethal at embryonic day 10.5–11.0 [73]. Nevertheless, the hearts of AKAP-Lbc null embryos displayed thin walls, were dilated, and developed a pericardial effusion. Accordingly, null myocytes displayed decreased sarcomere formation and reduced MEF2C expression.
Recently, signaling through AKAP-Lbc has been shown to be even more extensive. AKAP-Lbc can bind both the Ras effector Raf and the scaffold kinase suppressor of Ras (KSR-1) that anchors MEK1/2 and ERK1/2, thereby, bringing together the entire classical ERK1/2 cascade [83]. In HEK293 cells, AKAP-Lbc was required for efficient mitogen stimulation of ERK1/2 signaling. Furthermore, phosphorylation of KSR by PKA associated with the complexes sustained ERK1/2 activation. While this pathway has not been studied in myocytes, it will be important to study AKAP-Lbc activation of ERK1/2 signaling in the heart, especially in light of recent studies that ERK1/2 regulates the balance between concentric and eccentric cardiac myocyte growth [84].
mAKAPβ
mAKAPβ (AKAP6; AKAP100 is a C-terminal fragment of mAKAP) is a scaffold protein for a large signalosome located at the striated myocyte nuclear envelope that we have proposed serves as a gatekeeper for transcription factors involved in cardiac remodeling [85, 86]. The mAKAPβ signalosome contains all of the required machinery for cAMP synthesis, degradation and function, including AC5, PDE4D3, PKAII, and Epac1 [10, 26, 87, 88]. In addition to these enzymes, mAKAPβ also can bind the following molecules important for cardiac remodeling and contractility: calcineurin Aβ, protein phosphatase 2A (PP2A), NFATc transcription factor family members, MEK5, ERK5, and the type 2 ryanodine receptor (RyR2) [10, 89–93]. Recently, we have discovered mAKAPβ also contributes to the regulation of the transcription factor hypoxia-inducible factor 1α (HIF-1α) by binding HIF-1α and ubiquitin E3 ligases [94], while others have reported that mAKAPβ can also be co-immunoprecipitated with the sodium/calcium exchanger NCX1 and the CaN substrate myopodin [95, 96].
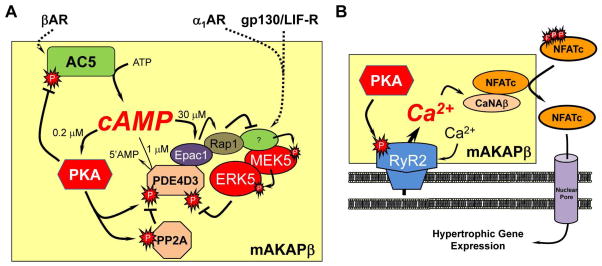
Work on the mAKAPβ signalosome has contributed importantly to our understanding of how AKAPs may modulate local cAMP fluxes (Figure 2). The direct binding of PDE4D3 to mAKAPβ was the first example of a PDE-AKAP complex [87]. As part of a negative feedback loop, PDE4D3 is activated by PKA phosphorylation at serine residue 54 (S54), resulting in increased cAMP degradation [16, 97]. PDE4D3 mediates the recruitment of an ERK5 signaling module to the complex [10]. Upon activation, ERK5 phosphorylates PDE4D3 S597, inhibiting PDE4D3 activity and increasing cAMP activation of PKA. Together these results suggest that upstream cAMP and ERK5 signals will synergistically activate mAKAPβ-bound PKA, a mechanism potentially important in the chronically stressed heart exposed to increased circulating catecholamine levels and IL-6 type cytokines. Besides ERK5, mAKAPβ-associated PDE4D3 binds Epac1 directly, thus establishing the mAKAPβ signalosome as the first identified to include two different cAMP effectors (PKA and Epac1) [10]. Through Rap1, Epac1 inhibits ERK5, as part of another negative feedback loop intrinsic to the mAKAPβ complex. Because Epac1 is a relatively low affinity cAMP target, Epac1 feedback may be important for homeostasis when sympathetic stimulation is maximal, such as during cardiac decompensation. Recently, we showed that PDE4D3 S54 is dephosphorylated by PP2A [98]. PP2A bound to mAKAPβ contains a B56δ-subunit that confers PKA activation, comprising an incoherent feedforward loop that opposes PDE4D3 activation. Moreover, we have also found that mAKAPβ-bound AC5 is inhibited by PKA phosphorylation, constituting yet another negative feedback loop [26]. Mathematical modeling suggests that negative feedback and incoherent feedforward loops are characteristic of systems that exhibit high sensitivity (the amplitude of the output in response to a stimulus) with precise adaptation (the ability of a signaling system to reset to baseline after termination of a stimulus) [99]. Through the organization of these interlinked feedback and feedforward loops, the mAKAPβ signalosome should confer tight control of local cAMP levels in response to stress-induced neuroendocrine and paracrine stimuli. cAMP fluxes local to mAKAPβ are likely to be autonomous of cAMP fluxes elsewhere in the myocyte that are regulated by signalosomes containing different sets of signaling enzymes.
Figure 2. Signal integration by the mAKAPβ signalosome.
The yellow boxes represent the mAKAPβ signalosome. A. There is evidence for three conjoined negative feedback loops intrinsic to the mAKAPβ complex that control local cAMP levels: (1) AC5, cAMP, PKA; (2) cAMP, PKA, PDE4D3; and (3) cAMP, Epac1, Rap1, MEK5, ERK5, PDE4D3. βAR stimulation will activate AC5, resulting in cAMP production and PKA activation. PKA phosphorylation inhibits AC5 and activates PDE4D3 activity, resulting in decreased cAMP accumulation [31, 97, 108]. The MEK5/ERK5 MAPK pathway is activated in myocytes by α1AR and gp130/LIF-R agonists [109]. Activation of ERK5 will lead to PDE4D3 inhibition and increased PKA activity [10]. When high cAMP levels activate the guanine nucleotide exchange factor Epac1, Rap1 will inhibit the ERK5 pathway, reversing the ERK5-mediated inhibition of PDE4D3 and limiting downstream signaling. In addition, there is an incoherent feedforward loop that will oppose PKA phosphorylation of PDE4D3 resulting from PKA phosphorylation and activation of PP2A in the complex. An incoherent feedforward loop is present when two pathways lead to the same effector with opposite results [99]. Compare PKA-PDE4D3 and PKA-PP2A-PDE4D3. B. Calcineurin Aβ (CaNAβ) may serve as an mAKAPβ signalosome effector. RyR2 bound to mAKAPβ is PKA phosphorylated when myocytes are stimulated by β-agonists, potentially increasing local Ca2+ levels [89]. Norepinephrine-treatment of myocytes results in CaNAβ recruitment into the complex, where it can catalyze the dephosphorylation and nuclear translocation of NFATc transcription factors [93]. While not illustrated in this Figure, ERK5 and HIF-1α are also potential effectors for mAKAPβ complexes.
An important substrate for mAKAPβ-bound PKA is RyR2 (Figure 2B), a Ca2+-induced Ca2+ release channel potentiated by PKA phosphorylation [90, 91]. RyR2 is important for excitation-contraction coupling at the sarcoplasmic reticulum (SR), but is also present within dyads formed between perinuclear transverse tubules and the outer nuclear membrane [25]. Since mAKAPβ is primarily located at the nuclear envelope through the binding of the KASH domain protein nesprin-1α [100], we propose that mAKAPβ complexes include a small subset of RyR2 molecules present within these perinuclear dyads [91]. There mAKAPβ-RyR2 complexes may modulate the activity of hypertrophic transcription factors that translocate to the nucleus. Upon norepinephrine stimulation, active calcineurin Aβ is recruited to mAKAPβ complexes where the phosphatase can promote the dephosphorylation of NFATc3 [93]. Accordingly, mAKAPβ RNAi inhibits NFATc nuclear translocation and transcriptional activity, as well as the adrenergic and cytokine-induced hypertrophy of cultured neonatal rat ventricular myocytes [10, 89, 93].
Although in vivo mAKAPβ function remains to be determined, it is interesting that AC5, calcineurin Aβ, NFATc2 and NFATc3 knock-out mice are resistant to pathologic cardiac hypertrophy [101–104]. In addition, a human nesprin-1 missense mutation has been described in a patient with dilated cardiomyopathy [105]. Conversely, unstressed PDE4D knock-out mice exhibit a progressive, exaggerated age-dependent cardiomyopathy [106]. mAKAPβ is one of the least abundant AKAPs in the heart (MSK, unpublished observations). In rescue experiments, we have found that myocytes expressing full-length mAKAPβ mutants lacking either the PKA or calcineurin Aβ binding domains have impaired hypertrophic signaling [89, 93]. It is remarkable that specific disruption of such a small pool of anchored PKA can have such an obvious effect on cellular phenotype. It is possible that in the future, drug therapy directed at specific AKAPs such as mAKAP may provide a strategy for the treatment or prevention of pathologic cardiac remodeling and heart failure in the absence of effects on cardiac contractility.
Summary
Through research on AKAPs, our view of the cell has changed dramatically. Traditionally, intracellular signal transduction was perceived to be driven primarily by the abundance of relevant molecules and the catalysis of specific post-translational modifications, as these molecules freely diffused within the cytosol. It has become clear that through scaffold and adaptor proteins that much of intracellular signaling actually occurs in defined compartments in which relevant molecules, that are either recruited or constitutively associated, interact due to specific protein-protein interactions. Thus, enzyme specificity is conferred as much by co-localization as by the intrinsic selectivity of the active site. Given that the inhibition of the catalytic activities of many enzymes is clinically problematic due to their pleiotropism, we propose that the disruption of unique protein-protein interactions is an alternative therapeutic strategy permitting the selective inhibition of cellular processes. While intracellular protein-protein interactions have not been traditionally considered feasible drug targets, recent work, including the inhibition of NOTCH transcriptional complexes by synthetic peptides, has shown that protein binding may be specifically inhibited in vivo with directed physiologic effects [107]. A more complete understanding of the structure-function relationships underlying AKAP complexes will result in the development of a new generation of therapeutics that moves clinical medicine beyond receptor ligands and enzyme inhibitors.
Research Highlights
In this review, we discuss A-kinase anchoring proteins expressed in the heart.
AKAPs are important for cAMP compartmentation.
AKAP scaffold proteins confer specificity and fidelity to cAMP signaling.
Acknowledgments
This work was supported by NIH grants HL82705 to KDK and HL075398 to MSK and American Heart Association awards to MK and JL.
Abbreviations
- AC
adenylyl cyclase
- AKAP
A-kinase anchoring protein
- CaN
calcineurin
- NFAT
nuclear factor of activated T-cell
- PDE
phosphodiesterase
- PKA
protein kinase A
- RyR2
ryanodine receptor
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 2.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17(1):5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes JS, Brunton LL, Mayer SE. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980;255(11):5113–9. [PubMed] [Google Scholar]
- 4.Keely SL. Activation of cAMP-dependent protein kinase without a corresponding increase in phosphorylase activity. Res Commun Chem Pathol Pharmacol. 1977;18(2):283–90. [PubMed] [Google Scholar]
- 5.Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. Hormonally specific expression of cardiac protein kinase activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1570–4. doi: 10.1073/pnas.76.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007 Nov;35(Pt 5):931–7. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- 7.Smith FD, Langeberg LK, Scott JD. The where’s and when’s of kinase anchoring. Trends Biochem Sci. 2006 Jun;31(6):316–23. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009 Apr;61(4):394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efendiev R, Samelson BK, Nguyen BT, Phatarpekar PV, Baameur F, Scott JD, et al. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J Biol Chem. 2010 May 7;285(19):14450–8. doi: 10.1074/jbc.M110.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005 Sep 22;437(7058):574–8. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaccolo M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol. 2006 Jul;85(7):693–7. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009 Sep;158(1):50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci U S A. 1996;93(1):295–9. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, et al. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res. 2006 Apr 28;98(8):1081–8. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, et al. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004 Jul 9;95(1):67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 16.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007 Apr 13;100(7):950–66. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 17.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006 Nov 10;99(10):1084–91. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010 Mar 26;327(5973):1653–7. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 19.Scott JD. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther. 1991;50(1):123–45. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- 20.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, et al. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008 Oct 10;103(8):836–44. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 21.Corbin JD, Sugden PH, Lincoln TM, Keely SL. Compartmentalization of adenosine 3′:5′-monophosphate and adenosine 3′:5′-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977;252(11):3854–61. [PubMed] [Google Scholar]
- 22.Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983;258(17):10233–9. [PubMed] [Google Scholar]
- 23.Tovey SC, Dedos SG, Taylor EJ, Church JE, Taylor CW. Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J Cell Biol. 2008 Oct 20;183(2):297–311. doi: 10.1083/jcb.200803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessauer CW. Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009 Nov;76(5):935–41. doi: 10.1124/mol.109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol. 2011 Mar;50(3):451–9. doi: 10.1016/j.yjmcc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, et al. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem. 2009 Aug 28;284(35):23540–6. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000 Sep;279(3):F400–16. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 28.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007 Jul;87(3):965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 29.Tang T, Lai NC, Hammond HK, Roth DM, Yang Y, Guo T, et al. Adenylyl cyclase 6 deletion reduces left ventricular hypertrophy, dilation, dysfunction, and fibrosis in pressure-overloaded female mice. J Am Coll Cardiol. 2010 Apr 6;55(14):1476–86. doi: 10.1016/j.jacc.2009.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9986–90. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, et al. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010 Sep 17;107(6):747–56. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor SS, Kim C, Cheng CY, Brown SH, Wu J, Kannan N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta. 2008 Jan;1784(1):16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarma GN, Kinderman FS, Kim C, von Daake S, Chen L, Wang BC, et al. Structure of D-AKAP2:PKA RI complex: insights into AKAP specificity and selectivity. Structure. 2010 Feb 10;18(2):155–66. doi: 10.1016/j.str.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newlon MG, Roy M, Morikis D, Hausken ZE, Coghlan V, Scott JD, et al. The molecular basis for protein kinase A anchoring revealed by solution NMR. Nat Struct Biol. 1999;6(3):222–7. doi: 10.1038/6663. [DOI] [PubMed] [Google Scholar]
- 35.Banky P, Roy M, Newlon MG, Morikis D, Haste NM, Taylor SS, et al. Related protein-protein interaction modules present drastically different surface topographies despite a conserved helical platform. J Mol Biol. 2003 Jul 25;330(5):1117–29. doi: 10.1016/s0022-2836(03)00552-7. [DOI] [PubMed] [Google Scholar]
- 36.Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Tasken K, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006 Nov 3;24(3):383–95. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Tasken K, et al. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem. 2006 Jul 28;281(30):21535–45. doi: 10.1074/jbc.M603223200. [DOI] [PubMed] [Google Scholar]
- 38.Torheim EA, Jarnaess E, Lygren B, Tasken K. Design of proteolytically stable RI-anchoring disruptor peptidomimetics for in vivo studies of anchored type I protein kinase A-mediated signalling. Biochem J. 2009 Nov 15;424(1):69–78. doi: 10.1042/BJ20090933. [DOI] [PubMed] [Google Scholar]
- 39.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, Damron DS, et al. AKAP-mediated targeting of protein kinase a regulates contractility in cardiac myocytes. Circ Res. 2001 Feb 16;88(3):291–7. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 40.McConnell BK, Popovic Z, Mal N, Lee K, Bautista J, Forudi F, et al. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem. 2009 Jan 16;284(3):1583–92. doi: 10.1074/jbc.M806321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel HH, Hamuro LL, Chun BJ, Kawaraguchi Y, Quick A, Rebolledo B, et al. Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase-anchoring peptide reduces cardiac function. J Biol Chem. 2010 Sep 3;285(36):27632–40. doi: 10.1074/jbc.M110.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992;267(24):16816–23. [PubMed] [Google Scholar]
- 43.Dell’Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5- bisphosphate. EMBO J. 1998;17(8):2246–60. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, et al. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12557–62. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisenhaus M, Allen ML, Yang L, Lu Y, Nichols CB, Su T, et al. Mutations in AKAP5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS One. 2010;5(4):e10325. doi: 10.1371/journal.pone.0010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser ID, Cong M, Kim J, Rollins EN, Daaka Y, Lefkowitz RJ, et al. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10(7):409–12. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 47.Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006 Nov 3;281(44):33537–53. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- 48.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, et al. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001 May 4;276(18):15192–9. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- 49.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006 Sep 15;23(6):925–31. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shcherbakova OG, Hurt CM, Xiang Y, Dell’Acqua ML, Zhang Q, Tsien RW, et al. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007 Feb 12;176(4):521–33. doi: 10.1083/jcb.200604167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, et al. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3322–7. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, et al. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19(1):185–96. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 53.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007 Jul 19;55(2):261–75. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009 Apr;89(2):411–52. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008 Feb 1;102(2):e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 56.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, et al. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J. 1998 Apr 15;17(8):2261–72. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and PKA to the C-terminus of theskeletal muscle Ca{super2+} channel and modulates its function. J Biol Chem. 2001;30:30. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 58.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, 3rd, Scheuer T, et al. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20(5):1017–26. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 59.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3(141):ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):13093–8. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007 Nov;8(11):1061–7. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A, Redden JM, Kapiloff MS, Dodge-Kafka KL. The large isoforms of A-kinase anchoring protein 18 mediate the phosphorylation of inhibitor-1 by protein kinase A and the inhibition of protein phosphatase 1 activity. Mol Pharmacol. 2011 Mar;79(3):533–40. doi: 10.1124/mol.110.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009 Sep;47(3):365–71. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295(5554):496–9. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20990–5. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terrenoire C, Houslay MD, Baillie GS, Kass RS. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem. 2009 Apr 3;284(14):9140–6. doi: 10.1074/jbc.M805366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piggott LA, Bauman AL, Scott JD, Dessauer CW. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):13835–40. doi: 10.1073/pnas.0712100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem. 2005 Sep 9;280(36):31347–52. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 69.Sumandea CA, Garcia-Cazarin ML, Bozio CH, Sievert GA, Balke CW, Sumandea MP. Cardiac troponin T, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J Biol Chem. 2011 Jan 7;286(1):530–41. doi: 10.1074/jbc.M110.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell MA, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M. The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys. 2006 Dec 15;456(2):204–15. doi: 10.1016/j.abb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Sarparanta J, Blandin G, Charton K, Vihola A, Marchand S, Milic A, et al. Interactions with M-band titin and calpain 3 link myospryn (CMYA5) to tibial and limb-girdle muscular dystrophies. J Biol Chem. 2010 Sep 24;285(39):30304–15. doi: 10.1074/jbc.M110.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakagami H, Kikuchi Y, Katsuya T, Morishita R, Akasaka H, Saitoh S, et al. Gene polymorphism of myospryn (cardiomyopathy-associated 5) is associated with left ventricular wall thickness in patients with hypertension. Hypertens Res. 2007 Dec;30(12):1239–46. doi: 10.1291/hypres.30.1239. [DOI] [PubMed] [Google Scholar]
- 73.Mayers CM, Wadell J, McLean K, Venere M, Malik M, Shibata T, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem. 2010 Apr 16;285(16):12344–54. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10140–5. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII- binding domain. J Biol Chem. 1992;267(19):13376–82. [PubMed] [Google Scholar]
- 76.Diviani D, Soderling J, Scott JD. AKAP-Lbc Anchors Protein Kinase A and Nucleates Galpha 12-selective Rho- mediated Stress Fiber Formation. J Biol Chem. 2001 Nov 23;276(47):44247–57. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 77.Baisamy L, Jurisch N, Diviani D. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem. 2005 Apr 15;280(15):15405–12. doi: 10.1074/jbc.M414440200. [DOI] [PubMed] [Google Scholar]
- 78.Cariolato L, Cavin S, Diviani D. AKAP-LBC anchors a PKN-based signaling complex involved in alpha1-adrenergic receptor-induced p38 activation. J Biol Chem. 2011 Jan 11; doi: 10.1074/jbc.M110.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004 Aug 24;14(16):1436–50. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 80.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004 Jul 21;23(14):2811–20. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008 Oct 24;32(2):169–79. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol Cell. 2004 Sep 24;15(6):889–99. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Smith FD, Langeberg LK, Cellurale C, Pawson T, Morrison DK, Davis RJ, et al. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat Cell Biol. 2010 Dec;12(12):1242–9. doi: 10.1038/ncb2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011 Jan 21;108(2):176–83. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kapiloff MS. Contributions of protein kinase A anchoring proteins to compartmentation of cAMP signaling in the heart. Mol Pharmacol. 2002 Aug;62(2):193–9. doi: 10.1124/mol.62.2.193. [DOI] [PubMed] [Google Scholar]
- 86.Bauman AL, Michel JJ, Henson E, Dodge-Kafka KL, Kapiloff MS. The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life. 2007 Mar;59(3):163–9. doi: 10.1080/15216540701358593. [DOI] [PubMed] [Google Scholar]
- 87.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001 Apr 17;20(8):1921–30. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci. 1999 Aug;112(Pt 16):2725–36. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 89.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005 Dec 1;118(Pt 23):5637–46. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 90.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000 May 12;101(4):365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 91.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001 Sep;114(Pt 17):3167–76. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 92.Michel JJ, Townley IK, Dodge-Kafka KL, Zhang F, Kapiloff MS, Scott JD. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol Cell. 2005 Dec 9;20(5):661–72. doi: 10.1016/j.molcel.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 93.Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K, et al. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010 Feb;48(2):387–94. doi: 10.1016/j.yjmcc.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal. 2008;1(51):ra18. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faul C, Dhume A, Schecter AD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol Cell Biol. 2007 Dec;27(23):8215–27. doi: 10.1128/MCB.00950-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM. Sodium/calcium exchanger (NCX1) macromolecular complex. J Biol Chem. 2003 Aug 1;278(31):28849–55. doi: 10.1074/jbc.M300754200. [DOI] [PubMed] [Google Scholar]
- 97.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996 Jul 12;271(28):16526–34. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- 98.Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, et al. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem. 2010 Apr 9;285(15):11078–86. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma W, Trusina A, El-Samad H, Lim WA, Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009 Aug 21;138(4):760–73. doi: 10.1016/j.cell.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res. 2005 Feb 15;303(2):388–99. doi: 10.1016/j.yexcr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 101.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, et al. Targeted Disruption of NFATc3, but Not NFATc4, Reveals an Intrinsic Defect in Calcineurin-Mediated Cardiac Hypertrophic Growth. Mol Cell Biol. 2002 Nov;22(21):7603–13. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, et al. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem. 2008 Aug 8;283(32):22295–303. doi: 10.1074/jbc.M801296200. [DOI] [PubMed] [Google Scholar]
- 103.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, et al. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4586–91. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007 Jul 27;130(2):247–58. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 105.Puckelwartz MJ, Kessler EJ, Kim G, Dewitt MM, Zhang Y, Earley JU, et al. Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell Cardiol. 2009 Nov 24; doi: 10.1016/j.yjmcc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005 Oct 7;123(1):25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009 Nov 12;462(7270):182–8. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, Ishikawa Y. Regulation of adenylyl cyclase by protein kinase A. J Biol Chem. 1995 Jun 26;270(21):12481–4. doi: 10.1074/jbc.270.21.12481. [DOI] [PubMed] [Google Scholar]
- 109.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001 Jun 1;20(11):2757–67. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]