Abstract
SLC9A9 (solute carrier family 9, member 9, also known as Na+/H+ exchanger member (NHE9)) is a membrane protein that regulates the luminal pH of the recycling endosome, an essential organelle for synaptic transmission and plasticity. SLC9A9 has been implicated in human attention deficit hyperactivity disorder (ADHD) and in rat studies of hyperactivity. We examined the SLC9A9 gene sequence and expression profile in prefrontal cortex, dorsal striatum and hippocampus in two genetic rat models of ADHD. We report two mutations in a rat model of inattentive ADHD, the WKY/NCrl rat, which affect the interaction of SLC9A9 with calcineurin homologous protein (CHP). We observed an age-dependent abnormal expression of SLC9A9 in brains of this inattentive model and in the Spontaneous Hypertensive Rat (SHR) model of ADHD. Our data suggest a novel mechanism whereby SLC9A9 sequence variants and abnormalities in gene expression could contribute to the ADHD-like symptoms of rat models and possibly the pathophysiology of ADHD in humans.
Keywords: SLC9A9, ADHD, CHP, RACK1
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a common, highly-heritable disorder (Faraone and Mick 2010; Gizer and others 2009). Genetic, brain imaging, and animal model studies have implicated numerous candidate genes in its etiology (Faraone and Mick 2010; Gizer and others 2009). One candidate gene is SLC9A9, a member of the Na+/H+ exchanger (NHE) family. SLC9A9 was first implicated in ADHD by a report of an extended family in which ADHD co-segregated with a pericentric inversion of Chromosome 3 that disrupted both DOCK3 and SLC9A9 (de Silva and others 2003). Subsequently, single-nucleotide polymorphisms (SNPs) in SLC9A9 were identified among the most significant findings in an analysis of 51 candidate genes from the International Multisite ADHD Genetics (IMAGE) project (Brookes and others 2006) and in the IMAGE genome-wide association study (GWAS) of ADHD symptoms, SLC9A9 achieved one of the lowest p values (~10−5; (Lasky-Su and others 2008)). SLC9A9 was also associated with ADHD in a subsequent association study (Markunas and others) but the association did not achieved genome-wide significance in a meta-analysis of GWAS (Neale and others 2010).
NHEs are large membrane-bound proteins that allow sodium or hydrogen ions to passively diffuse down their concentration gradients across the cell or organelle membrane. There are currently 10 known NHEs. SLC9A9 is primarily localized on the membranes of late recycling endosomes. Together with SLC9A6 (which is primarily localized on early/recycling endosomes), SLC9A9 regulates the pH of endosomal compartments (Nakamura and others 2005; Roxrud and others 2009). Endosomes play important roles in recycling neurotransmitter receptors and transporters, such as glutamate receptors and the dopamine transporter (DAT) (Loder and Melikian 2003; Park and others 2004). This recycling is a critical component of long-term potentiation (LTP) (Park and others 2004; Park and others 2006). Like other NHEs, SLC9A9 is predicted to have 12 transmembrane domains and a long intracellular C-terminal. The C-termini of the NHEs contain phosphorylation sites and binding domains for regulatory and signaling molecules (Slepkov and others 2007). Many NHE binding molecules, such as calcineurin homologous protein (CHP) (Lin and Barber 1996), receptor for activated C-kinase 1 (RACK1) (Ohgaki and others 2008), calmodulin (CaM)(Bertrand and others 1994) and phosphatidylinositol 4,5-bisphosphate (PIP2)(Aharonovitz and others 2000), participate in intracellular Ca2+ signaling cascades and protein phosphorylation/dephosphorylation, key cellular mechanisms believed to underlie synaptic transmission and plasticity.
The spontaneously hypertensive (SHR) rat obtained from Charles River, Germany (SHR/NCrl) is a well-validated animal model of the ADHD-Combined subtype (ADHD-C), with the Wistar-Kyoto strain obtained from Harlan, UK (WKY/NHsd) serving as its most appropriate control (Sagvolden 2000; Sagvolden and others 2009). A substrain, named the WKHA rat, created from a cross between SHR and WKY, displays high spontaneous activity but low systolic blood pressure. A single genome-wide significant locus (Act QTL) on Chromosome 8 that contains SLC9A9 showed significant linkage to hyperactivity (Moisan and others 2003). The Act QTL locus is homologous to the region of human Chromosome 3 where the pericentric inversion disrupting SLC9A9 segregated with ADHD (de Silva and others 2003). Moreover, the homologous region in mouse Chromosome 9 that contains SLC9A9 contains an activity-related QTL (Grisel and others 1997; Mathis and others 1995; Miner and Marley 1995).
We reported the behavioral and genetic characterization of a new rat model for the inattentive subtype of ADHD (WKY/NCrl, obtained from Charles River, Germany) (Sagvolden and others 2008). This rat, which shows impaired sustained attention, but normal activity level and impulsiveness, is genetically divergent from the common reference WKY strain (WKY/NHsd) used as a control for the SHR. The region of genetic divergence includes SLC9A9 (Sagvolden and others 2008).
The present study tested three hypotheses about SLC9A9: 1) that SLC9A9 gene expression would be dysregulated in WKY/NCrl and SHR/NCrl rats; 2) that SLC9A9 mutations would be found in these rats and 3) that these mutations would be associated with abnormal interactions of SLC9A9 protein with other proteins.
METHODS
Animals
We obtained rats from two sources: WKY/NCrl and SHR/NCrl rats from Charles River (Sulzfeld, Germany), and WKY/NHsd control rats from Harlan Europe, (Blacktorn, Bicester, UK). Animal procedures were approved by the Norwegian Animal Research Authority, and animals were housed and euthanized at the University of Oslo. Experiments were conducted in accordance with the laws and regulations controlling experimental procedures in live animals in Norway and the European Union. Brain parts were dissected and preserved in RNAlater or AllProtect (Qiagen, CA), and shipped at room temperature to SUNY Upstate Medical University. Samples were stored at −80°C upon receipt until use.
Expression analysis of SLC9A9 and SYP
15 adolescent (~28 days old, WKY/NCrl=6, SHR/NCrl=6 and WKY/NHsd=3) and 16 adult (~65 days old, WKY/NCrl=5, SHR/NCrl=3, and WKY/NHsd=8) rats were used for expression analysis of dissected medial prefrontal cortex, dorsal striatum, and hippocampus with real-time quantitative reverse-transcription PCR (qPCR). Two of these brain regions (prefrontal cortex, dorsal striatum) were chosen because they have been implicated in ADHD by a meta-analysis of structural imaging studies in humans (Valera and others 2007). Abnormal hippocampal structure has also been observed in ADHD patients (Plessen and others 2006) and in SHR rats (Amenta and others 1996), and there is an increasing recognition of the relevance of hippocampus in ADHD due to its essential role in learning and memory, attention (Volkow and others 2007), and decision-making (Ernst and others 2003).
Total RNA from all three brain areas were extracted using the RNeasy mini kit (Qiagen, Valencia, CA). Equal amounts of RNA from each brain regions from all three strains of rats were reverse-transcribed using Quantitect Reverse Transcription (RT) kit (Qiagen). Diluted RT reactions were used for qPCR in a Roche LightCycler® 480 Real-Time PCR system using Roche LightCycler 480 SYBR Green I Master reagents. Three stably expressed genes (CycA, Hprt1, and Ywhaz) were used as reference genes for normalization of gene expression (Bonefeld and others 2008). Relative expression levels (in log2 scale) were calculated based on differences in the number of cycles required to reach the threshold for target amplicon detection (Ct) versus the geometric mean of the three reference genes (the ΔCt method) and normalized within each brain region to the ΔCt value of the adolescent control WKY/NHsd group (ΔΔCt method).
We also examined the expression of a synaptic marker, the synaptophysin (SYP) gene. SYP gene was chosen because its expression is correlated with the basic differentiation process of the neurons such as proliferation, fiber outgrowth and the formation of synapses (Bergmann and others 1991; Bergmann and others 1993; Grabs and others 1994). Because developmental differences in neuronal numbers and synaptic connections between different ages and strains of animals could be a confounding factor for gross expression analysis, we calculated the SLC9A9/SYP ratio (a log2 difference) to estimate the relative amount of gene expression per overall synaptic density in each brain area. First, the level of SYP expression was normalized against the geometric mean of the 3 reference genes (ΔCt) method. Then, the SLC9A9/SYP ratio was calculated as the difference between the specific ΔCt of each individual gene v.s. the ΔCt of SYP (ΔΔCt). We analyzed SLC9A9 and SYP expression individually, as well as the SLC9A9/SYP ratios across all three brain regions.
Within each brain region, we assessed the effects of strain, age and the strain by age interaction using linear regression analysis in STATA 11.2. We followed up significant main effects or interactions by computing pairwise comparisons using Wald tests in STATA 11.2.
Genetic sequence analysis of gene SLC9A9
We used the whole genome genotyping data acquired from WKY/NCrl and SHR/NCrl rats (from Charles River, Sulzfeld, Germany), and WKY/NHsd control rats (from Harlan Europe, Blacktorn, Bicester, UK) to identify genetic divergence in the SLC9A9 gene region. These data were acquired using Affymetrix Targeted Genotyping Rat Panel (1.0 5K) as described previously (Sagvolden and others 2008). Four adult male rats in each strain that had been behaviorally phenotyped were used for genotyping. SNPs were used for cross-strain comparisons if they met two criteria: (1) complete concordance within each set of strain replicates, and (2) some degree of variation between the three strains.
Because the WKY/NCrl, SHR/NCrl, and WKY/NHsd rats showed genetic divergence in markers spanning the SLC9A9 locus (Sagvolden and others 2008), we sequenced the entire SLC9A9 gene in these three strains. PCR primers were designed using ExonPrimer (http://ihg2.helmholtz-muenchen.de/ihg/ExonPrimer.html) to amplify each of the exons and adjacent intronic regions with at least 100bp overlap. Template DNAs were selected from those used in the SNP genotyping scan. PCR products were cleaned and sent for sequencing (GeneWiz, Inc). Chromatograms were analyzed using Sequencher software V4.5 (GeneCodes) and compared with the rat reference sequences.
Effects of mutations on protein chemistry and protein-protein interactions
In silico analysis
To examine possible protein alterations produced by the sequence changes, SLC9A9 and NHE family protein sequences from selected species were downloaded from NCBI. Homology alignments were performed using ClustalX 2.0. Transmembrane domains of human SLC9A9 protein were predicted by TMHMM Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/). Glycosylation and phosphorylation were predicted by the YinOYang server (http://www.cbs.dtu.dk/services/YinOYang/). Deleage and Roux amino acid scales (Deleage and Roux 1987) was used to plot the conformational propensity for alpha-helix formation using a linear model with a 21 amino acid window.
Co-Immunoprecipitation
To express c-Myc-tagged SLC9A9 C-terminal (pMyc-SLC9A9C), we inserted a c-Myc tag sequence between Xho I and Hind III sites of the pdsRed1-N1 plasmid (Clontech). Rat SLC9A9 coding sequence for the complete C-terminal (residues 494–645 with stop codon) was amplified by PCR and directionally cloned between the EcoR I and Kpn I sites of modified c-Myc plasmid, thus allowing an expression of N-terminally c-Myc-tagged fusion protein. Mutations were introduced using the QuickChange® Site-Directed Mutagenesis Kit (Stratagene, TX) and verified by sequencing.
To express C-terminally Flag-tagged CHP and RACK1 proteins (pCHP-Flag and pRACK1-Flag), we replaced the original C-terminal dsRed tag coding sequences in pdsRed1-N1 with a Flag sequence between the AgeI and NotI sites. Rat CHP and RACK1 coding sequence were amplified by PCR with primers containing restriction sites. CHP was directionally cloned into the modified Flag plasmid between the EcoR1 and BamHI sites. RACK1 was cloned into the same plasmid between the EcoRI and SacII sites. All constructs were sequence-verified.
HEK 293 cells were maintained in DMEM medium with 10% Fetal Bovine Serum, 100U penicillin, 100μg streptomycin, and 1% GlutaMAX™-I supplement (Invitrogen). Cells were co-transfected with a combination of either pMyc-SLC9A9C and pCHP-Flag, or pMyc-SLC9A9C and pRACK1-Flag, using Lipofectamine 2000 reagent. Cells were harvested from 10cm culture dishes and washed twice with PBS buffer. After centrifugation, cell pellets were lysed on ice for 30 min with cold PBS buffer containing 1% Nonidet P-40, 1mM phenylmethylsulfonyl fluoride, and a protease inhibitor mixture. Following lysis, cells were centrifuged for 10min at 20,000g at 4°C. The resulting supernatants were mixed with Protein G agarose resins coupled with either Anti-c-Myc antibodies or normal mouse IgG (Pierce, Thermo Scientific). After incubation at 4°C overnight with gentle rotation, beads were washed four times with cold PBS buffer containing 0.1% Nonidet P-40, and immunoprecipitates were separated by SDS-PAGE. SLC9A9 C-terminal and Flag-tagged binding partners were detected by immunoblotting with horseradish peroxidase-conjugated anti-c-Myc IgG (ab1261, Abcam, MA) and anti-Flag IgG (Sigma, MO) respectively. Immunoreactive bands were quantified in Scion Image for both area and mean optical density (OD), corrected for background OD. The relative binding with the SLC9A9 C-terminal for CHP and RACK1 was determined by calculating a ratio of the product of area and corrected mean OD for each band detected with anti-Flag antibody compared to that for the anti-c-Myc blots for each co-IP sample. Ratios from three independent experiments were used in a two-tailed t-test.
RESULTS
Expression profiles of SYP and SLC9A9 in ADHD rat models
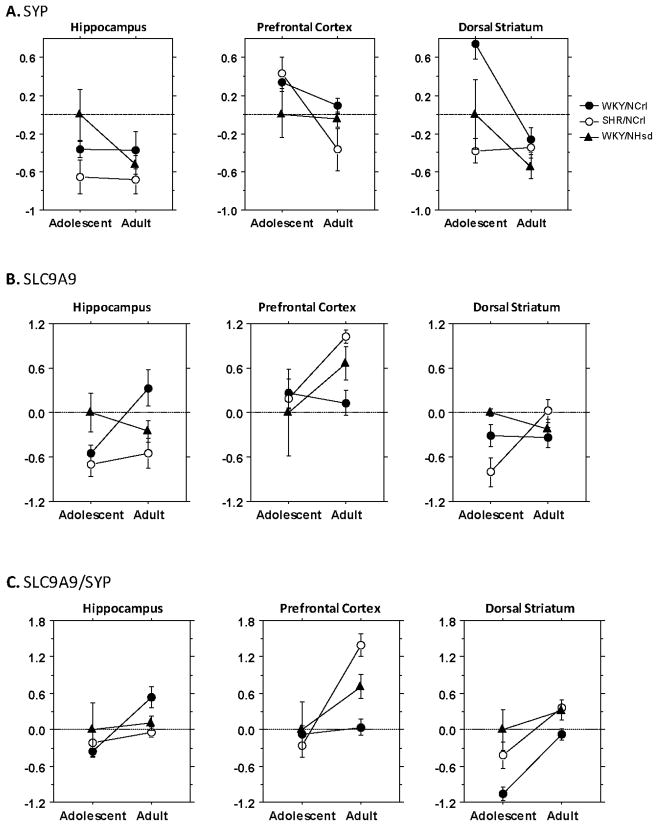
We first examined the expression of the SYP gene in each brain region and the correlation between SLC9A9 and SYP expression to determine if gross anatomical differences in neurons and synapses across different strains might confound our analyses. The expression of these two genes was significantly correlated (r=.40, p=.0001). We found a significant effect of strain on SYP expression in both dorsal striatum (F(2,26) = 9.56, p= 0.0008) and prefrontal cortex(F(2,26) =3.56; p=0.04). There was also a significant strain by age interaction in these two regions (dorsal striatum F(2, 26) = 4.82, p=0.02; prefrontal cortex F(2,26)=3.81, p=0.04). The effects of strain or strain by age interactions for the hippocampus were not significant (strain F(2, 26) = 2.10, p=0.1433; strain by age F(2, 26)=1.46, p=0.2515). Figure 1A shows that there was a similar magnitude of age-dependent decrease of SYP expression for the control WKY/NHsd rats in hippocampus (Fold Change (FC)= −.526, p=.047) and in dorsal striatum (FC= −.547, p=.1). SHR/NCrl rats lacked this pattern of decrease in these two regions, however they showed an abnormally large age-dependent decrease in SYP in the prefrontal cortex (t7=−2.9, p=.02), which the other strains did not (p’s>.05). The WKY/NCrl rats also lackedthe normal age-dependent decrease of SYP expression in hippocampus, but showed a larger age-dependent decrease in SYP (t7=−5.0, p=.001) in dorsal striatum.
Figure 1. Developmental expression profile of SYP and SLC9A9 in ADHD rat models.
A. Relative levels of SYP expression (log2 difference) in each brain regions were calculated using ΔΔCt methods with the adolescent WKY/NHsd rats as baseline level in each brain region. B. Relative levels of SLC9A9 expression (log2 difference) in each brain region were calculated using ΔΔCt methods with the adolescent WKY/NHsd rats as baseline level in each brain region. C. The log2 difference of SLC9A9 vs synaptaphysin (SLC9A9/SYP ratio) in each brain region was calculated using ΔΔCt methods.
Because SYP potentially could have confounded our analyses of SLC9A9 expression for prefrontal cortex and dorsal striatum, these analyses were statistically adjusted using SYP as a covariate. In dorsal striatum, we found a significant effect of strain (F(2,25)=3.51, p=0.045), and a significant effect of SYP expression (F(1, 25)=4.65, p=0.04) on SLC9A9 expression. The strain by age interaction was not significant (F(2,25)=2.6, p=0.09). For prefrontal cortex, the effect of SYP expression on the SLC9A9 expression was also significant (F(1, 25) =6.27, p=0.02), as well as the effect of strain by age interactions (F(2, 25) =4.53, p=0.02). In the hippocampus, age (F(1,25) =19.42, p=0.0002), strain by age interaction(F( 2, 25) = 3.85, p=0.035) and SYP expression(F(1,25) =14.50, p=0.0008) all showed a significant influence on the SLC9A9 expression.
SLC9A9 expression is plotted in two ways. Figure 1B plots the expression pattern of SLC9A9, unadjusted for SYP. Figure 1C plots the SYP adjusted expression of SLC9A9 using the SLC9A9/SYP ratios. The SLC9A9/SYP ratios show that the strain effect for dorsal striatum was primarily accounted for by decreased SLC9A9/SYP values for the WKY/NCrl compared to other strains. The interaction effects observed for hippocampus and prefrontal cortex were due to different effects. For both regions, there were no strain differences in adolescence. In adulthood, the hippocampal results showed an increase in SLC9A9/SYP for WKY/NCrl rats compared to WKY/NHsd controls (F(1, 14) =5.3, p=0.04) and the SHR/NCrl rats (F(1,14)=5.6, p=0.03) but the two latter strains did not differ from one another (F(1,14)=0.38, p=0.55). In contrast, in adulthood, the prefrontal cortex results showed that SLC9A9/SYP was increased for SHR/NCrl (F(1,14)=4.76, p=0.047) and reduced in WKY/NCrl rats (F(1,14)=7.49, p=0.02)compared with WKY/NHsd control rats. The difference between the two ADHD rat models was also highly significant (F(1,14)=17.46, p=0.0009).
Sequence variants of SLC9A9 in WKY/NCrl Rats
The gene for SLC9A9 lies within a region of Chromosome 8 that we previously identified in a genome-wide SNP scan as genetically divergent between the WKY/NCrl and WKY/NHsd strains (Supplementary Table 1; (Sagvolden and others 2008)). We resequenced all 18 exons and their flanking intronic regions, as well as 1Kb upstream of the start codon of SLC9A9. Consistent with our SNP panel results, The SHR/NCrl rats showed no sequence variations in the SLC9A9 coding region. We found five novel SNPs in the inattentive WKY/NCrl rats, including two nonsynonymous mutations in highly-conserved regions (V512G and K534R; Supplementary Figure 1A). These mutations were always found together. Retrospective examination of behavioral data for the one K534R/V512G heterozygote did not reveal any difference compared with K534R/V512G homozygotes, suggesting a likely dominant effect of the mutations (data available upon request). Although the SNP for the second mutation (K534R) lies at the exon and intron boundary, it did not change gene splicing. Only normally spliced transcripts were amplified from cDNAs of the WKY/NCrl rat brains and verified by sequencing using primers flanking exon 16 (data not shown).
Effects of SLC9A9 mutations on protein binding
The two non-synonymous mutations are located in the intracellular C-terminal juxtamembrane region of SLC9A9, where regulatory proteins normally bind. The two residues mutated in the inattentive WKY/NCrl rats are highly conserved across mammalian species (Supplementary Figure 1B). Specific binding partners of SLC9A9 in the region harboring the mutations have not been fully identified. This region shares some degree of homology with other members of the NHE family, whose binding partners are well-characterized. One binding partner that is essential for Na+/H+ exchanger function is CHP. The CHP-binding domain in SLC9A1 (510–540aa) forms an alpha-helix, inserting into a hydrophobic cleft formed by CHP (Ammar and others 2006; Pang and others 2001). To predict whether CHP also binds to SLC9A9, we first examined whether the SLC9A9 juxtamembrane region is able to form an alpha-helix secondary structure using an algorithm developed by Deleage and Roux (Deleage and Roux 1987). We found a high propensity for alpha-helix formation in the juxtamembrane region (Supplementary Figure 1C). The mutations slightly reduced the alpha helix conformational score. Alignment of this putative alpha-helix forming region with the CHP-binding domains in SLC9A1-5s reinforced the existence of conserved hydrophobic residues that may mediate an interaction of CHP with SLC9A9 (Supplementary Figure 1D).
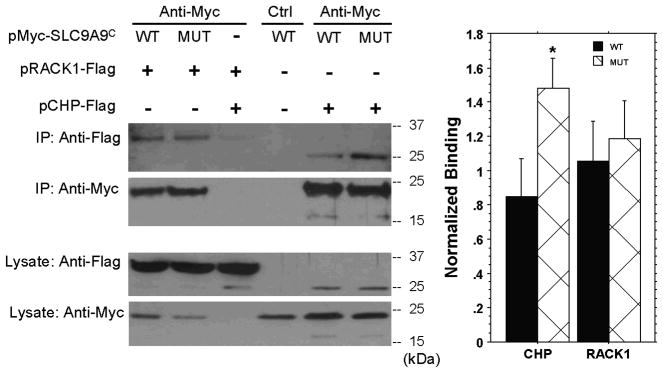
To test this possible interaction and examine the effects of the mutations on the interaction, we transiently co-expressed Myc-tagged SLC9A9 C-terminals (with or without the double mutations) and Flag-tagged CHP in HEK293 cells, and performed co-immunoprecipitation using an anti-Myc antibody. Since the two SLC9A9 mutations were always found together in WKY/NCrl rats, we tested the joint effect of both mutations on interactions between the C-terminal of SLC9A9 and CHP. We confirmed the interaction of the C-terminal of SLC9A9 with CHP. Furthermore, the mutations significantly increased the amount of CHP precipitated with SLC9A9 by almost two-fold (p=0.027 by two tailed t-test, Figure 2, last two lanes).
Figure 2.
Co-immunoprecipitation of SLC9A9 C-terminal with RACK1 or CHP in HEK293 cells. C-Myc-tagged SLC9A9 C-terminal (WT, wild type; MUT, with both mutations) and Flag-tagged binding partners (RACK1 and CHP) were co-expressed in HEK293 cells. The cell lysates were precipitated with either an anti-Myc antibody or equivalent mouse IgG as negative control (Ctrl). After extensive washes, the immunoprecipitate (IP) was eluted with SDS loading buffer and separated on SDS-PAGE gel. The same membrane was blotted with HRP conjugated anti-Flag and anti-Myc antibodies (the top two blots). Western blots of equal amount cell lysates were also done for both anti- Myc and anti-Flag antibodies to ensure the equal expression of both proteins (bottom two blots). The relative bindings of RACK1 or CHP to SLC9A9 are normalized and quantified in Scion Image (see Methods for details, * p=0.027, two tailed t-test).
Co-immunoprecipitation assays evaluated the effects of the joint mutations on the interaction with RACK1, the only previously described binding partner for SLC9A9. The K534R mutation is located within the binding region for RACK1, although multiple sites for RACK1 may exist in this region, which require further characterization (Ohgaki and others 2008). We confirmed the interaction of SLC9A9 with RACK1. However, the mutations had no effect on this interaction (Figure 2, first two lanes).
Although the main bands for the Myc-tagged SLC9A9 C-terminal appeared to be ~25kDa in SDS-PAGE in both cell lysates and immunoprecipitates, the predicted molecular weight of this peptide is 17.8kDa. We verified the specificity of the anti-Myc bands using another commercially available Myc antibody as well as an anti-SLC9A9 antibody (Abcam, data not shown). Predictions using the YinOYang web server indicated that the C-terminal of the SLC9A9 is heavily glycosylated and also contains several phosphorylation sites (data not shown). We suspect that the higher than expected molecular weight was due to post-translational modifications.
DISCUSSION
We found substantial strain differences in SYP expression for dorsal striatum and prefrontal cortex, but not hippocampus. We also found evidence for strain differences in SLC9A9 expression in each brain region and that these differences among strains were age dependent. After adjusting for potentially confounding differences between strains in neuronal numbers and synaptic connections as measured by the synaptic marker SYP, we found that the expression of SLC9A9 in ADHD rats, particularly the WKY/NCrl rats, significantly deviated from the control rats in the dorsal striatum at both ages, and in the hippocampus and the prefrontal cortex of the adult rats. For SHR/NCrl rats, the expression of SLC9A9 was elevated only in the prefrontal cortex at the adult age. We also found two co-transmitted mutations of SLC9A9 in the WKY/NCrl rats. The effects of mutations on the interaction of SLC9A9 with other proteins were predicted and confirmed with co-immunoprecipitation. It is not clear whether the SLC9A9 expression abnormalities in the WKY/NCrl rats were a compensatory response of the effect of mutations on the protein functions. Nevertheless, our results suggest that the gene expression and sequence abnormalities in SLC9A9 may play a role in the ADHD phenotype of the ADHD rat models.
Because SYP expression correlates with neuronal differentiation and synaptic formation (Bergmann and others 1991; Bergmann and others 1993; Grabs and others 1994), it has been used as a marker for quantitative measurements of synapses (Calhoun and others 1996; Mouton and others 1997). Overall the number of synapses that we measured by SYP may represent a net effect of dynamic postnatal synaptogenesis and pruning. We found a similar age-dependent decrease of SYP expression for the control WKY/NHsd rats in hippocampus and in dorsal striatum, indicating that active pruning is predominant in these areas. In contrast, for controls SYP expression was not changed between the two ages in prefrontal cortex, suggesting they may still be forming new synapses to counter balance the effect of pruning or that pruning was not robust during the two age points that we examined, which is consistent with the fact that prefrontal cortex matures later than other brain regions.
Examining the SYP expression profiles in two ADHD animal models suggests different developmental abnormalities in these two strains. SHR/NCrl rats lack the normal age-dependent decrease in SYP in both hippocampus and dorsal striatum, suggesting a defective pruning. In prefrontal cortex, SHR/NCrl rats showed a significantly more robust decrease in SYP with age. One possibility is that early postnatal pruning was delayed and followed by excessive or inappropriate pruning from adolescent to adulthood, resulting in a higher than normal number of synapses in adolescence and a lower than normal number of synapses in adulthood. Our SYP observations in SHR/NCrl rats are consistent with the anatomical alterations reported by others (Amenta and others 1996; Nelson and others 1993; Nelson and Boulant 1981; Nelson and Boulant 1983). WKY/NCrl rats were statistically no different from the controls in both the prefrontal cortex and the hippocampus, although there was some indication of a lack of hippocampal pruning. In dorsal striatum, however, WKY/NCrl rats significantly deviated from the two other strains in SYP expression, suggesting a delay in early postnatal pruning followed by excessive pruning. Overall, the SYP results suggest that anatomical defects during brain development and maturation may be associated with the ADHD phenotypes in animal models. These defects are more wide-spread in SHR brains, consistent with the presence of all three ADHD symptom clusters: hyperactivity, impulsivity and inattentiveness. The limited effects seen for the WKY/NCrl rats may explain why that strain only displays inattentive symptoms.
The significant correlation of SLC9A9 and SYP expression and the relative stability of the SLC9A9/SYP ratios over age in control rats suggest that SLC9A9 expression is proportional to the number of synapses. This is consistent with Morrow et al.’s (2008) study of hippocampal neurons, which suggested that SLC9A9 was one of the “activity-regulated” genes. Consistent with this idea, deleterious sequence variants of SLC9A9 have been found in patients with autism and epilepsy (Morrow and others 2008). Mutations of SLC9A6, a close family member that also resides in endosome membranes, were found in patients with Angelman Syndrome, involving autistic symptoms and epilepsy (Gilfillan and others 2008). SLC9A6 knockout mice showed increased seizure susceptibility. Mutations or targeted disruption of SLC9A1, an ubiquitous plasma membrane protein, produced seizures in mice (Bell and others 1999) (Cox and others 1997). Epilepsy often co-exists with ADHD and autism (Canitano 2007; Deonna and Roulet 2006; Gurrieri and others 1999; Hamoda and others 2009; Hesdorffer and others 2004). SHR rats also have increased susceptibility to seizures (Goldberg and others 1975), but we do not know whether WKY/NCrl rats are susceptible to seizures.
SLC9A9 may serve as a local signaling scaffold protein. Mammalian Na+/H+ exchangers have a long intracellular C-terminal, which recruits various signaling molecules, and may promote the assembly of local signaling complexes and coordinate divergent signaling pathways (Baumgartner and others 2004). In our study we showed that the SLC9A9 C-terminal interacts with the signaling molecules CHP and RACK1. These molecules and other binding partners of Na+/H+ exchangers (such as CAM and PIP2) are not only regulators of Na+/H+ exchanger activity, but also important signaling molecules in calcium signaling and protein phosphorylation. These signaling pathways are essential for synaptic transmission and plasticity. Mutations that affect the protein-protein interactions or expression of SLC9A9 may affect synaptic signaling and alter neuronal activities. In turn, the activity-dependent synaptogenesis and pruning can lead to changes in SYP.
A second mechanism underlying the role of SLC9A9 in neurotransmission is through the Na+/H+ exchanger activity of the SLC9A9. CHP interaction with Na+/H+ exchanger C-terminals plays an important role in regulating the activity of the exchanger (Pang and others 2001). The mutations that enhanced the interaction of CHP with SLC9A9 may influence the antiporter activity of the SLC9A9. In addition, expression abnormalities in SLC9A9 can also produce changes in the antiporter activity. By regulating endosomal pH, SLC9A9 modulates the functions of the endosome that are important in synaptic transmission. For example, recycling endosomes function as reservoirs for AMPA receptors (AMPARs), enabling their rapid insertion in the postsynaptic membrane and promoting dendritic spine formation during long term potentiation (LTP) (Park and others 2004),(Park and others 2006). Blocking the endosomal trafficking pathway prevents LTP. The dopamine transporter (DAT), a target for ADHD medications, is constitutively recycled through the endosome (Melikian and Buckley 1999). Impaired DAT internalization and recycling as a result of altered endosomal function could thus result in an excess of membrane-associated DAT. In turn, this could lead to hypodopaminergic synapses, one of the hypothesized neurochemical changes in ADHD (Brown and others 2010; Spencer and others 2005). In fact, we recently obtained evidence for increased DAT expression and activity in the nigrostriatal system of the WKY/NCrl rat as well as SHR/NCrl rat (Roessner and others), supporting the possibility that DAT regulation is abnormal in these models.
The implications of SLC9A9 variants for neuropsychiatric conditions extend beyond ADHD. Variants of SLC9A9 have been reported in patients with autism and epilepsy (Morrow and others 2008). These findings are notable for ADHD, given the known comorbidity and shared genetic and neuropathophysiological features between ADHD and autism (Corbett and Constantine 2006; Grady and others 2005; Kent and others 2008; Mulligan and others 2008; Nijmeijer and others; Reiersen and others 2007; Sinzig and Lehmkuhl 2007). Interestingly, epilepsy is also more frequent among ADHD patients compared with the general population (Hamoda and others 2009).
There are several limitations of the current study. First, the co-immunoprecipitation studies could not conclusively prove a direct binding between SLC9A9 and CHP. However, based on previous data regarding the interactions between CHP and other Na+/H+ exchanger members, and the sequence homology and structure prediction (supplementary figure 1), it is highly likely that SLC9A9 binds directly with CHP. Secondly, the sample sizes used in our expression studies are relatively small, particularly for the young WKY/NHsd and adult SHR/NCrl groups (represented by only 3 animals in each group). We only examined two different ages, and expression of SLC9A9 and SYP clearly various with age. It is worth noting that the expression in adult SHR rats may be influenced by hypertension developed later in life. Thus, hypertension could account for the SHR findings for PFC in adulthood. An increased sample size and more developmental time points, particularly the prenatal and early postnatal time points may reveal more significant findings. Finally, we could not draw conclusion that the abnormal expression or mutations of SLC9A9 is the causal variant for the ADHD phenotype in the WKY/NCrl rats, because that there may be other genetic causes, and that the expression abnormalities are merely a compensatory response to other genetic defects. Future studies utilizing transgenic or knockout animals would be useful to clarify these issues.
In summary, we found deleterious mutations of SLC9A9 in the inattentive WKY/NCrl rat and significantly dysregulated SLC9A9 expression in their brains and in the brains of SHR rats (who do not carry the mutations). Considering the complex network of signaling molecules that SLC9A9 may interact with and the importance of endosomal recycling in synaptic transmission, we hypothesize that this gene is critical for normal synaptic development and transmission.
In the context of prior human studies (cf. Introduction), our data raise the intriguing possibility that, for some cases of ADHD, upregulation of SLC9A9 could be one of pathophysiology that is potentially treatable with existing medications that inhibit sodium-hydrogen exchangers.
Supplementary Material
A, SLC9A9 sequence difference between rat strains. Novel SNPs were identified in the gene region. B, SLC9A9 sequences in selected orthologues were aligned in ClustalX 2.0 and partial alignments, including the last two transmembrane domains (indicated by symbols “^^^” on top) and the complete intracellular C-terminals, are shown. The alignment scores are plotted underneath the sequences to indicate conservation level. The residues that are mutated in WKY/NCrl rats are indicated by arrows. C, Conformational parameters for alpha-helix based on Deleage & Roux amino acid scales are plotted for wild type and mutant SLC9A9 (Deleage and Roux 1987). Arrows indicate the positions of two mutations. The positions for the last two transmembrane domains (TMs) are also indicated. The juxtamembrane region of rat SLC9A9, where the mutations are located, show a high propensity to form an alpha-helix. The two rat mutations slightly decreased this propensity, however mutant protein appears still highly capable of forming an alpha-helix. D, Sequence alignments of the alpha-helix region of the wild type SLC9A9 with the CHP-binding domains in SLC9A1-5s (Ammar and others 2006). Hydrophobic residues are highlighted in yellow. Mutation sites are bolded in red letters.
Acknowledgments
We thank Karen L Gentile and Lu Liu for technical assistance, Dr. Stephen Glatt and Cheryl Roe for statistical assistance, and Drs. Stewart N Loh and Mohamed Sherif for helpful comments and discussion. This study was supported by National Institutes of Health grant MH668877 to SV Faraone.
Footnotes
Conflict of interest
In previous years, Dr. Sagvolden received consulting fees or research support or has been on Advisory Boards or has been a speaker for: Shire, Janssen, and Eli Lilly. In the past year, Faraone has received consulting fees and has been on Advisory Boards for Shire Development and has received research support from Pfizer, Shire and the National Institutes of Health. In previous years, Dr. Faraone has received consulting fees or has been on Advisory Boards or has participated in continuing medical education programs sponsored by: Shire, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. In previous years he has received research support from Eli Lilly, Shire, Pfizer and the National Institutes of Health. Dr. Faraone receives royalties from a book published by Guilford Press: Straight Talk about Your Child’s Mental Health. Dr. Middleton, Dr Zhang-James, and Tania DasBanerjee reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Yanli Zhang-James, Email: zhangy@upstate.edu.
Tania DasBanerjee, Email: dast@upstate.edu.
Terje Sagvolden, Email: terje.sagvolden@medisin.uio.no.
Frank A. Middleton, Email: middletf@upstate.edu.
Stephen V Faraone, Email: sfaraone@childpsychresearch.org.
References
- Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na(+)/H(+) exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150(1):213–24. doi: 10.1083/jcb.150.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F, Strocchi P, Sabbatini M. Vascular and neuronal hypertensive brain damage: protective effect of treatment with nicardipine. J Hypertens Suppl. 1996;14(3):S29–35. doi: 10.1097/00004872-199610003-00006. [DOI] [PubMed] [Google Scholar]
- Ammar YB, Takeda S, Hisamitsu T, Mori H, Wakabayashi S. Crystal structure of CHP2 complexed with NHE1-cytosolic region and an implication for pH regulation. EMBO J. 2006;25(11):2315–25. doi: 10.1038/sj.emboj.7601145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner M, Patel H, Barber DL. Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol. 2004;287(4):C844–50. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276(4 Pt 1):C788–95. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Lahr G, Mayerhofer A, Gratzl M. Expression of synaptophysin during the prenatal development of the rat spinal cord: correlation with basic differentiation processes of neurons. Neuroscience. 1991;42(2):569–82. doi: 10.1016/0306-4522(91)90399-9. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Schuster T, Grabs D, Marqueze-Pouey B, Betz H, Traurig H, Mayerhofer A, Gratzl M. Synaptophysin and synaptoporin expression in the developing rat olfactory system. Brain Res Dev Brain Res. 1993;74(2):235–44. doi: 10.1016/0165-3806(93)90009-y. [DOI] [PubMed] [Google Scholar]
- Bertrand B, Wakabayashi S, Ikeda T, Pouyssegur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem. 1994;269(18):13703–9. [PubMed] [Google Scholar]
- Bonefeld BE, Elfving B, Wegener G. Reference genes for normalization: a study of rat brain tissue. Synapse. 2008;62(4):302–9. doi: 10.1002/syn.20496. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, Aneey R, Franke B, Gill M, Ebstein R, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006 doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Brown AB, Biederman J, Valera EM, Doyle AE, Bush G, Spencer T, Monuteaux MC, Mick E, Whitfield-Gabrieli S, Makris N, et al. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):365–75. doi: 10.1002/ajmg.b.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25(12):821–8. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Canitano R. Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry. 2007;16(1):61–6. doi: 10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ. Autism and attention deficit hyperactivity disorder: assessing attention and response control with the integrated visual and auditory continuous performance test. Child Neuropsychol. 2006;12(4–5):335–48. doi: 10.1080/09297040500350938. [DOI] [PubMed] [Google Scholar]
- Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, Aronson PS, Noebels JL, Frankel WN. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91(1):139–48. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- de Silva MG, Elliott K, Dahl HH, Fitzpatrick E, Wilcox S, Delatycki M, Williamson R, Efron D, Lynch M, Forrest S. Disruption of a novel member of a sodium/hydrogen exchanger family and DOCK3 is associated with an attention deficit hyperactivity disorder-like phenotype. J Med Genet. 2003;40(10):733–40. doi: 10.1136/jmg.40.10.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleage G, Roux B. An algorithm for protein secondary structure prediction based on class prediction. Protein Eng. 1987;1(4):289–94. doi: 10.1093/protein/1.4.289. [DOI] [PubMed] [Google Scholar]
- Deonna T, Roulet E. Autistic spectrum disorder: evaluating a possible contributing or causal role of epilepsy. Epilepsia. 2006;47(Suppl 2):79–82. doi: 10.1111/j.1528-1167.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160(6):1061–70. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33(1):159–80. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, Kroken M, Mattingsdal M, Egeland T, Stenmark H, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82(4):1003–10. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Milmore JE, Haubrich MK, Haubrich DR. Increased susceptibility to seizures and decreased catecholamine turnover in spontaneously hypertensive rats. Eur J Pharmacol. 1975;33(2):389–93. doi: 10.1016/0014-2999(75)90184-3. [DOI] [PubMed] [Google Scholar]
- Grabs D, Bergmann M, Schuster T, Fox PA, Brich M, Gratz M. Differential expression of synaptophysin and synaptoporin during pre- and postnatal development of the rat hippocampal network. Eur J Neurosci. 1994;6(11):1765–71. doi: 10.1111/j.1460-9568.1994.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Grady DL, Harxhi A, Smith M, Flodman P, Spence MA, Swanson JM, Moyzis RK. Sequence variants of the DRD4 gene in autism: further evidence that rare DRD4 7R haplotypes are ADHD specific. Am J Med Genet B Neuropsychiatr Genet. 2005;136(1):33–5. doi: 10.1002/ajmg.b.30182. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Belknap JK, O’Toole LA, Helms ML, Wenger CD, Crabbe JC. Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J Neurosci. 1997;17(2):745–54. doi: 10.1523/JNEUROSCI.17-02-00745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurrieri F, Battaglia A, Torrisi L, Tancredi R, Cavallaro C, Sangiorgi E, Neri G. Pervasive developmental disorder and epilepsy due to maternally derived duplication of 15q11-q13. Neurology. 1999;52(8):1694–7. doi: 10.1212/wnl.52.8.1694. [DOI] [PubMed] [Google Scholar]
- Hamoda HM, Guild DJ, Gumlak S, Travers BH, Gonzalez-Heydrich J. Association between attention-deficit/hyperactivity disorder and epilepsy in pediatric populations. Expert Rev Neurother. 2009;9(12):1747–54. doi: 10.1586/ern.09.128. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61(7):731–6. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- Kent L, Emerton J, Bhadravathi V, Weisblatt E, Pasco G, Willatt LR, McMahon R, Yates JR. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45(8):519–24. doi: 10.1136/jmg.2008.057729. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1345–54. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Natl Acad Sci U S A. 1996;93(22):12631–6. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278(24):22168–74. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markunas CA, Quinn KS, Collins AL, Garrett ME, Lachiewicz AM, Sommer JL, Morrissey-Kane E, Kollins SH, Anastopoulos AD, Ashley-Koch AE. Genetic variants in SLC9A9 are associated with measures of attention-deficit/hyperactivity disorder symptoms in families. Psychiatr Genet. 20(2):73–81. doi: 10.1097/YPG.0b013e3283351209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Neumann PE, Gershenfeld H, Paul SM, Crawley JN. Genetic analysis of anxiety-related behaviors and responses to benzodiazepine-related drugs in AXB and BXA recombinant inbred mouse strains. Behav Genet. 1995;25(6):557–68. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19(18):7699–710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LL, Marley RJ. Chromosomal mapping of the psychomotor stimulant effects of cocaine in BXD recombinant inbred mice. Psychopharmacology (Berl) 1995;122(3):209–14. doi: 10.1007/BF02246541. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Llamas B, Cook MN, Mormede P. Further dissection of a genomic locus associated with behavioral activity in the Wistar-Kyoto hyperactive rat, an animal model of hyperkinesis. Mol Psychiatry. 2003;8(3):348–52. doi: 10.1038/sj.mp.4001234. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–23. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Price DL, Walker LC. Empirical assessment of synapse numbers in primate neocortex. J Neurosci Methods. 1997;75(2):119–26. doi: 10.1016/s0165-0270(97)00058-7. [DOI] [PubMed] [Google Scholar]
- Mulligan A, Anney RJ, O’Regan M, Chen W, Butler L, Fitzgerald M, Buitelaar J, Steinhausen HC, Rothenberger A, Minderaa R, et al. Autism symptoms in Attention-Deficit/Hyperactivity Disorder: A Familial trait which Correlates with Conduct, Oppositional Defiant, Language and Motor Disorders. J Autism Dev Disord. 2008 doi: 10.1007/s10803-008-0621-3. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280(2):1561–72. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, Faraone SV, Nguyen TT, Schafer H, Holmans P, et al. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884–97. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DK, Coulson RL, Myers JH, Browning RA. Neuroanatomical differentiation in the brain of the spontaneously hypertensive rat (SHR). I. Volumetric comparisons with WKY control. Clin Exp Hypertens. 1993;15(5):867–94. doi: 10.3109/10641969309041647. [DOI] [PubMed] [Google Scholar]
- Nelson DO, Boulant JA. Altered CNS neuroanatomical organization of spontaneously hypertensive (SHR) rats. Brain Res. 1981;226(1–2):119–30. doi: 10.1016/0006-8993(81)91087-8. [DOI] [PubMed] [Google Scholar]
- Nelson DO, Boulant JA. Altered brainstem structure of spontaneously hypertensive (SHR) rats. Brain Res. 1983;261(1):145–50. doi: 10.1016/0006-8993(83)91294-5. [DOI] [PubMed] [Google Scholar]
- Nijmeijer JS, Arias-Vasquez A, Rommelse NN, Altink ME, Anney RJ, Asherson P, Banaschewski T, Buschgens CJ, Fliers EA, Gill M, et al. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. J Am Acad Child Adolesc Psychiatry. 49(7):675–85. doi: 10.1016/j.jaac.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki R, Fukura N, Matsushita M, Mitsui K, Kanazawa H. Cell surface levels of organellar Na+/H+ exchanger isoform 6 are regulated by interaction with RACK1. J Biol Chem. 2008;283(7):4417–29. doi: 10.1074/jbc.M705146200. [DOI] [PubMed] [Google Scholar]
- Pang T, Su X, Wakabayashi S, Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem. 2001;276(20):17367–72. doi: 10.1074/jbc.M100296200. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305(5692):1972–5. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52(5):817–30. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(7):795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48(5):464–72. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 167(4):1183–91. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Roxrud I, Raiborg C, Gilfillan GD, Stromme P, Stenmark H. Dual degradation mechanisms ensure disposal of NHE6 mutant protein associated with neurological disease. Exp Cell Res. 2009;315(17):3014–27. doi: 10.1016/j.yexcr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24(1):31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Dasbanerjee T, Zhang-James Y, Middleton F, Faraone S. Behavioral and genetic evidence for a novel animal model of Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Subtype. Behav Brain Funct. 2008;4:56. doi: 10.1186/1744-9081-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Woien G, Walaas SI, Storm-Mathisen J, Bergersen LH, Hvalby O, Jensen V, Aase H, Russell VA, et al. The spontaneously hypertensive rat model of ADHD--the importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57(7–8):619–26. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzig JK, Lehmkuhl G. Autism and ADHD - Are there Common Traits? Fortschr Neurol Psychiatr. 2007;75(5):267–74. doi: 10.1055/s-2005-915567. [DOI] [PubMed] [Google Scholar]
- Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J. 2007;401(3):623–33. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Faraone SV, Dougherty DD, Bonab AA, Fischman AJ. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57(11):1293–300. doi: 10.1016/j.biopsych.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Valera E, Faraone SV, Murray K, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1361–9. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):932–40. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, SLC9A9 sequence difference between rat strains. Novel SNPs were identified in the gene region. B, SLC9A9 sequences in selected orthologues were aligned in ClustalX 2.0 and partial alignments, including the last two transmembrane domains (indicated by symbols “^^^” on top) and the complete intracellular C-terminals, are shown. The alignment scores are plotted underneath the sequences to indicate conservation level. The residues that are mutated in WKY/NCrl rats are indicated by arrows. C, Conformational parameters for alpha-helix based on Deleage & Roux amino acid scales are plotted for wild type and mutant SLC9A9 (Deleage and Roux 1987). Arrows indicate the positions of two mutations. The positions for the last two transmembrane domains (TMs) are also indicated. The juxtamembrane region of rat SLC9A9, where the mutations are located, show a high propensity to form an alpha-helix. The two rat mutations slightly decreased this propensity, however mutant protein appears still highly capable of forming an alpha-helix. D, Sequence alignments of the alpha-helix region of the wild type SLC9A9 with the CHP-binding domains in SLC9A1-5s (Ammar and others 2006). Hydrophobic residues are highlighted in yellow. Mutation sites are bolded in red letters.