Abstract
Previous studies reveal age by valence interactions in attention and memory, such that older adults focus relatively more on positive and relatively less on negative stimuli than younger adults. In the current study, eyeblink startle response was used to measure differences in emotional reactivity to images that were equally arousing to both age groups. Viewing positive and negative pictures from the International Affective Picture System had opposite effects on startle modulation for older and younger adults. Younger adults showed the typical startle blink pattern, with potentiated startle when viewing negative pictures compared to positive pictures. Older adults, on the other hand, showed the opposite pattern, with potentiated startle when viewing positive pictures compared to viewing negative and neutral pictures. Potential underlying mechanisms for this interaction are evaluated. This pattern suggests that, compared with younger adults, older adults are more likely to spontaneously suppress responses to negative stimuli and process positive stimuli more deeply.
Keywords: aging, affective modulation, emotional reactivity, positivity effect, startle response
While increasing age is often associated with declines in physical and cognitive performance (e.g. processing speed, executive functioning), there is growing evidence to suggest that emotional well-being follows a different trajectory. Indeed, there are some indications that the ability to process emotional information and regulate emotions remains stable or increases across much of the adult lifespan (Ben-Zur, 2002; Carstensen, Fung, & Charles, 2003; Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Charles, Reynolds, & Gatz, 2001). In addition, as outlined below, recent findings have revealed that whereas emotional arousal can have similar effects on younger and older adults’ attention and memory, age differences are more likely to emerge when examining emotional valence (Mather & Carstensen, 2005). The present study examines age differences in physiological startle responses during positive and negative stimuli, with the prediction that older adults will show different effects of valence on startle due to a greater focus on positive over negative emotion compared with younger adults.
The Positivity Effect in Memory and Attention
Despite decades of intense interest in how aging affects memory, until recently little research had focused on aging and emotional memory. However, the past decade has seen a great deal of attention to the topic; it turns out that there are both surprising similarities and differences between younger and older adults. In terms of similarities, it appears that arousal continues to have a major impact on attention and memory as individuals increase in age. Like younger adults, older adults can detect arousing or threatening stimuli faster than other types of stimuli (Hahn, Carlson, Singer, & Gronlund, 2006; Knight et al., 2007; Leclerc & Kensinger, 2008; Mather & Knight, 2006). Older adults also show similar advantages in memory for emotionally arousing events or stimuli as do younger adults (e.g., Murphy & Isaacowitz, 2008).
However, age differences in the effects of emotion on initial attention and subsequent memory are more likely to emerge when comparing positive versus negative valence. Specifically, there appears to be a shift with age in the ratio of positive versus negative information attended to or remembered. This age by valence interaction is known as the “positivity effect,” and has been hypothesized to be the result of a greater focus among older adults on regulating emotion than among younger adults (Mather & Carstensen, 2005; Scheibe & Carstensen, 2010).
For instance, studies using attention probe and eye tracking methods reveal that, when shown two pictures or faces at the same time, older adults spend relatively more time looking at positive stimuli and relatively less of their time looking at negative stimuli compared with younger adults (Isaacowitz, Wadlinger, Goren & Wilson, 2006a, 2006b; Knight et al., 2007; Mather & Carstensen, 2003; Rösler et al., 2005). For older adults, this positivity effect in attention is most prominent when a sad mood is induced, consistent with the idea that they use selective attention to help regulate emotion (Isaacowitz, Toner, Goren, & Wilson, 2008).
The positivity effect has also been shown in older adults when testing memory recall of emotionally salient images. A number of studies have found age by valence interactions in the recall of pictures, in that older adults remember either more positive or fewer negative stimuli relative to younger adults (Charles, Mather, & Carstensen, 2003; Gruhn, Scheibe, & Baltes, 2007; Ko, Lee, Yoon, Kwon, & Mather, in press; Kwon, Scheibe, Samanez-Larkin, & Tsai, 2009; Langeslag & Van Strien, 2009; Mather & Knight, 2005; Tomaszczyk, Fernandes, & MacLeod, 2008). However, there is some disagreement in the literature as to whether older adults display a true positivity preference (better memory for positive stimuli over negative stimuli compared to younger adults), or whether the effects are due to older adults’ poorer memory for negative stimuli. A recent meta-analysis found that while younger adults showed a significantly stronger recognition memory preference for negative information compared to older adults, no age differences in positivity preference were found (Murphy & Isaacowitz, 2008). Furthermore, while many studies show less of a negativity effect in older compared to younger adults, age differences in the way valence influences memory have not always been found (Denburg, Buchanan, Tranel, & Adolphs, 2003; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002). Smaller sample sizes may also add variability to the findings, as the positivity effect age by valence interaction in memory tends to be a medium effect size (e.g., Charles et al., 2003).
Beyond the simple statistical power issue, whether or not age differences in the positivity effect in memory occur may be due to different factors. For example, older adults’ positivity effect in attention and memory appears most likely to arise when response biases or reconstructive memory processes can exert an influence (Fernandes, Ross, Wiegand, & Schryer, 2008; Thapar & Rouder, 2009), when gist rather than detailed memory is tested (Kensinger, Garoff-Eaton, & Schacter, 2007) and when cognitive control mechanisms needed to implement emotion regulation goals are available (Knight et al., 2007; Mather & Carstensen, 2005; Mather & Knight, 2005; Petrican, Moscovitch & Schimmack, 2008). These findings suggest that older adults’ positivity effect is the result of goal-directed processes that help direct attention and shape memory.
The nature of the stimuli eliciting emotion has also been shown to play a role in the differential reactivity of older and younger adults to positive and negative stimuli. For example, studies using mood induction procedures found that older adults experienced more intense sadness than younger adults when the experimental themes were highly relevant to them (Kunzmann & Gruhn, 2005). Moreover, age differences in pleasantness and arousal ratings to pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005) have also been found (Gruhn & Scheibe, 2008). Specifically, older adults on average rate IAPS negative pictures as more arousing compared with younger adults, while rating the positive pictures as less arousing. Given these findings, using images that both older and younger adults rate as equally intense is important when testing physiological differences in reactivity to valence for both age groups.
Startle Reflex Paradigm
The startle response is “an immediate reflex response to sudden, intense stimulation” (Landis & Hunt, 1939) and is typically measured using eyeblink magnitude, which is especially sensitive to startle stimuli and is likely to respond even when other reflexes are not triggered. The affective startle modification paradigm typically involves two stimuli, one that provokes an acoustic startle response (a loud burst of white noise) and a preceding stimulus that modulates this response (usually images evoking positive or negative emotions). A contraction of the orbicularis oculi muscle, which mediates the startle eyeblink response, occurs reflexively 30-50 ms following onset of the startling stimulus such as a loud burst of white noise (Berg & Balaban, 1999).
Unlike other physiological measures such as skin conductance that only measure arousal level, the eyeblink startle response differs during processing of negative and non-negative foreground pictures in a younger adult population. The magnitude of the startle eyeblink response elicited while viewing unpleasant pictures is typically larger than that elicited while viewing neutral or pleasant pictures. These effects are most reliable for highly arousing pictures (Cuthbert, Bradley, & Lang, 1996). According to the motivational priming hypothesis (Lang, 1995; Lang, Bradley, & Cuthbert, 1990), an unpleasant foreground stimulus activates the aversive motivational system. This activation of the aversive motivational system potentiates the startle response because the startle sound itself is unpleasant. Reacting to a pleasant foreground stimulus, on the other hand, inhibits the startle response by activating the appetitive motivational system. Thus, according to this framework by Lang and colleagues, negative stimuli prime and increase the startle blink response, while positive stimuli diminish the startle blink response. A number of studies have replicated this pattern with a younger adult population (see Bradley, Cuthbert, & Lang, 1999 for a review).
However, because most of the research has been conducted with younger adults, it is not clear whether older adults will show the same valence effects on startle. One study comparing age groups (Smith, Hillman, & Duley, 2005) found that older adults exhibited stronger eyeblink magnitudes than younger adults for negative stimuli, but also found that their older adult group reported greater overall ratings of arousal than younger adults. Since arousal level has been shown to play a role in startle magnitude (Cuthbert et al., 1996), it is difficult to determine whether the results are due to differences in reactivity to negative stimuli or to differences in arousal level. Thus, research is needed to see if there are age differences when subjective arousal responses are similar across the two age groups. In addition to arousal, other factors such as attention (Filion, Dawson, & Schell, 1998) and participant’s mood state (Bradley, et al., 1999) have been shown to affect startle to emotional stimuli.
The effect of age on affective startle modification is of particular interest given the positivity effect observed with other measures. More specifically, if older adults focus on regulating emotions by suppressing reactions to negative pictures while also enhancing reactions to positive stimuli, then we would anticipate a different pattern of startle modification than that pattern displayed by younger adults. First, consider the effects of emotion regulation on responses to negative pictures. Research with younger adults shows that asking them to suppress their emotional response to negative pictures reduces startle responses, whereas asking them to enhance their emotional response increases startle responses (Jackson, Malmstadt, Larson, & Davidson, 2000; Dillon & LaBar, 2005). Thus, if older adults spontaneously suppress reactions to negative images, they should show reduced startle in response to those pictures.
The degree to which individuals process emotional stimuli has also been shown to affect startle responses elicited during the presentation of those stimuli. For instance, younger adults asked to process adjectives deeply (thinking about its personal relevance, engaging in mental imagery about it, or categorizing it semantically) showed larger startle responses during presentation of the adjectives (the difference was especially large for positive adjectives) than when they were asked to process them shallowly (via passive viewing, rote rehearsal or lexical categorization; Herbert & Kissler, 2010). Young adults have also been found to exhibit a linear increase in startle magnitude as the perceptual load of neutral foreground stimuli increased (Thorne, Dawson, & Schell, 2006). All in all, these findings suggest that processing information deeply and in a purposeful manner can increase startle response compared to shallowly encoded information. These findings regarding the role of attention and depth of processing with younger adults together with findings reviewed earlier that older adults attend less to negative and more to positive information suggest that older adults should show larger startle to positive stimuli than to negative stimuli.
The Present Study
The current study examined the differential reactivity of older and younger adults to varying affective IAPS stimuli. The startle eyeblink response was used as the physiological measure of emotional reactivity due to its reliability in differentiating between negative and non-negative material in younger adults. The images used were taken from the IAPS and were selected based on high self-reported arousal for both younger and older adults. The aim was to control for age differences in arousal by choosing stimuli in each picture category that were rated equally arousing by older and younger adults. A surprise memory recall task was included to evaluate differential recall with age and as a measure of the depth of encoding for different types of stimuli.
In light of evidence indicating older adults’ greater focus on emotion regulation and their positivity effect, we predicted that the effects of picture category valence would differ for older adults and younger adults. Compared with younger adults, older adults should show diminished startle to negative images but enhanced startle to positive images. Furthermore, older adults should also exhibit enhanced startle to positive images compared to negative images, whereas younger adults are expected to exhibit the typical startle pattern (potentiated response to negative images compared to neutral and positive images).
Method
Participants
Sixty-nine younger and 56 older adult participants were recruited to take part in this study. All reported normal or corrected-to-normal vision as well as normal hearing capabilities. Participants who failed to reach 1 μV amplitudes on more than 50% of probed trials were considered non-responders and were excluded from further analyses. A total of 6 younger adults and 14 older adults from our sample were excluded from analyses due to the absence of reliable startle blink responses. Preliminary analyses also revealed that 18 younger and 2 older adults in our sample endorsed symptoms indicating a mildly elevated level of depression (16 or higher on the CES-D). To reduce the possible influence of clinical depression on the results (e.g., Kaviani et al., 2004), these participants were excluded from analyses. Therefore, 45 younger adult participants (32F, 13M) between the ages of 18 and 23, and 40 older adult participants (17F, 23M) between the ages of 65 and 88 were included in the analyses. Mean ages were 20.02 (SD = 1.06) for younger adults and 74.73 (SD = 6.87) for older adults. Out of the 45 younger adults, 19 were Caucasian, 11 were Asian, 7 were Latino, 6 were from other ethnic backgrounds, and 2 did not report their ethnicity. Out of the 40 older adults, 37 were Caucasian, 1 was Asian, 1 was from another ethnic background, and 1 did not report their ethnicity. Sex and ethnicity differences between groups were evaluated and are discussed in the results. Younger adults were undergraduate students attending the University of Southern California (USC) and were given experimental credit for participating in the study. Older adults were volunteers recruited through the USC Davis School of Gerontology, as well as USC alumni living in areas surrounding USC. Older adults (M = 17.63, SD = 1.54) reported having more education than younger adults 1(M = 14.46, SD = .84), t(61) = 10.41, p <.01, d = 2.56; however no differences were found between younger (M = 4.01, SD = .80) and older (M = 4.20, SD = .72) adults with regard to self-rated health in response to the question “How would you rate your overall health?”, t(83) = 1.14, p = .26, d = .25.
Questionnaires
CES-D
The Center for Epidemiological Studies – Depression Scale (CES-D) is a 20-item questionnaire developed to measure depressive symptoms (Radloff, 1977). Items regarding depressed mood, feelings of hopelessness and worthlessness, poor concentration, as well as other common depressive symptoms are included. Participants were asked to rate each item on a scale from 0 to 3 on the basis of how often they felt that way in the past two weeks, with a range of possible scores from 0 to 60. The CES-D has been used in multiple populations with high reliability and internal consistency (Radloff & Teri, 1986).
PANAS
The Positive and Negative Affective Schedule (PANAS) consists of 10 negative and 10 positive adjectives that describe different feelings and emotions (Watson, Clark, & Tellegen, 1988). Participants were asked to indicate to what extent they were feeling a certain way at the present moment. Responses were given on a Likert scale, ranging from 1 (very slightly or not at all) to 5 (extremely). The PANAS was given to participants at two different times during the procedure, once before the startle procedure and then again during the memory delay interval. The values given to each of the adjectives by each participant were summed separately by age (young and old), emotion (positive and negative), and time (at the beginning of the study and after viewing the IAPS). Results were grouped by age and positive versus negative emotion and were compared for time 1 and 2. This was done to evaluate any potential mood changes during the session.
Startle Response Measurement
Participants viewed 30 images (10 positive, 10 negative, 10 neutral) from the IAPS for six seconds each. Positive and negative images were selected based on arousal data collected in a previous study (Mather & Knight, 2005). Younger and older adults’ arousal ratings from the previous study for the chosen images did not statistically differ from each other. Neutral images were selected randomly from the full set of IAPS (Lang et al., 1990). The order of the negative and positive images was counterbalanced across individuals. These images were also counterbalanced within subjects so that no more than two images with the same picture type (negative, positive, neutral) were presented in a row. Intertrial intervals (ITIs) consisted of a 9-13 second blank white screen between image presentations. Eighteen pictures (six per picture type) were accompanied by a startle stimulus, which occurred 3.5 to 5.5 seconds after picture onset. Varying the interval between picture onset and startle onset within these intervals is a common procedure in startle research with IAPS (e.g., Patrick, Bradley, & Lang, 1993) and was included with the purpose of decreasing anticipation of the startle sound. In addition, six startle stimulus presentations were presented during the ITIs in order to measure baseline measurement of the startle eyeblink response. The startle eliciting stimuli were 110 dB white noise bursts 50 ms in duration with a near instantaneous rise/fall time presented binaurally through headphones.
Stimulus presentation and data acquisition were controlled through Contact Precision Instruments equipment and a computer running SAM2 software. The raw electromyographic data (filtered at 20 Hz high pass and 500 Hz low pass) were collected continuously throughout the session at a rate of 1000 Hz, and then software integrated for analysis using a 20-ms time constant. Electromyographic activity was measured from two Ag-AgCl electrodes, and the startle response amplitude recorded within a window of 20–200 ms following stimulus onset. In order to control for any possible baseline differences, standardized startle EMG T-scores were used to examine the effects of age on picture category.
Memory Delay Interval
Upon completion of the startle response procedure, examiners removed the headphones and all electrodes from the participant. During the next 15 min, participants had their hearing tested and filled out forms including the CES-D and a second PANAS. The 15-min interval was used as a delay period before the memory test.
Surprise Recall Task
Fifteen minutes following the end of the startle response procedure, participants were given a form that read, “Earlier, you participated in a task where you were asked to view various images on a computer screen. Please describe in writing, each of the pictures that you remember from the previous task. When you have finished, please alert the examiner.” Researchers read these instructions aloud to all participants and asked if they had any questions before proceeding. Participants were given as much time as they needed for this portion of the study.
Picture Ratings
After the recall task, participants were shown the images again and asked to rate them individually on both pleasantness and arousal using a computerized program. The computer screen presented each image on the screen one at a time, with two columns of buttons labeled from 1 – 9 on arousal (calm, unaroused to excited, stimulated) and pleasantness (extremely unpleasant to extremely pleasant). Upon completing this task, examiners debriefed participants and addressed any questions or concerns regarding the experiment.
Data Analysis
To test age differences in reactivity to positive, neutral, and negative images, EMG startle responses were standardized into T-scores for each subject individually and were analyzed using a 2 × 3 (age × picture category) analysis of variance. To test the main effects of physiological age on startle blink magnitude, we analyzed the age groups’ raw startle blink responses during their ITIs using an independent samples t-test. Age differences for the CES-D, PANAS, and self-reported pleasantness and arousal were also tested using independent samples t-tests.
A 2 (age) × 3 (valence) repeated measures ANOVA was conducted to determine age differences in memory recall to positive, negative, and neutral images. Two research assistants independently coded the descriptions written by participants by evaluating whether or not they could match them with an actual presented image. If both research assistants coded a description as matching the same image, then that description was counted as an accurate recall and included in the analyses. Any discrepancies were evaluated by a third independent rater. In the event of an unmatchable description, the description was not counted as an accurate recall and was excluded from analyses. Interrater reliability was high, with a Cohen’s Kappa value of .98.
The Wilk’s lambda statistic was used to evaluate repeated-measures picture category effects when the sphericity assumption was met. For tests where sphericity was violated, the Greenhouse-Geisser estimate was used in order to attain a more accurate significance value. Simple post-hoc contrasts and independent t-tests were conducted when significant main effects or interactions were found. A two-tailed significance level of p < .05 was used for all statistical measures.
Results
Self-report Measures
Mood Ratings
Table 1 presents the means and standard deviations of the self-reported measures. On the PANAS questionnaire given at the beginning of the procedure, independent samples t-tests revealed that older adults were in more positive moods than younger adults, t(83) = 2.88, p < .01, d = .45, as well as in less negative moods than younger adults, t(83) = 2.30, p < .05, d =.51. The PANAS was given a second time during the memory delay interval, where similar age differences for positive adjectives, t(83) = 2.07, p < .05, d = .45, and negative adjectives, t(83) = 3.67, p < .01, d = .81 were found. Paired samples t-tests revealed that older adults were in less negative moods, at time two compared to time one, t(39) = 3.20, p < .01, d = .51. There were no other time one vs. time two differences. In addition to the PANAS questionnaire, an independent samples t-test analysis of CES-D scores revealed that younger and older adults reported similar levels of depression, t(83) = 1.66, p = .10, d = .36.
Table 1.
Means and Standard Deviations for Self-report Measures and Recalled Images
| Age Group
|
||||
|---|---|---|---|---|
| Younger (n = 45)
|
Older (n = 40)
|
|||
| Demographic | M | SD | M | SD |
| Age (years)** | 20.02 | 1.06 | 74.73 | 6.87 |
| PANAS (1) +** | 25.09 | 7.91 | 31.13 | 11.28 |
| PANAS (1) -** | 13.47 | 4.01 | 11.85 | 2.06 |
| PANAS (2) +** | 25.66 | 9.13 | 29.93 | 9.85 |
| PANAS (2) -** | 13.42 | 4.07 | 10.90 | 1.63 |
| Valence Ratings (Negative) | 2.20 | .95 | 2.34 | 1.18 |
| Valence Ratings (Neutral)* | 5.02 | .28 | 5.27 | .63 |
| Valence Ratings (Positive) | 6.74 | .86 | 7.05 | .88 |
| Arousal Ratings (Negative) | 6.29 | 1.80 | 5.75 | 1.89 |
| Arousal Ratings (Neutral) | 1.29 | .48 | 1.48 | .94 |
| Arousal Ratings (Positive) | 4.86 | 1.67 | 5.07 | 1.79 |
| Negative Images Recalled | 5.80 | 1.73 | 5.43 | 1.80 |
| Neutral Images Recalled | 3.47 | 1.31 | 3.38 | 1.73 |
| Positive Images Recalled | 4.16 | 1.78 | 4.53 | 1.91 |
Note.
p < .05;
p < .01 for age group comparison.
Picture Affect Ratings
Main effects of both age, F(1, 83) = 6.22, p < .05, ηp 2 = .07 and picture category, F(1.38, 114.55) = 586.86, p < .01, ηp 2 = .88 were found when conducting a 2 (age) by 3 (picture category) repeated measures ANOVA. No interaction was found between age and picture category, F(1.38, 114.55) = .19, p = .75, ε = .69,ηp 2 = .00. Independent samples t-tests revealed that older adults rated the neutral images, t(83) = 2.38, p < .05, d = .51 as more pleasant than younger adults, with no age differences in pleasantness ratings for positive, t(83) = 1.61, p = .11, d = .36 or negative images, t(83) = .60, p = .55, d = .13. As expected, post-hoc contrasts revealed that positive images (M = 6.88, SD = .87) were rated by both younger and older adults as significantly more pleasant than neutral images (M = 5.14, SD = .49), F(1, 83) = 375.48, p < .01, ηp 2 = .82, as well as negative images (M = 2.26, SD = 1.06), F(1, 83) = 721.26, p < .001, ηp 2 = .90.
Picture Arousal Ratings
Main effects of picture category were found in a 2 (age) by 3 (picture category) repeated measures ANOVA, F(1.75, 145.17) = 384.47, p < .01, ε = .88, ηp 2 = .82. As expected, post-hoc contrasts revealed that neutral images (M = 1.38, SD = .74) were rated as less arousing than positive (M = 4.95, SD = 1.72), F(1, 83) = 422.90, p < .01, ηp 2 = .84, and negative images (M = 6.04, SD = 1.85), F(1, 83) = 524.39, p < .01, ηp 2 = .86. Negative images were also rated as more arousing than positive images, F(1, 83) = 53.92, p < .001, ηp 2 = .39. Importantly, no main effects of age, F(1, 83) = .03, p = .86, ηp2 < .01 or interaction effects were found, F(1.75, 145.17) = 3.01, p > .05, ηp 2 = .04, which indicates that younger and older adults in this study had similar arousal responses for each picture category.
Startle Reflex
Using raw ITI startle EMG data, independent samples t-tests revealed that older adults (M = 8.10, SD = 5.71) on average exhibited significantly smaller startle blink responses than younger adults did (M = 14.33, SD = 12.65), t(63) = 2.98, p < .01, d = .63. Due to the high levels of variability between participants in EMG responses, a common occurrence with startle data (e.g., Cuthbert et al., 1996; Patrick et al., 1993), all blink amplitude values were standardized using a within-subjects z-transformation (i.e., the difference between each participant’s EMG amplitude value on each trial and that participant’s mean value across all trials was divided by the standard deviation of all values). Scores were then subjected to a linear T-transformation resulting in a mean of 50 and a standard deviation of 10 for each participant. This helped to ensure that all participants contributed to group means equally.
Figure 1 presents the age differences in startle reactivity to the positive, neutral, and negative stimuli. No main effects of age, F(1, 83) = .11, p = .74, ηp 2 = .00 or picture category, F(2, 82) = .07, p = .94, ηp 2 = .00 were found when conducting a 2 (age) by 3 (picture category) repeated measures ANOVA. However, as anticipated, there was a significant interaction, F(2, 82) = 7.87, p < .01, ηp 2 = .16.2 Consistent with our hypothesis, independent samples t-tests indicated that younger adults exhibited larger startle blinks to negative images compared with older adults, t(83) = 3.40, p < .01, d = .74. Age differences in startle response to positive images were also found, such that when viewing positive images, older adults exhibited larger startle blinks than those of younger adults, t(83) = 2.30, p < .05, d = .50. Age differences in response to neutral images did not achieve significance, t(83) = 1.70, p = .09, d = .37. To examine the startle pattern for highly arousing stimuli, a 2 × 3 repeated measures ANOVA was conducted post-hoc on the startle responses for the two highest rated images on arousal for each participant, with results yielding the same age by valence interaction, F(2, 82) = 4.73, p < .05, ηp 2 = .10. The remaining startle reactivity analyses reported below were conducted using the full dataset with all startle responses.
Figure 1.
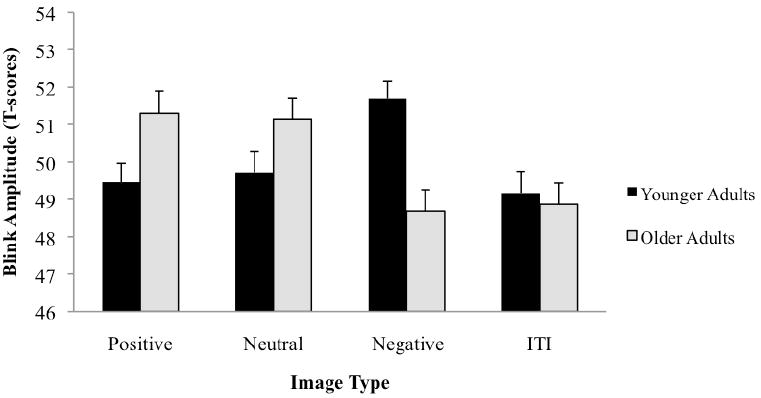
Mean magnitude of startle blink responses while viewing positive, neutral, and negative valenced images in younger and older adult groups (intertrial interval, ITI, responses are included for reference). Error bars represent standard errors.
To assess whether factors other than age may have contributed to this interaction effect, sex, self-reported pleasantness ratings, PANAS scores (initial mood state), and depression rating scores were included as covariates. The age by image category interaction remained significant even after controlling for these variables, F(2, 75) = 7.17, p < .01, ηp 2 = .16. Initial negative mood score was the only significant covariate in the analysis, F(2, 75) = 3.82, p < .05, ηp 2 = .09. Bivariate correlations revealed that negative mood state was negatively correlated with startle response to positive images, r = .35, p < .01. In other words, the increase in self-reported negative mood was associated with a decrease in startle reactivity to the positive images. None of the other specified covariates were significant (all F-values < 1.6). Age groups also differed in ethnicity, and because participants of Asian ethnicity have been shown to exhibit smaller blinks than non-Asian participants (Swerdlow, Talledo, & Braff, 2005), post-hoc analyses were conducted with only non-Asian participants (34 younger, 39 older). Results showed a similar pattern to the full dataset (although the previously significant negative mood state covariate did not reach significance in this model), and the interaction remained significant, F(2, 63) = 7.37, p < .01, ηp 2 = .19.
Paired samples t-tests were conducted to determine age differences in the startle pattern. As expected, analyses revealed that younger adults exhibited a potentiated startle response to negative images compared to positive images, t(44) = 2.30, p < .05, d = .53. In contrast, older adults exhibited an inhibited startle response to negative images compared to positive images, t(39) = 3.14, p < .01, d = .73. Furthermore, older adults exhibited smaller startle responses to negative compared to neutral images, t(39) = 2.62, p < .05, d = .67. Younger appeared to exhibit greater startle response to negative compared to neutral images, t(44) = 1.83, p = .08, d = .46; however this difference was not quite significant.
Memory Recall
A 2 (age) X 3 (valence) ANOVA was conducted to test age differences in the recall of positive, negative, and neutral images. No main effects of age, F(1, 76) = .66, p = .42, ηp 2 = .01 or valence, F(2, 75) = 2.05, p = .14, ηp 2 = .05 were found when sex, pleasantness ratings, initial PANAS scores, and depression ratings were indicated as covariates in the analyses. None of the covariates significantly contributed to the analyses, (all F-values < 1.1). In addition, although older adults recalled more positive pictures and fewer negative pictures than younger adults (Table 1), no interaction was found between age and valence, F(2, 75) = 1.16, p = .32, ηp 2 = .03.
Discussion
The present study tested age differences in emotional reactivity using stimuli that were equally arousing to both older and younger adults for each picture category. Based on previous findings of older adults’ positivity effect in attention, our main hypothesis predicted significant age differences in how viewing emotional pictures can affect startle, both between and within age groups.
Mechanisms Underlying Age Differences in Startle Reactivity
Compared with younger adults, older adults have been shown to attend to and more readily recall positive compared with negative material (Mather & Carstensen, 2005). In the present study, a similar age by valence interaction was found in startle responsivity, such that older adults exhibited smaller startle responses to negative images and larger startle responses to positive images than younger adults did.
According to Lang and colleagues (Lang, 1995; Lang et al., 1990), the startle reflex is an aversive or defensive response that is augmented in the context of an ongoing negative emotion but inhibited or attenuated in the context of a positive emotion. Negative emotions prime the defensive action disposition involved in startle responses, whereas positive emotions prime an opposing appetitive motivational system. In our study, younger adults displayed the typical startle pattern found in previous studies (see Bradley et al., 1999), namely a potentiated response to negative images compared with positive images. Our findings with younger adults are mostly consistent with the motivational priming hypothesis (although not entirely consistent, as the hypothesis would also predict that startle during viewing positive pictures should be lower than startle during neutral pictures or ITI, which is less commonly found).
However, in our study, older adults exhibited the opposite startle pattern to that typically found in younger adults, with amplified startle for positive pictures compared with negative pictures. While the motivational priming hypothesis can account for older adults’ reduced startle during negative pictures with its proposition that downward regulation of negative emotions should result in a decrease in startle magnitude, older adults’ enhanced startle during positive pictures cannot be accounted for by the motivational priming hypothesis, as there is no evidence that the positive pictures activated aversive or defensive responses among the older adults. Indeed, pleasantness ratings revealed no age differences for positive or negative images, and both younger and older adults rated the negative images as more negative than the positive images.
Mood state has also been shown to affect startle reactivity (reviewed by Bradley, et al., 1999; Ehrlichman, Kuhl, Zhu, & Warrenburg, 1997), therefore another possibility may be that older adults’ more positive mood at the beginning of the study could have affected how they responded to the emotion-laden images. Initial negative mood state was found to be a significant factor, in that increased negative mood at the beginning of the study was associated with smaller startle responses to positive images. Older adults reported less negative mood than younger adults, and they also responded more to the positive images than younger adults did. It is therefore possible that the participants’ initial mood affected how they reacted physiologically, at least with regard to positive stimuli. However, mood state cannot have been the only factor, as the age by valence interaction remained strong even after initial mood state was covaried out.
Thus, neither Lang’s motivational priming hypothesis nor age differences in mood or subjective ratings of images can fully explain the startle pattern displayed by the older adult group. Based on previous literature on aging and emotion, specifically the positivity effect (Mather & Carstensen, 2005), it is reasonable to suggest that emotion regulation may have played a role in these age differences. We posit that age differences in the startle response pattern may have been influenced by an underlying difference in how older adults regulate their emotions in comparison to younger adults.
In addition to past research on aging and emotion, research on the startle response and emotion regulation in younger adults also lends support to this idea. For example, previous research shows that instructing younger adults to suppress their emotional response to negative pictures reduces startle responses, whereas asking them to enhance their emotional response to positive pictures increases startle responses (Jackson et al., 2000; Dillon & LaBar, 2005). Our findings suggest that older adults in our study may have been spontaneously suppressing their reactions to the negative pictures as a way of regulating their emotions more than younger adults did. Older adults also exhibited larger startle responses to positive images than younger adults did, which would be consistent with the idea that older adults in our study were spontaneously enhancing their emotional response to the positive images compared to younger adults, who exhibited smaller responses.
Along with active enhancement and suppression of emotion, the depth of processing stimuli has also been shown to affect startle response (Herbert & Kissler, 2010; Thorne et al., 2006). Specifically, studies have found that the deeper the level of processing, the larger the startle response when viewing the encoded stimuli. In applying these findings to our present study, it is reasonable to suggest that older adults were processing positive stimuli more deeply than negative stimuli, whereas the same differential processing of positive and negative stimuli was not apparent in younger adults. Therefore, the difference in the extent to which younger and older adults process emotional stimuli may serve as another potential underlying mechanism for the resulting age differences in startle reactivity. Future studies may be able to manipulate these different aspects of attention and emotion regulation to see how they affect startle reactivity (see future studies section).
The mechanisms raised above may have also contributed to older adults’ self-reported decrease in negative mood after the startle procedure, in that while viewing emotional pictures, older but not younger adults may have been more influenced by the positive pictures than by the negative pictures. However, it is also possible that older adults enjoyed the tasks more than younger adults, which would lead to a similar effect. Similar mood-enhancing effects of experimental tasks were seen in a study of autobiographical memory in which both older adults in a control (no-focus) condition and participants induced to focus on their emotions found themselves in a better mood at the end of the session than at the beginning, whereas younger control participants and those in a memory focus condition did not show changes in mood (Kennedy, Mather, & Carstensen, 2004).
Memory Recall
In the present study, older and younger adults responded similarly when asked to recall images from memory. Although older adults recalled more positive and fewer negative images than younger adults, these differences were not significant. Given the strong interaction effects found with startle reactivity, the absence of interaction effects in memory recall, which tend to be in the medium effect size range (e.g., Charles et al., 2003), may have been due to a lack of power.
Limitations and Future Studies
The present study assessed age differences in emotional reactivity using self-report, physiological and memory measures, and should help to advance the understanding of both age differences in emotional reactivity as well as factors that influence the affective picture-startle paradigm. Still, there were some limitations to the study worth mentioning. With regards to recruitment, older adults in the study were required to have normal hearing and normal or corrected-to-normal vision, and were mostly USC alumni. Therefore, this sample may be comprised of healthier participants than the general older adult population. Having said this, our older adults may in fact be more comparable to our younger adult sample (comprised of current USC students) than a normal older adult population would be.
Additionally, images from the present study were selected from a subset of IAPS chosen in a previous study (Mather & Knight, 2005). This was done in order to find images that were similarly salient and arousing to both groups. However, because of the small sample of images to choose from, some the selected images were not as arousing to our participants as we had hoped. Despite these lower than expected arousal ratings, the typical startle pattern was found in younger adults, and significant valence effects were still found in both younger and older adult groups. Secondary analyses using participants’ startle data to selected highly arousing images were also conducted and confirmed the results from the complete set of stimuli.
Despite the noted limitations, the present study provides compelling new information that may inspire potential future research. For example, the present study found that younger adults startled more during negative images than older adults did, whereas older adults startled more during positive images than younger adults did. This finding suggests a difference in how both groups attend to and process emotional information. Future studies may be able to test and expand on this hypothesis by focusing on directly manipulating specific aspects of attention, such as depth of processing in both younger and older adults while viewing emotional images and measuring startle response. It would be interesting to determine whether asking older adults to enhance their emotions to negative information would lead to a pattern similar to younger adults in our study. Additionally, researchers could ask younger and older adults to process certain emotional stimuli more deeply than others, then compare startle reactivity under these conditions to a control for each age group. Furthermore, comparing a normal condition (eliciting startle during emotional images) with an increased cognitive load condition (adding a cognitively demanding task) would directly test the influence of cognitive control mechanisms and their impact on startle reactivity. Overall, research in this area may help to better quantify the role of emotion regulation and encoding depth on startle response, and to strengthen our knowledge of how physiological and emotion regulation processes interact and change over time.
Conclusions
Results from the present study provide new information on age differences in physiological reactivity that appear to be consistent with previous literature on aging and the positivity effect. Interestingly, while physiological age differences were found, no age differences in memory recall were found. Results also revealed interesting and novel information on age differences in the startle pattern, which indicates a need for further research on factors other than simple valence that can influence the affective modulation of the startle response. Positive and negative stimuli modulated eyeblink startle differently for older adults than for younger adults, in that older adults exhibited larger startle responses to positive compared to negative images, whereas younger adults exhibited the opposite pattern. These results are consistent with the idea that older adults process positive stimuli more deeply than negative stimuli when compared to younger adults.
Acknowledgments
We thank Anne M. Schell and Bob G. Knight for their valuable contribution and helpful feedback during earlier versions of this article and acknowledge NIH grants R01AG038043, RO1AG025340 and K02AG032309 for support.
Footnotes
Participants’ education levels were collected after the study had been completed. All participants were re-contacted through phone or email, and responses were collected from 28 younger and 35 older adults.
As a note, the 2 (age) × 3 (picture category) ANOVA remained significant even after including individuals with scores of 16 or higher on the CES-D (total 63 younger, 42 older adults), F(1, 102) = 6.00, p < .01, ηp 2 = .11. No main effects of age, F(1, 103) = .91, p = .34, ηp 2 = .01, or picture category, F(1, 102) = .7, p = .85, ηp 2 = .00 were found within this dataset, similar to the results found with the sample reported in the paper.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pag
Contributor Information
Michelle C. Feng, Department of Psychology, University of Southern California
Christopher G. Courtney, Department of Psychology, University of Southern California
Mara Mather, School of Gerontology and Department of Psychology, University of Southern California.
Michael E. Dawson, Department of Psychology, University of Southern California
Gerald C. Davison, School of Gerontology and Department of Psychology, University of Southern California
References
- Ben-Zur H. Coping, affect, and aging: The roles of mastery and self-esteem. Personality and Individual Differences. 2002;32:357–372. [Google Scholar]
- Berg KW, Balaban MG. Startle Elicitation: Stimulus parameters, recording techniques, and quantification. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification. Cambridge: Cambridge University Press; 1999. pp. 21–50. [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification. Cambridge: Cambridge University Press; 1999. pp. 157–186. [Google Scholar]
- Carstensen LL, Fung H, Charles S. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motivation and Emotion. 2003;27:103–123. [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology. 2001;80:136–151. [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan D, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal elderly persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dillon DG, LaBar KS. Startle modulation during conscious emotion regulation is arousal-dependent. Behavioral Neuroscience. 2005;119:1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Ehrlichman H, Kuhl SB, Zhu J, Warrenburg S. Startle reflex modulation by pleasant and unpleasant odors in a between-subjects design. Psychophysiology. 1997;34:726–729. doi: 10.1111/j.1469-8986.1997.tb02149.x. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Ross M, Wiegand M, Schryer E. Are the memories of older adults positively biased? Psychology and Aging. 2008;23:297–306. doi: 10.1037/0882-7974.23.2.297. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biological Psychology. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Gruhn D, Scheibe S. Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): Do ratings become more extreme with age? Behavior Research Methods. 2008;40:512–521. doi: 10.3758/brm.40.2.512. [DOI] [PubMed] [Google Scholar]
- Gruhn D, Scheibe S, Baltes PB. Reduced negativity effect in older adults’ memory for emotional pictures: The heterogeneity-homogeneity list paradigm. Psychology and Aging. 2007;22:644–649. doi: 10.1037/0882-7974.22.3.644. [DOI] [PubMed] [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychologica. 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Herbert C, Kissler J. Motivational priming and processing interrupt: Startle reflex modulation during shallow and deep processing of emotional words. International Journal of Psychophysiology. 2010;76:64–71. doi: 10.1016/j.ijpsycho.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson H. Looking while unhappy. Mood-congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19:848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006a;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye tracking study. Psychology and Aging. 2006b;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83:21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 2007;62:208–215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15:208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Ko SG, Lee TH, Yoon HY, Kwon JH, Mather M. How does context affect assessments of facial emotion? The role of culture and age. Psychology and Aging. doi: 10.1037/a0020222. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann U, Gruhn D. Age differences in emotional reactivity: The sample case of sadness. Psychology and Aging. 2005;20:47–59. doi: 10.1037/0882-7974.20.1.47. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Scheibe S, Samanez-Larkin GR, Tsai JL. Replicating the positivity effect in picture memory in Koreans: Evidence for cross-cultural generalizability. Psychology and Aging. 2009;24:748–754. doi: 10.1037/a0016054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C, Hunt WA. The startle pattern. New York: Holt, Rinehart & Winston; 1939. [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:371–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual (A-6) University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Langeslag SJE, van Strien JW. Aging and emotional memory: The co-occurrence of neurophysiological and behavioral positivity effects. Emotion. 2009;9:369–377. doi: 10.1037/a0015356. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008;23:209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: Threat detection is not impaired among older adults. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 2006;61:54–57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: A meta-analysis of memory and attention tasks. Psychology and Aging. 2008;23:263–286. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychology and Aging. 2008;23:585–594. doi: 10.1037/a0013176. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;3:385–401. [Google Scholar]
- Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clinical Gerontologist. 1986;5:119–135. [Google Scholar]
- Rösler A, Ulrich C, Billino J, Sterzer P, Weidauer S, Bernhardt T, Steinmetz H, Frolich L, Kleinschmidt A. Effects of arousing emotional scenes on the distribution of visuospatial attention: Changes with aging and early subcortical vascular dementia. Journal of Neurological Sciences. 2005;229:109–116. doi: 10.1016/j.jns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: recent findings and future trends. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 2010;65:135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DP, Hillman CH, Duley AR. Influences of age on emotional reactivity during picture processing. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 2005;60:49–56. doi: 10.1093/geronb/60.1.p49. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo JA, Braff DL. Startle modulation in Caucasian-Americans and Asian-Americans: a prelude to genetic/endophenotypic studies across the ‘Pacific Rim’. Psychiatric Genetics. 2005;15:61–65. doi: 10.1097/00041444-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rouder JN. Aging and recognition memory for emotional words: A bias account. Psychonomic Bulletin & Review. 2009;16:699–704. doi: 10.3758/PBR.16.4.699. [DOI] [PubMed] [Google Scholar]
- Thorne G, Dawson ME, Schell AM. Effects of perceptual load on startle reflex modification at a long lead interval. Psychophysiology. 2006;43:498–503. doi: 10.1111/j.1469-8986.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Tomaszczyk JC, Fernandes MA, MacLeod CM. Personal relevance modulates the positivity bias in recall of emotional pictures in older adults. Psychonomic Bulletin & Review. 2008;15:191–196. doi: 10.3758/pbr.15.1.191. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;65:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]


